Abstract
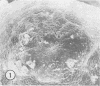
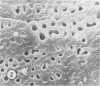
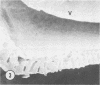
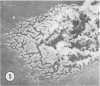
Aerobic heterotrophic and facultative anaerobic bacteria were isolated from all developmental stages of the sugar beet root maggot, Tetanops myopaeformis (von Röder). Two distinct bacterial symbiotic relationships were observed. Serratia liquefaciens and Serratia marcescens were found to be associated with all developmental stages. Bacterial symbiont transmission occurred from one generation to the next. Symbionts were transferred from the male reproductive system to the female reproductive system, where both an internal infiltration of the egg chorion and an external smearing of the eggs occurred during oviposition. Pseudomonas maltophilia was found in association with the larval gut and the inner surface of the puparium. Electron microscopy of the inner puparial surface revealed symbionts within the chitinous wall. In vitro symbiont chitinase production was found, using both nephelometric (turbidimetric) and N-acetylglucosamine assays. A relationship appeared to exist between adult fly emergence and enzymatic chitin degradation of the puparium by the bacterial symbionts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREENBERG B. Persistence of bacteria in the developmental stages of the housefly. III. Quantitative distribution in prepupae and pupae. Am J Trop Med Hyg. 1959 Nov;8:613–617. doi: 10.4269/ajtmh.1959.8.613. [DOI] [PubMed] [Google Scholar]
- HACKMAN R. H. Studies on chitin. I. Enzymic degradation of chitin and chitin esters. Aust J Biol Sci. 1954 May;7(2):168–178. doi: 10.1071/bi9540168. [DOI] [PubMed] [Google Scholar]
- JEUNIAUX C. [Research on chitinases. II. Purification of the chitinase from a streptomycete, and electrophoretic separation of distinct chitinolytic principles]. Arch Int Physiol Biochim. 1959 Oct;67:597–617. doi: 10.3109/13813455909067174. [DOI] [PubMed] [Google Scholar]
- KOCH A. Intracellular symbiosis in insects. Annu Rev Microbiol. 1960;14:121–140. doi: 10.1146/annurev.mi.14.100160.001005. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Bousch G. M., Baerwald R. J. Amino acid synthesis by Pseudomonas melophthora, bacterial symbiote of Rhagoletis pomonella (Diptera). J Insect Physiol. 1968 Apr;14(4):513–518. doi: 10.1016/0022-1910(68)90066-8. [DOI] [PubMed] [Google Scholar]
- Steinhaus E. A. THE MICROBIOLOGY OF INSECTS: With Special Reference to the Biologic Relationships between Bacteria and Insects. Bacteriol Rev. 1940 Mar;4(1):17–57. doi: 10.1128/br.4.1.17-57.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winicur S., Mitchell H. K. Chitinase activity during Drosophila development. J Insect Physiol. 1974 Sep;20(9):1795–1805. doi: 10.1016/0022-1910(74)90209-1. [DOI] [PubMed] [Google Scholar]