Abstract
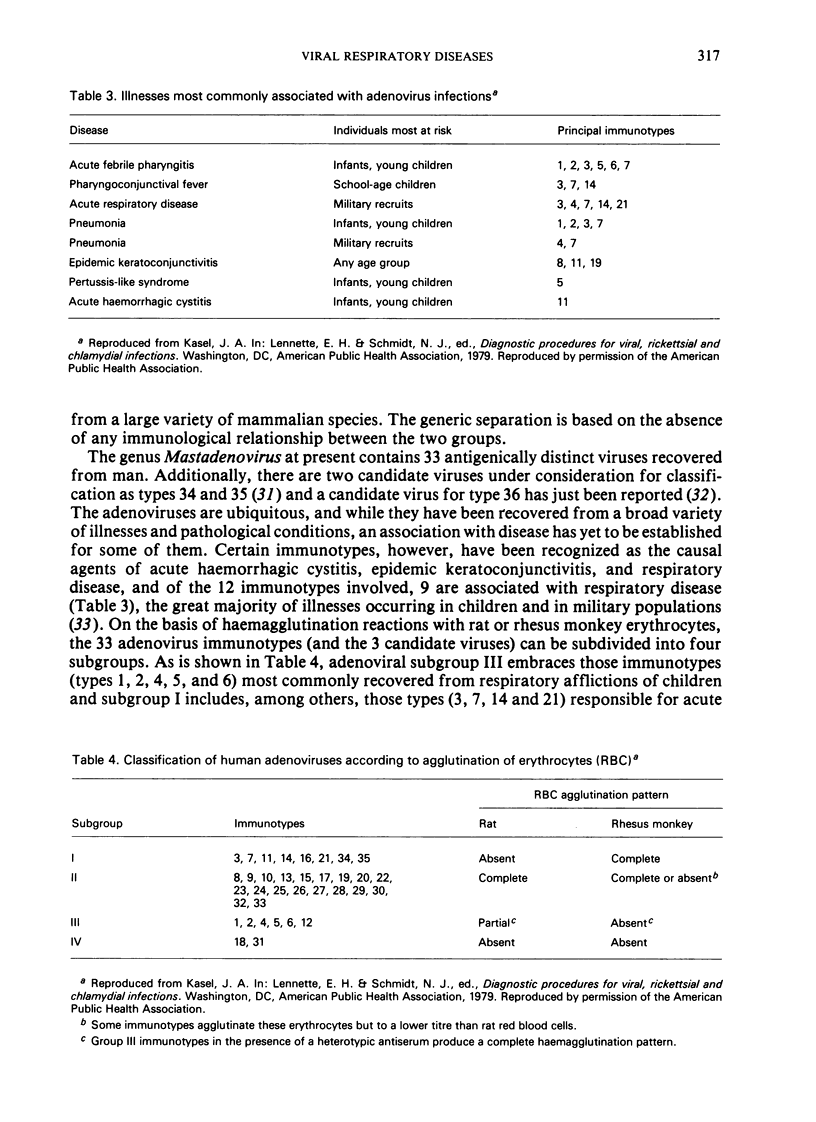
Acute respiratory diseases, most of which are generally attributed to viruses, account for about 6% of all deaths and for about 60% of the deaths associated with all respiratory disease. The huge cost attributable to viral respiratory infections as a result of absenteeism and the disruption of business and the burden of medical care makes control of these diseases an important objective. The viruses that infect the respiratory tract fall taxonomically into five viral families. Although immunoprophylaxis would appear to be the logical approach, the development of suitable vaccines has been confronted with numerous obstacles, including antigenic drift and shift in the influenzaviruses, the large number of antigenically distinct immunotypes among rhinoviruses, the occurrence after immunization of rare cases of a severe form of the disease following subsequent natural infection with respiratory syncytial virus, and the risk of oncogenicity of adenoviruses for man. Considerable expenditure on the development of new antiviral drugs has so far resulted in only three compounds that are at present officially approved and licensed for use in the USA. Efforts to improve the tools available for control should continue and imaginative and inventive approaches are called for. However, creativity and ingenuity must operate within the constraints imposed by economic, political, ethical, and legal considerations.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean W. J., Jr, Cox N. J., Kendal A. P. Recombination of human influenza A viruses in nature. Nature. 1980 Apr 17;284(5757):638–640. doi: 10.1038/284638a0. [DOI] [PubMed] [Google Scholar]
- Buynak E. B., Weibel R. E., Carlson A. J., McLean A. A., Hilleman M. R. Further investigations of live respiratory syncytial virus vaccine administered parenterally. Proc Soc Exp Biol Med. 1979 Feb;160(2):272–277. doi: 10.3181/00379727-160-40433. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Murphy B. R. Genetic approaches to control of influenza. Perspect Biol Med. 1979 Winter;22(2 Pt 2):S37–S48. [PubMed] [Google Scholar]
- Craighead J. E. Report of a workshop: disease accentuation after immunization with inactivated microbial vaccines. J Infect Dis. 1975 Jun;131(6):749–754. doi: 10.1093/infdis/131.6.749. [DOI] [PubMed] [Google Scholar]
- DeLong D. C., Reed S. E. Inhibition of rhinovirus replication in in organ culture by a potential antiviral drug. J Infect Dis. 1980 Jan;141(1):87–91. doi: 10.1093/infdis/141.1.87. [DOI] [PubMed] [Google Scholar]
- Dowdle W. R., Millar J. D., Schonberger L. B., Ennis F. A., LaMontagne J. R. Influenza immunization policies and practices in Japan. J Infect Dis. 1980 Feb;141(2):258–264. doi: 10.1093/infdis/141.2.258. [DOI] [PubMed] [Google Scholar]
- Glezen W. P., Paredes A., Taber L. H. Influenza in children. Relationship to other respiratory agents. JAMA. 1980 Apr 4;243(13):1345–1349. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- Gross P. A., Ennis F. A., Noble G. R., Gaerlan P. F., Davis W. J., Denning C. E. Influenza vaccine in unprimed children: improved immunogenicity with few reactions following one high dose of split-product vaccine. J Pediatr. 1980 Jul;97(1):56–60. doi: 10.1016/s0022-3476(80)80130-2. [DOI] [PubMed] [Google Scholar]
- Hall W. J., Hall C. B., Speers D. M. Respiratory syncytial virus infection in adults: clinical, virologic, and serial pulmonary function studies. Ann Intern Med. 1978 Feb;88(2):203–205. doi: 10.7326/0003-4819-88-2-203. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Hall W. J., Douglas R. G., Jr Therapeutic effects of aerosolized amantadine in naturally acquired infection due to influenza A virus. J Infect Dis. 1980 May;141(5):535–542. doi: 10.1093/infdis/141.5.535. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Clyde W. A., Jr, Denny F. W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979 Mar 8;300(10):530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- Hierholzer J. C., Hirsch M. S. Croup and pneumonia in human infants associated with a new strain of respiratory syncytial virus. J Infect Dis. 1979 Nov;140(5):826–828. doi: 10.1093/infdis/140.5.826. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson R. E. Epidemic mechanisms of type A influenza. J Hyg (Lond) 1979 Aug;83(1):11–26. doi: 10.1017/s002217240002578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. Ecology of influenza viruses in lower mammals and birds. Br Med Bull. 1979 Jan;35(1):29–33. doi: 10.1093/oxfordjournals.bmb.a071537. [DOI] [PubMed] [Google Scholar]
- Mathur A., Beare A. S., Reed S. E. In vitro antiviral activity and preliminary clinical trials of a new adamantane compound. Antimicrob Agents Chemother. 1973 Oct;4(4):421–426. doi: 10.1128/aac.4.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur U., Bentley D. W., Hall C. B. Concurrent respiratory syncytial virus and influenza A infections in the institutionalized elderly and chronically ill. Ann Intern Med. 1980 Jul;93(1):49–52. doi: 10.7326/0003-4819-93-1-49. [DOI] [PubMed] [Google Scholar]
- McClelland A. J. Vaccination against paramyxoviruses. Nature. 1980 Apr 3;284(5755):404–404. doi: 10.1038/284404a0. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Suffin S. C., Prevar D. A., Camargo E., Sly D. L., London W. T., Chanock R. M. Respiratory syncytial virus infection in owl monkeys: viral shedding, immunological response, and associated illness caused by wild-type virus and two temperature-sensitive mutants. Infect Immun. 1979 Dec;26(3):1009–1013. doi: 10.1128/iai.26.3.1009-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Charette R. P., Fox J. P., Cooney M. K., Hall C. E. Lack of effect of oral ribavirin in naturally occurring influenza A virus (H1N1) infection. J Infect Dis. 1980 May;141(5):548–554. doi: 10.1093/infdis/141.5.548. [DOI] [PubMed] [Google Scholar]
- Varma R. R., Riedel D. R., Komorowski R. A., Harrington G. J., Nowak T. V. Reye's syndrome in nonpediatric age groups. JAMA. 1979 Sep 28;242(13):1373–1375. [PubMed] [Google Scholar]
- Wigand R., Gelderblom H., Wadell G. New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol. 1980;64(3):225–233. doi: 10.1007/BF01322702. [DOI] [PubMed] [Google Scholar]
- Young J. F., Palese P. Evolution of human influenza A viruses in nature: recombination contributes to genetic variation of H1N1 strains. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6547–6551. doi: 10.1073/pnas.76.12.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]


