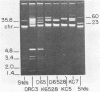
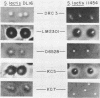
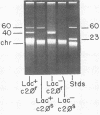
Abstract
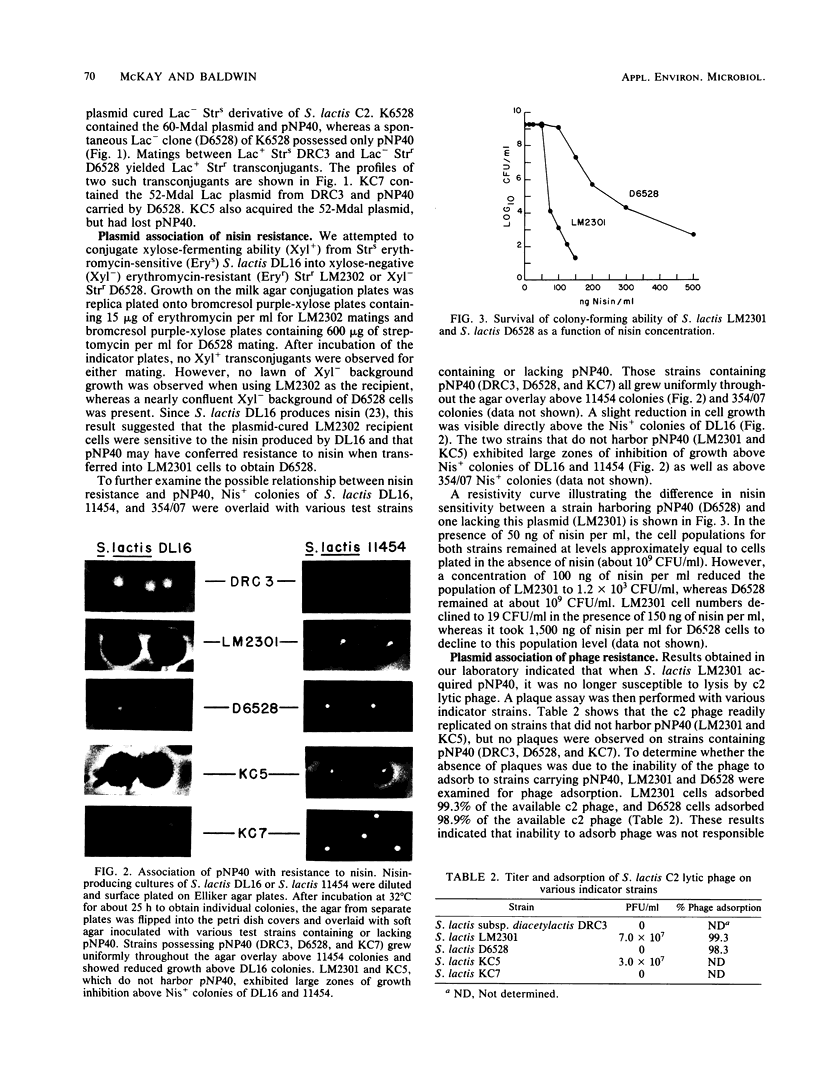
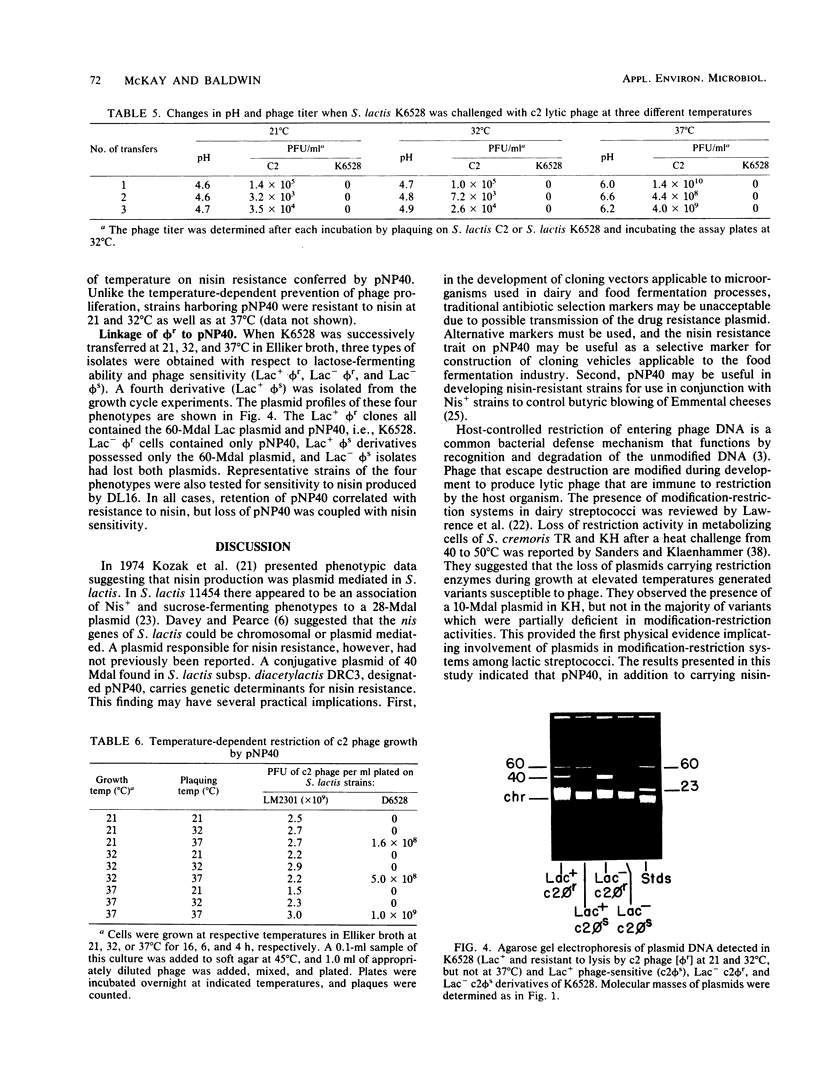
Streptococcus lactis subsp. diacetylactis DRC3 was examined for plasmid DNA and found to contain a previously unreported plasmid of 40 X 10(6) daltons. This plasmid, designated pNP40, was conjugally transferred to a plasmid-cured derivative of S. lactis C2. Transconjugants containing pNP40 acquired resistance to nisin produced by strains of S. lactis and to commercially available nisin when assay plates were incubated at 21, 32, and 37 degrees C. In addition, c2 phage growth was completely restricted in transconjugants containing pNP40 at 21 and 32 degrees C, but not at 37 degrees C. This result suggests that pNP40 may be coding for a temperature-sensitive enzyme that restricts phage growth at 21 and 32 degrees C, but not at 37 degrees C. Eight consecutive transfers of a transconjugant containing pNP40 in Elliker broth at 37 degrees C resulted in 100% loss of resistance to c2 phage when colonies were tested at 32 degrees C. These phage-sensitive isolates had lost pNP40 and had also become sensitive to nisin. This result suggests that pNP40 may also be thermosensitive in its replication. The finding of a phage resistance determinant located on a conjugative plasmid should prove useful in constructing phage-resistant variants for dairy fermentation processes.
Full text
PDF
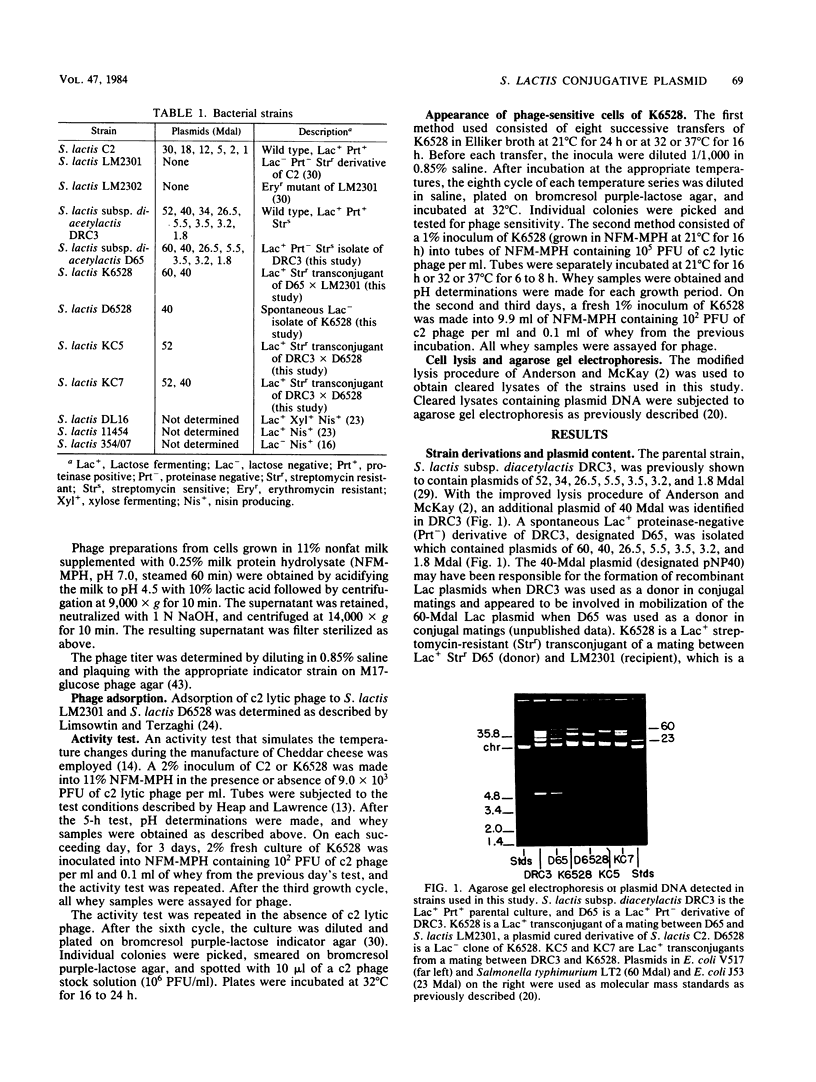



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Davey G. P., Pearce L. E., Thomas T. D. Plasmid linkage of the D-tagatose 6-phosphate pathway in Streptococcus lactis: effect on lactose and galactose metabolism. J Bacteriol. 1983 Jan;153(1):76–83. doi: 10.1128/jb.153.1.76-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies F. L., Gasson M. J. Reviews of the progress of dairy science: genetics of lactic acid bacteria. J Dairy Res. 1981 Jun;48(2):363–376. doi: 10.1017/s0022029900021798. [DOI] [PubMed] [Google Scholar]
- DiJoseph C. G., Kaji A. Change in the cell envelope of Escherichia coli carrying the thermosensitive drug resistance factor, Rts 1, at the nonpermissive temperature. Antimicrob Agents Chemother. 1975 Oct;8(4):504–509. doi: 10.1128/aac.8.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G. The thermosensitive lesion in the replication of the drug resistance factor, Rts1. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2515–2519. doi: 10.1073/pnas.71.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. G., Zajdel J., Dobrzański W. T. Possible plasmid nature of the determinant for production of the antibiotic nisin in some strains of Streptococcus lactis. J Gen Microbiol. 1975 May;88(1):189–192. doi: 10.1099/00221287-88-1-189. [DOI] [PubMed] [Google Scholar]
- Ishaq M., Kaji A. Mechanism of T4 phage restriction by plasmid Rts 1. Cleavage of T4 phage DNA by Rts 1-specific enzyme. J Biol Chem. 1980 May 10;255(9):4040–4047. [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar W., Rajchert-Trzpil M., Dobrzański W. T. The effect of proflavin, ethidium bromide and an elevated temperature on the appearance of nisin-negative clones in nisin-producing strains of Streptococcus lactis. J Gen Microbiol. 1974 Aug;83(2):295–302. doi: 10.1099/00221287-83-2-295. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Induction of prophage in Streptococcus lactis C2 by ultraviolet irradiation. Appl Microbiol. 1973 Apr;25(4):682–684. doi: 10.1128/am.25.4.682-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., de Vos W. M., Gavrieli J. Plasmid DNA in Streptococcus cremoris Wg2: Influence of pH on Selection in Chemostats of a Variant Lacking a Protease Plasmid. Appl Environ Microbiol. 1982 Jun;43(6):1272–1277. doi: 10.1128/aem.43.6.1272-1277.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. H., McKay L. L. Distinct galactose phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus lactis. J Bacteriol. 1982 Feb;149(2):420–425. doi: 10.1128/jb.149.2.420-425.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl Environ Microbiol. 1980 Sep;40(3):500–506. doi: 10.1128/aem.40.3.500-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherwitz K. M., Baldwin K. A., McKay L. L. Plasmid linkage of a bacteriocin-like substance in Streptococcus lactis subsp. diacetylactis strain WM4: transferability to Streptococcus lactis. Appl Environ Microbiol. 1983 May;45(5):1506–1512. doi: 10.1128/aem.45.5.1506-1512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]