Abstract
Background and Purpose
Transient ischemic attacks have long been regarded as a risk factor for the incidence of stroke but may reduce the severity of stroke by inducing ischemic tolerance. The present objective was to develop an ischemic preconditioning (IPC) model of delayed tolerance in the mouse based on repetitive, transient middle cerebral artery occlusion (MCAO).
Methods
Mice anesthetized with halothane or isoflurane underwent IPC, which consisted of repetitive MCAO at 45-minute intervals by the intraluminal filament technique. A 90-minute test MCAO was performed 24 to 96 hours later.
Results
Using an IPC of 2 5-minute MCAO episodes, the reduction in infarct volume from the test MCAO was maximal with a 72-hour delay in striatum (70%) and cerebral cortex (64%) when halothane was used for surgical anesthesia. With isoflurane anesthesia, the reduction in infarct volume was less prominent in striatum (34%) and not significant in cortex (9%) despite similar levels of arterial pressure and decreases in cortical perfusion. Neuronal cell death was rare 6 days after this IPC stimulus alone with halothane or isoflurane. Increasing the severity of IPC to 3 5-minute bouts or 1 15-minute bout of MCAO in the presence of isoflurane anesthesia augmented the reduction in infarct volume in striatum and cortex, but it also augmented selective neuronal cell death in striatum after the IPC stimulus alone.
Conclusions
These data demonstrate that a repetitive focal IPC stimulus can be titrated to induce delayed tolerance in both striatum and cortex of the mouse without inducing neuronal death by itself.
Keywords: anesthesia, ischemic preconditioning, ischemic tolerance, middle cerebral artery, transient ischemic attack
Ischemic preconditioning (IPC) confers delayed tolerance over a period of a few days.1 Understanding which proteins are upregulated or downregulated during delayed tolerance can provide insight into the mechanisms of ischemic injury and provide therapeutic targets.2 Also, IPC may be of clinical relevance for transient ischemic attacks (TIAs). Although TIAs may be associated with increased risk of a subsequent stroke, a TIA occurring before a stroke could render delayed tolerance.3,4 Short episodes of IPC stimuli may mimic some of the characteristics of clinical TIA and lead to delayed tolerance.
Animal models of IPC often use global conditioning stimuli, such as systemic hypoxia or common carotid artery occlusion, followed by a focal ischemic test.5,6 Limited work has been done with focal IPC localized to the same region as the subsequent ischemic stroke.7 In rat, a focal model of repetitive IPC in which the middle cerebral artery (MCA) was occluded for 3 10-minute bouts with 45-minute reperfusion intervals was found to produce delayed tolerance to 100 to 120 minutes of MCA occlusion (MCAO) 72 hours later.8,9 Because of the availability of genetically altered mouse strains, models of focal IPC in the mouse are useful. For example, rapid tolerance dependent on nitric oxide has been described in the mouse using 3 5-minute periods of MCAO 30 minutes before permanent MCAO.10,11 Moreover, the delayed genomic response to IPC induced 72 hours after a single 15-minute period of MCAO has been described in the mouse.2 Although it is usually stated that the conditioning stimulus is sublethal, brief periods of focal ischemia can produce delayed neuronal death.12,13 Indeed, 3 10-minute bouts of MCAO in the rat or 3 5-minute bouts of MCAO in the mouse has been reported to produce delayed death of striatal neurons.9,10 The purpose of the present study was to develop a model of delayed tolerance in the mouse using a repetitive focal ischemic conditioning stimulus that by itself did not produce neuronal injury. Volatile anesthetics were used during the conditioning and test stimuli to permit rapid recovery after anesthesia and restoration of cardiovascular regulation. Because 3- to 4-hour exposure to halothane or isoflurane without ischemia can provide tolerance to focal cerebral ischemia induced 1 day later,14,15 both halothane and isoflurane anesthesia were evaluated as anesthetics during induction of IPC and compared with groups preconditioned with anesthesia alone or anesthesia plus sham surgery.
Materials and Methods
All experiments were conducted in accordance with the guidelines of the National Institutes of Health for the care and use of animals in research and were approved by the institutional animal care and use committee. Anesthesia was induced in ≈200 male C57BL/6 mice (20 to 28 g; 8 to 10 weeks old; Charles River Laboratories; Wilmington, Del) with either 5% halothane or 5% isoflurane. When unconscious, the anesthetic was administered at a nominal concentration of 2% in 25% to 30% O2 via face mask with spontaneous ventilation for a brief period during incision of the skin and isolation of the carotid artery. Anesthesia was then maintained with a nominal inspired concentration of either 1% halothane or 1.2% isoflurane for the remainder of the procedure. The concentration was increased if spontaneous movement occurred or ventilatory frequency increased. The duration of anesthesia was the same for groups exposed to halothane or isoflurane. Rectal temperature was maintained at ≈37°C during the surgery. The intraluminal filament model of MCAO was used for both the conditioning and test ischemia. A laser-Doppler flow (LDF) probe was secured on the skull over the lateral parietal cortex for monitoring perfusion in the ischemic cortical core. The right common carotid artery (CCA) was exposed through a 15-mm submandibular midline incision. The external carotid artery (ECA) was carefully separated from the adjacent vagus nerve and muscle to prepare a site for coagulation and division located 3 to 5 mm distal to the CCA bifurcation. The proximal end of the CCA was temporarily ligated using a 6−0 suture. A 7−0 nylon monofilament with a silicone-coated tip was inserted from the distal end of the isolated ECA, gently introduced into the internal carotid artery (ICA), and advanced approximately 6 to 8 mm past the CCA bifurcation to the origin of the MCA. Adequacy of MCAO was confirmed by LDF monitoring. Reperfusion of the MCA was achieved by withdrawal of the filament into the stump of the ECA and release of the CCA ligature. The neck incision was closed with suture, and anesthesia was discontinued.
Preconditioning MCAO
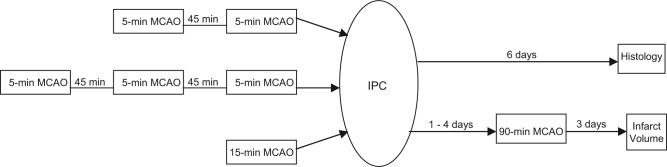
Preconditioning stimuli included (1) a single 15-minute period, (2) 3 periods of 5-minute duration, or (3) 2 periods of 5-minute duration (Figure 1). The interval of reperfusion between repetitive MCAOs was 45 minutes, as has been used in the rat.8,9 The mice remained anesthetized during the reperfusion periods between bouts of MCAO. In surgical sham preconditioning groups, mice underwent the same duration of anesthesia and the same surgical procedures that were performed in the IPC groups, with the exception that the intraluminal filament was not advanced to the origin of the MCA. In anesthesia-preconditioned groups, mice were exposed to the same anesthetic concentrations for the same duration (total of 90 minutes) used in the IPC experiments (but without undergoing surgery) 3 days before the test MCAO.
Figure 1.
Schematic diagram of timing for different ischemic preconditioning (IPC) protocols and test middle cerebral artery occlusion (MCAO).
Test MCAO
Under the same anesthetic that was used for preconditioning, the neck incision was reopened 1 to 4 days later, the CCA was ligated, and the suture was reintroduced into the ICA from the previous ECA arteriotomy by loosening the silk suture. The filament was advanced to the origin of the MCA to produce 90 minutes of MCAO. After the decrease in LDF was monitored for 10 minutes, the incision was closed, anesthesia was discontinued, and neurologic deficit was assessed. To establish reperfusion, the mouse was briefly anesthetized, the monofilament was withdrawn, and the CCA ligature was removed. In addition to preconditioning groups, naïve groups underwent the 90-minute test MCAO only (without prior surgery or anesthesia). Neurologic deficits were scored 3 days after the test MCAO on a 0 to 4 scale.11
Analysis of Injury
Because a reduction in infarct volume seen at 1 day after the test MCAO may not be sustained at 3 days,9 infarct volume was evaluated 3 days after the test MCAO by standard volumetric analysis of anterior and posterior views of 5 coronal slabs stained with triphenyltetrazolium chloride (TTC), with correction for swelling.
Injury from IPC alone was evaluated 6 days after the conditioning stimulus in separate cohorts by TTC staining, hematoxylin and eosin (H&E) staining, or terminal transferase-mediated biotin-dUTP nick-end labeling (TUNEL) staining. The 6-day period was chosen to correspond to a 3-day interval between the conditioning and test stimuli plus the 3-day interval for reperfusion after the test MCAO. Paraffin-embedded coronal sections (8 μm), fixed with paraformaldehyde and stained with H&E, were observed under oil immersion microscopy for eosinophilic cytoplasm and shrunken, pyknotic nuclei. For quantification of cells undergoing cell death in additional cohorts, deparaffinized sections were TUNEL stained using diaminobenzidine as a chromogen. Positive cell counts were measured in 5 microscopic fields at 200 power in both lateral frontoparietal cortex and striatum on 3 coronal sections (bregma 1.7 mm, 0.7 mm and to 1.3 mm) by an observer who was not aware of the treatment group. At this power, most of the structures of interest were covered by the 5 fields.
Statistical Analysis
Statistical analysis used analysis of variance. Post hoc comparisons with the respective sham groups were made by applying the false discovery rate procedure for multiple t tests.16 Differences of P<0.05 were considered to be significant. All data are presented as mean±SEM.
Results
Outcome With Conditioning MCAO Only
Different preconditioning paradigms were screened with TTC staining (n=6 per group) to determine whether IPC alone caused injury. Mice subjected to a single episode of 15 minutes of MCAO and recovered for 6 days showed pale staining with TTC in ipsilateral striatum compared with contralateral striatum when either halothane or isoflurane (Figure 2A) anesthesia was used. Staining was bilaterally symmetric in the cerebral cortex. Preliminary experiments with 3 bouts of MCAO of 10-minute duration under halothane also produced pale TTC staining in ipsilateral striatum (not shown). When the duration of the 3 bouts of MCAO was reduced to 5 minutes, striatal infarcts were not observed, but the staining remained lighter on the ipsilateral side with either halothane or isoflurane anesthesia (Figure 2B). In contrast, differences between ipsilateral and contralateral TTC staining could not be detected by visual inspection 6 days after 2 bouts of 5 minutes of MCAO with either halothane or isoflurane anesthesia (Figure 2C). To verify the results of TTC staining, analysis of H&E-stained sections was performed (n=3 per group). At 6 days after 1 bout of 15 minutes of MCAO (Figure 2D) or 3 bouts of 5 minutes of MCAO (Figure 3E), eosinophilic cells with pyknotic nuclei were present in striatum. In lateral cerebral cortex, neurons with dark eosinophilic staining but normal-sized nuclei were occasionally observed, but neurons with shrunken cytoplasm and pyknotic nuclei were scarce. After 2 bouts of 5-minute MCAO, normal cytomorphologic staining of neurons was observed with normal-sized nuclei in both striatum (Figure 2F) and cortex. Cells with positive TUNEL staining were evident in striatum 6 days after 1 bout of 15 minutes of MCAO (Figure 2G) or 3 bouts of 5 minutes of MCAO (Figure 2H) but were rare after 2 bouts of 5 minutes of MCAO (Figure 2I). In cerebral cortex, few neurons were TUNEL-positive with any of the preconditioning (Figure 2J). TUNEL staining did not differ between halothane and isoflurane groups (Figure 2J). Therefore, 2 episodes of 5 minutes of MCAO were selected as the preconditioning stimulus in subsequent experiments.
Figure 2.
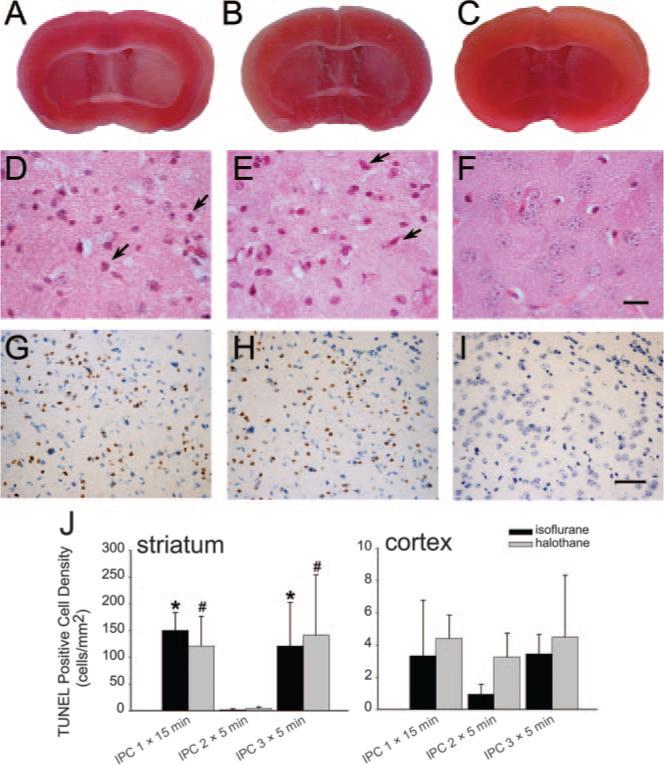
Brain slices stained with triphenyltetrazolium chloride 6 days after 1 15-minute period (A) and 3 5-minute periods (B) of middle cerebral artery occlusion (MCAO) show lighter staining in striatum on ischemic side (right) compared with nonischemic side (left), but no asymmetry in staining was observed after two 5-minute periods of MCAO (C). Striatal sections stained with hematoxylin-eosin show some eosinophilic cells with shrunken, pyknotic nuclei (arrows) 6 days after 1 15-minute period of MCAO (D) or 3 5-minute periods of MCAO (E) but intact neurons after 2 5-minute periods of MCAO (F; scale bar=10 μm). Striatal sections stained for TUNEL (brown) and cresyl violet show TUNEL-positive cells 6 days after 1 15-minute period of MCAO (G) or 3 5-minute periods of MCAO (H), but not after 2 5-minute periods of MCAO (I; scale bar=40 μm). All examples in A to I were from isoflurane groups. Similar results were seen in halothane groups. J, Mean±SEM of TUNEL-positive cell density (n=4 in halothane IPC 2X5 minutes and IPC 3X5 minutes; n=3 in other groups) in striatum and cortex (note scale difference). P<0.05 from IPC 2×5 minutes for isoflurane (*) or halothane (#).
Figure 3.
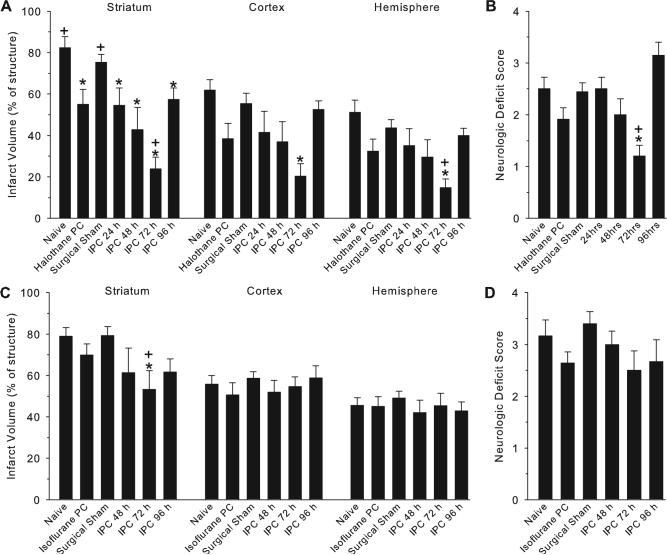
Infarct volume (±SEM) in striatum, cortex, and hemisphere in groups with halothane (A) and isoflurane (C) anesthesia and neurologic deficit scores in groups with halothane (B) and isoflurane (D) anesthesia. Measurements were made 3 days after 90 minutes of MCAO in groups with no prior surgery (naïve), prior anesthesia alone, sham surgery, or IPC, consisting of 2 5-minute periods of MCAO conducted at either 24, 48, 72, or 96 hours before the test MCAO. *P<0.05 from sham surgical group, +P<0.05 from anesthetic preconditioning group.
Effect of Interval Between Conditioning and Test MCAO
Overall mortality was 8.8% from the IPC surgical procedure and 6.9% from the test MCAO. Infarct volume measured 3 days after 90 minutes of MCAO in mice that underwent sham preconditioning surgery (n=9 halothane; n=10 isoflurane) was similar to naïve mice (n=6) without prior surgery when either halothane (Figure 3A) or isoflurane (Figure 3C) anesthesia was used. With 2 episodes of 5 minutes of MCAO performed under halothane anesthesia as the IPC stimulus, infarct volume in striatum was significantly decreased from the sham surgical group when the interval between the conditioning and test MCAO was 1 (n=6), 2 (n=7), 3 (n=10), or 4 (n=7) days (Figure 3A). The minimum striatal infarct was seen with a 3-day interval. In cerebral cortex and the entire hemisphere, infarct volume was significantly decreased only in the 3-day-interval group.
Preconditioning with halothane alone (n=11) 3 days before the test MCAO reduced infarct volume in striatum compared with surgical sham and naïve groups, but infarct volume remained significantly greater than that seen in the 3-day-interval IPC group. Preconditioning with halothane alone did not produce a statistically significant effect on cortical or hemispheric infarcts.
With 2 episodes of 5 minutes of MCAO performed under isoflurane anesthesia, infarct volume in striatum was significantly decreased when the interval between the conditioning and test MCAO was 3 days (n=6) but not when the interval was 2 (n=6) or 4 (n=6) days (Figure 3C). However, no significant reduction in infarct volume was seen in cerebral cortex or the entire hemisphere. Preconditioning with isoflurane alone (n=11) had no effect.
The neurologic deficit score at 3 days after the test MCAO was significantly improved in the IPC halothane group with a 3-day interval between the IPC and test MCAO, but not in the remaining groups (Figure 3B).
Physiological Effects of IPC
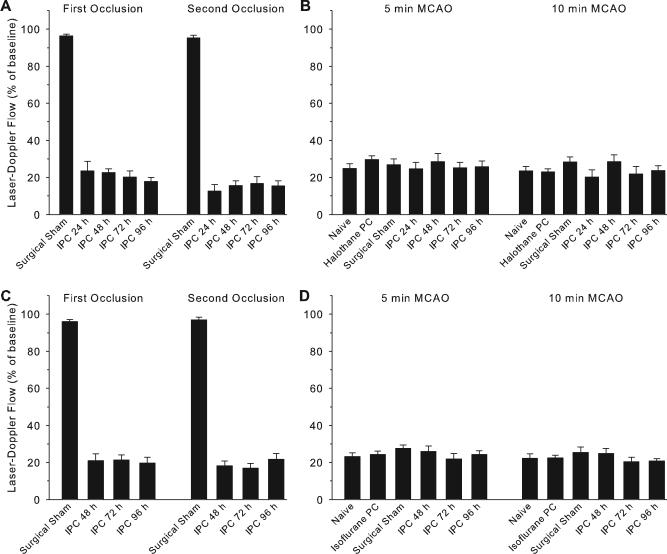
During the first and second MCAO conditioning episodes, LDF over the lateral cortex decreased to approximately 20% of baseline in each of the groups with different intervals between the conditioning and test stimuli (Figure 4). The decrease in LDF at 5 and 10 minutes of the test MCAO was not different from that of the sham preconditioning group. Moreover, the decreases in LDF were similar under halothane and isoflurane anesthesia.
Figure 4.
Laser-Doppler flow (±SEM) measured as a percent of baseline over lateral cortex in groups with halothane (A, B) and isoflurane (C, D) anesthesia. Panels A and C show measurements made during the first and second 5-minute MCAO of the IPC stimulus or equivalent times in sham groups. Panels C and D show measurements made at 5 and 10 minutes of the test MCAO conducted in mice with no prior surgery (naïve), prior anesthesia alone, sham surgery, or IPC at either 24, 48, 72, or 96 hours before the test MCAO.
To determine whether the enhanced tolerance seen under halothane anesthesia could have been attributed to a lower arterial pressure leading to a more severe IPC stimulus, arterial pressure and blood gases were monitored in separate cohorts during 2 bouts of 5 minutes of MCAO. Mean arterial blood pressure, blood gases, pH, hemoglobin concentration, and glucose concentration were not different between halothane (n=6) and isoflurane (n=6) groups at baseline or after the second occlusion (Table). Changes in LDF during MCAO and reperfusion were also similar between anesthetic groups.
Table.
Arterial Blood Analysis and Temperature During Baseline and 5-Minute Middle Cerebral Artery Occlusion in Mice Anesthetized With Halothane or Isoflurane
| Halothane | Isoflurane | |
|---|---|---|
| Laser-Doppler flow, % of baseline | ||
| First occlusion | 22±3 | 21±2 |
| First reperfusion | 108±7 | 102±5 |
| Second occlusion | 20±3 | 20±2 |
| Second reperfusion | 107±5 | 100±6 |
| Mean arterial pressure, mm Hg | ||
| Baseline | 77±1.5 | 78±1.6 |
| Second reperfusion | 78±1.1 | 78±1.2 |
| PaCO2, mm Hg | ||
| Baseline | 41.8±1.1 | 38.8±1.8 |
| Second reperfusion | 45.0±1.6 | 43.2±1.8 |
| PaO2, mm Hg | ||
| Baseline | 139±5 | 134±4 |
| Second reperfusion | 137±6 | 125±2 |
| pH | ||
| Baseline | 7.33±0.01 | 7.38±0.02 |
| Second reperfusion | 7.30±0.01 | 7.32±0.03 |
| Glucose, mg/dL | ||
| Baseline | 131±4 | 127±6 |
| Second reperfusion | 133±5 | 123±7 |
| Hemoglobin, g/dL | ||
| Baseline | 13.6±0.6 | 13.2±0.4 |
| Second reperfusion | 12.7±0.6 | 11.8±0.3 |
| Rectal temperature, °C | 36.9±0.1 | 37.0±0.1 |
Data are mean±SEM (n=6).
Comparison With Other IPC Stimuli
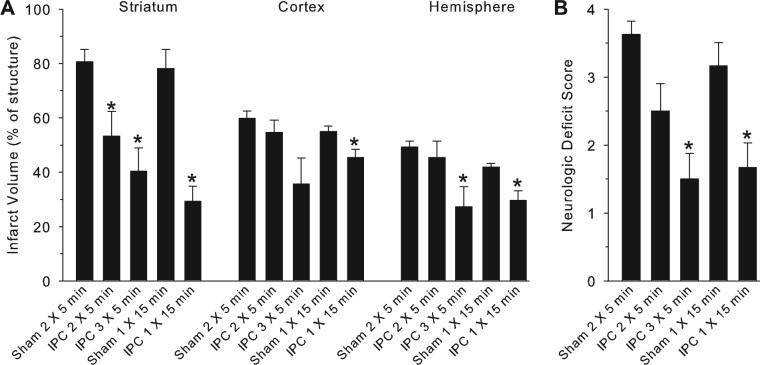
Because the 2 bouts of 5 minutes of MCAO produced only moderate tolerance when isoflurane was used, comparisons were made with more severe IPC stimuli to determine whether greater tolerance was feasible using MCAO as a conditioning stimulus in the presence of isoflurane. With 2 bouts of 5 minutes of MCAO (n=8), results were similar to the previous cohort with isoflurane anesthesia; infarct volume was reduced in striatum but not in cortex compared with a surgical sham group (n=8). Increasing the IPC to 3 bouts of 5 minutes of MCAO (n=6) produced significant reductions in infarct volume in the striatum and entire hemisphere, a marginal reduction in cortex (P<0.06), and improved deficit scores (Figure 5). To test the effect of a single bout of 15 minutes of MCAO, a separate surgical sham group (n=6) was performed because this procedure required a shorter duration of anesthesia. Infarct volumes were similar in the 2 sham groups. A single bout of 15 minutes of MCAO as an IPC stimulus (n=6) produced substantial reduction in infarct volume in striatum, a moderate reduction in cortex, and improved deficit scores (Figure 5).
Figure 5.
Infarct volume (A) in striatum, cortex, and hemisphere and neurologic deficit score (B) 3 days after 90 minutes of MCAO in groups with IPC consisting of 2 or 3 5-minute periods of MCAO or 1 15-minute period of MCAO conducted 72 hours before the test MCAO. Separate sham groups were conducted for the repetitive IPC and single IPC groups because of different anesthetic durations. Isoflurane anesthesia was used for all groups. *P<0.05 from respective sham group.
Discussion
The major finding of this study is that repetitive focal ischemic preconditioning stimuli can be titrated in the mouse to produce delayed ischemic tolerance without producing neuronal injury by itself. Two occlusions of the MCA for 5-minute durations were sufficient to reduce infarct size from a subsequent occlusion of the MCA for 90-minute duration. Tolerance was maximal with a 3-day interval between the preconditioning and test stimuli and was prominent in highly vulnerable striatum. Unexpectedly, protection was greater with the use of halothane than with isoflurane during each surgical procedure.
Time Delay
Rapid tolerance to focal cerebral ischemia has been demonstrated with 3 5-minute bouts of focal cerebral ischemia as a conditioning stimulus in the mouse.10,11 With 3 1-minute periods of bilateral carotid occlusion in the mouse, delayed tolerance to subsequent MCAO for 20 minutes was present with a 1-day delay and lost with a 3-day delay.5 In the present model with 2 5-minute periods of MCAO, delayed tolerance to subsequent MCAO for 90 minutes was maximal with a 3-day delay and diminished with a 4-day delay. Others using a single period of 15 minutes of MCAO in the mouse2 or 3 10-minute periods of MCAO in the rat8 also found tolerance to subsequent MCAO after a 3-day delay, and the tolerance induced by 3 10-minute periods of MCAO or 3 3-minute periods of MCAO in the rat was also maximal at 3 days.9,17 Bilateral carotid occlusion was reported to decrease LDF to approximately 10% of baseline,5 whereas LDF decreased to approximately 20% of baseline in the ischemic core in our model. Most likely, the delay in altered protein expression responsible for tolerance depends on the severity of the conditioning stimulus and regional location of the stimulus.
Regional Tolerance
Studies of IPC have often not separately quantified striatal and cortical tolerance to subsequent MCAO, although tolerance in cortex has been reported to be more prominent than in striatum.2,9 Because MCAO is generally thought to produce more severe ischemia in striatum,9 less protection in striatum by IPC may be attributed to the greater ischemic insult during the test MCAO. However, tolerance to 90 minutes of MCAO in the present study was found to be robust in striatum with each of the conditioning stimuli, including 2 or 3 bouts of 5 minutes of MCAO or a single bout of 15 minutes of MCAO. Robust striatal protection to 2 hours of MCAO was also found in the rat with an IPC of 3 10-minute bouts of MCAO.8 Thus, IPC can induce tolerance in the striatum to subsequent transient MCAO that would normally injure 80% of striatum. However, longer durations of the test MCAO were not evaluated to determine when tolerance might be lost. Another limitation is that infarction may expand beyond the 3-day assessment timepoint selectively in IPC groups.
In cerebral cortex, infarct volume was reduced by as much as 64% with 2 5-minute bouts of MCAO under halothane anesthesia and a 3-day delay. This reduction in cortical infarct volume was comparable to the 70% reduction seen in striatum. However, cortex was not significantly protected by 2 5-minute bouts of MCAO under isoflurane anesthesia. Increasing the severity of IPC under isoflurane anesthesia produced marginal cortical protection with 3 5-minute bouts and significant cortical protection with 1 15-minute bout. The ability to induce tolerance in a specific region likely depends on using a conditioning stimulus that maintains a balance between being sufficiently severe to induce a specific pattern of proteins and not inducing cell death by itself. The severity of ischemia during the various IPC challenges may have been greater in striatum than in cortex and may have consequently led to a greater alteration of protein expression.
Effect of Anesthetics
The tolerance evoked by 2 bouts of 5 minutes of MCAO was greater when halothane was used during the IPC and test MCAO than when isoflurane was used. We considered the possibility that halothane may have generated a more severe IPC insult by producing greater hypotension than isoflurane. However, measurement of arterial blood pressure and blood gases in separate nonsurvival cohorts of mice did not reveal any significant differences between the two anesthetics. The percent decrease in cortical LDF was also equivalent with the 2 anesthetics during each IPC occlusion and during the test occlusion. Therefore, the present findings do not indicate a difference in the severity of the IPC insult. In spontaneously hypertensive rats, part of the IPC-induced tolerance has been attributed to improvement in blood flow over a period of several hours of permanent MCAO.18 In the present model with transient MCAO, the decrease in LDF over lateral cortex in the ischemic core during the test MCAO was similar to that during each IPC occlusion. Although improved blood flow distribution in penumbra by IPC cannot be excluded, differences in intraischemic regional blood flow determined by autoradiography could not be detected during acute occlusion in rats previously conditioned with 3 10-minute bouts of MCAO.8,9
Exposure to volatile anesthetics without surgery can produce a form of chemical preconditioning. For example, exposure of rats to isoflurane for 3 hours produced tolerance to permanent MCAO induced within 24 hours.14 Furthermore, exposure of male mice, but not female mice, to isoflurane for 4 hours produced tolerance to 2 hours of transient MCAO 24 hours later.15 In the present study on male mice, preconditioning with isoflurane alone or with sham surgery had no effect on infarct volume compared with the naïve group with no anesthetic or surgical preconditioning. The 90-minute isoflurane exposure required for performing the 2 5-minute bouts for IPC may not have been sufficient to alter gene induction. In addition, preconditioning from isoflurane exposure was reported to be diminished after a 48-hour delay.14 The anesthetic preconditioning effect with isoflurane may have been lost by the 72-hour delay used in the present study. In the case of preconditioning with halothane alone, infarct volume was reduced in striatum, but only a nonsignificant trend was seen in cortex. Apparently, the 90-minute duration was closer to the threshold necessary for altering gene induction with halothane than with isoflurane, or the gene alteration persists for a longer duration. Interestingly, the preconditioning effect of halothane on striatal infarction was lost in those mice undergoing sham surgery, thereby suggesting that the endocrine response associated with surgical stress or local release of inflammatory cytokines mitigates the protective effect of anesthetic preconditioning. Thus, in analyzing the genomic response to IPC, the genetic temporal profile induced by anesthetics and surgical stress must be considered. Nevertheless, IPC still had a greater effect than the corresponding halothane or isoflurane preconditioning. Because halothane is no longer manufactured or used clinically in the United States, the ability to use halothane during IPC may become limited, at least in this country.
Lethal IPC
Brief, repetitive bouts of global ischemia in gerbils can produce a cumulative effect on brain injury.19 Focal tolerance may be limited if the IPC insult itself induces cell death. Indeed, substantial pallor in TTC-stained striatum occurred after 3 10-minute bouts of MCAO. Others have reported neuronal cell death in striatum after 3 5-minute bouts of MCAO in the mouse10 and after 3 10-minute bouts in the rat.9 At 6 days after IPC, selective striatal cell death was evident in the present study with TTC staining, H&E histology, and TUNEL staining when 3 5-minute bouts or 1 15-minute bout of MCAO were used but was absent with 2 5-minute bouts. Therefore, it is possible to elicit protection in striatum with an IPC stimulus that, by itself, either produces moderate selective cell death or produces no detectable cell death. However, analyzing the pattern of increased and decreased gene expression in a region where selective neurons are destined to die by the IPC alone would confound interpretation of the results. In contrast to striatum, cortical neurons did not display pyknotic nuclei and TUNEL staining was sparse after 3 5-minute bouts or 1 15-minute bout of MCAO. Presumably, sparse cell death would have a minor effect on IPC analysis of gene expression on tissue sampled exclusively from cortex as used by others.2
In summary, the present study demonstrates that brief repetitive focal ischemia can be used as a preconditioning stimulus in the mouse for inducing tolerance to severe transient focal cerebral ischemia 72 hours later in cortex and striatum. Two episodes of only 5-minute duration, which by themselves did not cause significant cell death in either region, were effective in producing tolerance. Unexpectedly, tolerance was greater when halothane was used for surgical anesthesia. Although tolerance could be augmented by increasing the IPC severity under isoflurane anesthesia, selective neuronal injury in striatum produced by the more severe IPC stimuli alone limits their use to cerebral cortex for investigation of mechanisms of tolerance produced by TIA.
Acknowledgments
The authors thank Ellen Gordes for her technical assistance and Tzipora Sofare for her editorial assistance.
This work was supported in part by National Institutes of Health grants NS39148 and NS38684.
Footnotes
Disclosures
None.
References
- 1.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K. ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- 2.Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- 3.Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, Einhaupl KM. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30:1851–1854. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- 4.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- 5.Cho S, Park EM, Zhou P, Frys K, Ross ME, Iadecola C. Obligatory role of inducible nitric oxide synthase in ischemic preconditioning. J Cereb Blood Flow Metab. 2005;25:493–501. doi: 10.1038/sj.jcbfm.9600058. [DOI] [PubMed] [Google Scholar]
- 6.Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- 7.Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- 8.Alkayed NJ, Goyagi T, Joh HD, Klaus J, Harder DR, Traystman RJ, Hurn PD. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33:1677–1684. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Graham SH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:566–577. doi: 10.1097/00004647-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Atochin DN, Clark J, Demchenko IT, Moskowitz MA, Huang PL. Rapid cerebral ischemic preconditioning in mice deficient in endothelial and neuronal nitric oxide synthases. Stroke. 2003;34:1299–1303. doi: 10.1161/01.STR.0000066870.70976.57. [DOI] [PubMed] [Google Scholar]
- 12.Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Katchanov J, Waeber C, Gertz K, Gietz A, Winter B, Bruck W, Dirnagl U, Veh RW, Endres M. Selective neuronal vulnerability following mild focal brain ischemia in the mouse. Brain Pathol. 2003;13:452–464. doi: 10.1111/j.1750-3639.2003.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 15.Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1377–1386. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1–R8. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 17.Chimon GN, Wong PT. Ischemic tolerance and lipid peroxidation in the brain. Neuroreport. 1998;9:2269–2272. doi: 10.1097/00001756-199807130-00023. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Nowak TS., Jr CBF changes associated with focal ischemic preconditioning in the spontaneously hypertensive rat. J Cereb Blood Flow Metab. 2006;26:1128–1140. doi: 10.1038/sj.jcbfm.9600269. [DOI] [PubMed] [Google Scholar]
- 19.Tomida S, Nowak TS, Jr, Vass K, Lohr JM, Klatzo I. Experimental model for repetitive ischemic attacks in the gerbil: the cumulative effect of repeated ischemic insults. J Cereb Blood Flow Metab. 1987;7:773–782. doi: 10.1038/jcbfm.1987.133. [DOI] [PubMed] [Google Scholar]