Abstract
The fungus-growing ant–microbe mutualism is a classic example of organismal complexity generated through symbiotic association. The ants have an ancient obligate mutualism with fungi they cultivate for food. The success of the mutualism is threatened by specialized fungal parasites (Escovopsis) that consume the cultivated fungus. To defend their nutrient-rich garden against infection, the ants have a second mutualism with bacteria (Pseudonocardia), which produce antibiotics that inhibit the garden parasite Escovopsis. Here we reveal the presence of a fourth microbial symbiont associated with fungus-growing ants: black yeasts (Ascomycota; Phialophora). We show that black yeasts are commonly associated with fungus-growing ants, occurring throughout their geographical distribution. Black yeasts grow on the ants' cuticle, specifically localized to where the mutualistic bacteria are cultured. Molecular phylogenetic analyses reveal that the black yeasts form a derived monophyletic lineage associated with the phylogenetic diversity of fungus growers. The prevalence, distribution, localization and monophyly indicate that the black yeast is a fifth symbiont within the attine ant–microbe association, further exemplifying the complexity of symbiotic associations.
Keywords: host–microbe interaction, mutualism, microbial ecology
1. Introduction
Symbiosis, first defined as ‘the living together of unlike named organisms’ (deBary 1879), has received increasing interest over the past 15 years for two important reasons: (i) recognition of the ubiquity and importance of symbiotic associations and (ii) advances in molecular biology facilitating sampling and identification of microbes. Because animals and plants survive amidst environments rich in microbes, it is challenging to delineate a truly symbiotic host–microbe association from the countless allochthonous microbes that hosts interact with.
The mutualism between attine ants and the fungi they culture for food is a well-studied symbiosis. Recently, it was discovered that this symbiosis is more than just a mutualism: the ants' fungal cultivar hosts specialized pathogenic fungi (Escovopsis; Currie et al. 1999a). To combat the antagonistic fungi, ants engage in a second mutualism with actinomycetous bacteria (Pseudonocardia) that produce secondary metabolites that specifically inhibit the growth of Escovopsis (Currie et al. 1999b). Attine ants, their fungal cultivars, bacterial mutualists and cultivar antagonists have coevolved for over 50 Myr (Chapela et al. 1994; Mueller et al. 1998; Currie et al. 2003, 2006), suggesting that complex networks of symbionts are not only successful, but perhaps more common than we currently recognize.
Here we describe a fifth symbiont of the fungus-growing ant–microbe symbiosis, a black yeast (figure 1a) closely related to the genus Phialophora. We establish that black yeasts are symbionts of fungus-growing ants through intense sampling, isolations and culture-independent amplification of the microbe from ants across the geographical and phylogenetic distribution of fungus growers, and on the finding that it consistently grows on a specialized location on the ant's cuticle. We discuss potential origins of the symbiosis and the importance of additional layers of complexity in symbioses.
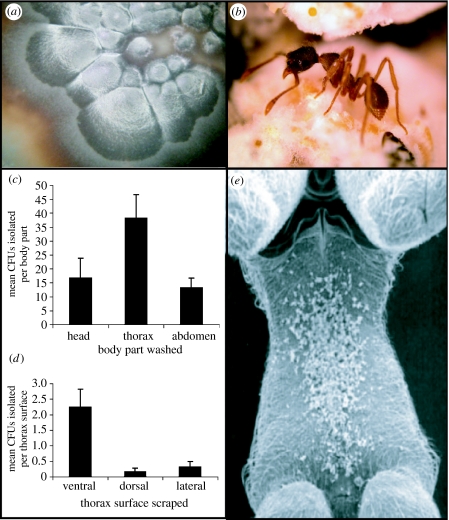
Figure 1.
Black yeast symbiont associated with fungus-growing ants. (a) Photo of a black yeast colony growing on nutrient agar. (b) Photo of Apterostigma pilosum, a fungus grower from which black yeasts are isolated. (c) Black yeast growth on ant body parts. Black yeast is most frequently isolated from ant thoraxes. (d) Black yeast growth on thorax surfaces. Black yeast growth is concentrated on the metapleura (ventral) surface of the thorax. (e) Scanning electron micrograph of A. pilosum thorax (metapleura) on which antibiotic-producing Pseudonocardia and black yeasts grow; Pseudonocardia seen here as white tufts on the ants' cuticle. CFUs, colony forming units.
2. Material and methods
(a) Study organisms
Black yeasts isolated and amplified in this study were obtained from colonies of fungus-growing ants collected between 2002 and 2006 in Panama, Ecuador and Peru. To assess the prevalence of black yeasts in fungus-growing ant populations, we intensively sampled Apterostigma spp. (figure 1b) in Gamboa and Pipeline Road in the Panama Canal zone in 2003.
Colonies were maintained in dual plastic chambers placed on mineral oil islands, to avoid horizontal transfer of microbes among colonies. Ants were watered and fed a mixture of oats, corn meal, tea leaves and oak catkins weekly, or watered and fed a variety of leaf material three times weekly. Voucher samples of ants from each colony collected were taken stored at −80°C in ethanol, and transferred to Ted Schultz, Smithsonian Institution, for long-term storage.
(b) Isolation of microbes
Black yeast isolations were performed by scraping ants with a sterile metal utensil. Scraped materials were spread on chitin agar and incubated at room temperature for four weeks. Individual colony forming units of black yeast were then transferred to potato dextrose agar (Difco).
(c) Localization of black yeasts on Apterostigma
To determine whether black yeasts associated with Apterostigma spp. workers occupy a specific niche on the ants' body, we carried out isolations from different body parts. The head, thorax and abdomen were separated (n=27). Each body part was placed in 500 μl of sterile double-distilled water in a 1.5 ml tube, vortexed for approximately 30 s, and 100 μl was spread on chitin agar. We further investigated the ant's thorax for localized growth of black yeast by scraping the dorsal, ventral and lateral surfaces of the thorax (n=27), and streaking scraped material on chitin agar as above.
(d) DNA extraction, amplification and sequence analysis
DNA was extracted from pure cultures and directly from ants as previously described (Cafaro & Currie 2005). The universal fungal primers NL1 and NL4 (O'Donnell 1993) were used to amplify 28S ribosomal genes from pure culture isolates. Black yeast DNA was amplified directly from ants with NL4 and a primer designed to specifically amplify ant-associated black yeasts (5′-GAGTCGAGTTGTTTGGGAATGC-3′). Amplification reactions were standardized to a final volume of 40 μl (Cafaro & Currie 2005). Cycle parameters were 95°C for 4 min, 35 cycles of 94°C for 45 s, 51°C for 50 s, 72°C for 140 s, 72°C for 4 min and hold at 4°C. PCR products were cleaned using Wizard PCR Preps DNA Purification System (Promega). Sequencing reactions contained 2 μl of BigDye Terminator v. 3.1 (Applied Biosystems), 3 μl of dilution buffer (Applied Biosystems), 15 pmol of primer and 0.2 μg of template DNA in a final reaction volume of 20 μl. Cycle conditions followed Cafaro & Currie (2005). Excess dye was removed using CleanSeq (Agencourt Biosciences). Samples were resuspended in 50 μl of double-distilled water and sequenced at the UW-Madison Biotech facility.
BLAST searches were preformed with raw sequences to determine whether amplicon data corresponded to Phialophora as suggested by morphological observations. Sequences were combined and edited using Sequencher and MacClade (Maddison & Maddison 2000). To represent taxonomic diversity of black yeast, out-group sequences were downloaded from GenBank. Bayesian analysis was preformed in MrBayes v. 3.1 (Ronquist & Huelsenbeck 2003). Four analyses with 2 million generations each were conducted. Log-likelihood scores consistently stabilized after 5000 generations; therefore, the initial 5000 generations from each run were discarded. One of every 100 generations was sampled to calculate the posterior probability for each branch in the tree. Sequences are available in GenBank (see table 1 in the electronic supplementary material).
3. Results
(a) Prevalence and distribution of the black yeast in Apterostigma
Intensive sampling of ant populations in the Panama Canal zone revealed that black yeasts have a high prevalence within (n=69, 94.2% of ants per nest) and among (n=23, 100% of nests) Apterostigma pilosum colonies. Black yeasts were also isolated from Apterostigma colonies (Apterostigma dentigerum and A. pilosum species complex) collected in other regions of Panama and in Peru and Ecuador, indicating that the ant-associated black yeast has a broad geographical distribution.
(b) Localization of black yeasts on A. pilosum
Microbial isolations from different parts of the ant's bodies show that the black yeast is concentrated on the thorax (analysis of variance (ANOVA), d.f.=80, F=4.29, p<0.017; figure 1c), specifically on the metapleural plate under the ant's forelegs (ANOVA, d.f.=80, F=11.69, p<0.001; figure 1d). This is also the location of bacterial mutualist growth in Apterostigma (Currie et al. 2006; figure 1e).
(c) Black yeasts across the phylogenetic diversity of fungus-growing ants
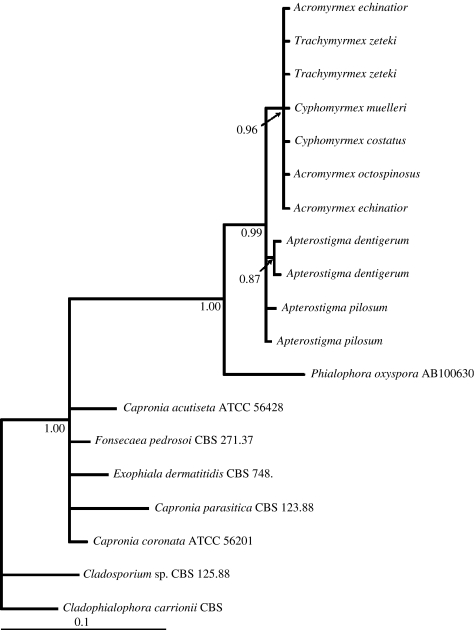
Although black yeast is frequently cultured from Apterostigma workers, extensive attempts to isolate it from other genera of fungus-growing ants were unsuccessful. Attempts included using multiple nutrient sources, and various environmental conditions (A.E.F. Little 2003–2005, unpublished data). Culture-independent methods, however, yielded sequences of black yeasts from worker ants of species spanning the diversity of fungus growers. A molecular phylogeny, based on sequences of a conserved genetic region (28S), generated from culture and culture-independent methods, indicates that black yeasts associated with fungus-growing ants form a derived monophyletic group (figure 2).
Figure 2.
Bayesian molecular phylogeny of black yeasts associated with fungus-growing ants based on 28S rDNA. Numbers on branches represent posterior probabilities. Tree was rooted using Cladophialophora carrionii. Sequences from taxa with accession numbers were obtained from GenBank, all others are named for ants from which the black yeast sequences were amplified.
4. Discussion
Multiple lines of evidence indicate that the black yeast is a fifth symbiont in the fungus-growing ant symbiosis. First, black yeasts are commonly associated with colonies of fungus-growing ants. Second, black yeasts were present in every population of Apterostigma spp. we sampled across much of the geographical distribution of the ants. Third, black yeast growth is concentrated on the specialized location on the ants' cuticle where bacterial mutualists are maintained. Fourth, ant-associated black yeasts form a derived monophyletic group, indicating that the association originated once and has been maintained over evolutionary time. Collectively, these data support our hypothesis that black yeasts are specialized symbionts of fungus-growing ants.
The mutualism between attine ants, their fungal cultivar, bacterial mutualists and garden pathogens probably originated 50–65 Myr ago (Chapela et al. 1994; Mueller et al. 1998). Our finding that ant-associated black yeasts form a monophyletic group supports a single evolutionary origin in the symbiosis. The amplification of black yeasts from fungus-growing ants in phylogenetically basal and derived genera (Apterostigma and Acromyrmex, respectively) suggests that black yeasts may have been present in early stages of the symbiosis. We suggest four possible scenarios regarding the evolutionary origin of black yeasts within the symbiosis, as follows. (i) Some Phialophora species live endophytically in plants (Latch et al. 1984); thus, ants may have acquired the black yeast by manuring their gardens with vegetation containing Phialophora endophytes. (ii) Black yeasts may have been associated with ancestral ants of fungus growers, although no such association has been described. (iii) Similarly, the black yeast may have undergone a host shift, jumping from another host insect to an ant; indeed, some black yeasts are associated with bark beetle galleries (Kerrigan & Rogers 2003). (iv) Alternatively, a black yeast–Pseudonocardia association could have predated the ant–fungus association, as Phialophora spp. and Pseudonocardia spp. grow together in some soils (Sivasithamparam et al. 1987). The general association between Phialophora spp. and Pseudonocardia spp. in the soil may have become specialized, thus when the ant–bacterial interaction originated, the ant–black yeast association may have developed simultaneously. Further studies to tease apart the interactions between black yeast and the ant–microbe mutualism may shed light on the origin of the symbiosis, and additional phylogenetic studies using more variable loci may indicate whether black yeasts have codiversified with the ant–microbe symbionts.
In nature, black yeasts are found in soil, plants, water and decaying wood, where they act as secondary saprophytes and oligotrophs. They also cause disease in humans and plants (deHoog 1977; Chen et al. 1996). The black yeast symbionts of fungus-growing ants have the potential to directly and indirectly affect each member of the symbiosis. Exploration of black yeast's role within the ant–microbe symbiosis indicates that they antagonize the ants' mutualistic bacteria, which inhibits the ants' ability to suppress garden infection by Escovopsis (Little & Currie submitted).
Our results further indicate that it is important to recognize and study symbioses as potential complex networks of interacting species, rather than binary relationships. Evidence of symbiotic networks extends well beyond the fungus-growing ant system, for example, bacteriophage were recently described as important members of the Wolbachia–arthropod symbiosis (Bordenstein et al. 2006), and virus-infected fungal endophytes were shown to confer heat tolerance to their plant hosts (Marquez et al. 2007). A re-evaluation of other well-studied symbioses using molecular tools to identify as yet unculturable microbes will undoubtedly yield findings of new symbiotic complexity that are critical to their host's ecological and evolutionary success.
Acknowledgments
This work was supported by NSF (IRCEB DEB-0110073 to C.R.C., DDIG DEB-0608078 to C.R.C. and A.E.F.L.). We thank STRI and ANAM (Republic of Panama) for their logistical support and permits, and M. Bergsbaken, M. Cafaro, P. Foley, M. Leone, O. Arosemana, K. Boomsma, E. Caldera, N. Gerardo, A. Pinto and M. Poulsen for their support and comments on this study.
Supplementary Material
Table 1 in the electronic supplementary material. Isolate and sequence details for samples used in phylogenetic analysis
References
- Bordenstein S.R, Marshall M.L, Fry A.J, Kim U, Wernegreen J.J. The tripartite associations between bacteriophage Wolbachia, and arthropods. PLoS Pathog. 2006;2:e43. doi: 10.1371/journal.ppat.0020043. doi:10.1371/journal.ppat.0020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro M.J, Currie C.R. Phylogenetic analysis of mutualistic filamentous bacteria associated with fungus-growing ants. Can. J. Microbiol. 2005;51:441–446. doi: 10.1139/w05-023. doi:10.1139/w05-023 [DOI] [PubMed] [Google Scholar]
- Chapela I.H, Rehner S.A, Schultz T.R, Mueller U.G. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science. 1994;266:1691–1694. doi: 10.1126/science.266.5191.1691. doi:10.1126/science.266.5191.1691 [DOI] [PubMed] [Google Scholar]
- Chen W.D, Gray L.E, Grau C.R. Molecular differentiation of fungi associated with brown stem rot and detection of Phialophora gregata in resistant and susceptible soybean cultivars. Phytopathology. 1996;86:1140–1148. doi:10.1094/Phyto-86-1140 [Google Scholar]
- Currie C.R, Mueller U.G, Malloch D. The agricultural pathology of ant fungus gardens. Proc. Natl Acad. Sci. USA. 1999a;96:7998–8002. doi: 10.1073/pnas.96.14.7998. doi:10.1073/pnas.96.14.7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie C.R, Scott J.A, Summerbell R.C, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999b;398:701–704. doi:10.1038/19519 [Google Scholar]
- Currie C.R, Wong B, Stuart A.E, Schultz T.R, Rehner S.A, Mueller U.G, Sung G.H, Spatafora J.W, Straus N.A. Ancient tripartite coevolution in the attine ant–microbe symbiosis. Science. 2003;299:386–388. doi: 10.1126/science.1078155. doi:10.1126/science.1078155 [DOI] [PubMed] [Google Scholar]
- Currie C.R, Poulsen M, Mendenhall J, Boomsma J.J, Billen J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science. 2006;311:81–83. doi: 10.1126/science.1119744. doi:10.1126/science.1119744 [DOI] [PubMed] [Google Scholar]
- deBary A. Trubner; Strassburg, Germany: 1879. Die Erscheinung der symbios. [Google Scholar]
- deHoog G.S. The black yeasts and allied hyphomycetes. Stud. Mycol. 1977;15:1–233. [Google Scholar]
- Kerrigan J, Rogers J.D. Microfungi associated with the wood-boring beetles Saperda calcarata (poplar borer) and Cryptorhynchus lapathi (poplar and willow borer) Mycotaxon. 2003;86:1–18. [Google Scholar]
- Latch G.C.M, Christensen M.J, Samuels G.J. Five endophytes of Lolium and Festuca in New Zealand. Mycotaxon. 1984;20:535–550. [Google Scholar]
- Little, A. E. F. & Currie, C. R. Submitted. Indirect interaction web reveals how black yeast symbionts compromise the efficiency of antibiotic defenses in fungus-growing ants. [DOI] [PubMed]
- Maddison D.R, Maddison W.P. Sinauer; Sunderland, MA: 2000. MacClade. [Google Scholar]
- Marquez L.M, Redman R.S, Rodriguez R.J, Roossinck M.J. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science. 2007;315:513–515. doi: 10.1126/science.1136237. doi:10.1126/science.1136237 [DOI] [PubMed] [Google Scholar]
- Mueller U.G, Rehner S.A, Schultz T.R. The evolution of agriculture in ants. Science. 1998;281:2034–2038. doi: 10.1126/science.281.5385.2034. doi:10.1126/science.281.5385.2034 [DOI] [PubMed] [Google Scholar]
- O'Donnell K. Fusarium and its near relatives. In: Reynolds D.R, Taylor J.W, editors. The fungal holomorph: mitotic, meiotis and pleomorphic speciation in fungal systematics. CAB International; Wallingford, UK: 1993. [Google Scholar]
- Ronquist F, Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. doi:10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Sivasithamparam K, MacNish G.C, Fang C.S, Parker C.A. Microflora of soil and wheat rhizosphere in a field following fumagation. Aust. J. Soil Res. 1987;25:491–498. doi:10.1071/SR9870491 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 in the electronic supplementary material. Isolate and sequence details for samples used in phylogenetic analysis