Abstract
The timing of sexual maturation in non-avian dinosaurs is not known. In extant squamates and crocodilians it occurs in conjunction with the initial slowing of growth rates as adult size is approached. In birds (living dinosaurs) on the other hand, reproductive activity begins well after somatic maturity. Here we used growth line counts and spacing in all of the known brooding non-avian dinosaurs to determine the stages of development when they perished. It was revealed that sexual maturation occurred well before full adult size was reached—the primitive reptilian condition. In this sense, the life history and physiology of non-avian dinosaurs was not like that of modern birds. Palaeobiological ramifications of these findings include the potential to deduce reproductive lifespan, fecundity and reproductive population sizes in non-avian dinosaurs, as well as aid in the identification of secondary sexual characteristics.
Keywords: development, reproduction, histology, Aves, Theropoda
1. Introduction
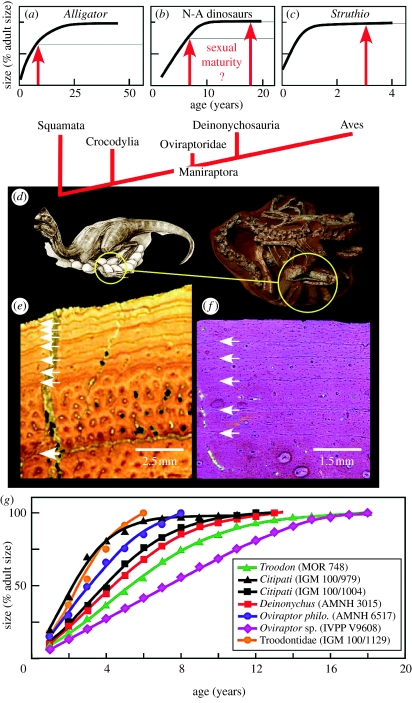
Evidence ranging from fossil eggs to skeletal anatomy, soft tissues and behaviour now documents the derivation of birds from theropod dinosaurs (Gauthier & Gall 2001; Currie et al. 2004). Nevertheless, many questions remain, especially with regard to their reproductive biology (Horner 2000). Information on the timing of sexual maturity in non-avian dinosaurs in particular could substantially advance our understanding of individual, population and species biology; however variance in timing among living reptilian groups leaves the condition in these animals ambiguous. Specifically, in the successive extant outgroups to Dinosauria, Crocodylia (alligators and crocodiles) and Squamata (lizards, snakes and amphisbaenians), sexual maturity coincides with a substantial decrease from more rapid juvenile growth rates (figure 1a; Andrews 1982; Shine & Charnov 1992). This developmental milestone occurs well before somatic maturity is attained. For instance, male American alligators (Alligator mississippiensis) sexually mature by 10 years of age at a length of approximately 2.5 m, but can reproduce for decades and grow to 4 m in length (figure 1a; Chabreck & Joanen 1979; Wilkinson & Rhodes 1997). Conversely, living birds (Aves/Neornithes) stand out among extant reptilian taxa in showing delayed sexual maturation that occurs only after reaching full adult size (Ricklefs 1968; figure 1c). This occurs even in relatively large taxa. For example, American bald eagles (Haliaeetus leucocephalus) reach full size in less than a year, but sexually mature 4–7 years later (Buehler 2000). Whether the peculiar avian pattern reflects their dinosaurian ancestry or an avian innovation remains unknown (figure 1b). To address this issue, we documented the age and developmental stage for all seven of the known brooding non-avian dinosaur specimens (Dong & Currie 1995; Norell et al. 1995; Varricchio et al. 1997; Grellet-Tinner & Makovicky 2006). Data from these reproductively active individuals were used to reveal the timing of sexual maturity in extinct dinosaurs and elucidate the origin of the modern avian condition.
Figure 1.
(a–c) Reptilian growth curves and relationships. Arrows indicate size/timing of sexual maturity. (d) Nesting individuals (e.g. Citpati IGM 100/1004) with eggs (circled) were studied. (e) Troodon (MOR 748) metatarsal showing tightly spaced peripheral growth lines and avascular lamellar bone indicating negligible growth. (f) Oviraptor (AMNH 6517) phalanx showing moderately spaced growth lines, peripherally decreasing vascularization, and woven bone. Growth was slowed, but still active. (g) Growth curves for reproductively active dinosaurs. Oviraptor (AMNH 6517) and Troodontidae (IGM 100/1129) perished prior to attaining full asymptotic size (=rates<3% yr−1). Troodon: %L, (1.238/1+e−034(Age−4.26))−0.22; Citipati 100/979, (1.278/1+e−0.77(Age−1.58))−0.29; Citpati 100/1004, (1.230/1+e−0.46(Age−3.20))−0.21; Deinonychus, (1.273/1+e−0.40(Age−3.45))−0.25; Oviraptor p., (1.53/1+e−0.47(Age−1.98))−0.45; Troodontidae, (2.76/1+e−0.25(Age−2.84))−0.90; Oviraptor sp., (0.379/1+e−0.77 (Age−12.71))−0.63; R2, 0.988–0.999. N-A, non-avian dinosaur.
2. Material and methods
Our sample included four oviraptorids (Oviraptor philoceratops; Oviraptor sp.; Citipati osmolskae (n=2)) and one deinonychosaur (Troodontidae nov. sp.) from Mongolia and China (table 1). All were buried by mass flows emanating from destabilized dunes (Loope et al. 1998) that captured them in brooding postures (Loope et al. 1998; Clark et al. 1999; figure 1d). We also examined two North American deinonychosaurs, Deinonychus antirrhopus and Troodon formosus, closely associated with eggs. The former preserves the eggshell in contact with its gastral basket (belly ribs; Grellet-Tinner & Makovicky 2006), while the latter was found overlying an egg clutch (Varricchio et al. 1997). Although their sex remains unknown, all of these specimens are assumed to be the parents of the associated eggs (Varricchio & Jackson 2004). We ascertained ages for these dinosaurs via multiple element osteohistological analysis that included counts of clearly demarcated growth lines (Chinsamy-Turan 2005; figure 1e,f). Based upon anatomical and phylogenetic grounds, these lines are assumed to reflect an annual genesis (Chinsamy-Turan 2005; Erickson 2005). If necessary, minor back-calculations of growth lines were made to account for rings effaced owing to medullar expansion (Erickson & Tumanova 2000). These had no bearing on assessments of development stage during which the dinosaurs perished (see below).
Table 1.
Specimens sampled and longevity estimates. (AMNH, American Museum of Natural History, New York; IGM, Geological Institute, Mongolian Academy of Sciences, Ulaan Baatar, Mongolia; MOR, Museum of the Rockies, Montana State University, Bozeman, Montana; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, PR China.)
| taxon | specimen | elements sampled | longevity (years) |
|---|---|---|---|
| Oviraptor philoceratops | AMNH 6517 | dorsal rib | 8 |
| manal phalanx II | 8 | ||
| Deinonychus antirrhopus | AMNH 3015 | gastralia | 13–14 |
| gastralia | 13 | ||
| dorsal rib | 13 | ||
| dorsal rib | —a | ||
| Troodontidae nov. sp. (to be described) | IGM 100/1129 | fibula | 6 |
| femur | 6 | ||
| Citipati osmolskae | IGM 100/979 | fibula | 13 |
| femur | —a | ||
| dorsal rib | —a | ||
| Citipatiosmolskae | IGM 100/1004 | fibula | 13 |
| dorsal rib | —a | ||
| femur | 13 | ||
| Troodon formosus | MOR 748 | metatarsal IV | 18 |
| femur | —a | ||
| Tibia | 18 | ||
| Oviraptor sp. | IVPP V9608 | manal phalanx IV | 18 |
Poor growth line preservation.
Proportions of the growth zone widths relative to the total amount of bone deposited at the site of sectioning were used to estimate annual increases in whole-body linear dimensions (e.g. total length; Sander 2000). For individuals where longevity was determined from several bones (table 1), mean proportions of annual growth were used to describe development. (Note: the differences between curves were negligible.) Age versus body size plots were then made (figure 1g). We used von Bertalanffy and logistic least-squares regression to describe the patterns (Ricklefs 1967). The latter provided the best statistical fit and allowed us to pinpoint the developmental stage when each animal perished (Erickson & Tumanova 2000). Following standardization to 100% adult size (figure 1a–c,g), the growth curves for the non-avian dinosaurs were compared with those for extant non-avian reptiles (Chabreck & Joanen 1979; James 1991; Wilkinson & Rhodes 1997) and birds (Cilliers et al. 1995).
The osteohistological and growth curve patterns were contrasted with expectations based upon maturation patterns in living reptilian species. Specifically, if the avian pattern (somatic maturity followed considerably later by sexual maturity) were inherited from theropod ancestors, our entire sample of actively reproducing theropods would be predicted to exhibit external fundamental systems (tightly packed growth lines that develop throughout theropod skeletons as full adult size is attained; Chinsamy-Turan 2005; Erickson 2005) and asymptotic growth curves. Conversely, if the primitive reptilian condition (sexual maturity coincides with the beginning of the transition to the growth asymptote) were retained, we would expect some individuals to be somatically immature, while others would show external fundamental systems and growth asymptotes.
3. Results
The brooding dinosaurs range in age from 6 to 18 years (figure 1g). Five specimens exhibit external fundamental systems and growth curves with substantial asymptotes indicating attainment of full adult size (figure 1e,g). In two specimens (Oviraptor philoceratops and Troodontidae nov. sp.), one from each clade (Oviraptoridae and Deinonychosauria, respectively), external fundamental systems are absent and their growth curves are not asymptotic (figure 1f,g). Though nesting, these individuals were still actively growing.
A comparison of these results with the expectations based upon maturation patterns in living squamates and crocodilian reptiles (the primitive character state) versus that in birds (derived character state) revealed conformity with the former.
4. Discussion
Our findings point to somatic and sexual maturity occurring simultaneously among brooding Deinonychosauria and Oviraptoridae, the two clades of non-avian dinosaurs considered most closely related to birds (Norell et al. 2006; figure 1). Viewed in a broader phylogenetic context it can be inferred that this condition probably characterized the non-avian dinosaur radiation as a whole. The non-avian dinosaur groups that were not examined in the present research, namely more basal saurischians and Ornithischia, are bracketed by the clades Squamata, Crocodylia, Oviraptoridae and Deinonychosauria—all of which show early sexual maturation. The palaeobiological ramifications of this inference are considerable. For example, by coupling data from growth curves (Sander 2000; Erickson et al. 2004) with nest characteristics (e.g. clutch sizes; Varricchio & Jackson 2004), and/or population level sampling (Chinsamy 1994) including survivorship curves (Erickson et al. 2006), it becomes possible to deduce non-avian dinosaur reproductive lifespan (e.g. approx. 7–9 years in Citipati osmolskae), fecundity (e.g. approx. 140–180 eggs in C. osmolskae), reproductive population sizes (e.g. approx. 46–83% among tyrannosaurs; Erickson et al. 2006), etc. Furthermore, this maturity information can aid in the identification of secondary sexual characteristics that are integral for understanding reproductive behaviour and characterizing species variability.
Our findings also show that, despite the presence of many modern avian anatomical features such as hollow bones and feathers (Gauthier & Gall 2001; Currie et al. 2004), the life history of non-avian theropods differed substantially from extant birds. Additionally, they suggest that late avian maturation evolved no earlier than the genesis of the first bird, Archaeopteryx lithographica in the Jurassic period. Intracortical growth lines in bones from some of the earliest birds suggest that these animals, like their dinosaurian ancestors, took more than 1 year to reach adult size (Chinsamy 2002). Conversely, living birds typically explode to adult size in just a matter of weeks. This affords them, among other things, early thermal independence (endothermy) and/or rapid attainment of physical capacities to dissuade/escape predators (Brooke & Birkhead 1991). Substantial resources are subsequently allotted to reproductive development. These unprecedented growth rates probably evolved in the Cretaceous period when modern avian bone structure devoid of intracortical growth lines appears among ornithurine birds, the group inclusive of living forms (Chinsamy 2002). We hypothesize that this developmental shift favouring rapid somatic growth led to the decoupling of somatic and sexual maturity seen in birds today.
Acknowledgments
We thank Carl Mehling, Jack Horner and Fang Zheng for access to specimens in their care and Philip Currie, Pete Makovicky and Scott Yerby for their helpful input. This work was generously supported by grants from the National Science Foundation: EAR 0207744 (GME and MAN) and DBI 0446224 (GME). Mick Ellison provided illustrations and photographs of Citipati.
References
- Andrews R.M. Patterns of growth in reptiles. In: Gans C, Pough F.H, editors. Biology of the reptilia. vol. 13. Academic Press; New York, NY: 1982. pp. 273–320. [Google Scholar]
- Brooke M, Birkhead T, editors. The Cambridge encyclopedia of ornithology. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- Buehler D.A. Bald eagle. In: Poole A, Gill F, editors. The birds of North America. vol. 506. The Birds of North America, Inc; Philadelphia, PA: 2000. [Google Scholar]
- Chabreck R.H, Joanen T. Growth rates of American alligators in Louisiana. Herpetologica. 1979;35:51–57. [Google Scholar]
- Chinsamy, A. 1994 Dinosaur bone histology: implications and inferences. In Dino fest (eds G. D. Rosenberg & D. L. Wolberg). Special Publication, no. 7, pp. 213–227. Knoxville, KY: Paleontological Society.
- Chinsamy A. Bone microstructure of early birds. In: Chiappe L.M, Witmer L.M, editors. Mesozoic birds: above the heads of dinosaurs. University of California Press; Berkeley, CA: 2002. pp. 421–431. [Google Scholar]
- Chinsamy-Turan A. Johns Hopkins University Press; Baltimore, MD: 2005. The microstructure of dinosaur bone. [Google Scholar]
- Cilliers S.C, Du Preez J.J, Maritz J.S, Hayes J.P. Growth curves of ostriches (Struthio camelus) from Oudtshoorn in South Africa. Anim. Sci. J. 1995;61:161–164. [Google Scholar]
- Clark J.M, Norell M.A, Chiappe L.M. An oviraptorid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avian-like brooding position over an oviraptorid nest. Am. Mus. Novit. 1999;3265:1–36. [Google Scholar]
- Currie P.J, Koppelhus E.B, Shugar M.A, Wright J.L, editors. Feathered dragons: studies on the transition from dinosaurs to birds. Indiana University Press; Bloomington, IN: 2004. [Google Scholar]
- Dong Z.-M, Currie P.J. On the discovery of an oviraptorid skeleton on a nest of eggs at Bayan Mandahu, Inner Mongolia, People's Republic of China. Can. J. Earth Sci. 1995;33:631–636. [Google Scholar]
- Erickson G.M. Assessing dinosaur growth patterns: a microscopic revolution. Trends Ecol. Evol. 2005;20:677–684. doi: 10.1016/j.tree.2005.08.012. doi:10.1016/j.tree.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Erickson G.M, Tumanova T.A. Growth curve of Psittacosaurus mongoliensis Osborn inferred from long bone histology. Zool. J. Linn. Soc. 2000;130:551–566. doi:10.1006/zjls.2000.0243 [Google Scholar]
- Erickson G.M, Makovicky P.J, Currie P.J, Norell M.A, Yerby S.A, Brochu C.A. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature. 2004;430:772–775. doi: 10.1038/nature02699. doi:10.1038/nature02699 [DOI] [PubMed] [Google Scholar]
- Erickson G.M, Currie P.J, Inouye B.D, Winn A.A. Tyrannosaur life tables: the first look at non-avian dinosaur population biology. Science. 2006;313:213–217. doi: 10.1126/science.1125721. doi:10.1126/science.1125721 [DOI] [PubMed] [Google Scholar]
- Gauthier J, Gall L.F, editors. New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History; New Haven, CT: 2001. [Google Scholar]
- Grellet-Tinner G, Makovicky P. A possible egg of the dromaeosaur Deinonychus antirrhopus: phylogenetic and biological implications. Can. J. Earth Sci. 2006;43:705–719. doi:10.1139/E06-033 [Google Scholar]
- Horner J.R. Dinosaur reproduction and parenting. Annu. Rev. Earth Planet. Sci. 2000;28:19–45. doi:10.1146/annurev.earth.28.1.19 [Google Scholar]
- James C.D. Growth rates and ages at maturity of sympatric scincid lizards (Ctenotus) in central Australia. J. Herpetol. 1991;25:284–295. doi:10.2307/1564586 [Google Scholar]
- Loope D.B, Dingus L, Swisher C.C, III, Minjin C. Life and death in a Late Cretaceous dune field, Nemegt Basin, Mongolia. Geology. 1998;26:27–30. doi:10.1130/0091-7613(1998)026<0027:LADIAL>2.3.CO;2 [Google Scholar]
- Norell M.A, Clark J.M, Chiappe L.M, Dashzeveg D. A nesting dinosaur. Nature. 1995;378:774–776. doi:10.1038/378774a0 [Google Scholar]
- Norell M.A, Clark J.M, Turner A.H, Makovicky P.J, Barsbold R, Rowe T. A new dromaeosaurid from Ukhaa Tolgod (Omnogov, Mongolia) Am. Mus. Novit. 2006;3545:1–51. doi:10.1206/0003-0082(2006)3545[1:ANDTFU]2.0.CO;2 [Google Scholar]
- Ricklefs R.E. A graphical method of fitting equations to growth curves. Ecology. 1967;48:978–983. doi:10.2307/1934545 [Google Scholar]
- Ricklefs R.E. Patterns of growth in birds. Ibis. 1968;110:419–451. [Google Scholar]
- Sander P.M. Longbone histology of the Tendaguru sauropods: implications for growth and biology. Paleobiology. 2000;26:466–488. doi:10.1666/0094-8373(2000)026<0466:LHOTTS>2.0.CO;2 [Google Scholar]
- Shine R, Charnov E.L. Patterns of survival, growth, and maturation in snakes and lizards. Am. Nat. 1992;139:1257–1269. doi:10.1086/285385 [Google Scholar]
- Varricchio D.J, Jackson F.D. Two eggs sunny-side up: reproductive physiology in the dinosaur Troodon formosus. In: Currie P.J, Koppelhus E.B, Shugar M.A, Wright J.L, editors. Feathered dragons. Indiana University Press; Bloomington, IN: 2004. pp. 215–233. [Google Scholar]
- Varricchio D.J, Jackson F, Borkowski J, Horner J.R. Nest and eggs clutches of the dinosaur Troodon formosus and the evolution of avian reproductive traits. Nature. 1997;385:247–250. doi:10.1038/385247a0 [Google Scholar]
- Wilkinson P.M, Rhodes W.E. Growth rates of American alligators in coastal South Carolina. J. Wildlife Manage. 1997;61:397–402. doi:10.2307/3802596 [Google Scholar]