Abstract
Recombination is thought to occur only rarely in animal mitochondrial DNA (mtDNA). However, detection of mtDNA recombination requires that cells become heteroplasmic through mutation, intramolecular recombination or ‘leakage’ of paternal mtDNA. Interspecific hybridization increases the probability of detecting mtDNA recombinants due to higher levels of sequence divergence and potentially higher levels of paternal leakage. During a study of historical variation in Atlantic salmon (Salmo salar) mtDNA, an individual with a recombinant haplotype containing sequence from both Atlantic salmon and brown trout (Salmo trutta) was detected. The individual was not an F1 hybrid but it did have an unusual nuclear genotype which suggested that it was a later-generation backcross. No other similar recombinant haplotype was found from the same population or three neighbouring Atlantic salmon populations in 717 individuals collected during 1948–2002. Interspecific recombination may increase mtDNA variability within species and can have implications for phylogenetic studies.
Keywords: mitochondrial DNA, interspecific recombination, Atlantic salmon, Salmo salar, brown trout, Salmo trutta
1. Introduction
Recombination of mitochondrial DNA (mtDNA) occurs commonly in plants and fungi but was traditionally thought to be rare or absent in animal mtDNA (Birky 2001). However, there is evidence that at least some of the molecular machinery required for recombination does exist in animal mitochondria (Thyagarajan et al. 1996). In recent years, there have been a number of examples from natural populations of mtDNA recombinants in species including nematodes (Lunt & Hyman 1997), butterflies (Andolfatto et al. 2003), fish (Hoarau et al. 2002), humans (Kraytsberg et al. 2004), scorpions (Gantenbein et al. 2005) and lizards (Ujarvi et al. 2007). Moreover, surveys of mtDNA sequence databases have revealed previously undetected recombination events (Ladoukakis & Zouros 2001; Piganeau et al. 2004; Tsaousis et al. 2005).
In order for recombinant mtDNA molecules to be detected, a state of cellular heteroplasmy is required, i.e. more than one mitochondrial lineage needs to be present in a cell (through mutation, intramolecular recombination or ‘leakage’ of paternal mtDNA), otherwise recombinant molecules will be identical to parental molecules. Animal mtDNA has conventionally been assumed to have strict maternal inheritance and consequently cells are generally homoplasmic (Birky 2001). However, paternal leakage of mtDNA from sperm, demonstrated in several species (e.g. Gyllensten et al. 1991; Magoulas & Zouros 1993; Schwartz & Vissing 2002; Kvist et al. 2003), can cause heteroplasmy and increase the probability of detection of recombination events. Sperm contain mitochondria, and it is thought that up to 100 functional mitochondria (and their genomes) enter the oocyte cytoplasm at fertilization in mammals (Sutovsky et al. 2000). In several mammalian species (including mice, cattle and humans), it has been shown that the mitochondria are tagged with ubiquitin which allows recognition by oocyte proteolytic enzymes and commits sperm-derived mitochondria to destruction, before or during the third proteolytic cleavage (Sutovsky et al. 2000).
Rokas et al. (2003) theorized that survival of paternal mitochondria may be higher in interspecific crosses than within-species matings because molecular recognition systems may be relaxed in such crosses. Studies have demonstrated that in mice there is a higher probability of paternal leakage in interspecific when compared with intraspecific crosses (Shitara et al. 1998). Sutovsky et al. (2000) suggested this may be because the ubiquitin-activating and ubiquitin-conjugating enzymes may not be directly compatible across species. High levels of divergence between haplotypes, for example at the interspecific level, would aid in detection of recombination events. Here, we report an example of interspecific mtDNA recombination identified as part of a study of long-term temporal genetic changes in four Spanish Atlantic salmon (Salmo salar) populations.
2. Material and methods
Scale samples were collected from angled adult Atlantic salmon in the rivers Asón, Nansa, Pas and Deva in northern Spain during 1948–2002. Variation in mtDNA was assayed through a combination of restriction fragment length polymorphism (RFLP) analysis and direct DNA sequencing (Ciborowski et al. 2007) and individuals were genotyped at five polymorphic microsatellite loci (EST47, SsaA124, SsaD486, Ssosl85 and Sasa-UBA-3UTR; K.L. Ciborowski et al. 2007, unpublished data). To test whether the individual carrying the putative recombinant mtDNA haplotype was an F1 hybrid, the 5S rDNA locus was amplified (Pendas et al. 1995). Small amounts of DNA from the scale sample for this individual precluded any longer range PCRs of mtDNA.
Sequences were searched for similarity with existing sequences in GenBank using BLAST (Altschul et al. 1990). Possible recombination events were detected using a bootscanning method implemented in Simplot (Lole et al. 1999). The recombinant sequence was queried with a sliding window size of 200 bp and step size of 20 bp against the most common S. salar sequence in the sample (GenBank Accession DQ237848), a Salmo trutta sequence from the River Nansa, northern Spain (EF513754) and an Oncorhynchus mykiss (rainbow trout) sequence (L29771). The integrity of the open reading frame in the recombinant sequence was checked using the vertebrate mitochondrial genetic code and the program Translate (http://bioinformatics.org/sms2/translate.html).
3. Results and discussion
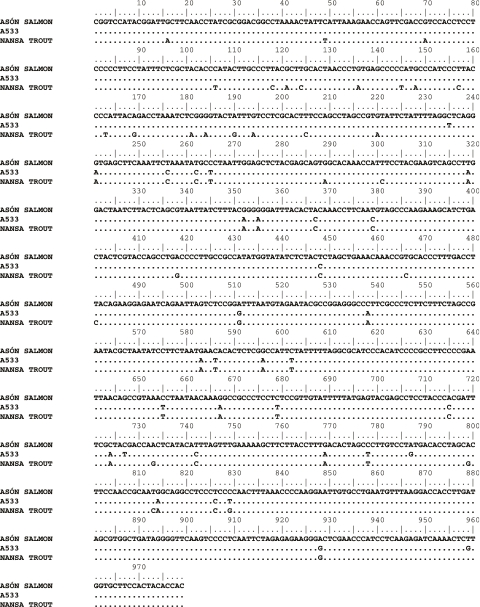
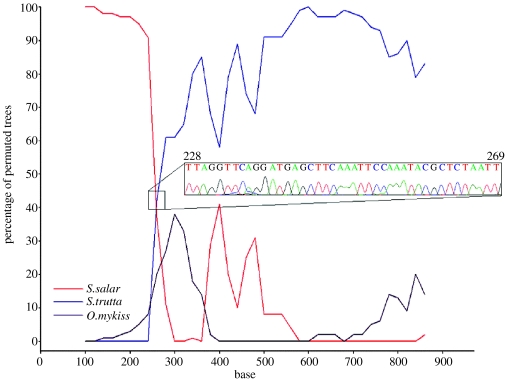
We found a unique and anomalous mtDNA ND1 gene RFLP pattern in an individual (A533) caught in the River Asón in 1953. Direct sequencing of the mtDNA for this individual (EF513755) and a subsequent BLAST search returned homologies with both Atlantic salmon and brown trout (S. trutta) mtDNA ND1 sequences. A visual inspection of an alignment of the sequence from A533, a representative Spanish Atlantic salmon mtDNA ND1 sequence (from Ciborowski et al. 2007) and a Spanish brown trout mtDNA ND1 sequence, suggested two separate regions (figure 1). The 5′ region of the A533 sequence appeared to have high similarity with the Atlantic salmon sequence, and then abruptly to have similarity with the brown trout sequence in the 3′ region. Using a bootscanning method for detecting recombination events (Lole et al. 1999), recombination between Atlantic salmon and brown trout in the mtDNA ND1 gene was detected around position 240 (figure 2). No evidence of brown trout sequence was detected in any of the other Atlantic salmon samples (n=717) and the absence of ambiguous peaks in the sequencing chromatogram (figure 2 inset) argues against contamination of the sample by DNA from both species. The recombinant sequence is also unlikely to be derived from a nuclear-encoded mitochondrial pseudogene (i.e. a Numt) as we found no evidence for amplification of more than one sequence from any other individual, either from RFLP patterns (n=717) or direct sequencing (n=55; Ciborowski et al. 2007), and Numts are unknown from fish species in general (Bensasson et al. 2001; Venkatesh et al. 2006).
Figure 1.
Alignment of the mtDNA ND1 sequence from A533 with that of an Atlantic salmon and brown trout, both also from northern Spanish populations.
Figure 2.
Bootscan using the mtDNA ND1 sequence from A533 as a query with those from Atlantic salmon (S. salar), brown trout (S. trutta) and rainbow trout (O. mykiss) as references. The ‘percentage of permuted trees’ is a bootstrap value for trees which have query and reference sequences as sister taxa. Inset: experimental chromatogram for the 228–269 bp region.
Amplification of the 5S rDNA locus—which has fixed variants of differing size in Atlantic salmon and brown trout (Pendas et al. 1995)—from A533 yielded only a PCR product of the expected size for Atlantic salmon. However, the nuclear genotype of A533 was anomalous, i.e. at five microsatellite loci typed, eight of the alleles found in this individual were not found in a sample of Atlantic salmon from the same river in the same decade (n=31–36 individuals per locus). Collectively, the evidence suggests that A533 was not an F1 hybrid, but the result of a later-generation backcross. Without similar data from the sympatric trout population, it is not possible to definitively assign the relative contributions of Atlantic salmon and brown trout to the nuclear genome of A533.
Atlantic salmon and brown trout are sympatric in the River Asón, and hybridization between the species has been reported at relatively high frequencies in Spanish rivers (García de Leániz & Verspoor 1989). While the recombinant mtDNA haplotype had an intact open reading frame (in the sequenced section, at least) and could have produced a functional protein, in general, Atlantic salmon×brown trout hybrids display reduced survival and fecundity. While in some situations, hybrids can produce viable backcross offspring, they are generally triploid and therefore infertile (Galbreath & Thorgaard 1995), limiting the possibility of introgression of genes from one species to the other (Verspoor & Hammar 1991). As A533 was angled as an adult salmon, it appears that its viability was not overly compromised. However, if A533 was infertile (its microsatellite genotype did not suggest that it was triploid) or male, the recombinant mtDNA haplotype would not have been inherited by future generations via this individual. Even if A533 was fertile and female the low-frequency recombinant haplotype would have been liable to loss through genetic drift. Indeed, no other such recombinant mtDNA ND1 haplotype was observed in similar samples collected from 1950 to 2002 in the River Asón or three neighbouring Atlantic salmon populations over the same time span (Ciborowski et al. 2007).
To our knowledge, this is one of only a few clear examples of interspecific mtDNA recombination in the literature (Piganeau et al. 2004; Tsaousis et al. 2005). Given the rare and fleeting nature of the occurrence of the recombinant haplotype identified here, it would have gone undetected in typical surveys of mtDNA variability within species, which are often less intensive and limited to contemporary samples. Interspecific recombination may increase intraspecific mtDNA diversity but, given reduced viability and fecundity in Atlantic salmon×brown trout hybrids and backcrosses, it is an unlikely mechanism for doing so in these species. Although our detection of the interspecific recombination event was aided by relatively high sequence divergence (5.62%) between Atlantic salmon and brown trout at this region of the mtDNA ND1 gene and the relatively long tracts of sequence involved, interspecific recombination between shorter sections of mtDNA genomes showing lower levels of divergence may go undetected and have an insidious effect on phylogenetic studies involving mtDNA. Importantly, interspecific mtDNA recombination may be another consideration in evaluating the usefulness of mtDNA barcoding for species identification (Rokas et al. 2003; Rubinoff et al. 2006).
Acknowledgments
This study was funded by a NERC studentship to K.L.C.
References
- Altschul S.F, Gish W, Miller W, Myers E.W, Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andolfatto P, Scriber J.M, Charlesworth B. No association between mitochondrial DNA haplotypes and a female-limited mimicry phenotype in Papilio glaucus. Evolution. 2003;57:305–316. doi: 10.1111/j.0014-3820.2003.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Bensasson D, Zhang D.-X, Hartl D.L, Hewitt G.M. Mitochondrial pseudogenes: evolution's misplaced witnesses. Trends Ecol. Evol. 2001;16:314–321. doi: 10.1016/s0169-5347(01)02151-6. doi:10.1016/S0169-5347(01)02151-6 [DOI] [PubMed] [Google Scholar]
- Birky C.W. The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu. Rev. Genet. 2001;35:125–148. doi: 10.1146/annurev.genet.35.102401.090231. doi:10.1146/annurev.genet.35.102401.090231 [DOI] [PubMed] [Google Scholar]
- Ciborowski K.L, Consuegra S, García de Leániz C, Wang J, Beaumont M.A, Jordan W.C. Stocking may increase mitochondrial DNA diversity but fails to halt the decline of endangered Atlantic salmon populations. Conserv. Gen. 2007 doi:10.1007/s10592-007-9286-2 [Google Scholar]
- Galbreath P.F, Thorgaard G.H. Sexual maturation and fertility of diploid and triploid Atlantic salmon×brown trout hybrids. Aquaculture. 1995;137:299–311. doi:10.1016/0044-8486(95)01115-3 [Google Scholar]
- Gantenbein B, Fet V, Gantenbein-Ritter I.A, Balloux F. Evidence for recombination in scorpion mitochondrial DNA (Scorpiones: Buthidae) Proc. R. Soc. B. 2005;272:697–704. doi: 10.1098/rspb.2004.3017. doi:10.1098/rspb.2004.3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García de Leániz C, Verspoor E. Natural hybridization between Atlantic salmon, Salmo salar, and brown trout, Salmo trutta, in northern Spain. J. Fish Biol. 1989;34:41–46. doi:10.1111/j.1095-8649.1989.tb02956.x [Google Scholar]
- Gyllensten U, Wharton D, Josefsson A, Wilson A.C. Paternal inheritance of mitochondrial-DNA in mice. Nature. 1991;352:255–257. doi: 10.1038/352255a0. doi:10.1038/352255a0 [DOI] [PubMed] [Google Scholar]
- Hoarau G, Holla S, Lescasse R, Stam W.T, Olsen J.L. Heteroplasmy and evidence for recombination in the mitochondrial control region of the flatfish Platichthys flesus. Mol. Biol. Evol. 2002;19:2261–2264. doi: 10.1093/oxfordjournals.molbev.a004049. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Schwartz M, Brown T.A, Ebralidse K, Kunz W.S, Clayton D.A, Vissing J, Khrapko K. Recombination of human mitochondrial DNA. Science. 2004;304:981. doi: 10.1126/science.1096342. doi:10.1126/science.1096342 [DOI] [PubMed] [Google Scholar]
- Kvist L, Martens J, Nazarenko A.A, Orell M. Paternal leakage of mitochondrial DNA in the great tit (Parus major) Mol. Biol. Evol. 2003;20:243–247. doi: 10.1093/molbev/msg025. doi:10.1093/molbev/msg025 [DOI] [PubMed] [Google Scholar]
- Ladoukakis E, Zouros E. Recombination in animal mitochondrial DNA: evidence from published sequences. Mol. Biol. Evol. 2001;18:2127–2131. doi: 10.1093/oxfordjournals.molbev.a003755. [DOI] [PubMed] [Google Scholar]
- Lole K.S, Bollinger R.C, Paranjape R.S, Gadkari D, Kulkarni S.S, Novak N.G, Ingersoll R, Sheppard H.W, Ray S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt D.H, Hyman B.C. Animal mitochondrial DNA recombination. Nature. 1997;387:247. doi: 10.1038/387247a0. doi:10.1038/387247a0 [DOI] [PubMed] [Google Scholar]
- Magoulas A, Zouros E. Restriction-site heteroplasmy in anchovy (Engraulis encrasicolus) indicates incidental biparental inheritance of mitochondrial DNA. Mol. Biol. Evol. 1993;10:319–325. [Google Scholar]
- Pendas A.M, Moran P, Martinez J.L, García-Vázquez E. Applications of 5S rDNA in Atlantic salmon, brown trout, and in Atlantic salmon×brown trout hybrid identification. Mol. Ecol. 1995;4:275–276. doi: 10.1111/j.1365-294x.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Piganeau G, Gardner M, Eyre-Walker A. A broad survey of recombination in animal mitochondria. Mol. Biol. Evol. 2004;21:2319–2325. doi: 10.1093/molbev/msh244. doi:10.1093/molbev/msh244 [DOI] [PubMed] [Google Scholar]
- Rokas A, Ladoukakis E, Zorros E. Animal mitochondrial DNA recombination revisited. Trends Ecol. Evol. 2003;18:411–417. doi:10.1016/S0169-5347(03)00125-3 [Google Scholar]
- Rubinoff D, Cameron S, Will K. A genomic perspective on the shortcomings of mitochondrial DNA for “barcoding” identification. J. Hered. 2006;97:581–594. doi: 10.1093/jhered/esl036. doi:10.1093/jhered/esl036 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Vissing J. Paternal inheritance of mitochondrial DNA. New Engl. J. Med. 2002;347:576–580. doi: 10.1056/NEJMoa020350. doi:10.1056/NEJMoa020350 [DOI] [PubMed] [Google Scholar]
- Shitara H, Hayashi J, Takahama S, Kaneda H, Yonekawa H. Maternal inheritance of mouse mtDNA in interspecific hybrids: segregation of the leaked paternal mtDNA followed by the prevention of subsequent paternal leakage. Genetics. 1998;148:851–857. doi: 10.1093/genetics/148.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P, Moreno R.D, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol. Reprod. 2000;63:582–590. doi: 10.1095/biolreprod63.2.582. doi:10.1095/biolreprod63.2.582 [DOI] [PubMed] [Google Scholar]
- Thyagarajan B, Papua R.A, Campbell C. Mammalian mitochondria possess homologous DNA recombination activity. J. Biol. Chem. 1996;271:27 536–27 543. doi: 10.1074/jbc.271.44.27536. doi:10.1074/jbc.271.44.27536 [DOI] [PubMed] [Google Scholar]
- Tsaousis A.D, Martin D.P, Ladoukakis E.D, Posada D, Zouros E. Widespread recombination in published animal mtDNA sequences. Mol. Biol. Evol. 2005;22:925–933. doi: 10.1093/molbev/msi084. doi:10.1093/molbev/msi084 [DOI] [PubMed] [Google Scholar]
- Ujarvi B, Dowton M, Madsen T. Mitochondrial DNA recombination in a free-ranging Australian lizard. Biol. Lett. 2007;3:189–192. doi: 10.1098/rsbl.2006.0587. doi:10.1098/rsbl.2006.0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh B, Dandona N, Brenner S. Fugu genome does not contain mitochondrial pseudogenes. Genomics. 2006;87:307–310. doi: 10.1016/j.ygeno.2005.11.007. doi:10.1016/j.ygeno.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Verspoor E, Hammar J. Introgressive hybridisation in fishes: the biochemical evidence. J. Fish Biol. 1991;39:309–334. doi:10.1111/j.1095-8649.1991.tb05094.x [Google Scholar]