Abstract
The rostral part of the dorsal midbrain, known as the superior colliculus in mammals or the optic tectum in birds, receives a substantial retinal input and plays a diverse and important role in sensorimotor integration. However, little is known about the development of specific subtypes of neurons in the tectum, particularly those which contribute tectofugal projections to the thalamus, isthmic region, and hindbrain. Here we show that two homeodomain transcription factors, Brn3a and Pax7, are expressed in mutually exclusive patterns in the developing and mature avian midbrain. Neurons expressing these factors are generated at characteristic developmental times, and have specific laminar fates within the tectum. In mice expressing βgalactosidase targeted to the Pou4f1 (Brn3a) locus, Brn3a-expressing neurons contribute to the ipsilateral but not the contralateral tectofugal projections to the hindbrain. Using misexpression of Brn3a and Pax7 by electroporation in the chick tectum, combined with GFP reporters, we show that Brn3a determines the laminar fate of subsets of tectal neurons. Furthermore, Brn3a regulates the development of neurons contributing to specific ascending and descending tectofugal pathways, while Pax7 globally represses the development of tectofugal projections to nearly all brain structures.
Keywords: Brn3, Brn3a, Pou4f1, POU-domain, Pax7, superior colliculus, midbrain, tectofugal, tectospinal, tectobulbar
INTRODUCTION
The outer layers of the rostral part of the dorsal midbrain, known as the optic tectum in non-mammalian vertebrates and the superficial part of the superior colliculus (SC) in mammals, constitutes one of the principal retino-recipient areas of the central nervous system. In all vertebrates, midbrain regions deep to the retino-recipient layers play key roles in the integration of visual stimuli with auditory and tactile inputs, and govern motor responses to these stimuli, such as saccadic eye movements (King, 2004). The tectum is highly laminated, with superficial layers that receive direct sensory input from the retina, and deeper layers that perform the integration of multiple sensory modalities and output functions. In birds and reptiles, the lamination of the tectum is especially distinct, and in birds fifteen principal tectal layers have been described, designated from superficial to deep as L1-L15. Although retinal fibers directly innervate only the most superficial seven layers, tectal neurons residing as deep as L13 receive retinal synaptic input via extensive dendritic arbors which extend into the superficial layers (Karten et al., 1997; Luksch et al., 1998).
Retinal inputs to the tectum/SC are organized in a precise topographic map, in which the temporal-nasal and dorsal-ventral axes of the retina correspond to the anterior-posterior and lateral-medial axes of the midbrain. Extensive investigation of the developmental patterning of this map has revealed gradients of transcription factors and cell surface receptors in the retina and tectum which guide its formation (McLaughlin and O’Leary, 2005; Thanos and Mey, 2001).
The anatomy of the tectal efferents, or tectofugal pathways, has also been extensively studied (Harting, 1977; Harting et al., 1980; Holcombe and Hall, 1981a; Holcombe and Hall, 1981b; Hunt and Kunzle, 1976; Reiner and Karten, 1982). Tectal efferents include ascending pathways to the ventral lateral geniculate, the nucleus rotundus of the thalamus and the pretectum, and descending pathways which includeprojections to the isthmic nuclei, the crossed tectobulbar tract (CTB), and ipsilateral tectopontine tract (ITP). Neurons contributing to the major tectofugal systems originate in multiple layers, but are concentrated predominantly in L10 and L13-15. Specifically, the ascending projections to the nucleus rotundus receive major inputs from L13 (Karten et al., 1997). Tectofugal neurons innervating the isthmic nuclei reside predominantly in L10 (Wang et al., 2006; Wang et al., 2004), while the CTB receives inputs primarily from L13-15, and the ITP originates from multiple layers (Reiner and Karten, 1982). However, compared to the retinotectal inputs, much less is known about the mechanisms which guide the development of tectal output pathways.
Development of the laminar tectum is preceded by patterning events which establish the tectal compartment along the rostrocaudal and dorsoventral axes of the early embryo. Along the rostrocaudal axis, the midbrain is patterned by signals from the midbrain-hindbrain junction, particularly FGF8, which induces graded expression of the homeodomain transcription factor engrailed (Liu and Joyner, 2001; Nakamura, 2001; Prakash and Wurst, 2004). Less is known about the dorsoventral patterning of the midbrain, but prior work has shown that the ventral signal Shh is sufficient to suppress the development of the tectal lamina, and induce a pattern of gene expression characteristic of the tegmentum (Agarwala et al., 2001; Fedtsova and Turner, 2001; Watanabe and Nakamura, 2000).
The neuroepithelium of the developing tectal compartment is also characterized by the expression of the paired-homeodomain transcription factors Pax3 and Pax7. Ventralization of the midbrain by Shh represses Pax7 expression (Blaess et al., 2006; Fedtsova and Turner, 2001), while Shh blockade induces ectopic Pax7 expression (Bayly et al., 2007), and early mis-expression of Pax7 in the diencephalon and ventral midbrain can induce the expression of other tectal markers (Matsunaga et al., 2001). Pax7 expression persists in laminar pattern a subset of tectal neurons (Thomas et al., 2004), but its role in postmitotic neurons is largely unknown. In prior work, we have shown that the POU-domain transcription factor Brn3a is also expressed in a lamina-specific pattern in the mature tectum (Fedtsova and Turner, 2001). However, little is known about the transcriptional mechanisms controlling the development of the tectal layers or the specification of functional subclasses of tectal neurons.
Here we use the expression of green fluorescent protein (GFP) at specific stages in the developing chick tectum to define the distinct temporal and lamina-specific generation of Brn3a- and Pax7-expressing tectal neurons, and the timing of the generation of neurons contributing to specific tectofugal pathways. Using transgenic mice expressing a LacZ marker targeted to the Brn3a locus, we show that that Brn3a-expressing neurons contribute to the descending ITP but not the CTB. Loss of Brn3a function in these mice also demonstrates that this factor is also necessary for the development of the rubrospinal tract in the midbrain tegmentum. Induced expression of Brn3a at appropriate developmental stages in the chick alters the laminar fate of developing tectal neurons, and prevents neurons from projecting via the CTB, but has little effect on tectothalamic pathways. In contrast to Brn3a-expressing tectal neurons, Pax7-expressing neurons do not appear to contribute to tectothalamic or tectoisthmic pathways. Misexpression of Pax7 prevents neurons from projecting to the nucleus rotundus, and also to the major descending tectofugal pathways. Together these results begin to define a program for the transcriptional regulation of neuronal diversity in the tectum/superior colliculus.
MATERIALS AND METHODS
Animals
Two strains of transgenic mice were employed in these studies, both of which lack a functional Brn3a gene. Mice bearing a null allele of Brn3a (Eng et al., 2001; Xiang et al., 1996) and a tauLacZ expression cassette replacing the Brn3a coding sequence (Quina et al., 2005) have been previously described. Embryos with the genotypes Brn3atauLacZ/+ (control) and Brn3atauLacZ/- (knockout) were generated by interbreeding mice heterozygous for the two mutant alleles. Following mouse matings, noon of the day of the detection of a mucous plug was designated embryonic day 0.5. (E0.5), and embryos were also staged according to the method of Theiler (Theiler, 1972). Mice and embryos were genotyped for the Brn3a null allele, Brn3a wildtype allele, and tauLacZ knockin allele by PCR.
Fertile White Leghorn chicken eggs were obtained from a local source, and were incubated in a humidified atmosphere at 38°C. Embryos were staged according to the system of Hamburger (Hamburger and Hamilton, 1951). Embryos of appropriate developmental ages were used for electroporation, with E3 corresponding to st19, E4 to st24, E5 to st27, and E6 to st29. Cholera toxin B-subunit (CTxB) injections were performed in 3-day-old chicks, and analysis was done four days after injection, in 7-day-old animals. Immunohistochemical analysis of the tectal lamina of mature animals was performed in 5- week-old chickens. To the extent possible, nomenclature and abbreviations for anatomical structures are drawn from standard atlases (Paxinos and Watson, 2005; Puelles et al., 2007).
Immunohistochemistry/immunofluorescence
Immunostaining of both mouse and chicken tissue was performed on paraformaldehyde-fixed cryosections. Early chicken embryos and isolated E12 chicken brains were fixed by immersion in 4% paraformaldehyde in PBS at 4C. The time of fixation varied from 40 minutes for E3 embryos to 4 hours for E12 brains. Hatchling chicks and 5- week chicken brains were fixed by perfusion with 4% paraformaldehyde in PBS, followed by immersion in the same solution for 2 hours. Antibodies used were: rabbit anti-Brn3a (Fedtsova and Turner, 1995), rabbit anti-GFP (Abcam), chicken anti-GFP (Avian Laboratories), mouse monoclonal anti-Pax7 (Developmental Studies Hybridoma Bank), goat anti-beta-galactosidase (Biogenesis), rabbit anti-intermediate neurofilament (Novus Biologicals). Secondary anti-mouse, anti-rabbit and anti-chicken fluorescent antibodies conjugated with Alexa Fluor 488, Alexa Fluor 594, and Alexa Fluor 647 were obtained from Molecular Probes.
Electroporation
Eggs were prepared for electroporation using standard methods, by cutting a 2 × 3 cm window in the eggshell and the withdrawal of a small amount of albumen (Nakamura and Funahashi, 2001). Electroporation in ovo was then performed in 3-, 4-, 5-, and 6-day embryos. A solution containing the expression plasmid at 0.33 μg/μl and 0.015% Fast Green in distilled water was injected into the lumen of the mesencephalon with a drawn glass pipette. Electrophoresis of the plasmid in ovo was performed by application of electric pulses using microelectrodes (BTX Genetrodes Model 516) and a square-wave electroporator (BTX, model T820), with the following settings: electrode gap width 4-6 mm, field strength 25 V/cm, pulse length 50 ms, pulse interval 1 sec, with five pulses applied in a unipolar direction. After electroporation, embryos were sealed with tape and returned to the incubator until the appropriate stage for analysis.
Plasmids for electroporation were designated pTS-GFP, pTS-Brn3a and pTS-Pax7. The pTS parent vector contains a CMV enhancer, a chick βactin promoter, a multiple cloning site, IRES sequence, eGFP expression cassette, and a βglobin polyadenylation signal, and is derived from the vector pMES (Swartz et al., 2001). Derivation of pTS from pMES involved replacement of the multiple cloning site and modification of restriction sites flanking the CMV enhancer. The expression cassette encoding the full mouse Brn3a open reading frame has been described (Gruber et al., 1997) and the coding sequence corresponds to Entrez accession NP_035273. The expression clone for chick Pax7 was a gift of Atsushi Kawakami (Kawakami et al., 1997).
Retrograde tracing
Cholera toxin B-subunit (CTxB) was used as a retrograde tracer to examine the tectofugal pathways in 3-day old chicks. Specimens were analyzed 4 days after injection, at day 7 post-hatching. CTxB was injected into the posterolateral and central anterior subdivisions of the nucleus rotundus, and the isthmic nucleus semilunaris as described in a previous study (Wang et al., 2004). Briefly, animals were anesthetized using a mixture of ketamine and xylazine, placed in a stereotaxic head holder, the skull exposed, and a hole made above the injection site. A solution of 1% CTxB (List Laboratories, Campbell, CA) in phosphate buffered saline was injected through a glass micropipette using a pressure device (PicoSpritzer II; General Valve, Fairfield, NJ). After injection, the micropipette was withdrawn from the brain and the wound was closed.
After four days, animals were anesthetized with an overdose of anesthesia and transcardially perfused with 0.9% saline followed by chilled 4% paraformaldehyde. The brain was removed from the skull, postfixed overnight in the paraformaldehyde solution, and then transferred to 30% sucrose until no longer buoyant. Frozen sections were cut at 30 μm on a freezing sliding microtome. Alternating series of sections were collected and stained for standard immunoperoxidase or immunofluorescence for CTxB using goat anti-CTxB primary antibody (List Laboratories).
For standard immunoperoxidase staining, sections were then incubated in avidin-biotin-peroxidase complex solution (ABC Elite kit; Vector Laboratories, Burlingame, CA) diluted 1:100 in PBS with 0.3% Triton X-100 for 1 hour at room temperature. Sections were incubated for 3-5 minutes in 0.025% 3-3-diaminobenzidine (DAB; Sigma) with 0.01% hydrogen peroxide in PB. Sections were mounted on gelatin-coated slides and stained with 0.05% osmium tetroxide for 30 seconds. Sections were then dehydrated, cleared, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
RESULTS
Generation of Brn3a and Pax7 tectal neurons and the tectofugal tracts
In order to better understand the role of transcription factors in specifying tectal identity and axon pathfinding we turned to the avian brain, where the functional architecture of the tectal lamina and the tectofugal projections have been extensively studied. Examination of the adult chick brain (Figure 1A-C) reveals that both Brn3a and Pax7 expressing neurons are enriched in specific lamina. Brn3a cells are concentrated in layer 8 (L8), the inner part of L10, and L13, while Pax7 neurons are most abundant in L4, L6, L8, and the outer part of L10. Remarkably, even where Brn3a and Pax7 cells closely intermingle (L8, L10), cellular co-expression is not observed. Because Pax7 is expressed throughout the tectal neuroepithelium prior to cell cycle exit (Fedtsova and Turner, 2001), it may be inferred that some tectal neurons, including all of the Brn3a-expressing cells, switch off Pax7 expression during development, and some do not.
Figure 1. Lamina-specific expression of Brn3a and Pax7 in the chick tectum.
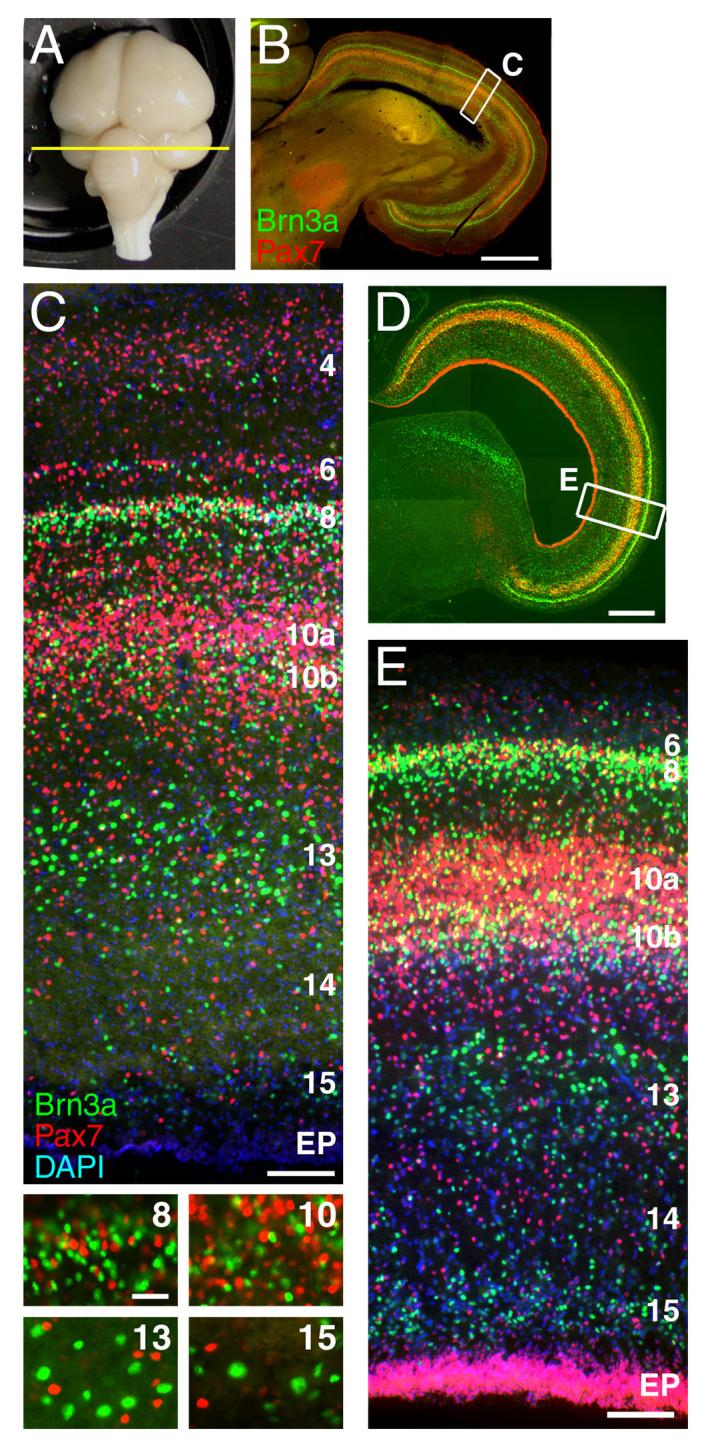
The expression patterns of Brn3a and Pax7 was examined in a 5 week-old chick (A-C) and in an E12 chick embryo (D-E). (A) An intact 5 week chick brain showing the plane of section in subsequent views. (B) Low magnification view of the tectum with immunofluorescent staining for Brn3a and Pax7. (C) Laminar distribution of Brn3a and Pax7 neurons in the mature tectum (rotated relative to B, as shown by inset box). High magnification views of layers 8, 10, 13 and 15 demonstrate that Brn3a and Pax7 are not co-expressed at the cellular level. (D,E) Expression of Brn3a and Pax7 at E12, at which time the laminar expression pattern of these markers has been established, and all of the principal deep layers of the tectum can be identified. In C and E counterstaining with DAPI is used to identify all nuclei. Tectal lamina are designated numerically; EP, ependymal layer. View E is rotated relative to D, as shown by inset box. The nomenclature for the tectal layers used here, derived from the work of Ramon y Cajal (1995), is directly related to an alternate system in which layer 1 corresponds to the stratum opticum (SO), layers 2-11 to the stratum griseum et fibrosum superficiale (SGFS), sublayers a-j, layer 13 to the stratum griseum centrale (SGC), layer 14 to the stratum album centrale (SAC) and layer 15 to the stratum griseum et fibrosum periventriculare (SGFP, LaVail and Cowan, 1971a). Scale: B, 1mm; C, 100μm (insets, 25μm); D, 500μm; E, 50μm.
In order to find a suitable endpoint for developmental studies of neuronal subtypes in the tectum, we examined the expression of Brn3a and Pax7 at various embryonic stages. Prior studies using tritiated thymidine (LaVail and Cowan, 1971a; LaVail and Cowan, 1971b; Wu et al., 2000) and data from the present study using BrdU incorporation (data not shown) indicate that neurogenesis in the tectum is largely complete by E6. However, subsequent neuronal migration and the generation of axon tracts and glia are necessary to complete the laminar architecture. By E12 it is possible to clearly distinguish L8-L15, which include all of the neurons giving rise to tectofugal projections, and their complements of Brn3a and Pax7-expressing neurons (Figure 1D,E). Thus E12 is a suitable stage for studies of layer-specific tectal neurogenesis.
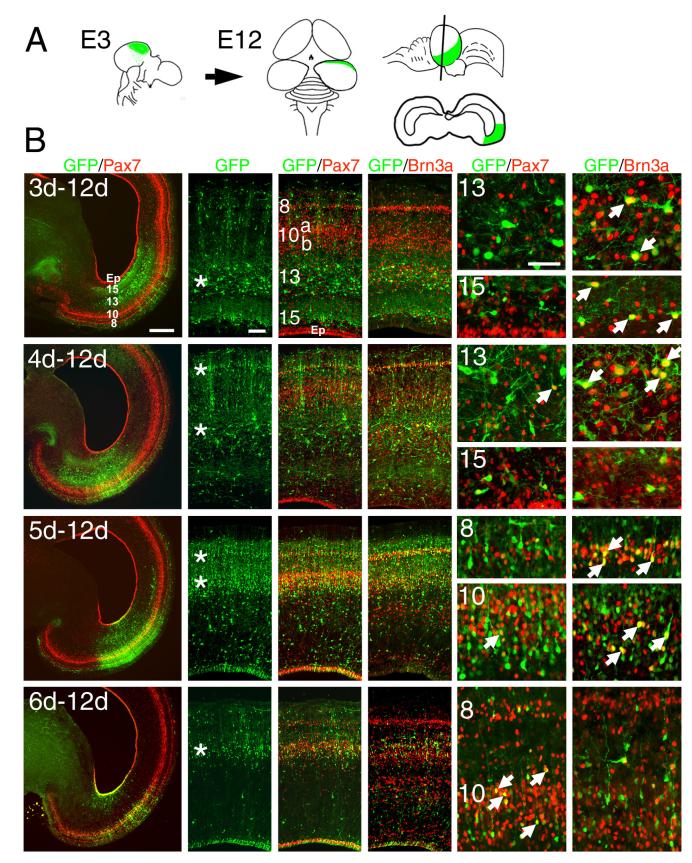
Next we sought to establish the relationship between the birthdate of tectal neurons, their laminar position, and transcription factor expression. Electroporation of a marker plasmid into the neuroepithelium can be used to establish the time of cell-cycle exit of newly generated neurons in a manner analogous to 3H-thymidine or BrdU incorporation. When a plasmid is injected into the lumen of the neural tube and an electrical field is applied, only dividing neuroepithelial cells exposed to the ventricular space are transfected (Figure S1). The strongest labeling will identify neurons which exit the cell cycle shortly after the time of electroporation, while cells that continue to divide will dilute the signal. Neurons which have previously exited the cell cycle and no longer contact the ventricle will not be accessible to labeling.
In order to establish the birthdates of Brn3a and Pax7-expressing tectal neurons, a GFP expression plasmid was electroporated into the tectum at each stage from E3 to E6, and tecta were examined at E12 (Figure 2). The applied electrical field directed the plasmid into the dorsolateral tectum, generally in a zone between ten and sixty degrees from the midline axis (Figure 2A). However, due to extensive cellular proliferation in the midbrain during the course of development, and the resulting rotation of the tectum which shifts the early rostral tectum laterally (Thanos and Mey, 2001), the labeled zone resided in a lateral position by E12. The neurons heavily labeled with the GFP marker at E3 migrated predominantly to L13-L15. Many of these early-born neurons expressed Brn3a, and had large soma and nuclei characteristic of tectal ganglion cells, a class of tectofugal neurons with extensive dendritic arbors (Karten et al., 1990). At E4, neurogenesis continued in L13-15, and a significant number of strongly labeled neurons also began to appear in L8. Many of the L13 neurons generated at this stage were Brn3a positive, and small, scattered GFP-labeled neurons in these layers were noted to express Pax7. By E5, generation of Brn3a-expressing neurons in the deep layers was largely complete, but active generation of Brn3a positive cells was observed in L8 and the inner part of L10. At E6, the generation of Brn3a-expressing cells in L10 was largely complete, and the most prominent newly generated neurons were Pax7-expressing cells of the outer part of L10. This pattern of neurogenesis at E5-E6 supports the idea that the outer and inner sublayers of L10, which have been termed L10a and L10b (Wang et al., 2006), are developmentally and functionally distinct.
Figure 2. Birthdating of Brn3a and Pax7 expressing tectal neurons.
A plasmid encoding a GFP reporter was electroporated unilaterally into the tectal neuroepithelium at each day from E3 to E6, and the electroporated tecta were analyzed at E12 for GFP, Brn3a and Pax7 expression. In each case, strong expression of the GFP marker indicates neurons which exited the cell cycle (i.e. were “born”) shortly after electroporation. (A) Overall strategy for the electroporation experiments. Rotation of the tectum between E6 and E12 results in a shift of the dorsal/rostral electroporated area at E3-E5 to a lateral position by E12 (Thanos and Mey, 2001). However, the region of electroporation can be recognized at any stage by the GFP-labeled radial processes of a subset of the electroporated cells, and a fraction of labeled cells that remain in the ependymal zone. (B) Brn3a and Pax7 expressing neurons generated at E3-E6, and analyzed at E12. In the views showing GFP expression alone, asterisks indicate the layers with the greatest numbers of neurons born soon after the electroporation. In the high magnification views, arrows indicate cells co-expressing GFP and the indicated transcription factor. The predominant pattern of neurogenesis is for newly born cells to be generated first in the in deep layers (L13-L15) at E3-E4, then in superficial layers (L8) at E4-E5, and finally in the intermediate layer (L10) at E5-E6. In layer 10, the peak generation of Brn3a-expressing cells (E5) appears to precede that of the Pax7-expressing population (E6). Data shown are representative of at least 5 cases electroporated at each developmental stage. Scale: B, low power, 500μm; medium power, 100μm; high power, 50μm.
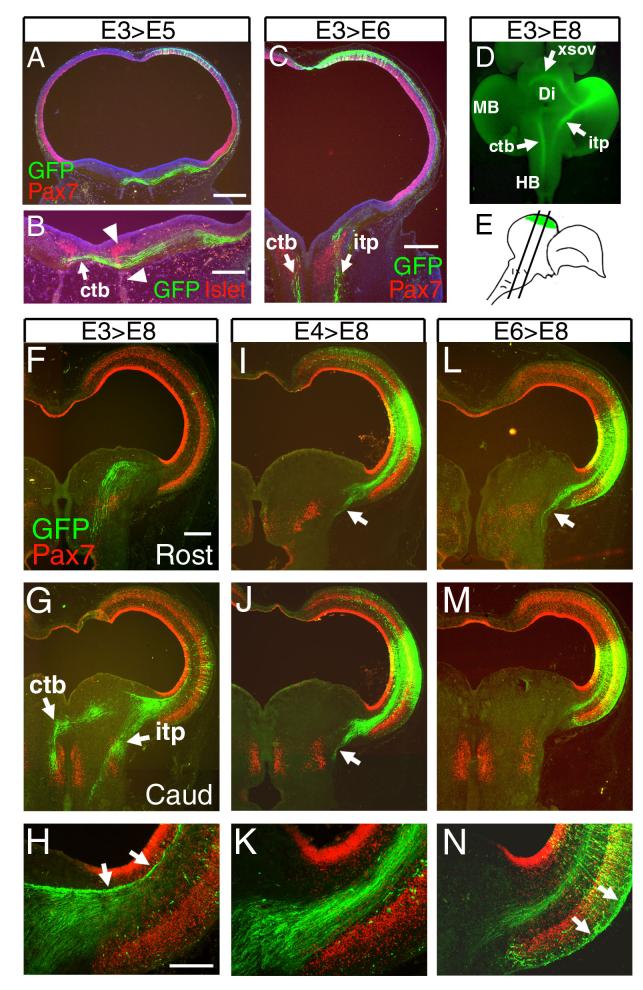
We next used electroporation of a GFP expression plasmid to determine the birthdates of neurons that contribute to specific tectofugal tracts (Figure 3). We first examined the hindbrain of embryos electroporated at E3 and fixed after 2-3 days of incubation in order to reveal the initial trajectories of axons contributing to the crossed tectobulbar tract (CTB) and the ipsilateral tectopontine tract (ITP). Tectofugal neurons born on E3 extend fibers across the midline by E5 (Figure 3A,B). By E6, the CTB and ITP can be distinguished in their characteristic medial and lateral positions in the hindbrain (Figure 3C).
Figure 3. Genesis of the descending tectofugal tracts.
Neurons contributing to the tectofugal tracts were marked by the electroporation of a plasmid encoding a GFP reporter into the tectal neuroepithelium at various stages from E3 to E6. (A,B) Embryos electroporated at E3 and examined at E5. Axons of the crossed tectobulbar (CTB) pathway are strongly labeled, and have crossed the midline by E5. (B) Co-labeling with GFP and Islet1/2 shows the relationship of the decussation of the CTB (arrow) to the developing oculomotor neurons and tract (arrowheads). (C) Embryos electroporated at E3 and examined at E6. Descending fibers of the CTB and ipsilateral tectopontine tract (ITP) can be easily discerned by this stage. (D) Ventral, whole-mount view of GFP fluorescence in a brain electroporated at E3 and examined at E8, showing the electroporated region of the tectum and projections to hindbrain and diencephalon. (E) Diagram depicting the region of electroporation and plane of section for subsequent views of the midbrain and hindbrain. (F-N) Descending tectofugal axons from embryos electroporated at stages from E3-E6, and examined at E8. (F-H) In embryos electroporated at E3, labeled axons are prominent in both the CTB and ITP. Some of the early-generated neurons contribute to axons which fasciculate in the deepest tectal lamina, adjacent to the ependymal layer (arrows, H). (I-K) In embryos electroporated at E4 axons of the ITP continue to be strongly labeled (arrows I,J), but the generation of neurons contributing to the CTB is nearly complete. Similar results were obtained at E5 (not shown). (L-N) By E6 the generation of neurons contributing to the descending pathways is nearly complete. Late born neurons migrate extensively and send axons tangentially, superficial to layer 10 (arrows, N). Views A-C are counterstained with DAPI to reveal all nuclei. In F-H, the cell bodies of most of the electroporated neurons are out of the plane of section. In low power views of the tectum at E8 (F,G,I,J,L,M), GFP expression does not appear lamina-specific as it does in Figure 2 (E12) because the migration of neurons to specific layers is ongoing at E8, and because the long exposure times needed to reveal distant axon projections obscure the lamina-specific expression of GFP within the tectum. 10, future tectal layer 10; Ctb, crossed tectobulbar tract; Di, diencephalon; Ep, ependymal layer; HB, hindbrain; itp, ipsilateral tectopontine tract; MB, midbrain; xsov, ventral supraoptic decussation. Data shown are representative of at least 5 cases at E3 and E4 and 3 cases at E6. Scale: A, 400μm; B, 200μm; C, 400μm; F, 500μm; H, 200μm.
To obtain a more complete picture of the genesis of the neurons contributing to the tectofugal pathways, tecta were electroporated at each developmental day from E3 to E6, and examined at E8 for the descending CTB and ITP (Figure 3D-N) and at E12 for the ascending pretectal and tectothalamic tracts (Figure 4). Neurons contributing to the CTB were heavily labeled by electroporation at E3 (Figure 3D,G,H). Early-born neurons also made a substantial contribution to the ITP (Figure 3D,G). By E4, neurons contributing to the CTB could no longer be labeled (Figure 3 I,J), but generation of ITP neurons continued until E6 (Figure 3L-N). ITP projections originating from neurons born at E3-E4 tended to take a more medial course than the axons of later-born neurons contributing to this tract, consistent with prior observations that the ITP is heterogeneous.
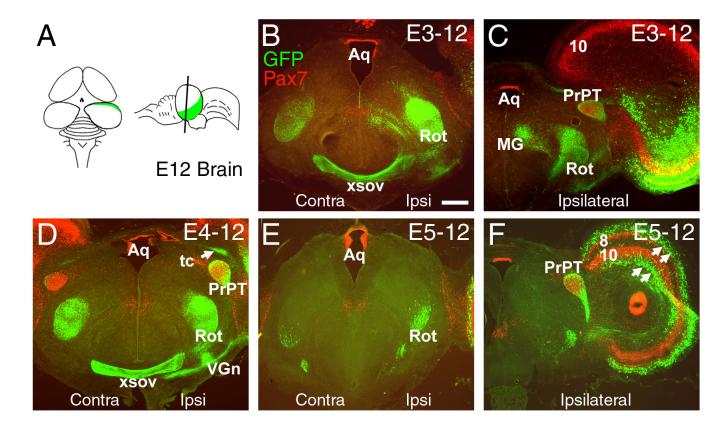
Figure 4. Genesis of the ascending tectofugal tracts.
A plasmid expressing a GFP reporter was electroporated into the tectal neuroepithelium at various stages from E3 to E5, and the ascending tectofugal tracts were examined at E12. (A) Plane of section for subsequent views. (B, C) Electroporation at E3 results in heavy labeling of the ipsilateral nucleus rotundus, and moderate labeling of the contralateral nucleus rotundus and the pretectal nucleus. Labeling of the medial geniculate nucleus (nucleus ovoidalis) in embryos electroporated at E3 varied between cases and probably results from incidental labeling of the inferior colliculus. Section (C) is caudal to (B). (D) Electroporation at E4 heavily labels the ipsilateral and contralateral nucleus rotundus, the pretectal nucleus, the ventral geniculate nucleus, the xsov and the tectal commissure. (E,F) Electroporation at E5 shows that generation of neurons contributing to the nucleus rotundus is nearly complete at this stage; some fibers innervating the area surrounding the principal pretectal nucleus continue to be heavily labeled. Section (F) is caudal to (E). Arrows in (F) indicate tectal neurons which have migrated laterally away from the area of electroporation in distinct zones superficial to layer 8 and deep to layer 10. Aq, aqueduct; MG, medial geniculate nucleus (nucleus ovoidalis); PrPT, principal pretectal nucleus; Rot, nucleus rotundus; 8, 10, tectal lamina; tc, tectal commissure; VGn, ventral geniculate nucleus (pregeniculate nucleus); xsov, ventral supraoptic decussation. Data shown represent at least 3 cases at each developmental stage. Scale 400μm.
Labeling of the tectal projections to the thalamus and pretectum was observed from E3 to E5 (Figure 4), with a peak in all structures at E4 (Figure 4D). Electroporation at E3 preferentially labeled the ipsilateral nucleus rotundus (Figure 4B), while labeling at E5 was weak in the rotundus but remained strong in the area surrounding the principal pretectal nucleus (Figure 4E,F). Because the pretectal nucleus and the subprectal nucleus have been previously shown to be innervated by collateral fibers of L13 tectal neurons which also innervate the nucleus rotundus (Karten et al., 1997), these staged electroporations may reveal previously unrecognized diversity in the tectal neurons projecting to this area. Taken together, these data demonstrate that tectal neurogenesis takes place primarily between E3 and E6 of development and consists of the sequential generation of neurons with specific laminar fates, transcription factor identities, and targets of innervation.
Tectofugal tracts in Brn3a null mice
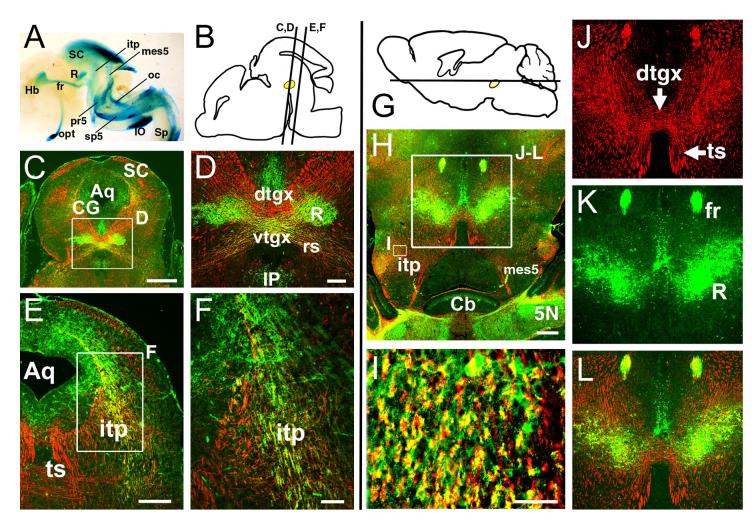
To begin to understand the function of these transcription factors in midbrain development, we first took a tract-tracing approach using a mouse transgenic model in which the Brn3a coding sequence has been replaced with a marker consisting of a fusion product of bovine tau and LacZ (Quina et al., 2005). Staining of intact preparations of the CNS of E16.5 Brn3atauLacZ/+ embryos for βgalactosidase (βgal) activity (Figure 5A) reveals the superior colliculus and descending tectofugal tracts, as well as the habenula and habenulopeduncular pathway (fasciculus retroflexus), the red nucleus, the olivocerebellar pathway, and peripheral sensory fibers innervating the trigeminal nucleus.
Figure 5. Tectofugal projections of Brn3a neurons in the mouse.
Mice expressing a LacZ marker targeted to the Brn3a locus were used to examine the tectofugal pathways. In all immunofluorescence views, βgalactosidase expressed from the Brn3a/tauLacZ allele is labeled in green, and intermediate neurofilament immunoreactivity appears in red. In accordance with accepted terminology in rodents (Paxinos and Watson, 2005) the crossed descending tract from the tectum is here referred to as the tectospinal tract (TS) rather than the CTB. (A) An E16.5 Brn3atauLacZ/+ embryo, hemisected at the midline, and stained for βgalactosidase activity with X-gal. The major tracts formed by Brn3a-expressing neurons, including the ITP and tract/neurons of the mesV, are shown. Brn3a neurons also contribute to the retinothalamic/retinocollicular pathway, the habenulopeduncular tract, the olivocerebellar tract, and trigeminal afferent fibers. (B) Sagital view of an E16.5 brain, showing the planes of section in views C-F. Location of the red nucleus is shown in yellow. (C,D) Coronal section of the E16.5 tectum at the level of the red nucleus. βgalactosidase immunoreactive fibers contribute to the rubrospinal tract but not the decussating fibers of the CTB. The inset box in C appears enlarged in D. (E,F) More rostral view of the tectofugal pathways showing βgalactosidase expression in the ITP, but not the TS. The inset box in E appears enlarged in F. (G) Schematic of the postnatal (P10) brain showing plane of section for subsequent views. (H-L) Horizontal section of the postnatal brain of a Brn3atauLacZ/+ mouse at the level of the dorsal tegmental decussation shows expression of βgalactosidase in the red nucleus and ITP, but not the decussating fibers of the TS. The small inset box in H appears enlarged in I to show co-localization of neurofilament and LacZ staining in the ITP; the large inset box in H appears enlarged in J-L. 5N, trigeminal nucleus; Aq, aqueduct; Cb, cerebellum; CG, central gray; dtgx, dorsal tegmental decussation; fr, fasciculus retroflexus; Hb, habenula; IO, inferior olive; IP, interpeduncular nucleus; itp, ipsilateral tectopontine tract; mes5, mesencephalic trigeminal (and associated tract); oc, olivocerebellar tract; opt, optic tract; pr5, principal tract of trigeminal; R, red nucleus; rs, rubrospinal tract; Sp, spinal cord; sp5, spinal tract of trigeminal; ts, tectospinal tract; vtgx, ventral tegmental decussation. Scale: C, 1μm; D,E 200μm; F, 50μm; H, 400μm; I, 50μm; J, 400μm.
We then examined the tectofugal pathways of Brn3atauLacZ/+ mice in detail at E16.5, using immunofluorescence for the βgal marker and intermediate neurofilaments to trace developing fiber tracts. At this stage, the dorsal tegmental decussation (dtgx) of the CTB and the ventral tegmental decussation (vtgx) of the rubrospinal tract can be discerned at the tegmental midline (Figure 5C,D). However, only the vtgx/rubrospinal tract exhibits βgal labeling, consistent with intense labeling of cell bodies in the red nucleus. βgal expressing neurons can also be identified in the deep tectal lamina, and send their fibers only to the uncrossed tectobulbar tract (Figure 5E,F). In the postnatal (P10) brain, Brn3a/LacZ expression is maintained in the uncrossed tectobulbar tract which appears as diffuse fibers in the lateral tegmentum (Figure 5H,I). As in the embryonic brain, βgal immunoreactivity was not observed in the dtgx or in the CTB (Figure 5J-L).
To examine the effect of loss of Brn3a function on the development of CNS pathways, Brn3atauLacZ/+ mice were bred with Brn3a+/-mice bearing a conventional targeted deletion of Brn3a (Xiang et al., 1996) to produce Brn3atauLacZ/- null mice, which have known abnormalities of the peripheral sensory nervous system (Eng et al., 2001; Huang et al., 1999) and generally die within 12 hours of birth. Because of this neonatal lethality, E18.5 was the latest stage at which the tectofugal projections could be examined in these mice. In the midbrain tegmentum, the extent of the red nucleus and vtgx were markedly reduced, and the rubrospinal tract was nearly absent (Figure S1 C-F,G,I). In contrast, extensive βgal labeling persisted in the fibers of the ITP (Figure S1 H,J), demonstrating that the neurons of this major descending pathway express Brn3a, but do not require it to generate or maintain their projections, at least at embryonic stages.
Brn3a controls the laminar fate and projections of tectal neurons
To further delineate the role of Brn3a in determining the phenotype of tectal neurons, we used electroporation of a Brn3a-IRES-GFP expression plasmid to mis-express Brn3a in the developing chick tectum. For studies of the laminar fate of tectal neurons, electroporation was performed at E3, and the midbrain was examined at E12. Mis-expression of Brn3a resulted in a large increase in GFP-expressing neurons in tectal lamina 13-15, where Brn3a immunoreactive neurons born on E3 normally appear (Figure 6A-F), but the overall laminar architecture of the tectum was not altered (Figure 6C,D).
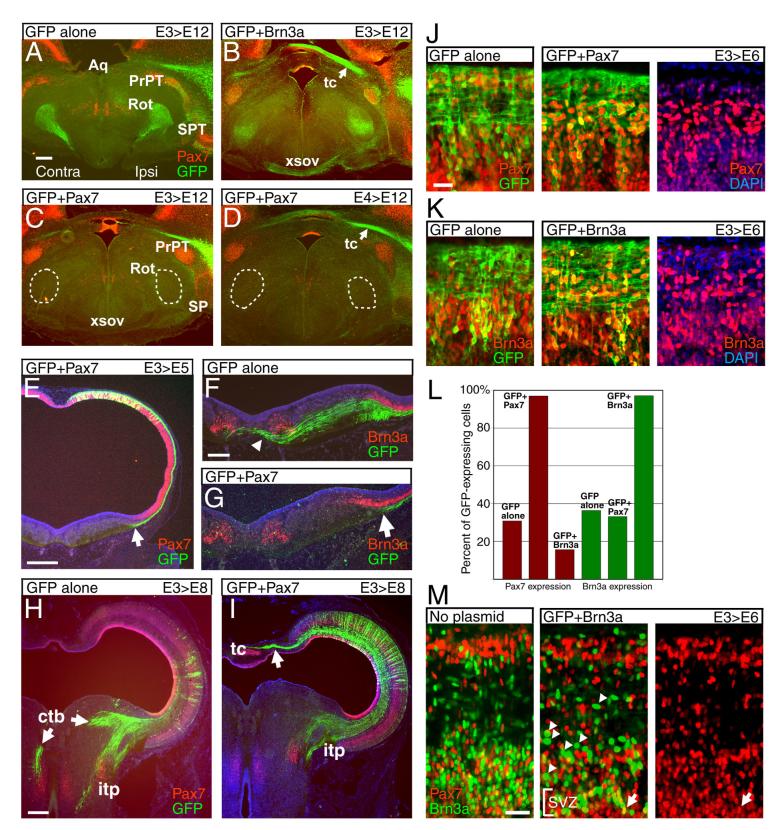
Figure 6. Brn3a determines the laminar fate and projections of tectal neurons.
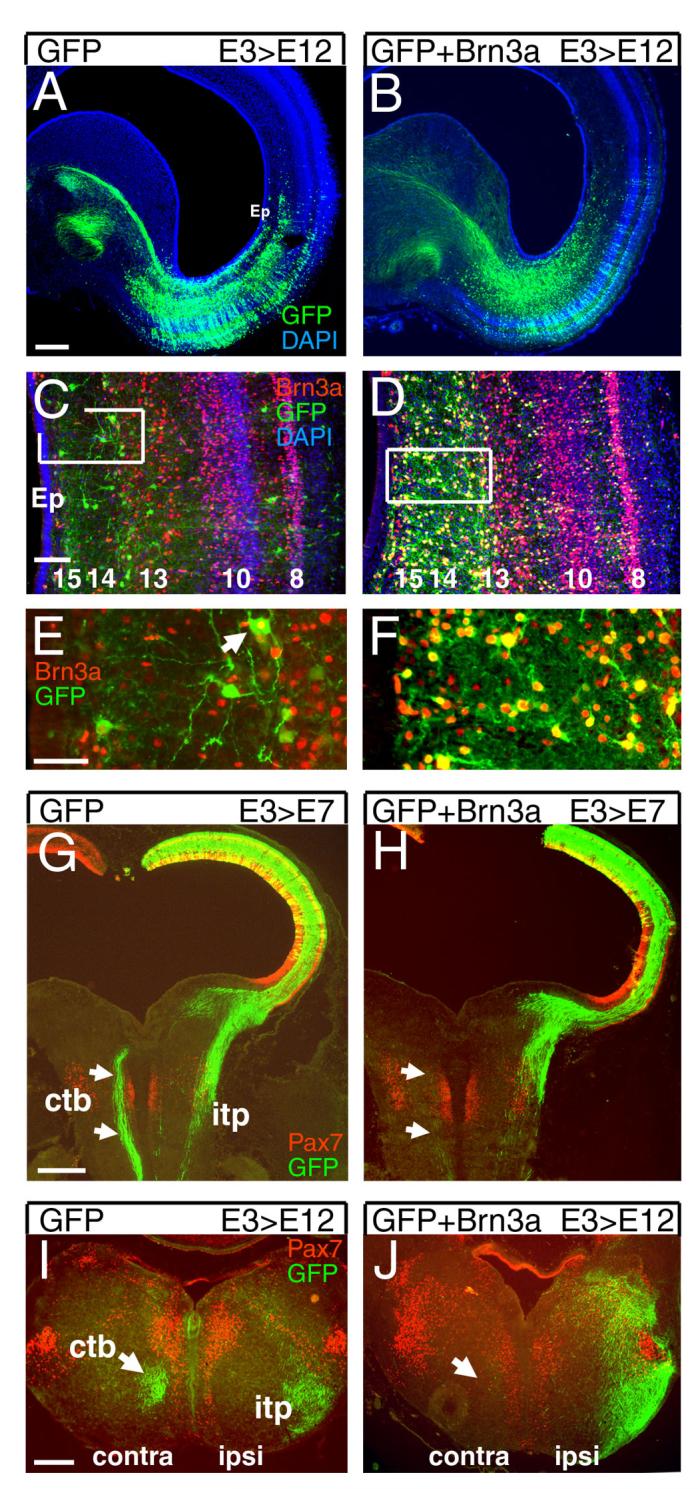
(A-F) Effect of Brn3a on laminar fate in the tectum. Tecta were electroporated with a plasmid encoding GFP alone (A,C,E) or GFP and Brn3a (B,D,F), at E3, and examined by immunofluorescence at E12. In control embryos, a fraction of cells in L13-15 are strongly labeled at this stage, some of which express Brn3a (arrow, E; see also Figure 2). Co-electroporation of Brn3a results in a large increase in the number of GFP-positive neurons in L13-15, nearly all of which express Brn3a, but the overall laminar architecture is not affected. (G-J) Effect of Brn3a on development of the tectobulbar tracts. (G,H) Embryos electroporated at E3 and examined at E7 show that mis-expression of Brn3a prevents labeled neurons from projecting via the CTB. Plane of section is similar to that shown in Figure 3E. (I,J) Cross section of the rostral hindbrain of embryos electroporated at E3 and examined at E12. Mis-expression of Brn3a eliminates projection of labeled neurons via the CTB, and increases labeling of the ITP. Ctb, crossed tectobulbar tract; itp, ipsilateral tectopontine tract. Data shown are representative of at least 5 cases. Scale A,G, 400μm; C, 100μm; E, 50μm.
We then examined the effect of Brn3a mis-expression on the descending tectofugal projections. Because neurons contributing to both the CTB and ITP are generated at E3, plasmids encoding GFP and Brn3a were electroporated at this stage, and the descending tracts were analyzed at E7 and E12. Examination of the descending tracts at E7 shows that expression of GFP alone results in robust labeling of the CTB, while co-expression of Brn3a results in the nearly complete elimination of midline crossing by the projections of the labeled neurons, with preservation of the uncrossed projections (Figure 6G,H). Transverse sections at E12 show that in addition to loss of the labeled fibers in the CTB, mis-expression of Brn3a results in an expansion of the ITP outside its usual domain in the ventrolateral hindbrain (Figure 6I,J).
Pax7 restricts the development of tectofugal projections
Brn3a expression is clearly associated with classes of neurons which project from the midbrain to other CNS regions. Data presented here for the mouse show that Brn3a-expressing neurons contribute to the ITP and rubrospinal tract. Prior retrograde labeling studies in the chick have shown that Brn3a-expressing neurons in L10 project to the isthmic nuclei (Wang et al., 2006) and Brn3a expression in neurons projecting to the nucleus rotundus will be described elsewhere. Given that Brn3a is expressed in several classes of tectofugal neurons, we wished to determine whether Pax7 is also expressed in neurons which project outside the midbrain.
Injections of cholera toxin subunit B (CTxB, Figure 7) were performed in tectorecipient areas of 3 day-old chicks, followed by localization of the transported label and Pax7 protein in the tectum by immunofluorescence. As expected, CTxB injections in the posterolateral (Figure 7A, C) and anterior-central (Figure B,D) nucleus rotundus labeled numerous neurons with large soma located in L13, characteristic of the tectal ganglion cells known to project to this nucleus. The labeled tectal neurons projecting to the nucleus rotundus did not express Pax7. CTxB injections in the isthmic nucleus semilunaris (SLu) labeled numerous neurons in the inner part of L10, and injections in the zona incerta of the ventral thalamus labeled tectal neurons in L13-15, and in both cases the labeled cells were also Pax7 negative (Figure 7E,F).
Figure 7. Retrograde tracing of tectofugal tracts.
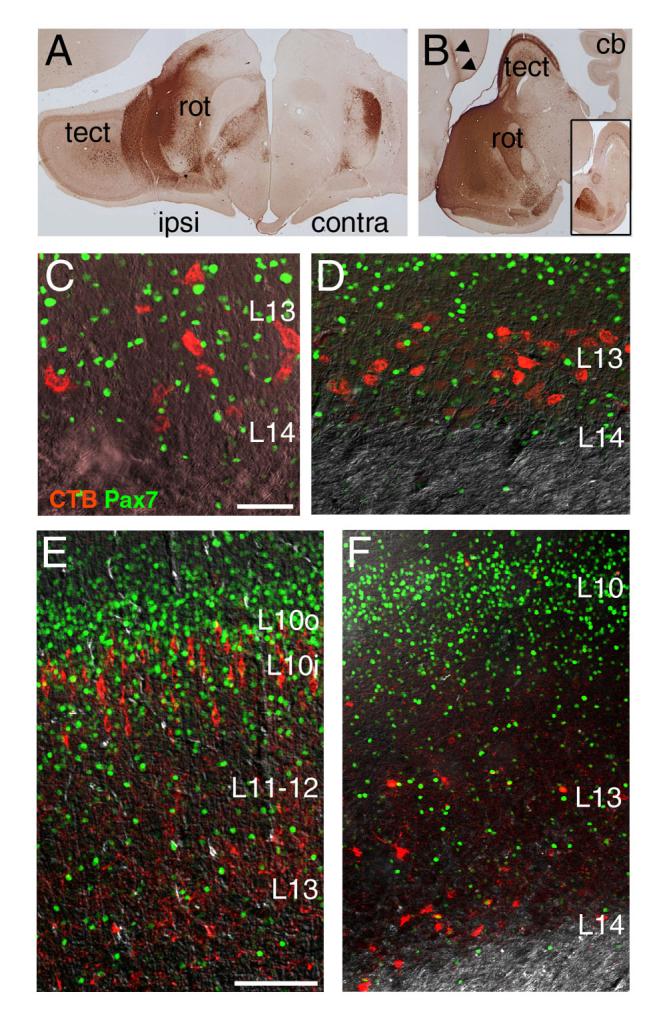
CTxB was injected into brain regions of 3-day old chicks known to receive tectal input. The brain was then perfusion fixed and sectioned, and the immunostaining was performed for the transported CTxB label and Pax7. (A) Injection site in the posterolateral nucleus rotundus, coronal view. (B) Injection site in the central-anterior nucleus rotundus, sagital view. An equivalent section on the side opposite to the injection is shown in the inset. (C,D) CTxB label and Pax7 expression in the tectum of cases injected in A and B, respectively. Pax7-expressing tectal neurons are not labeled from the rotundus. (E,F) CTxB label and Pax7 expression in the tectum following injection of the label in the isthmic nucleus semilunaris (E) and the zona incerta of the ventral thalamus (F). Pax7 neurons are very rarely labeled from these sites. Cb, cerebellum; tect, tectum; rot, nucleus rotundus. Scale: C, 40μm; E, 100μm.
In order to test the effect of Pax7 on the laminar identity of tectal neurons and the development of tectofugal projections, we used a Pax7-IRES-GFP expression plasmid to mis-express Pax7 in the developing tectum. Misexpression of Pax7 at E3 or E4 had little effect on the number of GFP-labeled neurons, or their laminar distribution (data not shown). However, in embryos electroporated at E3 or E4 and examined at E12, misexpression of Pax7 profoundly reduced labeling of the rostral projections to the nucleus rotundus and area surrounding the pretectal nucleus (Figure 8A,C,D). Labeling of the ventral supraoptic decussation (XSOV) carrying axons to the contralateral nucleus rotundus and pretectal nucleus was also nearly eliminated, but labeling of the dorsal tectal commissure was not affected. In contrast to the profound effects of Pax7 on the tectothalamic projections, misexpression of Brn3a had little effect on projections to the nucleus rotundus, pretectal nucleus, or XSOV (Figure 8B).
Figure 8. Pax7 inhibits the development of tectofugal projections.
The developing chick tectum was electroporated with plasmids encoding GFP alone or GFP plus Pax7, and the effects on the ascending and descending tectofugal projections were analyzed. (A-D) Embryos electroporated at E3 or E4 and analyzed at E12. Electroporation of GFP plus Brn3a (B) has no profound effect on projections to rostral targets compared to GFP alone (A), compare also Figure 4 (B-D). Electroporation of Pax7 at E3 (C) or E4 (D)prevents labeled neurons from projecting to the nucleus rotundus (circled) or the area around the pretectal nucleus. Consistent with the loss of rotundal labeling, labeling of the xsov is eliminated, while GFP expression in the tc appears unchanged. (E-G) Embryos electroporated at E3 and analyzed at E5. In both control and experimental embryos GFP-labeled projections from early-born neurons follow a circumferential path within the tectum (arrowheads, E; compare Figure 3A). However, labeled axons in tecta electroporated with GFP alone project across the midline (arrowhead, F), while axons of Pax7 electroporated neurons stop at the tectal border (arrows, E,G). Brn3a-expressing neurons in the red nucleus provide a landmark for the dorsal tegmental decussation. (H-I) Embryos electroporated at E3 and analyzed at E8. In Pax7 electroporated embryos, labeled fibers in the CTB is are absent, and labeling of the ITP is markedly reduced. In H most of the electroporated area of the tectum is out of the plane of section. (J-M) Cross regulation of Pax7 and Brn3a. Embryos were electroporated with Pax7 and Brn3a expression plasmids, or GFP alone, at E3 and analyzed at E6. Neurons mis-expressing Pax7 or Brn3a are concentrated in the deep part of the postmitotic layer. The neuroepithelial layer is not shown. (J,K) Electroporation of a Pax7 or Brn3a expression plasmid results in transcription factor co-expression in nearly 100% of the GFP-positive cells (see also L). (L) Fractional expression of Pax7 and Brn3a in electroporated neurons. Mis-expression of Brn3a reduces the expression of Pax7 in the electroporated neurons, but mis-expression of Pax7 has little effect on Brn3a. The electroporated plasmids are noted at the top of each column. (M) Mis-expression of Brn3a rarely results in Pax7/Brn3a co-expression. Arrowheads show examples of cells misexpressing Brn3a, which are characteristically large, with strong Brn3a signals, and are numerous in the section (see also K). Arrow indicates lone cell co-expressing Brn3a and Pax7; in some other cases nuclear overlap gives appearance of co-expression. Aq, aqueduct; ctb, crossed tectobulbar pathway; dtgx, dorsal tegmental decussation; itp, ipsilateral tectopontine pathway; PrPT, principal pretectal nucleus; R, red nucleus; Rot, nucleus rotundus; SPT, subpretectal nucleus; tc, tectal commissure; xsov, ventral supraoptic decussation. (A-I) show representative data from at least 3 cases for each developmental stage and expression plasmid. (L) Represents mean values from >100 GFP+ cells counted in each of three samples. Scale: A,E,H 400μm; F, 200μm; J,M 25μm.
We next examined the effect of Pax7 misexpression at earlier developmental stages. In normal embryos electroporated at E3, the axons of early neurons can be seen to follow a circumferential route toward the floor of the mid/hindbrain, and by E5 early axons contributing to the CTB have crossed the midline (Figure 8F). In embryos co-electroporated with Pax7, labeled axons followed their normal circumferential pathway, demonstrating that Pax7 did not prevent axonogenesis (Figure 8E). However, the axons of Pax7 electroporated neurons stopped near the border of the tectal compartment, defined by the limit of Pax7 expression in the neuroepithelial layer, and did not enter the tegmentum (Figure 8E,G). In embryos examined at E8, Pax7 electroporation eliminated labeled axons in the CTB, and markedly reduced labeling of the ITP, although a small number of axons projected a short distance into the ventral hindbrain (Figure 8H,I).
In normal embryos, co-expression of Pax7 and Brn3a is only transiently observed in developing neurons immediately adjacent to the neuroepithelial layer, not in the mature tectal lamina. This mutually exclusive expression of Brn3a and Pax7 suggests the possibility of a direct repressive relationship between these transcription factors. To examine the regulatory interrelationship between Brn3a and Pax7, we first examined the efficiency of the mis-expression of Brn3a and Pax7 in electroporated embryos. In embryos electroporated at E3 and examined at E6, co-electroporation of Brn3a or Pax7 with GFP resulted in expression of the appropriate transcription factor in nearly 100% of the GFP-positive neurons (Figure 8J-K). Electroporation of Brn3a reduced the fractional expression of Pax7 by about half, from 31% to 15% (Figure 8L). Furthermore, in Brn3a-electroporated embryos, Pax7 co-expression was rarely observed in cells with the strongest Brn3a signal (Figure 8M). In contrast, mis-expression of Pax7 did not have a significant effect on the fraction of transfected neurons expressing Brn3a. These results suggest that direct or indirect repression of Pax7 by Brn3a is part of a mechanism through which distinct subsets of tectal neurons are generated.
DISCUSSION
Neuroanatomical and functional studies over several decades have established the avian tectum as one of the most intricately structured brain regions, with laminar architecture and connectivity similar in complexity to that of the neocortex. The superior colliculus of the mammalian midbrain, although less elegantly structured, appears to share many anatomical and functional attributes in common with the tectum of birds. These studies of the role of Brn3a and Pax7 in the developing tectum begin to link prior studies of midbrain neuroanatomy and development with current models of the generation of neuronal diversity at the transcriptional level.
Understanding the development of the tectum must begin with principles of early midbrain development that appear to be conserved across all vertebrate species. Beginning at the neural plate/neural tube stage, the early midbrain is patterned in two dimensions by signals from the midbrain/hindbrain boundary (MHB, or isthmus, Joyner et al., 2000; Liu and Joyner, 2001; Nakamura, 2001; Prakash and Wurst, 2004) and from the floor plate of the neural tube (Agarwala et al., 2001; Fedtsova and Turner, 2001). The principal diffusible signal from the MHB is FGF8, which induces rostrocaudal pattering of engrailed expression in the midbrain compartment (Crossley et al., 1996), while the diffusible signal from the floorplate is Shh. The intersection of these spatially graded signals produces a pattern which has been described as a grid (Ye et al., 1998), or arcs (Sanders et al., 2002) in the ventral midbrain, and the characteristic neuronal types of the tegmentum, such as oculomotor neurons and dopaminergic neurons, develop at characteristic coordinates on this initial map.
Unlike the tegmentum, which is composed of morphologically distinct nuclei, the principal part of the tectum develops as a continuous laminated structure. One hallmark of the early tectal compartment is the expression of Pax3 and Pax7 throughout the dividing neuronal precursors of the ventricular layer. Ectopic expression of Shh in the dorsal midbrain represses the expression of Pax3 and Pax7, and induces the differentiation of tegmental neurons which are positionally appropriate with respect to the rostrocaudal axis (Fedtsova and Turner, 2001; Watanabe and Nakamura, 2000). Conversely, misexpression of Pax7 in the early ventral midbrain can induce tectal markers in the tegmentum (Matsunaga et al., 2001). The expression of Pax3 and Pax7 in the midbrain is biphasic, occurring universally in neural precursors, but only in a subset of differentiated neurons (Thomas et al., 2006). The data presented here establish a role for this postmitotic phase of Pax7 expression.
Development of the laminar tectum
The histiogenesis of the laminar tectum has been studied for over a century (Ramón y Cajal, 1909). As tectal development progresses, a period of very active neurogenesis from approximately E3 to E6 is followed by extensive cell migration events which reorganize the tectum into distinct layers, most of which can be recognized by E12. Two systems of nomenclature have been used to designate 15 primary layers in mature tectum of the chick (Figure 1). Classic birthdating studies using cumulative tritiated thymidine labeling have demonstrated that the neurons in the deepest tectal lamina (13-15, or SGC, SAC and SGFP) are generated first, followed by the superficial layers (2-8, or sublamina a-g of the SGFS), and finally by the intermediate layers (9-12, or sublamina h-j of the SGFS, LaVail and Cowan, 1971a; LaVail and Cowan, 1971b; Wu et al., 2000). More recent studies using the electroporation of a βgalactosidase marker at E3 and E6 are largely consistent with these results (Sugiyama and Nakamura, 2003).
In the current study, we have used staged electroporation of a GFP marker to examine systematically the relationship between the timing of tectal neurogenesis, laminar fate, and transcription factor identity. Electroporation at E3-E4 labels predominantly L13-15, and the early-labeled cells in L13 clearly include the large, morphologically distinct tectal ganglion cells, many of which express Brn3a. At E5, L4-L10 are intensely labeled, while by E6, neurogenesis is waning, and labeling is predominantly in L10. These results are in general accord with the prior cumulative labeling studies.
The use of transcription factors to identify specific subpopulations of tectal neurons to adds a new molecular face to the complexity of the developing laminar tectum, which has previously been the subject of detailed morphological studies. For example, L10 neurons generated at E5 reside predominantly in the inner sublayer of L10 (L10b), and express Brn3a, while L10 neurons generated at E6 reside predominantly in the outer sublayer of L10 (L10a), and express Pax7. The application of morphological criteria across developmental time has previously led to the identification of Type I and Type II tectal neuroblasts based on patterns of ventricular detachment at the time of cell cycle exit, and subsequent morphology (Puelles and Bendala, 1978). Based on the classes of neurons generated from Type I and Type II neuroblasts and the timing of their generation, Brn3a-expressing neurons in L13 likely develop from Type I neuroblasts. The later-developing Pax7 neurons in the more superfical lamina may develop from Type II neuroblasts.
The laminar fate of tectal neurons is determined at least in part by their complement of transcriptional regulators. Misexpression of Brn3a at E3 has a dominant effect on the laminar fate of differentiating neuroblasts, causing a large increase in the number of GFP-labeled neurons in L13-15 relative to a GFP control plasmid. In contrast, obvious effects of Pax7 mis-expression on lamination were not observed. In prior work, the groucho-related factor Grg4 has been shown to be expressed specifically in the superficial tectal layers, and misexpression of this factor also causes a shift in neuronal migration (Sugiyama and Nakamura, 2003). Other transcription factors with layer-specific patterns of expression in the tectum include Lhx1/5 (Lim1/2, Fedtsova and Turner, 2001), Sox2, Sox14, and En2 (Sugiyama and Nakamura, 2003), but their functions remain to be defined.
Development of the descending tectofugal projections
Prior studies have described the early development of the tectofugal projections to the hindbrain, which are among the first long-range projections to appear in the embryo. The existing nomenclature of these pathways is confusing and non-systematic. Here we have used the term “crossed tectobulbar tract” (CTB) to designate the contralateral descending projections (Hellmann et al., 2004; Reiner and Karten, 1982). This tract has also been called the “tectospinal tract” (Shepherd and Taylor, 1995). We have used “ipsilateral tectopontine tract” (ITP) to designate the uncrossed projections (Reiner and Karten, 1982). Theses have also been called the “tectobulbar tract” (Shepherd and Taylor, 1995), but elsewhere “tectobulbar tract” has been used as a generic term for both parts of the descending tectofugal pathway (e.g. Treubert-Zimmermann et al., 2002). ITP is used here as a term of convenience, but this is clearly a heterogenous collection of tracts which needs further resolution. The decending projections of the superior colliculus have also been well described in the rat (Redgrave et al., 1987). In rodents, the crossed pathway is referred to as the tectospinal tract (Paxinos and Watson, 2005), which crosses via the ventral tegmental decussation and joins the predorsal bundle of the contralateral hindbrain. The heterogeneous uncrossed projection does not have a systematic name in rodents, and here ITP is used for convenience.
Tectobulbar fibers can be detected in the chick midbrain from E3, where they course circumferentially around the developing tectum. Once reaching the tegmentum, a subset of these axons then turn caudally before reaching the midline, while another group cross to the contralateral side before descending (Kroger and Schwarz, 1990; Shepherd and Taylor, 1995). The CTB and ITP have been shown to originate in separate populations of neurons distributed throughout the tectal hemispheres (Kroger and Schwarz, 1990). Circumferential descending axons also take crossed and uncrossed pathways in the developing mouse midbrain, where they can be detected from E10-10.5 (Mastick and Easter, 1996).
Past studies have begun to reveal the mechanisms guiding the specific formation of the tectofugal projections. Axons growing from early rat tectal explants, destined to join the ITP, are repelled by the midbrain floorplate (Tamada et al., 1995). In chick, a role for Sema3a in repelling axons of the ITP at the midline has been described (Henke-Fahle et al., 2001). A second class of cell surface molecules, the cadherins, are expressed in specific laminar patterns in the tectum (Miskevich et al., 1998; Redies and Takeichi, 1993) and distinguish axons in the tectobulbar, tectoisthmic and tectothalamic tracts (Wohrn et al., 1999). Forced expression of cadherins affects pathway selection by tectofugal axons in a manner consistent with homophilic cadherin interactions (Treubert-Zimmermann et al., 2002).
In the present study we have used electroporation of a GFP expression plasmid to trace the developing tectobulbar pathways, and determine the birthdate of neurons contributing to the CTB and ITP. Birthdating of the descending tectofugal tracts indicates that neurons contributing to the CTB are generated in a relatively short interval, primarily on E3, while neurons contributing to the ITP are generated over the entire period of tectal neurogenesis. Retrograde tracing in the adult pigeon has shown that neurons contributing to the CTB are found predominantly in layers 13-15, tectal gray and tegmentum. Retrograde labeling of the ITP has indicated that this tract originates in multiple tectal lamina (Hellmann et al., 2004; Reiner and Karten, 1982), and further studies with more restricted injections are needed to better define its origin. These anatomical studies are also are consistent with our laminar birthdating data, in that neurons in the deep layers giving rise to the CTB are generated predominantly on E3-E4, while the neurons contributing to the ITP are contained in multiple layers generated throughout neurogenesis. Together these results indicate significant anatomical and developmental heterogeneity in the neurons which contribute to the ITP.
In GFP-electroporated embryos, fibers of the CTB have crossed the midline by E5, and by E6 the descending CTB and ITP tracts can be clearly discerned in the hindbrain. Brn3a and Pax7 have profound and distinct effects on the phenotype of neurons contributing to these pathways. Misexpression of Brn3a in E3 embryos prevents neurons from projecting via the CTB, but the ITP projections appear to be increased, and their territory in the hindbrain is expanded. In neurons mis-expressing Pax7, early axons follow their normal circumferential path around the tectum, but are arrested at the boundary of the tectal compartment, and do not project into the tegmentum. Both the CTB and ITP fail to form in Pax7 electroporated embryos. However, at E8, a few GFP-labeled fibers penetrate a short distance into the ventral compartment. This may be due the fact that expression of electroporated Pax7 appears to be less persistent than that of GFP (data not shown), allowing some escape from the repression of ventral axon growth.
Transgenic tract tracing in Brn3a/tauLacZ mice demonstrates that Brn3a-expressing tectal neurons contribute to the ITP, but not the tectospinal tract (CTB), consistent with the effect of Brn3a electroporation in the chick. However, βgal expressing fibers of the ITP are present in Brn3atauLacZ/- (null) embryos, indicating that while Brn3a is sufficient to induce neurons to project via the ITP rather than the CTB, but is not necessary for ITP formation. In contrast, loss of Brn3a had a severe effect on the developing red nucleus and its projection, the rubrospinal tract (Figure S1). Partial redundancy with a closely related Pou4-class transcription factor, Brn3b, which is extensively expressed in the superior colliculus but not in the red nucleus (Turner et al., 1994; Xiang et al., 1996) may explain the differential effect of the loss of Brn3a on these structures.
Development of the ascending tectofugal projections
In birds, the largest and best characterized ascending tectofugal projection innervates the nucleus rotundus of the thalamus, part of the retinal-tectal-thalamic-telencephalic visual pathway. Tectal neurons residing mainly in L13 project bilaterally to the rotundus, with the contralateral component crossing via the ventral supraoptic decussation (Benowitz and Karten, 1976; Hunt and Kunzle, 1976). Collateral branches from the same neurons also innervate the subpretectal nucleus and the region surrounding the pretectal nucleus (Karten et al., 1997). L13 neurons projecting to the nucleus rotundus are designated “tectal ganglion cells”, with characteristic large soma and extensive dendritic fields, and which function in motion detection (Frost and Nakayama, 1983; Luksch et al., 2001). Tectal ganglion cells can be differentiated into subtypes characterized by their dendritic arborizations within different tectal lamina and by projections to different compartments of the rotundus (Hellmann and Gunturkun, 2001; Karten et al., 1997; Luksch et al., 1998). Although both ascending and descending fibers arise from L13, distinct populations of neurons within this layer contribute to these pathways (Hellmann et al., 2004; Karten et al., 1997).
Electroporation of Brn3a did not have a profound effect on the development of the ascending tectal projections. GFP labeling in the nucleus rotundus, subpretectal nucleus, the area surrounding the principal pretectal nucleus and the ventral supraoptic decussation appeared similar to control electroporations. Consistent with this, Brn3a is normally expressed in neurons with the characteristic morphology of tectal ganglion cells projecting to the rotundus (e.g. Figure 2B), and retrograde tracing studies to be published elsewhere confirm Brn3a expression these neurons (HK, data not shown). Misexpression of Pax7, in contrast, eliminated labeled projections to the nucleus rotundus bilaterally, and to all of the collateral targets of this pathway. Among the tectofugal projections, only the dorsal projection via the tectal commissure appears to be formed normally by the Pax7 electroporated neurons.
Transcriptional regulation of neuronal phenotypes in the tectum
These studies identify distinct roles for Brn3a and Pax7, and begin to define a mechanism for the generation of neuronal diversity in the tectum at the transcriptional level. Brn3a expression characterizes important classes of tectofugal neurons, including L10 neurons projecting to the isthmic nuclei Ipc and SLu (Wang et al., 2006), L13 neurons projecting to the nucleus rotundus, and neurons in deep tectal layers that project to the ITP. Mis-expression of Brn3a blocks projections to the CTB, the only class of tectofugal neurons identified here which do not include at least a subset of Brn3a expressing cells. In contrast, misexpression of Pax7 inhibits axon growth into both ascending and descending ventral pathways. Only the dorsal tectal projection, via the tectal commissure, appears to be unaffected by Pax7 misexpression. Thus the best generalization regarding the axons of Pax7-expressing neurons may be that they are restricted to the dorsal compartment, which is also the anatomical limit of Pax7 expression, and cannot project via ventral pathways.
The effects of nuclear transcription factors such as Brn3a and Pax7 on laminar fate and pathway selection must be mediated by the downstream targets regulated by these factors. The regulatory targets of Brn3a and Pax7 in the midbrain are unknown, but recent studies of Brn3a in developing sensory neurons have identified multiple downstream genes with known roles in cell migration, axon growth, and neurotransmitter identity (Eng et al., 2007; Eng et al., 2004; Lanier et al., 2007). Similar roles are likely for Brn3a and Pax7 in the midbrain, where these factors are likely to regulate a coordinated program of gene expression which governs several aspects of tectal neuron phenotype.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Agnieszka Brzozowska-Prechtl for technical assistance and Tamara Stawicki for work in development of electroporation plasmids. We also thank Iain Dykes for comments on the manuscript. Special thanks go to Harvey Karten for extensive assistance with avian neuroanatomy, sharing of unpublished data, and comments on the manuscript. Mouse monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, maintained under contract NO1-HD23144 from the NICHD. Supported in part by Department of Veterans Affairs MERIT funding, and NIH awards HD33442 and MH065496 (E.E.T). E.E.T. and N.F are NARSAD Investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agarwala S, Sanders TA, Ragsdale CW. Sonic hedgehog control of size and shape in midbrain pattern formation. Science. 2001;291:2147–50. doi: 10.1126/science.1058624. [DOI] [PubMed] [Google Scholar]
- Bayly RD, Ngo M, Aglyamova GV, Agarwala S. Regulation of ventral midbrain patterning by Hedgehog signaling. Development. 2007;134:2115–2124. doi: 10.1242/dev.02850. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Karten HJ. Organization of the tectofugal visual pathway in the pigeon: a retrograde transport study. J Comp Neurol. 1976;167:503–20. doi: 10.1002/cne.901670407. [DOI] [PubMed] [Google Scholar]
- Blaess S, Corrales JD, Joyner AL. Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development. 2006;133:1799–809. doi: 10.1242/dev.02339. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;380:66–8. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Eng S, Gratwick K, Rhee J, Fedtsova N, Gan L, Turner E. Defects in sensory axon growth precede neuronal death in Brn3a-deficient mice. J. Neuroscience. 2001;21:541–49. doi: 10.1523/JNEUROSCI.21-02-00541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng SR, Dykes IM, Lanier J, Fedtsova N, Turner EE. POU-domain factor Brn3a regulates both distinct and common programs of gene expression in the spinal and trigeminal sensory ganglia. Neural Develop. 2007;2:3. doi: 10.1186/1749-8104-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng SR, Lanier J, Fedtsova N, Turner EE. Coordinated regulation of gene expression by Brn3a in developing sensory ganglia. Development. 2004;131:3859–70. doi: 10.1242/dev.01260. [DOI] [PubMed] [Google Scholar]
- Fedtsova N, Turner E. Brn-3.0 Expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mechanisms of Development. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Fedtsova N, Turner EE. Signals from the ventral midline and isthmus regulate the development of Brn3.0-expressing neurons in the midbrain. Mech Dev. 2001;105:129–44. doi: 10.1016/s0925-4773(01)00399-9. [DOI] [PubMed] [Google Scholar]
- Frost BJ, Nakayama K. Single visual neurons code opposing motion independent of direction. Science. 1983;220:744–5. doi: 10.1126/science.6836313. [DOI] [PubMed] [Google Scholar]
- Gruber C, Rhee J, Gleiberman A, Turner E. POU-domain factors of the Brn-3 class recognize functional DNA elements which are distinctive, symmetrical, and highly conserved in evolution. Mol. Cell Bio. 1997;17:2391–2400. doi: 10.1128/mcb.17.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harting JK. Descending pathways from the superior collicullus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta) J Comp Neurol. 1977;173:583–612. doi: 10.1002/cne.901730311. [DOI] [PubMed] [Google Scholar]
- Harting JK, Huerta MF, Frankfurter AJ, Strominger NL, Royce GJ. Ascending pathways from the monkey superior colliculus: an autoradiographic analysis. J Comp Neurol. 1980;192:853–82. doi: 10.1002/cne.901920414. [DOI] [PubMed] [Google Scholar]
- Hellmann B, Gunturkun O. Structural organization of parallel information processing within the tectofugal visual system of the pigeon. J Comp Neurol. 2001;429:94–112. doi: 10.1002/1096-9861(20000101)429:1<94::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hellmann B, Gunturkun O, Manns M. Tectal mosaic: organization of the descending tectal projections in comparison to the ascending tectofugal pathway in the pigeon. J Comp Neurol. 2004;472:395–410. doi: 10.1002/cne.20056. [DOI] [PubMed] [Google Scholar]
- Henke-Fahle S, Beck KW, Puschel AW. Differential responsiveness to the chemorepellent Semaphorin 3A distinguishes Ipsi- and contralaterally projecting axons in the chick midbrain. Dev Biol. 2001;237:381–97. doi: 10.1006/dbio.2001.0376. [DOI] [PubMed] [Google Scholar]
- Holcombe V, Hall WC. The laminar origin and distribution of the crossed tectoreticular pathways. J Neurosci. 1981a;1:1103–12. doi: 10.1523/JNEUROSCI.01-10-01103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe V, Hall WC. Laminar origin of ipsilateral tectopontine pathways. Neuroscience. 1981b;6:255–60. doi: 10.1016/0306-4522(81)90061-0. [DOI] [PubMed] [Google Scholar]
- Huang E, Zang K, Schmidt A, Saulys A, Xiang M, Reichardt L. POU domain factor Brn-3a controls the differentiation and survival of trigeminal neurons by regulating Trk receptor expression. Development. 1999;126:2869–82. doi: 10.1242/dev.126.13.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Kunzle H. Observations on the projections and intrinsic organization of the pigeon optic tectum: an autoradiographic study based on anterograde and retrograde, axonal and dendritic flow. J Comp Neurol. 1976;170:153–72. doi: 10.1002/cne.901700203. [DOI] [PubMed] [Google Scholar]
- Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12:736–41. doi: 10.1016/s0955-0674(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Cox K, Mpodozis J. Two distinct populations of tectal neurons have unique connections within the retinotectorotundal pathway of the pigeon (Columba livia) J Comp Neurol. 1997;387:449–65. [PubMed] [Google Scholar]
- Karten HJ, Keyser KT, Brecha NC. Biochemical and morphological heterogeneity of retinal ganglion cells. In: Cohen B, Bodis-Wollner I, editors. Vision and the brain: the organization of the central visual system. Raven Press; New York: 1990. p. xxxiii.p. 364. [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H. Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66:119–30. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol. 2004;14:R335–8. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Kroger S, Schwarz U. The avian tectobulbar tract: development, explant culture, and effects of antibodies on the pattern of neurite outgrowth. J Neurosci. 1990;10:3118–34. doi: 10.1523/JNEUROSCI.10-09-03118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier J, Quina LA, Eng SR, Cox E, Turner EE. Brn3a target gene recognition in embryonic sensory neurons. Dev Biol. 2007;302:703–16. doi: 10.1016/j.ydbio.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail JH, Cowan WM. The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res. 1971a;28:391–419. doi: 10.1016/0006-8993(71)90053-9. [DOI] [PubMed] [Google Scholar]
- LaVail JH, Cowan WM. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971b;28:421–41. [PubMed] [Google Scholar]
- Liu A, Joyner AL. Early anterior/posterior patterning of the midbrain and cerebellum. Annu Rev Neurosci. 2001;24:869–96. doi: 10.1146/annurev.neuro.24.1.869. [DOI] [PubMed] [Google Scholar]
- Luksch H, Cox K, Karten HJ. Bottlebrush dendritic endings and large dendritic fields: motion-detecting neurons in the tectofugal pathway. J Comp Neurol. 1998;396:399–414. [PubMed] [Google Scholar]
- Luksch H, Karten HJ, Kleinfeld D, Wessel R. Chattering and differential signal processing in identified motion-sensitive neurons of parallel visual pathways in the chick tectum. J Neurosci. 2001;21:6440–6. doi: 10.1523/JNEUROSCI.21-16-06440.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick GS, Easter SS., Jr. Initial organization of neurons and tracts in the embryonic mouse fore- and midbrain. Dev Biol. 1996;173:79–94. doi: 10.1006/dbio.1996.0008. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Role of Pax3/7 in the tectum regionalization. Development. 2001;128:4069–77. doi: 10.1242/dev.128.20.4069. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O’Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–55. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Miskevich F, Zhu Y, Ranscht B, Sanes JR. Expression of multiple cadherins and catenins in the chick optic tectum. Mol Cell Neurosci. 1998;12:240–55. doi: 10.1006/mcne.1998.0718. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Regionalisation and acquisition of polarity in the optic tectum. Prog Neurobiol. 2001;65:473–88. doi: 10.1016/s0301-0082(01)00015-6. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Funahashi J.-i. Introduction of DNA into Chick Embryos by in Ovo Electroporation. Methods. 2001;24:43–48. doi: 10.1006/meth.2001.1155. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam; Boston: 2005. [Google Scholar]
- Prakash N, Wurst W. Specification of midbrain territory. Cell Tissue Res. 2004;318:5–14. doi: 10.1007/s00441-004-0955-x. [DOI] [PubMed] [Google Scholar]
- Puelles L, Bendala MC. Differentiation of neuroblasts in the chick optic tectum up to eight days of incubation: a Golgi study. Neuroscience. 1978;3:307–25. doi: 10.1016/0306-4522(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S. The chick brain in stereotactic coordinates. Elsevier; New York: 2007. [Google Scholar]
- Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O’Leary DD, Goulding M, Turner EE. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci. 2005;25:11595–604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du syst`eme nerveux de l’homme & des vertebres. A. Maloine; Paris: 1909. [Google Scholar]
- Ramón y Cajal S. Histology of the nervous system of man and vertebrates. Oxford University Press; New York: 1995. [Google Scholar]
- Redgrave P, Mitchell IJ, Dean P. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm- agglutinin conjugated horseradish peroxidase. Exp Brain Res. 1987;68:147–67. doi: 10.1007/BF00255241. [DOI] [PubMed] [Google Scholar]
- Redies C, Takeichi M. Expression of N-cadherin mRNA during development of the mouse brain. Dev Dyn. 1993;197:26–39. doi: 10.1002/aja.1001970104. [DOI] [PubMed] [Google Scholar]
- Reiner A, Karten HJ. Laminar distribution of the cells of origin of the descending tectofugal pathways in the pigeon (Columba livia) J Comp Neurol. 1982;204:165–87. doi: 10.1002/cne.902040206. [DOI] [PubMed] [Google Scholar]
- Sanders TA, Lumsden A, Ragsdale CW. Arcuate plan of chick midbrain development. J Neurosci. 2002;22:10742–50. doi: 10.1523/JNEUROSCI.22-24-10742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd IT, Taylor JS. Early development of efferent projections from the chick tectum. J Comp Neurol. 1995;354:501–10. doi: 10.1002/cne.903540403. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Nakamura H. The role of Grg4 in tectal laminar formation. Development. 2003;130:451–62. doi: 10.1242/dev.00232. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE. EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development. 2001;128:4669–80. doi: 10.1242/dev.128.23.4669. [DOI] [PubMed] [Google Scholar]
- Tamada A, Shirasaki R, Murakami F. Floor plate chemoattracts crossed axons and chemorepels uncrossed axons in the vertebrate brain. Neuron. 1995;14:1083–93. doi: 10.1016/0896-6273(95)90347-x. [DOI] [PubMed] [Google Scholar]
- Thanos S, Mey J. Development of the visual system of the chick. II. Mechanisms of axonal guidance. Brain Res Brain Res Rev. 2001;35:205–45. doi: 10.1016/s0165-0173(01)00049-2. [DOI] [PubMed] [Google Scholar]
- Theiler K. The house mouse; development and normal stages from fertilization to 4 weeks of age. Springer-Verlag; Berlin, New York: 1972. [Google Scholar]
- Thomas M, Beazley L, Ziman M. A multiphasic role for Pax7 in tectal development. Exp Brain Res. 2006;169:266–71. doi: 10.1007/s00221-005-0335-0. [DOI] [PubMed] [Google Scholar]
- Thomas M, Lazic S, Beazley L, Ziman M. Expression profiles suggest a role for Pax7 in the establishment of tectal polarity and map refinement. Exp Brain Res. 2004;156:263–73. doi: 10.1007/s00221-003-1775-z. [DOI] [PubMed] [Google Scholar]
- Treubert-Zimmermann U, Heyers D, Redies C. Targeting axons to specific fiber tracts in vivo by altering cadherin expression. J Neurosci. 2002;22:7617–26. doi: 10.1523/JNEUROSCI.22-17-07617.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner E, Jenne K, Rosenfeld M. Brn-3.2: A POU-domain Transcription Factor with Distinctive CNS Expression and Regulation by Retinoic Acid. Neuron. 1994;12:205–18. doi: 10.1016/0896-6273(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol. 2006;494:7–35. doi: 10.1002/cne.20821. [DOI] [PubMed] [Google Scholar]
- Wang Y, Major DE, Karten HJ. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus) J Comp Neurol. 2004;469:275–97. doi: 10.1002/cne.11007. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakamura H. Control of chick tectum territory along dorsoventral axis by Sonic hedgehog. Development. 2000;127:1131–40. doi: 10.1242/dev.127.5.1131. [DOI] [PubMed] [Google Scholar]
- Wohrn JC, Nakagawa S, Ast M, Takeichi M, Redies C. Combinatorial expression of cadherins in the tectum and the sorting of neurites in the tectofugal pathways of the chicken embryo. Neuroscience. 1999;90:985–1000. doi: 10.1016/s0306-4522(98)00526-0. [DOI] [PubMed] [Google Scholar]
- Wu CC, Russell RM, Karten HJ. Ontogeny of the tectorotundal pathway in chicks (Gallus gallus): birthdating and pathway tracing study. J Comp Neurol. 2000;417:115–32. [PubMed] [Google Scholar]
- Xiang M, Lin G, Zhou L, Klein WH, Nathans J. Targeted deletion of the mouse POU-domain gene Brn-3a causes a selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc. Nat. Acad. Sci. 1996;93:11950–11955. doi: 10.1073/pnas.93.21.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–66. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.