Abstract
Dipeptidyl peptidase IV converts neuropeptide Y1-36 (Y1-receptor agonist released from renal sympathetic nerves) to neuropeptide Y3-36 (selective Y2-receptor agonist). Previous studies suggest that Y1, but not Y2, receptors enhance renovascular responses to angiotensin II in kidneys from genetically-susceptible animals. Therefore, we hypothesized that inhibition of dipeptidyl peptidase IV with sitagliptin (antidiabetic drug) would augment the ability of exogenous and endogenous neuropeptide Y1-36 to enhance renal vascular responses to angiotensin II in kidneys from spontaneously hypertensive rats. This hypothesis was tested using three protocols in isolated, perfused kidneys.
Results from Protocol 1:
Exogenous neuropeptide Y1-36 enhanced renovascular responses to angiotensin II; This effect of neuropeptide Y1-36 was blocked by BIBP3226 (selective Y1 receptor antagonist); Exogenous neuropeptide Y3-36 did not enhance renovascular responses to angiotensin II.
Results from Protocol 2:
Sitagliptin augmented the ability of exogenous neuropeptide Y1-36 to enhance renovascular responses to angiotensin II; This effect of sitagliptin was blocked by BIBP3226.
Results from Protocol 3:
Renal sympathetic nerve stimulation enhanced renovascular responses to angiotensin II; This enhancement was augmented by sitagliptin and abolished by BIBP3226.
Conclusion:
Neuropeptide Y1-36 via Y1 receptors enhances renovascular responses to angiotensin II in kidneys from genetically hypertensive animals. Sitagliptin, by blocking dipeptidyl peptidase IV, prevents metabolism of neuropeptide Y1-36 and thereby increases the effects of neuropeptide Y1-36 released from renal sympathetic nerves on Y1 receptors leading to augmentation of neuropeptide Y1-36-induced enhancement of the renovascular effects of angiotensin II. The renal effects of dipeptidyl peptidase IV inhibitors in hypertensive, diabetic patients merit a closer examination.
Keywords: neuropeptide Y; CD26; receptors, neuropeptide Y; rats, inbred SHR; sympathetic nervous system; kidney; renal circulation
INTRODUCTION
Our previously published results indicate that activation of renovascular Y1 receptors (Y1Rs) markedly enhances renovascular responses to physiological levels of angiotensin II (Ang II) in kidneys of spontaneously hypertensive rats (SHR)1. In contrast, kidneys from normotensive Wistar-Kyoto rats (WKY) are resistant to this interaction1. Our studies also show that unlike Y1Rs, renovascular Y2 receptors (Y2Rs) exert little effect on Ang II-induced renovascular responses in kidneys from either SHR or WKY1. Although we do not know precisely why SHR, but not WKY, kidneys are susceptible to Y1R-induced enhancement of Ang II-mediated renal vasoconstriction, these findings indicate that endogenous agonists of Y1Rs, but not Y2Rs, would potentiate Ang II-induced renal vasoconstriction in genetically-susceptible kidneys, provided Y1R agonists reach the renal microcirculation.
In this regard, there are two endogenous agonists of Y1Rs that would be presented to the kidney microcirculation, i.e., peptide YY1-36 (PYY1-36) and neuropeptide Y1-36 (NPY1-36). Both are pancreatic polypeptide-fold peptides. A fatty meal releases PYY1-36 into the systemic circulation from endocrine L-cells in the small bowel, colon and rectum producing physiologically active levels of PYY1-36 in plasma that are 500% to 1000% above basal circulating levels2-8, and this circulating PYY1-36 would be delivered promptly to the renal microcirculation via the blood stream (humoral Y1R-agonist input to kidney microcirculation). Renal sympathetic nerves release NPY1-36 in response to central nervous system-mediated activation of the renal sympathetic nerves (neural Y1R-agonist input to kidney microcirculation), resulting in high local levels of NPY1-36 in sympathetically-innervated renal microvessels during renal sympathetic activation9. Because both PYY1-36 and NPY1-36 are potent Y1R agonists10, 11, physiological processes that increase PYY1-36 release from the gut, NPY1-36 release from renal sympathetic nerves or both simultaneously would activate Y1Rs in the renal microcirculation, which in genetically-susceptible kidneys would enhance Ang II-induced renal vasoconstriction.
It is conceivable, however, that renal dipeptidyl peptidase IV (DPP IV, also called CD26) limits stimulation of renal Y1Rs by physiological processes that increase the exposure of the renal microcirculation to PYY1-36 and NPY1-36. DPP IV is an ecto-enzyme that is anchored to the cell surface and converts PYY1-36 to PYY3-3612 and NPY1-36 to NPY3-3612 by cleaving two amino acids from the N-terminus of PYY1-36 and NPY1-36. Whereas PYY1-36 and NPY1-36 are potent Y1R agonists, PYY3-36 and NPY3-36 are inactive at Y1Rs but are potent and selective Y2R agonists10, 11. These facts suggest the hypothesis that DPP IV in the renal vasculature or glomeruli is a determinant of the extent to which PYY1-36 and NPY1-36 enhance renovascular responses to Ang II in genetically-susceptible kidneys.
In support of this concept, our recently published study13 demonstrates that in SHR kidneys: 1) PYY1-36 enhances renovascular responses to Ang II, whereas PYY3-36 has little effect in this regard; 2) P32/98 (DPP IV inhibitor) augments the ability of PYY1-36 to enhance renovascular responses to Ang II; 3) DPP IV is expressed in preglomerular microvessels and glomeruli; 4) kidneys metabolize arterial PYY1-36 to PYY3-36 via a mechanism blocked by P32/98; and 5) freshly isolated preglomerular microvessels and glomeruli convert PYY1-36 to PYY3-36, and this conversion is inhibited by P32/98. These results confirm that DPP IV is expressed in the renal microcirculation and that inhibition of this ecto-enzyme causes arterial PYY1-36 to more effectively enhance Ang II-induced renal vasoconstriction in genetically-susceptible kidneys.
Although the aforementioned study supports the hypothesis that renal DPP IV importantly modifies the effects of humoral Y1R-agonist input to the kidney microcirculation, this study does not address whether renal DPP IV significantly influences the effects of neural Y1R-agonist input to the kidney. This is an important unanswered question because previously published results indicate that renal sympathetic nerve stimulation enhances renovascular responses to Ang II in the SHR, but not WKY, kidneys via a Y1R mechanism14. Therefore, one aspect of the present study was to determine whether inhibition of DPP IV alters the ability of exogenous NPY1-36 and endogenous NPY1-36 (i.e., that released from renal sympathetic nerves in response to periarterial renal nerve stimulation) to enhance renovascular responses to Ang II.
Another important unanswered question is whether clinically available DPP IV inhibitors affect the ability of exogenous and endogenous Y1R agonists to enhance renovascular responses to Ang II. P32/98 is a DPP IV inhibitor that is available from chemical supply companies, but is not used clinically, nor is P32/98 as potent as clinically employed DPP IV inhibitors. Another aspect of the present study was to examine the effects of sitagliptin (Januvia®), a potent, selective and recently FDA-approved and marketed DPP IV inhibitor, on the interaction between exogenous and endogenous NPY1-36 on Ang II-induced renal vasoconstriction in kidneys from genetically hypertensive rats.
METHODS
Animals
Studies utilized adult (14-16 weeks-of-age) male SHR obtained from Taconic Farms (Germantown, NY). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Isolated, Perfused Kidney Preparation
SHR were anesthetized with Inactin (90 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO), and the left kidney was isolated and perfused with Tyrode's solution using a Hugo Sachs Elektronik-Harvard Apparatus GmbH (March-Hugstetten, Germany) kidney perfusion system as previously described15. Briefly, all branches of the left renal artery and vein were ligated. A polyethylene-50 cannula was placed into the left renal artery, and a polyethylene-90 cannula was placed into the left renal vein. The left kidney was removed, attached to the perfusion system and allowed to stabilize for an hour before the experimental protocol. Kidneys were perfused (single pass mode) at a constant flow (5 ml/min), and perfusion pressure was monitored with a pressure transducer.
Protocol 1
Kidneys were isolated from adult SHR and perfused in vitro as described above. After a 1-hour stabilization period, renovascular responses (i.e., changes in perfusion pressure) to 0.3 nmol/L of Ang II were assessed by infusing Ang II into the perfusate for 10 minutes and noting the difference between the perfusion pressure just before the Ang II infusion compared to the perfusion pressure at the end of the Ang II infusion. The infusion of Ang II was stopped, and 10 minutes later the kidneys were exposed to NPY1-36 or NPY3-36 (6 nmol/L) for 20 minutes. Ten minutes into the treatments with either NPY1-36 or NPY3-36, Ang II was infused once again for 10 minutes to obtain another renovascular response to Ang II, but this time in the presence of either NPY1-36 or NPY3-36. Some kidneys were treated with BIBP3226 (1 μmol/L), a highly selective Y1R antagonist10, beginning 20 minutes before the first renovascular response to Ang II and continuing until the end of the protocol. The effect of NPY1-36 or NPY3-36 on the renovascular response to Ang II was calculated by subtracting the renovascular response to Ang II before treatment with either NPY1-36 or NPY3-36 from the renovascular response to Ang II after treatment with either NPY1-36 or NPY3-36. Ang II, NPY1-36, NPY3-36 and BIBP3226 were obtained from Sigma-Aldrich.
Protocol 2
Kidneys were isolated from adult SHR and perfused in vitro as described above. After a 1-hour stabilization period, the renovascular response (as defined above) to Ang II (0.1 nmol/L) was assessed by infusing Ang II into the perfusate for 10 minutes. The infusion of Ang II was stopped, the kidney was exposed to 0.1 nmol/L of NPY1-36 for 20 minutes, and 10 minutes into the treatment with 0.1 nmol/L of NPY1-36, the renovascular response to Ang II was again obtained by infusing Ang II (0.1 nmol/L) for 10 minutes. The infusion of Ang II was again stopped, and the concentration of NPY1-36 was increased to 0.3 nmol/L for 20 minutes, and 10 minutes into this higher concentration of NPY1-36, the renovascular response to Ang II (0.1 nmol/L) was again obtained by infusing Ang II (0.1 nmol/L) for 10 minutes. This procedure was repeated as the concentration of NPY1-36 was increased to 1 nmol/L and finally to 3 nmol/L. Some kidneys were pretreated for 10 minutes before the first response to Ang II with either sitagliptin (1 μmol/L) or sitagliptin + BIBP3226 (1 μmol/L), and these treatments were maintained throughout the experiment. The fold-increase in the renovascular response to Ang II was calculated by dividing the renovascular response before administering NPY1-36 into the renovascular response to Ang II in the presence of each concentration of NPY1-36.
Protocol 3
Kidneys were isolated from adult SHR and perfused in vitro as described above. Care was taken not to damage the periarterial renal sympathetic nerves during isolation by leaving as much tissue as possible around the artery and avoiding trauma to the nerves. Immediately after initiating perfusion of the kidney, a platinum bipolar electrode was positioned around the renal artery close to the kidney for renal nerve stimulation, and the electrode was connected to a Grass stimulator (model SD9E; Grass Instruments, Quincy, MA). The tissues around the electrode were kept moist with Tyrode's solution. After a 1-hour stabilization period, prazosin (30 nmol/L) was infused into the renal artery to block α1-adrenoceptors so that renal nerve stimulation would not cause direct vasoconstriction and would not increase basal renovascular tone14. Some kidneys also were pretreated with sitagliptin (1 μmol/L). The infusions of prazosin or prazosin plus sitagliptin were continued for the duration of the experiment. Ten minutes after initiating the infusions of prazosin or prazosin plus sitagliptin, a sham periarterial renal nerve stimulation was performed by going through the motions of activating the stimulator while not actually activating the stimulator. Two minutes into the sham renal nerve stimulation, Ang II (0.1 nmol/L) was infused into the renal artery for 7 minutes to obtain a renovascular response to Ang II (as defined above). This first renovascular response to Ang II was designated the period 1 response, which was a basal/control response. At the end of the Ang II infusion, kidneys were allowed to recover for 10 minutes, and then were subjected to renal nerve stimulation (biphasic, 5 Hz, 1-ms pulse duration, 35 V) for 9 minutes. Two minutes into the renal nerve stimulation, Ang II (0.1 nmol/L) was infused once again for 7 minutes to obtain another renovascular response to Ang II. This second renovascular response to Ang II was designated the period 2 response, which was a response to Ang II in the presence of renal nerve stimulation. At this point, both the Ang II infusion and the renal nerve stimulation were discontinued, and an infusion of BIBP3226 (1 μmol/L) was initiated and continued until the end of the protocol. After a 20-minute stabilization period, the kidneys were once again subjected to renal nerve stimulation (biphasic, 5 Hz, 1-ms pulse duration, 35 V) for 9 minutes. Two minutes into the renal nerve stimulation, Ang II (0.1 nmol/L) was infused again for 7 minutes to obtain a third renovascular response to Ang II. This third response to Ang II was designated the period 3 response, which was a response to Ang II in the presence of renal nerve stimulation plus BIBP3226.
Extraction of Sitagliptin
Sitagliptin is not available from chemical supply companies. However, because sitagliptin phosphate monohydrate is highly water soluble, we purchased Januvia® tablets containing 100 mg of sitagliptin phosphate monohydrate from the University of Pittsburgh Medical Center hospital pharmacy and dissolved these tablets in Tyrode's solution. In this regard, we added a 100-mg Januvia® tablet to 10 ml of Tyrode's solution, and placed this preparation in the refrigerator for 1 hour to allow tablet dissolution to occur. The preparation was then vortexed at room temperature and then centrifuged to remove any particulates. An appropriate volume of this preparation (based on the labeled amount of sitagliptin in the tablet) was then diluted in Tyrode's solution to give the desired final concentration of sitagliptin in the perfusate. The solution was then tested by ion-trap mass spectrometry. The mass spectrum of the extract was characterized by a single peak at 408 m/z corresponding to protonated sitagliptin (407 + 1 = 408).
Statistical Analysis
Data were analyzed by one-factor analysis of variance, repeated measures two-factor analysis of variance, Fisher's Least Significant Difference (LSD) test or Mann-Whitney U test as appropriate. The criterion of significance was p<0.05. All data are presented as means ± SEM.
RESULTS
Protocol 1
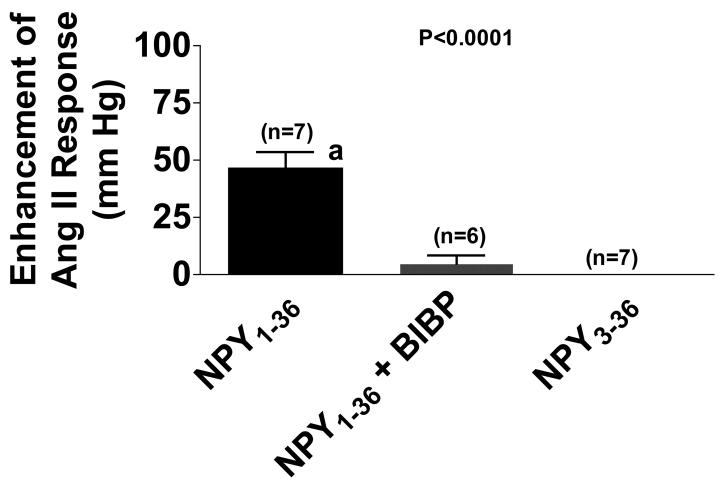
As shown in Figure 1, the Ang II-induced change in perfusion pressure was enhanced by 47 ± 7 mm Hg (n=7) by NPY1-36 (6 nmol/L). Unlike NPY1-36, NPY3-36 (n=7) had little effect on Ang II-induced changes in perfusion pressure (Figure 1). Moreover, the ability of NPY1-36 to potentiate renovascular responses to Ang II was blocked by pretreating the kidneys for 20 minutes with BIBP3226 (1 μmol/L; n=6; Figure 1), a highly selective Y1-receptor blocker10.
Figure 1.
Bar graph shows enhancement of angiotensin II (Ang II)-induced changes in renal perfusion pressure by neuropeptide Y1-36 (NPY1-36; 6 nmol/L), without and with BIBP3226 (BIBP; 1 μmol/L), and by neuropeptide Y3-36 (NPY3-36; 6 nmol/L). The effect of NPY1-36 or NPY3-36 on the renovascular response to Ang II was calculated by subtracting the renovascular response to Ang II before treatment with either NPY1-36 or NPY3-36 from the renovascular response to Ang II after treatment with either NPY1-36 or NPY3-36. The concentration of Ang II was 0.3 nmol/L. Basal perfusion pressure (48 ± 2 mm Hg) was similar among the three groups and was not affected by NPY1-36, NPY3-36 or BIBP3226. The basal response to Ang II (before treatments) was similar among the three groups and was 31 ± 3 mm Hg. The p-value is from 1-factor analysis of variance. aIndicates p<0.05 versus NPY1-36 (Fisher's LSD test). Values represent means ± SEM for the indicated sample size.
Protocol 2
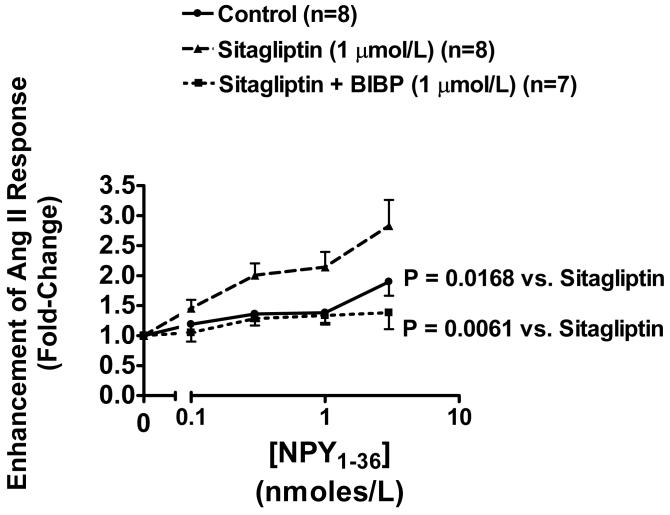
In this protocol, we examined the effects of increasing concentrations of NPY1-36 (0.1, 0.3, 1 and 3 nmol/L) in the absence and presence of sitagliptin (1 μmol/L) or sitagliptin plus BIBP3226 (1 μmol/L) on vasoconstrictor responses to a very low, physiologically-relevant concentration (100 pmol/L) of Ang II. The basal response to 100 pmol/L of Ang II was similar in all three groups and averaged 7 ± 1 mm Hg (n=23). As shown in Figure 2, at concentrations of 1 nmol/L or less, NPY1-36 had little effect on the vasoconstrictor response to Ang II, and at 3 nmol/L the renovascular response to Ang II was enhanced by only 1.90-fold (n=8). In marked contrast, in sitagliptin-treated kidneys (Figure 2), the vasoconstrictor response to Ang II was concentration-dependently (from 0.1 to 3 nmol/L) enhanced by NPY1-36 and at 3 nmol/L the renovascular response to Ang II was enhanced by 2.83-fold (n=8). The relationship between concentration of NPY1-36 and fold-enhancement was statistically-significantly greater in sitagliptin-treated kidneys (n=8) compared with the non-sitagliptin-treated kidneys (p=0.0168). Importantly, in kidneys treated with both sitagliptin and BIBP326, NPY1-36 had little effect on Ang II-induced renovascular responses regardless of the concentration of NPY1-36 (Figure 2; n=7). The relationship between concentration of NPY1-36 and fold-enhancement was statistically-significantly less in BIBP3226 plus sitagliptin-treated kidneys (n=7) compared with sitagliptin-treated kidneys (p=0.0061).
Figure 2.
Line graph illustrates the fold-increase in the renovascular response to Ang II (100 pmol/L) induced by increasing concentrations of NPY1-36. The fold-increase was calculated by dividing the renovascular response to Ang II before administering NPY1-36 into the renovascular response to Ang II in the presence of each concentration of NPY1-36. Experiments were performed in the absence and presence of sitagliptin (1 μmol/L) or sitagliptin plus BIBP3226 (1 μmol/L). Basal perfusion pressure was 48 ± 2 mm Hg was similar among the three groups and was not affected by NPY1-36, sitagliptin or BIBP3226. The basal renovascular response to Ang II was 7 ± 1 mm Hg and was not affected by sitagliptin or sitagliptin plus BIBP3226. The p-value is for the treatment (sitagliptin or sitagliptin plus BIBP3226) term in the repeated measures 2-factor analysis of variance. Values represent means ± SEM for the indicated sample size.
Protocol 3
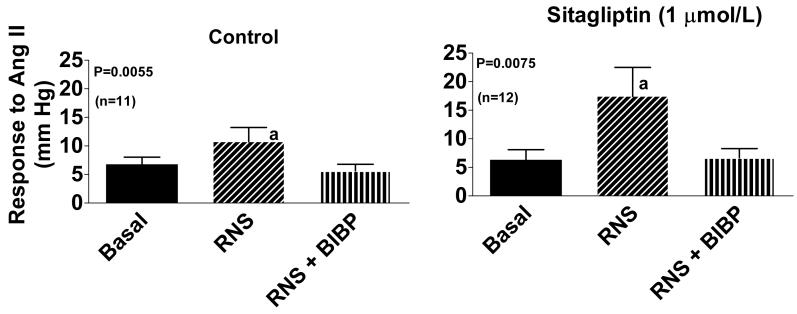
In the third protocol, we examined the effects of renal nerve stimulation in control kidneys (n=11) versus kidneys pretreated with sitagliptin (1 μmol/L; n=12) on renovascular responses to a very low, physiologically-relevant concentration of Ang II (100 pmol/L) before and after BIBP3226 (1 μmol/L). The basal response to 100 pmol/L of Ang II was similar in both groups and averaged 6 ± 1 mm Hg (n=23). Renal nerve stimulation did not influence basal renal perfusion pressure because the kidneys were pretreated with prazosin to block the ∀1-adrenoceptor-mediated effects of norepinephrine. As shown in Figure 3, in both non-sitagliptin-treated control kidneys and in sitagliptin-treated kidneys, renal nerve stimulation significantly (p<0.05) enhanced Ang II-induced renovascular responses. Also, in both non-sitagliptin-treated control kidneys and in sitagliptin-treated kidneys BIBP3226 blocked all effects of renal nerve stimulation on Ang II-induced renovascular responses. Notably, the fold-enhancement of Ang II-induced renovascular responses was significantly (p=0.0227) greater in sitagliptin-treated versus non-sitagliptin-treated kidneys (3.60-fold versus 1.51-fold).
Figure 3.
Bar graph shows the renovascular response to angiotensin II (Ang II) in the absence of renal nerve stimulation and BIBP3226 (Basal), during periarterial renal nerve stimulation (RNS; 5 Hz, 1-ms pulse duration, 35 V) and during RNS in the presence of BIBP3226 (RNS + BIBP; 1 μmol/L). In the left panel, kidneys were not pretreated with sitagliptin, whereas in the right panel, kidneys were pretreated with sitagliptin (1 μmol/L) throughout the experiment. The concentration of Ang II was 0.1 nmol/L. Basal perfusion pressure in the presence of prazosin (42 ± 2 mm Hg) was similar among the three groups and was not affected by RNS or BIBP3226. The p-values are from 1-factor analysis of variance. aIndicates p<0.05 versus Basal (Fisher's LSD test). Values represent means ± SEM for the indicated sample size.
DISCUSSION
The results from the first protocol of the present study demonstrate that a high concentration of exogenous NPY1-36 markedly enhances renovascular responses to Ang II in SHR kidneys. This finding supports the concept that if endogenous concentrations of NPY1-36 achieve a high enough level, endogenous NPY1-36 also should enhance renovascular responses to Ang II in kidneys from genetically-susceptible kidneys. Importantly, BIBP3226, a selective Y1R antagonist, abrogates the effects of a high concentration of exogenous NPY1-36 on renovascular responses to Ang II. This finding implies that the effect of NPY1-36 on renovascular responses to Ang II is mediated by activation of Y1Rs and not by other Y-receptor subtypes. Consistent with this interpretation, a high concentration of exogenous NPY3-36, a peptide that selectively activates Y2Rs and is inactive at Y1Rs, is not efficacious with regard to enhancing Ang II-induced renal vasoconstriction. Because DPP IV metabolizes NPY1-36 to NPY3-3612, this latter finding implies that renovascular DPP IV has the potential to minimize the renovascular impact of endogenous NPY1-36 in genetically-susceptible kidneys by converting the efficacious NPY1-36 to the non-efficacious NPY3-36.
The results from the second protocol of the present study are consistent with the hypothesis that renovascular DPP IV minimizes the renovascular impact of endogenous NPY1-36 in genetically-susceptible kidneys. In this regard, we observe that very low concentrations of exogenous NPY1-36 have little effect on renovascular responses to Ang II in genetically-susceptible kidneys. In contrast, when very low concentrations of exogenous NPY1-36 are administered to kidneys pretreated with sitagliptin, a potent and clinically useful DPP IV inhibitor, even these minute concentrations of exogenous NPY1-36 enhance renovascular responses to Ang II. This finding strongly suggests that renovascular DPP IV inactivates NPY1-36 so that low concentrations cannot enhance the renovascular effects of Ang II; however, when DPP IV is inhibited, this inactivation is impaired and so even low concentrations of NPY1-36 potentiate renovascular responses to Ang II.
Although the first and second protocols are consistent with the concept that endogenous NPY1-36 may regulate renovascular responses to Ang II in genetically-susceptible kidneys, these experiments do not directly address this critical issue. As discussed above, the main source of renal NPY1-36 is neural, i.e., NPY1-36 is a co-transmitter in renal sympathetic nerve varicosities9. Therefore, a straightforward approach to test the role of endogenous NPY1-36 with regard to its interaction with Ang II is to examine renovascular responses to Ang II in the absence and presence of renal sympathetic nerve stimulation. However, renal nerve stimulation normally increases renovascular tone, and increases in renovascular tone would be expected to enhance responses to any vasoconstrictor. Therefore, such experiments would be impossible to interpret. However, an experimental design around the confounding variable of changes in basal renovascular tone is to conduct the renal nerve stimulation in the presence of an ∀1-adrenoceptor antagonist, such as prazosin, so that NPY1-36 is released upon nerve stimulation but the co-released norepinephrine cannot increase basal renovascular tone.
Protocol three demonstrates that renal nerve stimulation in the presence of prazosin does indeed enhance the renovascular response to Ang II without changing basal renovascular tone. Moreover, this protocol shows that the effect of renal nerve stimulation on the renovascular response to Ang II is entirely blocked by BIBP3226. Thus it is clear that Y1Rs mediate the effect of renal nerve stimulation on renovascular responses to Ang II, a finding consistent with our previously published results14. Because NPY1-36 is the only known Y1R agonist released from renal sympathetic nerves, it is nearly certain that the enhancement of renovascular responses to Ang II by renal nerve stimulation is mediated entirely by endogenous NPY1-36 activating Y1Rs.
As mentioned above, an implication of protocols one and two is that renovascular DPP IV inactivates NPY1-36 so that low concentrations cannot enhance the renovascular effects of Ang II; however, when DPP IV is inhibited, this inactivation is impaired and so even low concentrations of NPY1-36 potentiate renovascular responses to Ang II. If this conclusion can be applied to endogenous NPY1-36, then sitagliptin, a DPP IV inhibitor, should augment the effects of renal nerve stimulation on renovascular responses to Ang II. Importantly, the results of protocol three are entirely consistent with this hypothesis, i.e., renal nerve stimulation enhances renovascular responses to Ang II more so in kidneys treated with sitagliptin compared with kidneys not treated with sitagliptin. The fact that BIBP3226 blocks the ability of sitagliptin to augment the interaction between renal nerve stimulation and Ang II is entirely consistent with the effects of sitagliptin being mediated by reducing metabolism of endogenous NPY1-36.
In the present study does not examine the interaction between NPY1-36 and Ang II in WKY rats because such an interaction cannot be demonstrated. In this regard, our previous studies show that activation of Y1Rs, even with high concentrations of potent Y1R agonists such as (Leu31,Pro34)-NPY1 or PYY1-3613, has little or no ability to enhance renovascular responses to Ang II in WKY kidneys. Our previous results also demonstrate that renal nerve stimulation does not enhance renovascular responses to Ang II in WKY kidneys14. Thus, with respect to the interaction between Ang II and NPY1-36, it is a moot point as to whether DPP IV regulates the levels of NPY1-36 in WKY kidneys because the peptide cannot significantly alter Ang II-induced renal vasoconstriction regardless of whether or not it is metabolized by DPP IV. The ability of Y1Rs to enhance renovascular responses in SHR kidneys, but not WKY kidneys, is due to the fact that Y1Rs signal via the Gi pathway. In this regard, activation of the Gi pathway enhances renovascular responses to Ang II in SHR, but not WKY, kidneys15. Why this is the case is presently unknown, but under investigation. Nonetheless, whether renovascular or glomerular DPP IV expression is increased in hypertension is an interesting and important question that deserves investigation because DPP IV has many substrates and alters the levels of a large array of polypeptides.
Perspective
The FDA recently approved sitagliptin, a potent inhibitor of DPP IV, for the treatment of type 2 diabetes, and other similar compounds are in the development and approval pipeline. Given the epidemic of type 2 diabetes and the emerging controversy regarding the safety of PPAR( agonists (the thiazolidinediones) in type 2 diabetics, it is likely that DPP IV inhibitors will become widely used in type 2 diabetics, many of whom will have hypertension as a co-morbidity. The results of the present study support the hypothesis that DPP IV inhibitors, in the appropriate genetic setting, may augment the ability of endogenously released NPY1-36 to enhance renovascular responses to Ang II. In this highly vulnerable patient population, this could impair renal function, increase arterial blood pressure and inadvertently lead to an increased risk of stroke and myocardial infarction. Counteracting this possible adverse effect of DPP IV inhibitors on renal function is the fact that, as shown by Girardi and colleagues, DPP IV forms a physical complex with the sodium hydrogen exchanger type 3 (NHE3) in the proximal tubular brush border so that inhibition of DPP IV leads to a reduction in NHE3-mediated transport16-18. Thus, the renal effects of DPP IV inhibitors in hypertensive, diabetic patients will be complicated and perhaps strongly dependent on the genetic predisposition of the patient. Certainly, the renal effects of DPP IV inhibitors in various patient populations merit a closer examination. In the meanwhile, it may be prudent to consider the concomitant use of an angiotensin receptor blocker or renin inhibitor (but perhaps not an angiotensin converting enzyme inhibitor which possibly could increase the risk of angioedema in patients treated with DPP IV inhibitors19) in type 2 diabetics treated with DPP IV inhibitors until this issue is resolved.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH grants HL69846 and DK068575.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Hypertension, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Hypertension. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosures: NONE
REFERENCES
- 1.Dubinion JH, Mi Z, Zhu C, Gao L, Jackson EK. Pancreatic polypeptide-fold peptide receptors and angiotensin II-induced renal vasoconstriction. Hypertension. 2006;47:545–551. doi: 10.1161/01.HYP.0000197033.54756.83. [DOI] [PubMed] [Google Scholar]
- 2.Pappas TN, Debas HT, Chang AM, Taylor IL. Peptide YY release by fatty acids is sufficient to inhibit gastric emptying in dogs. Gastroenterology. 1986;91:1386–1389. doi: 10.1016/0016-5085(86)90191-5. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DN, Ballantyne GH, Adrian TE, Bilchik AJ, McMillen MA, Modlin IM. Adaptive increase in peptide YY and enteroglucagon after proctocolectomy and pelvic ileal reservoir construction. Dis Colon Rectum. 1991;34:119–125. doi: 10.1007/BF02049984. [DOI] [PubMed] [Google Scholar]
- 4.Fu-Cheng X, Anini Y, Chariot J, Castex N, Galmiche JP, Roze C. Mechanisms of peptide YY release induced by an intraduodenal meal in rats: neural regulation by proximal gut. Pflugers Arch- Eur J Physiol. 1997;433:571–579. doi: 10.1007/s004240050316. [DOI] [PubMed] [Google Scholar]
- 5.Anini Y, Fu-Cheng X, Cuber JC, Kervran A, Chariot J, Roz C. Comparison of the postprandial release of peptide YY and proglucagon-derived peptides in the rat. Pflugers Arch- Eur J Physiol. 1999;438:299–306. doi: 10.1007/s004240050913. [DOI] [PubMed] [Google Scholar]
- 6.MacIntosh CG, Andrews JM, Jones KL, Wishart JM, Morris HA, Jansen JB, Morley JE, Horowitz M, Chapman IM. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am J Clin Nutr. 1999;69:999–1006. doi: 10.1093/ajcn/69.5.999. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira FV, Pera M, Kelly KA. Enhancing release of peptide YY after near-total proctocolectomy: jejunal pouch vs. ileal pouch-distal rectal anastomosis. J Gastrointest Surg. 2001;5:108–112. doi: 10.1016/s1091-255x(01)80020-2. [DOI] [PubMed] [Google Scholar]
- 8.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [see comment] [DOI] [PubMed] [Google Scholar]
- 9.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med. 2003;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- 11.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 12.McIntosh CH, Demuth HU, Pospisilik JA, Pederson R. Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents? Regul Pept. 2005;128:159–165. doi: 10.1016/j.regpep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Jackson EK, Zhang M, Liu W, Mi Z. Inhibition of renal dipeptidyl peptidase IV enhances peptide YY1-36-induced potentiation of angiotensin II-mediated renal vasoconstriction in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2007;323:431–437. doi: 10.1124/jpet.107.126847. [DOI] [PubMed] [Google Scholar]
- 14.Dubinion JH, Mi Z, Jackson EK. Role of renal sympathetic nerves in regulating renovascular responses to angiotensin II in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2006;317:1330–1336. doi: 10.1124/jpet.106.101279. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Zhu C, Jackson EK. alpha 2-Adrenoceptors potentiate angiotensin II- and vasopressin-induced renal vasoconstriction in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2003;305:581–586. doi: 10.1124/jpet.102.047647. [DOI] [PubMed] [Google Scholar]
- 16.Girardi AC, Degray BC, Nagy T, Biemesderfer D, Aronson PS. Association of Na(+)-H(+) exchanger isoform NHE3 and dipeptidyl peptidase IV in the renal proximal tubule. J Biol Chem. 2001;276:46671–46677. doi: 10.1074/jbc.M106897200. [DOI] [PubMed] [Google Scholar]
- 17.Girardi ACC, Fukuda LE, Rossoni LV, Malnic G, Reboucas NA. Dipeptidyl peptidase IV inhibition downregulates Na+-H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294:F414–422. doi: 10.1152/ajprenal.00174.2007. [DOI] [PubMed] [Google Scholar]
- 18.Girardi ACC, Knauf F, Demuth H-U, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. American Journal of Physiology - Cell Physiology. 2004;287:C1238–1245. doi: 10.1152/ajpcell.00186.2004. [DOI] [PubMed] [Google Scholar]
- 19.Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, Adam A, Brown NJ. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]