Abstract
The fields of tissue engineering and regenerative medicine aim at promoting the regeneration of tissues or replacing failing or malfunctioning organs, by means of combining a scaffold/support material, adequate cells and bioactive molecules. Different materials have been proposed to be used as both three-dimensional porous scaffolds and hydrogel matrices for distinct tissue engineering strategies. Among them, polymers of natural origin are one of the most attractive options, mainly due to their similarities with the extracellular matrix (ECM), chemical versatility as well as typically good biological performance. In this review, the most studied and promising and recently proposed naturally derived polymers that have been suggested for tissue engineering applications are described. Different classes of such type of polymers and their blends with synthetic polymers are analysed, with special focus on polysaccharides and proteins, the systems that are more inspired by the ECM. The adaptation of conventional methods or non-conventional processing techniques for processing scaffolds from natural origin based polymers is reviewed. The use of particles, membranes and injectable systems from such kind of materials is also overviewed, especially what concerns the present status of the research that should lead towards their final application. Finally, the biological performance of tissue engineering constructs based on natural-based polymers is discussed, using several examples for different clinically relevant applications.
Keywords: natural origin polymers, biomacromolecules, tissue engineering, biomedical applications, biodegradable
1. Introduction
In the new paradigms of regenerative medicine, the use of materials in contact with biological materials (cells, tissues/organs, physiological fluids and biomolecules) is a current illustration of the need of interdisciplinary scientific approaches that combine the most recent advances in materials science and technology, basic sciences and life sciences. In tissue engineering, matrices are developed to support cells, promoting their differentiation and proliferation towards the formation of a new tissue. Such strategies allow for producing hybrid constructs that can be implanted in patients to induce the regeneration of tissues or replace failing or malfunctioning organs. Different materials have been proposed to be used in the processing of scaffolds, namely biodegradable polymers. Natural-based polymers offer the advantage of being similar to biological macromolecules, which the biological environment is prepared to recognize and deal with metabolically. Owing to their similarity with the extracellular matrix (ECM), natural polymers may also avoid the stimulation of chronic inflammation or immunological reactions and toxicity, often detected with synthetic polymers. In this review, the different natural-based materials that have been proposed to be used in tissue engineering strategies will be overviewed. An important aspect is also addressed, which is the processing of such kind of materials into porous matrices, a task that usually needs other technologies rather than those usually employed in the processing of conventional synthetic polymers. There are also clinical needs for processing biomaterials into other shapes, including nano/microparticles (for control release application), or into two-dimensional structures (e.g. membranes as wound dressing). Non-invasive materials containing cells and bioactive agents are very attractive approaches in tissue engineering and regeneration contexts, which will also be discussed. Section 7 is devoted to the clinical applications of such materials and their in vivo performance for different cases.
2. Natural-based polymeric systems
The design and selection of a biomaterial is a critical step in the development of scaffolds for tissue engineering. Generally, the ideal biomaterial should be non-toxic, biocompatible, promoting favourable cellular interactions and tissue development, while possessing adequate mechanical and physical properties. In addition, it should be biodegradable and bioresorbable to support the reconstruction of a new tissue without inflammation (Kim et al. 2000). On the other hand, novel concepts of tissue engineering are imposing new and more specific requirements on macromolecular components. Living organisms are able to synthesize a vast variety of polymers, which can be divided into major classes according to their chemical structure: (i) polysaccharides, (ii) proteins, and (iii) polyesters. Nowadays, with the advances in biotechnology, natural polymers can be obtained by the fermentation of micro-organisms (Widner et al. 2005) or produced in vitro by enzymatic processes (Kobayashi et al. 2003). However, the largest amount is still extracted from plant (Franz & Blaschek 1990; Morrison & Karkalas 1990; Stephen et al. 1990) and animal (Izydorczyk et al. 2005) sources or from algae (Percival & McDowell 1990).
2.1 Materials inspired by the extracellular matrix
The ECM is the optimized milieu that nature has been developing to maintain homeostasis and to direct tissue development. Therefore, a great effort has been made to mimick the ECM to guide morphogenesis in tissue repair and tissue engineering (Hubbell 2003). A strategy has been proposed to isolate the main constituents of the ECM and directly use them after purification, with or without further modifications. As ECM plays an instructive role in cell activities, the hypothesis here is that such biomolecules would maintain the biological information and other physico-chemical features, which would preserve a potential space for new tissue development after cell seeding. This would help to overcome one of the main drawbacks in the use of synthetic materials, which lack cell recognition signals. A description of some protein-based biomaterials isolated from ECM follows. These materials are typically extracted from blood plasma and the skeleton. Other functional proteins, including growth factors, enzymes and interleukins, which are used essentially as ingredients to be incorporated into biomaterials, will not be discussed here.
Collagen is the most abundant protein in the body. More than 20 genetically distinct forms have been identified, type I being the most abundant and most investigated for biomedical applications (Hayashi 1994). Characteristics such as high mechanical strength, good biocompatibility, low antigenicity and ability of crosslinking enable the tailoring of the mechanical, degradation and water uptake properties. To obtain matrices with adequate mechanical properties, chemical glycation procedures or heat treatments have been proposed (Hubbell 2003). Combinations of collagen with other materials have also been prepared. For example, collagen microsponges may be easily impregnated into previously prepared synthetic polymeric scaffolds, which will increase their mechanical performance (Chen et al. 2000). On the other hand, growth factors and other active agents can be combined with collagen-based systems, including scaffolds and gels to prolong their release rate and increase their therapeutic effect on tissue engineering approaches (Geiger et al. 2003; Wallace & Rosenblatt 2003).
Fibronectin is a multifunctional component of the ECM, known to induce cell attachment and spreading through its cell binding site and related synergy sites. The ability of such glycoprotein (a disulphide-bonded dimer of 220–250 kDa subunits) to serve as a substrate for cell adhesion is based on the biological activity of several modules: the RGD tripeptide, arginine-glycine-aspartic acid, in the tenth Fn3 module plays here an important role (Ruoslahti & Pierschbacher 1987) and has been incorporated onto the surface of numerous biomaterials; several strategies have been summarized by Hubbell (2003). One of the suggested strategies was to deposit layers of oriented fibronectin to enhance the availability of its cell binding site (Calonder et al. 2005). On an oriented fibronectin layer, compared with an isotropic layer, human umbilical vein endothelial cells spread significantly faster in a more spherical way.
Glycosaminoglycans (GAGs) are linear chains consisting of the repeating unit of a disaccharide, generically a hexosamine (glucosamine or galactosamine) and a uronic acid component (Hayashi 1994). With the exception of hyaluronic acid, such chains are attached to a central protein to form the proteoglycans. Owing to their ionic character, GAGs are able to absorb large quantities of water, and this osmotic swelling provides compressive strength.
Fibrin plays an important role in haemostasis and spontaneous tissue repair (it naturally forms during blood coagulation). Fibrin is a complex network formed by polymerization of fibrinogen in the presence of the enzyme thrombin. Fibrinogen can be isolated from the blood plasma of the patient, limiting its potential for disease transmission and immunogenic reactions. Fibrin is not a regular component of the ECM, but is found as a temporary matrix that will be further replaced by the ECM, and is currently used as fibrin glue in clinical applications. Fibrin has been a useful cell delivery matrix for cartilage tissue engineering, especially in combination with other biodegradable substances, such as alginate (Perka et al. 2000) or hyaluronic acid (Park et al. 2005b). It has also been used in the regeneration of the skin, with considerable success, and even in the loading and posterior release of growth factors (see Hubbell 2003 and references therein).
Hydrogels enabling enzymatically mediated cell migration are part of an interesting strategy to mimic the ECM, whose aim is to develop matrices that could promote cell ingrowth through proteolytic degradation of the matrix, usually requiring the action of metalloproteinases that are secreted by cells. Very elegant works have reported the use of conjugates of poly(ethylene glycol) and specific peptides that can be hydrolysed under the presence of enzymes involved in cell migration (Mann et al. 2001; Lutolf et al. 2003a). Growth factors can be entrapped that can also promote mesenchymal stem cell (MSC) infiltration and corresponding differentiation (Lutolf et al. 2003a,b). Instead of peptide segments, denaturated fibrinogen segments were combined with poly(ethylene glycol) to form biosynthetic hybrid hydrogels (Almany & Seliktar 2005).
Self-assembling materials, also aimed at mimicking the ECM, have been proposed as hydrogels for tissue engineering applications. Peptide-amphiphilic-based nanostructured fibrous scaffolds were produced by pH-induced self-assembly that could also induce biomineralization (Hartgerink et al. 2001). A three-dimensional network based on nanofibres formed by the self-assembly of peptide-amphiphilic molecules was also used to encapsulate neural progenitor cells, showing the ability to induce very rapid differentiation into neurons, while discouraging the development of astrocytes (Silva et al. 2004).
2.2 Other protein-derived biomaterials
Animal- or vegetal-derived proteins have been shown to have potential to be used as scaffolds for tissue engineering applications. Silk proteins, for example, contain a highly repetitive primary sequence that leads to a high content of β-sheets, responsible for the good mechanical properties of silk fibres. It has been reported that silk fibroin may have potential to be used in tissue engineering applications, where mechanically robust, long-term degradable materials are needed (Altman et al. 2003). For example, highly porous silk scaffolds were combined with adult MSCs for in vitro cartilage tissue engineering (Wang et al. 2005). Additionally, casein and soybean protein-based materials were found to be promising materials for use in different biomedical applications, including the production of scaffolds for tissue engineering (Vaz et al. 2003). The major drawback of such materials is the possibility of eliciting some level of foreign body response following implantation in vivo, which can be minimized, for instance, by purification.
2.3 Polysaccharides
Polysaccharides consist of monosaccharides linked together by O-glycosidic linkages. Differences in the monosaccharide composition, linkage types and patterns, chain shapes and molecular weight dictate their physical properties, including solubility, flow behaviour, gelling potential and/or surface and interfacial properties. Polysaccharides are derived from renewable resources, namely plants, animals and micro-organisms, and are therefore widely distributed in nature. They perform different physiological functions and may offer a variety of potential applications in the fields of tissue engineering and regenerative medicine.
2.3.1 Plant polysaccharides
Starch is a mixture of glycans that plants synthesize as their principal food reserve. It is deposited in the chloroplasts of plant cells as insoluble granules composed of α-amylose (normally 20–30%) and amylopectin (normally 70–80%; Morrison & Karkalas 1990). α-Amylose is a linear polymer of several thousands of glucose residues linked by α(1→4) bonds. Amylopectin, consisting mainly of α(1→4)-linked glucose residues, is a branched molecule with α(1→6) branch points at every 24–30 glucose residues in average (table 1). Amylopectin molecules contain up to 106 glucose residues, making them some of the largest molecules in nature (Voet et al. 1999).
Table 1.
Examples of some polysaccharides available in nature, their relevant properties and applications in the fields of tissue engineering and regenerative medicine.
| polysaccharide | source | repeating unit | relevant properties | examples of proposed applications |
|---|---|---|---|---|
| starch | plant (e.g. corn, rice, potato, wheat, tapioca, etc.; Morrison & Karkalas 1990) | 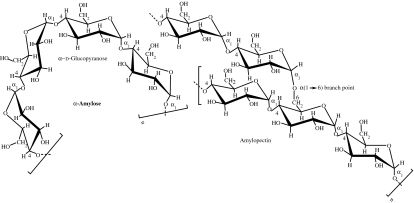 |
starch is a highly available material and can be easily modified (crosslinked, oxidized and acetylated; Xie et al. 2005); it can be converted into a thermoplastic (Rodriguez-Gonzalez et al. 2004), or blended with synthetic polymers to improve the weakness of starch and thus obtain better mechanical properties (Ciardelli et al. 2005); amylose forms gels and films; it is enzymatically degraded by α-amylase (Azevedo et al. 2003), which exists in serum | starch-based scaffolds produced by melt-based (Gomes et al. 2001, 2003; Salgado et al. 2004a) and rapid prototyping (Lam et al. 2002; Pfister et al. 2004) techniques for bone and other TE applications |
| cellulose | plant (cotton, wood, straw, etc.; Franz & Blaschek 1990); microbial (bacterial cellulose, e.g. Acetobacter xylinum; Svensson et al. 2005) | 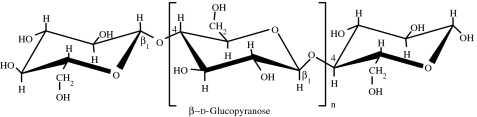 |
cellulose can be converted into different derivatives (carboxymethylcellulose, cellulose nitrate, cellulose acetate, cellulose xanthate; Franz & Blaschek 1990) that can be easily moulded or drawn into fibres (Entcheva et al. 2004) | cellulose acetate scaffolds for cardiac TE (Entcheva et al. 2004) and bacterial cellulose for cartilage TE (Svensson et al. 2005) |
| arabinogalactan (larch gum) | plant (extracted from the heartwood of the western larch Larix occidentalis; Chandrasekaran & Janaswamy 2002) | 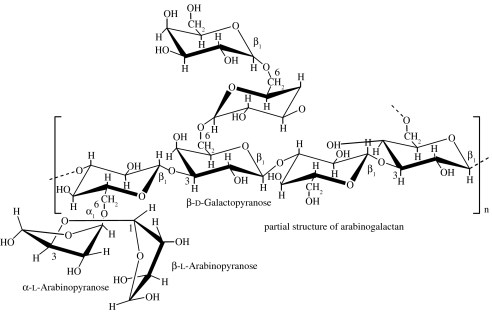 |
this polymer is a highly branched polysaccharide with high water solubility (70% in water; Stephen et al. 1990; Ehrenfreund-Kleinman et al. 2002) | arabinogalactan-based sponges for TE (Ehrenfreund-Kleinman et al. 2002) |
| alginic acid | brown algae (Phaeophyceae, mainly Laminaria); microbial (bacteria Pseudomonas mendocina, Azotobacter vinelandii; Percival & McDowell 1990) | 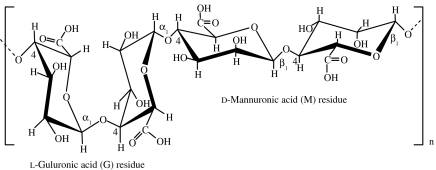 |
simple gelation with divalent cations (e.g. Ca2+, Mg2+, Ba2+, Sr2+); it is the size and proportion of the G blocks in the polymer chain that determines the formation and strength of gels formed with calcium (Percival & McDowell 1990) | cell encapsulation matrix (Jen et al. 1996; Rowley et al. 1999; Kampf 2002); injectable cell delivery vehicle (Bouhadir et al. 2001); alginate scaffolds for hepatic TE (Glicklis et al. 2000); alginate hydrogels for cartilage TE (Awad et al. 2004; Mouw et al. 2005) |
| agar | red algae: Rhodophyceae (Gelidium and Gracilaria spp.; Percival & McDowell 1990) | 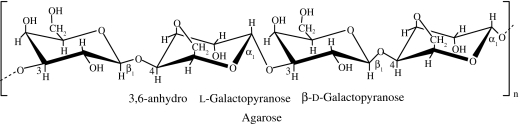 |
agarose forms thermally reversible gels (cold setting gels at approx. 38°C); however, the melting temperature is much higher, approximately 85°C; this gives agar gel a very large gelling/melting hysteresis (Izydorczyk et al. 2005) | agarose gels/sponges for cartilage (Awad et al. 2004; Miyata et al. 2004; Mouw et al. 2005; Ng et al. 2005), disc TE (Gruber et al. 2006) and nerve regeneration (Balgude et al. 2001); production of anatomically shaped, engineered cartilage constructs using chondrocyte-seeded agarose hydrogels (Mauck et al. 2003); cell encapsulation matrix (Jen et al. 1996; O'Connor et al. 2001; Kampf 2002) |
| carrageenan | red algae: Rhodophyceae (Chondrus crispus, Eucheuma cottonii, Eucheuma spinosum; Percival & McDowell 1990) | 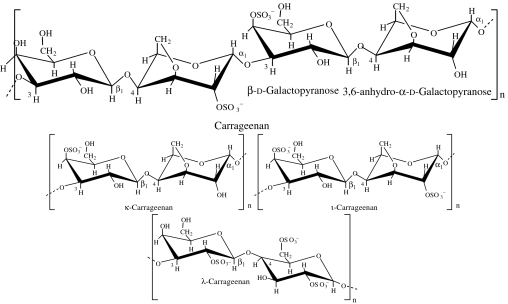 |
κ-carrageenan gels in the presence of K+ ions to form strong crisp gels; ι-carrageenan gels in the presence of Ca2+ ions to form elastic gels; λ-carrageenan is non-gelling but forms viscous solutions (Percival & McDowell 1990) | carrageenan-based polyelectrolyte for cell encapsulation (Prokop et al. 1998a,b; Bartkowiak & Hunkeler 2001; Shumilina & Shchipunov 2002) |
| chitin | animal (crustacean shells, exoskeletons of insects and other arthropods); microbial (fungal cell walls; Lezica & Quesada-Allué 1990) |
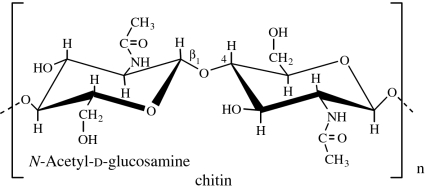 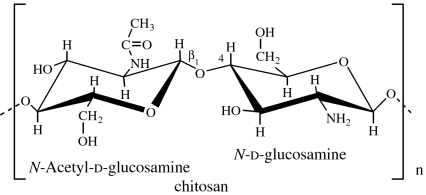
|
chitin is insoluble in most common solvents, but can be converted by chemical reactions into soluble derivatives (Kurita 2001); chitin derivatives are enzymatically degraded by lysozyme, which exists in blood serum and phagocytic cells, but the rate of degradation depends on the DA (Freier et al. 2005a) | chitosan fibre mesh scaffolds produced by wet spinning for bone TE (Tuzlakoglu et al. 2004); chitosan sponges as scaffolding materials for bone formation and cartilage TE (Nettles et al. 2002; Seol et al. 2004); chitin-based tubes for nerve regeneration (Freier et al. 2005b); chitosan particles agglomerated scaffolds for cartilage and osteochondral TE (Malafaya et al. 2005); injectable chitosan-based cell delivery vehicle for cartilage TE (Chenite et al. 2000; Hoemann et al. 2005) |
| hyaluronic acid | animal (synovial fluid, vitreous humour of the eye, umbilical tissue; Drury & Mooney 2003); microbial (fermentation Bacillus subtilis; Leach et al. 2003; Widner et al. 2005) | 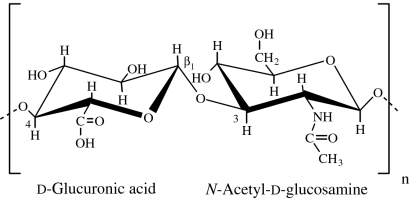 |
hyaluronan is soluble in water and can form hydrogels by covalent and photocrosslinking with hydrazide derivatives, by esterification and annealing; it is enzymatically degraded by hyaluronidase, which exists in cells and serum (Drury & Mooney 2003) | prototype benzylic ester of hyaluronic acid scaffold for ligament TE (Cristino et al. 2005); hyaluronan-based sponges for the treatment of osteochondral defects (Solchaga et al. 2000), flat sheets of non-woven hyaluronan-based scaffold as vascular graft (Arrigoni et al. 2006); photocrosslinked hyaluronic acid hydrogels as TE scaffolds (Leach et al. 2003) |
| dextran | microbial (bacterium Leuconostoc mesenteroides; Naessens et al. 2005) | 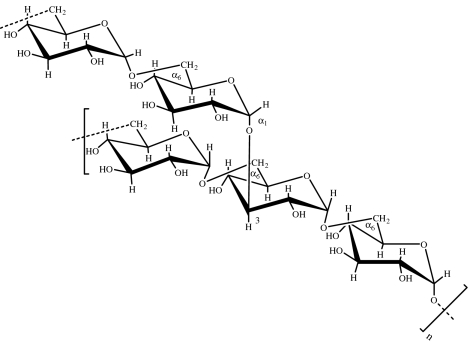 |
dextran is soluble in both water and organic solvents (e.g. dimethyl sulphoxide; Jiang et al. 2004); it is stable under mild acidic and basic conditions, and contains a large number of hydroxyl groups available for modification/conjugation with other molecules (Mehvar 2000) | porous hydrogels as scaffolds for TE applications (Levesque et al. 2005) |
| gellan gum | microbial (bacterium Sphingomonas elodea; Ciardelli et al. 2005) | 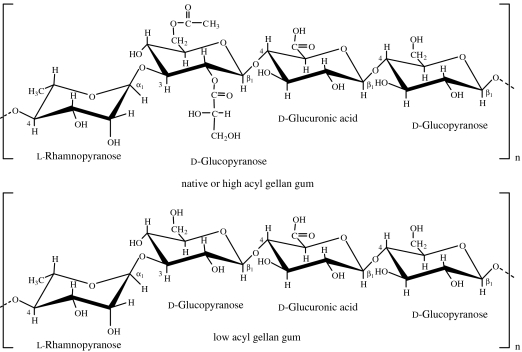 |
high acyl (HA) gellan gum gives soft, elastic, transparent and flexible gels at polymer concentrations higher than 0.2%; HA gels set and melt at approximately 70–80°C with no thermal hysteresis; however, low acyl (LA) gellan gum can form hard, non-elastic and brittle gels in the presence of cations, including Ca2+, Mg2+, Na+, K+ and H+; the gel strength of LA gellan gum increases with increasing ion concentration; cation concentration can also affect gel setting and melting temperatures (Izydorczyk et al. 2005) | laser sintered polycaprolactone/gellan as TE scaffolds (Cascone et al. 2001; Ciardelli et al. 2005) |
| pullulan | microbial (fungus Aureobasidium pullulans; Izydorczyk et al. 2005) |  |
pullulan dissolves readily in water (Gupta & Gupta 2004) to form a stable viscous solution that does not gel; it has adhesive properties and can be used to form fibres, compression mouldings and strong, oxygen-impermeable films; pullulan is easily derivatized to control its solubility or provide reactive groups (Na et al. 2003) | carboxylated pullulan derivatives as extracellular matrix for TE applications (e.g. endothelial cells; Na et al. 2003) |
Cellulose, the primary structural component of plant cell walls, is a linear polysaccharide of d-glucose units linked by β(1→4) glycosidic bonds (table 1). The fully equatorial conformation of β-linked glucose residues stabilizes the chair structure, minimizing its flexibility. This highly cohesive, hydrogen-bonded structure gives cellulose fibres the exceptional strength and makes them water insoluble despite their hydrophilicity (Voet et al. 1999). Cellulosic materials exhibit, however, poor degradation in vivo (Entcheva et al. 2004).
2.3.2 Exudate gums
Exudate gum polysaccharides are produced at the surface of a plant, usually as a result of trauma or stress (physical injury and/or fungal attack). These exudates are complex, uronic acid-containing polysaccharides; some are even associated with proteins (Stephen et al. 1990). Most gums are soluble in water and have high viscosity. Major use of gums is in the food and pharmaceutical industries, where their emulsifying, stabilizing, thickening and gel-forming properties are the main physical requirements (Stephen et al. 1990). The gums with major commercial value are gum arabic, larch gum, gum karaya, gum ghatti and gum tragacanth (Stephen et al. 1990). Arabinogalactan is a major d-galactan obtained from soft woods such as larch (larch gum). It is composed of β(1→3)-linked d-galactose units, each containing a side chain at position C6. It is extracted from the Larix tree and available in 99.9% pure form with reproducible molecular weight and physico-chemical properties. The high solubility in water, biocompatibility, biodegradability and the ease of chemical modification in aqueous media make it an attractive polymer for the synthesis of scaffolds for application in tissue engineering.
Gum arabic is an exudate gum obtained from Acacia trees and consists of a variable mixture of arabinogalactan oligosaccharides, polysaccharides and glycoproteins. It is an acidic arabinogalactan with a complex structure. The main chain of this polysaccharide consists of β(1→3) and β(1→6)-linked d-galactose units along with β(1→6)-linked d-glucopyranosyl uronic acid units. Though gum arabic has high water solubility (up to 50% w/v) and relatively low viscosity (Izydorczyk et al. 2005), it exhibits emulsification, encapsulation and film-forming abilities.
2.3.3 Algal polysaccharides
Alginate, the monovalent form of alginic acid, is a linear polymer of β(1→4)-linked d-mannuronic acid and α(1→4)-linked l-guluronic acid, which occurs combined with calcium and other bases in the cell walls and intracellular matrix of brown seaweeds, constituting the main structural component (Percival & McDowell 1990). The residues are present in the blocks of each monomer, separated by regions in which they are randomly arranged or alternating. The proportions of mannuronic (M) and guluronic (G) residues (table 1), and the lengths of the blocks, can vary considerably, depending on the source of the alginate. The polymer undergoes ionotropic gelation in the presence of divalent cations and gelling depends on the ion binding (Mg2+≪Ca2+<Sr2+<Ba2+; Izydorczyk et al. 2005). One of the drawbacks of alginate hydrogels is that the degradation occurs via a slow and unpredictable dissolution process in vivo (Rowley et al. 1999; Bouhadir et al. 2001; Boontheekul et al. 2005).
Galactans, the major polysaccharides of the red algae Rhodophyceae, comprise agars, carrageenans and related hybrid polysaccharides. Agar is made of two components: agarose (a non-sulphated fraction) and agaropectin (a mixture of various sulphated molecules). The separation of agarose from the polysaccharide mixture is based on the differences in solubility and chemical reactivity, which is associated with the anionic character of agaropectins (Percival & McDowell 1990). Agarose is a linear polysaccharide based on the (1→3)-β-d-galactopyranose-(1→4)-3,6-anhydro-α-l-galactopyranose unit (table 1). This arrangement allows the chains to join together and adopt a double helix. The two chains are wrapped together so tightly that any gaps are closed, trapping any water inside the helix.
Carrageenan polysaccharides are linear polymers consisting of chains of (1→3)-linked β-d-galactose and (1→4)-linked α-d-galactose units, which are variously substituted and modified into the 3,6-anhydro derivative, depending on the source and extraction conditions (table 1; Thanh et al. 2002). All carrageenans are highly flexible molecules, which at higher concentrations wind around each other to form double helical structures. This gives them the ability to form a variety of different gels at room temperature. κ- and ι-carrageenans form thermoreversible gels on cooling in the presence of appropriate counterions. A particular advantage is that they are thixotropic (Bartkowiak & Hunkeler 2001), i.e. they thin under shear stress and recover their viscosity once the stress is removed.
2.3.4 Animal polysaccharides
Chitin is the principal structural component of the exoskeletons of invertebrates, such as crustaceans and insects, and is also present in the cell walls of most fungi and many algae. It is a homopolymer of β(1→4)-linked N-acetyl-d-glucosamine residues (GlcNAc; Lezica & Quesada-Allué 1990). Chitin can be converted into soluble derivatives such as chitosan, carboxymethyl chitin and glycochitin, among others (Kurita 2001).
Chitosan is soluble in diluted acids. The number fraction (%) of GlcNAc residues in the polymer chain is designated by degree of acetylation (DA) and influences the chitosan physico-chemical properties, such as solubility, reactivity, biodegradability and cell response (Khor & Lim 2003; Freier et al. 2005a). Water-soluble derivatives (e.g. carboxymethyl chitin) can also be obtained, extending the domain of applications of chitin, especially in the biomedical field. Chitin derivatives possess many unique properties, such as biocompatibility, biodegradability, hydrophilicity, adsorption capability and high reactivity. Moreover, chitin-based polymers are materials with great versatility to be processed in different forms (fibres, sponges, membranes, beads and hydrogels; Khor & Lim 2003; Di Martino et al. 2005; Yi et al. 2005).
Glycosaminoglycans are unbranched polysaccharides that consist of alternating uronic acid and hexosamine residues. The extracellular spaces, in particular those of connective tissues such as cartilage, tendon, skin and blood vessel walls, contain collagen and other proteins embedded in a gel-like matrix that is composed largely of GAGs.
Hyaluronic acid, also known as hyaluronan or hyaluronate, is an important GAG component of connective tissue, synovial fluid (the fluid that lubricates joints) and the vitreous humour of the eye (Drury & Mooney 2003). Hyaluronic acid is a linear polysaccharide composed of 250–25 000 β(1→4)-linked disaccharide units, which consists of d-glucuronic acid and N-acetyl-d-glucosamine (GlcNAc) linked by β(1→3) bond (table 1). The disaccharide units of hyaluronic acid are extended, forming a rigid molecule whose numerous repelling anionic groups bind cations and water molecules. In solution, hyaluronate occupies a volume approximately 1000 times that in its dry state. Hyaluronate solutions exhibit clear viscoelastic properties that make them excellent biological absorbers and lubricants. In addition, hyaluronic acid can be easily and controllably produced in large scales through microbial fermentation, enabling the scale-up of derived products and avoiding the risk of animal-derived pathogens. Chondroitin-4-sulphate and chondroitin-6-sulphate differ only in the sulphation of their N-acetylgalactosamine (GalNAc) residues. Dermatan sulphate is derived from chondroitin by enzymatic epimerization of C5 of glucuronate residues to form iduronate residues. Keratan sulphate is the most heterogeneous of the major GAGs, in that its sulphate content is variable and contains small amounts of fucose, mannose, GlcNAc and sialic acid. Heparin also consists of a variably sulphated repeating disaccharide unit, with an average of 2.5 sulphate residues per disaccharide unit, which makes it the most highly charged polymer in mammalian tissues.
2.3.5 Microbial polysaccharides
Dextran is a branched bacterial polysaccharide, produced from sucrose via the action of dextransucrase enzyme (Naessens et al. 2005), consisting of α(1→6)-linked d-glucose residues with some degree of branching via α(1→3) linkages (table 1). The molecular weight and degree of branching is dependent on the source of dextrans and may vary from 0.5 to 60%. Both the degree of branching and molecular weight distribution affect its physico-chemical properties (Mehvar 2000).
Gellan gum is a high molecular weight bacterial exopolysaccharide. It is a linear anionic heteropolysaccharide composed of the tetrasaccharide (1→4)-l-rhamnose-α(1→3)-d-glucose-β(1→4)-d-glucuronic acid-β(1→4)-d-glucose as a repeating unit (table 1). In its native, or high acyl form, two acyl substituents d-acetate and d-glycerate are present. Both substituents are located in the same glucose residue. The high acyl form produces transparent, soft, elastic and flexible gels that are resistant to heat and acid, whereas the low acyl form produces firm, non-elastic brittle gels. Gel formation is due to a conformational heat reversible transition from a state of single random macromolecules to a more ordered state, where macromolecules pair with each other to form double helices (Cascone et al. 2001).
Pullulan is an extracellular microbial homopolysaccharide of glucose, produced from starch by fermentation, and consists in repeating units of maltotriose (α(1→4)-linked) joined by α(1→6) linkages (table 1). Some advantages of pullulan are its non-toxicity and lack of immunogenicity (Gupta & Gupta 2004).
Xanthan gum is an anionic polysaccharide produced from glucose via fermentation by the bacterium Xanthomonas campestris (Rosalam & England 2006). The structure of xanthan is based on a cellulosic backbone of β(1→4)-linked glucose units that have trisaccharide side chains of d-mannose-β(1→4)-d-glucuronic acid-β(1→2)-d-mannose linked to C3 every second glucose unit in the main chain. Some terminal mannose units can contain a pyruvate group and mannose residues attached to backbone units are variably acetylated. It has unique rheological properties (high viscosity even at low concentrations) and forms hydrogels by annealing the solution in sol state and subsequent cooling (Iseki et al. 2001).
Cellulose can be produced by the bacterium Gluconacetobacter xylinus (table 1). Bacterial cellulose (BC) has unique properties, including biocompatibility, high water-holding capacity, high crystallinity, a fine fibre network and high tensile strength in the wet state. In addition to its cost-efficient and relatively simple production, it has the advantage of in situ mouldability (Svensson et al. 2005).
Table 1 summarizes the polysaccharides that are currently used in the field of tissue engineering and regenerative medicine. However, there is a wide variety of other potentially useful polysaccharides exhibiting a diversity of structures and functions (e.g. pseudoplastic behaviour, gelation, water binding, etc.), such as laminarin, gluco- and galactomannans, exudate and mucilage gums, xanthan gum, levan, curdlan, among many others, which remain unexploited within the biomedical field. This situation may be associated with their current unavailability, difficulty of their isolation/purification and processing and/or lack of information related to their biocompatibility and toxicity.
An important aspect to consider when using natural materials is that they can induce an undesirable immune response due to the presence of impurities and endotoxins, depending on the source of the material. Additionally, their properties may differ from batch to batch during large-scale isolation procedures due to inability to control the processing techniques. Nevertheless, as knowledge about these natural materials is widening, new approaches, including methods for production, purification, control of material properties (mechanical and degradation rate) and for enhancing material biocompatibility, are being developed for designing better scaffolding materials to support the development of more natural and functional tissues.
2.4 Naturally derived polyesters
Polyhydroxyalkanoates (PHAs) are degradable, biocompatible, thermoplastic polyesters derived from micro-organisms, used as a reserve of carbon and energy. A great variety of materials of this family can be produced, but the use of PHAs in tissue engineering has been mainly restricted to two polymers, namely poly(hydroxybutyrate), PHB, and poly(hydroxybutyrate-co-valerate), PHBV (Williams et al. 1999; Chen & Wu 2005); the copolymerization produces less crystalline, more flexible and more readily processable materials than pure PHB. Previous works have shown that such materials can be used as cell support for tissue engineering application (e.g. Deng et al. 2002).
3. Processing of tissue engineering scaffolds
Successful tissue engineering strategies require the development of an adequate scaffold that supports the regeneration process. The scaffold provides a three-dimensional structure and template upon which the tissue-specific cells attach, proliferate and produce ECM. The scaffold should provide sufficient surface area for the initial cell seeding and subsequent proliferation and colonization of the construct. Furthermore, the necessary diffusion of nutrients and metabolites in a three-dimensional environment demands highly porous scaffolds (Karageorgiou & Kaplan 2005). Ideally, the material should degrade in agreement with the deposition of ECM.
3.1 Particle leaching
Particle leaching is one of the most widely used processing methods to obtain controlled size of the porosity of scaffolds (Riddle & Mooney 2004). The process is based on the dispersion of a porogen agent (such as salt, sugar or others) either in a liquid, particulate or powder-based material (Hou et al. 2003; Lee et al. 2004c). The liquid may be solidified by solvent evaporation, crosslinking or other reactions, and the powder may be compacted using pressure and temperature. After the shaping process, the porogen agent is dissolved by immersion in a solvent specific to it and porosity is created (Lee et al. 2003). Besides the uneven permeability caused by the poor dispersion of the porogen, it is relevant to highlight its adverse effect over the mechanical properties of the porous structure (Zhang et al. 2005). Advantages of this process include its simplicity, versatility and easiness of control of the pore size and geometry. The pore geometry is obtained by the selection of the shape of the porogen agent, whereas the pore size is controlled by sieving the porogen particles to a specific dimension range. It is, however, difficult by this method to accurately design the interconnectivity of the pores (Moore et al. 2004).
3.2 Freeze drying
Freeze drying is a commonly used process to stabilize and preserve heat-sensitive bioproducts. The method is based on the formation of ice crystals that induce porosity through ice sublimation and desorption. The kinetics of the freezing stage controls the porosity and the interconnectivity of foams (Liapis et al. 1996). It is possible to control the porosity level of the foams by varying the freezing time and the annealing stage (Hottot et al. 2004). Values of porosity up to 90% with different interconnectivities are common in freeze-dried structures. The main difficulty associated with this process is to ensure structural stability and adequate mechanical properties of the porous constructs after subsequent hydration. This limitation hinders its use when the application involves conditions with mechanical stress, even at low-to-moderate levels. Our group developed bioactive chitosan/hydroxyapatite scaffolds using this method (Malafaya & Reis 2003). Another study (Oliveira et al. 2006) developed bilayered scaffolds based on chitosan and hydroxyapatite aimed at osteochondral application, where the porous morphology of the chitosan layer was induced by freeze drying.
3.3 Phase separation
Phase separation of polymeric solutions can be induced by various techniques. An extensive review of the processes available has been published elsewhere (vandeWitte et al. 1996). Thermal-induced phase separation is based on the decrease in solubility associated with temperature increase. After demixing is induced, the solvent is removed by extraction, evaporation or freeze drying (Yang et al. 2004). In air-casting precipitation, a polymer solution is prepared by dissolving a polymer in a mixture of a volatile solvent and a less volatile non-solvent. Upon evaporation of the solvent, the solubility of the polymer decreases and the phase separation takes place (Zeman & Fraser 1993). Immersion precipitation is the casting of a polymer solution as a thin film on a support or by extruding the solution through a die followed by subsequent immersion in a non-solvent bath (Cheng et al. 1995).
Several natural-based biodegradable polymers and composites have been processed by this route, including chitosan/nano-hydroxyapatite (Almany & Seliktar 2005), chitosan-gelatine/hydroxyapatite (Zhao et al. 2002) and alginate/hydroxyapatite (Lin & Yen 2004) composites.
3.4 Fibre meshes and fibre bonding
Fibre meshes produced from melt- or solution-spun fibres have also been investigated as TE scaffolds. Our group has developed starch-based scaffolds (Gomes et al. 2003; Pavlov et al. 2004) by melt-spinning blends of starch with ethylene vinyl alcohol copolymer, poly(ϵ-caprolactone) (PCL) and polylactide (PLA) into fibre bundles. In this methodology, fibre mesh scaffolds are produced by applying a heat treatment to bond fibre bundles (Pavlov et al. 2004). For these scaffolds, cell survival was shown to be highly dependent on scaffold porosity, which is believed to be related to the more efficient diffusion of nutrients within the scaffold (Gomes et al. 2004a). Our group has yet proposed another method for the production of fibre mesh scaffolds from non-fusible materials, by directly producing fibre bundles by wet spinning (Tuzlakoglu et al. 2004).
3.5 Melt processing
The non-thermoplastic behaviour of natural polymers has limited the application of melt-based processing methods for the production of TE scaffolds to these materials. However, several melt-based processing methodologies, such as compression moulding combined with particulate leaching, extrusion and injection moulding with blowing agents, have been proposed within our group. Foaming during melt extrusion or injection moulding is based on the use of physical or chemical blowing agents that are responsible for inducing porosity. Research on these processing routes has mainly been focused on the use of chemical blowing agents. So far, TE scaffolds based on thermoplastic blends of starch with ethylene vinyl alcohol copolymer and cellulose acetate have been produced by melt extrusion, employing endothermic chemical blowing agents based on mixtures of citric acid and sodium bicarbonate (Gomes et al. 2002). These scaffolds have been reported to be highly biocompatible (Salgado et al. 2002) and to exhibit adequate properties, both in terms of porosity and pore geometry, for supporting cell growth and apparent bone formation (Salgado et al. 2004b). A similar strategy has been applied for the production of TE scaffolds based on injection moulding (Gomes et al. 2001; Neves et al. 2005). Compared with extrusion, injection moulding uses the combined effect of heat and shear to plasticize thermoplastic formulations. More recently, physical blowing agents such as supercritical CO2 and water have been used to produce TE scaffolds by injection moulding (Haugen et al. 2005; Leicher et al. 2005).
3.6 Batch foaming
Traditional methods for the production of microporous materials are based on phase separation of a homogeneous polymer solution by temperature quench or by the addition of an anti-solvent. Alternatively, it is possible to produce porous morphologies by first saturating a polymer with a high pressure or supercritical gas (usually CO2) at controlled temperature and submitting it to pressure quenching induced by rapid depressurization. The production of TE scaffolds by batch foaming is based on the saturation of a polymer with pressurized gas, followed by a rapid pressure reduction that causes thermodynamic instability and subsequent nucleation and growth of gas pores. Mooney and co-workers (Harris et al. 1997, 1998) pioneered the use of this strategy for the development of TE scaffolds based on PLA, PGA and poly(lactic-co-glycolic acid) (PLGA) in combination with particle salt leaching. Recently, anisotropic composites based on PLA and hydroxyapatite (HA) were produced (Mathieu et al. 2005, 2006) by a different method based on the saturation of the polymer above its melting temperature with supercritical CO2 followed by rapid depressurization, which resulted in porosity levels up to 87.8% (Mathieu et al. 2006). As opposed to the use of high pressure or supercritical, other foaming methods have also been investigated, which rely on the use of chemical foaming agents. Silk fibroin scaffolds were developed using ammonium bicarbonate as a porogen agent (Nazarov et al. 2004). In this case, a solution of silk fibroin solution containing ammonium bicarbonate was dried and stabilized with ethanol, before being foamed in hot water. An alternative batch foaming method for the production of scaffolds is based on the supersaturation of a polymer paste containing sodium bicarbonate salt particles with citric acid (Jun Jin & Park 2001). This methodology has been applied to the fabrication of galactose-conjugated PLGA scaffolds (Tae Gwan 2002) and PLGA scaffolds incorporating dexamethasone (Yoon et al. 2003).
3.7 Electrospinning
Electrospinning (or electrostatic spinning) can be used to produce polymeric nanofibre non-woven membranes. The process is controlled by a high intensity electric field created between two electrodes bearing electric charges of opposite polarity. One electrode is placed in the polymer solution and the other is placed in the collector. The polymer solution is pumped from a needle forming a drop of solution. When the electric field produces a force in the droplet that is able to overcome the surface tension of the solution, a jet of polymer is ejected, producing the fibres. The solvent starts to evaporate at the instant of jet formation and continues after the nanofibres are deposited in the collector. The characteristics of the nanofibres and meshes depend on various properties of the solution and on the processing parameters. This process gained a renewed interest in the early 1990s for the development of nanostructured membranes aimed at biomedical applications. Most of the work in this field uses biodegradable synthetic polymers (such as PLLA and PCL) to produce non-woven membranes for various tissue engineering or drug delivery applications (Li & Xia 2004). Extensive reviews of the electrospinning process can be found elsewhere (Reneker & Chun 1996). Different modifications of the process have been proposed in order to produce non-woven meshes or fibres with specific tailored morphologies, such as hollow fibres (Yu et al. 2004), aligned nanofibres (Li et al. 2004; Xu et al. 2004) or continuous nanofibre yarns (Ko et al. 2003).
3.8 Rapid prototyping
The difficulties and limitations of the previous processing routes have led to the investigation of alternative processing methodologies based on solid freeform fabrication (SFF) or rapid prototyping (RP). One of the main advantages of the latter is the possible integration of RP fabrication with computer-assisted design and medical imaging acquisition and processing techniques for the production of anatomically adapted scaffolds, featuring customized internal architectures. Although the integration of the previously mentioned technologies for the production of TE scaffolds is not exactly novel, only recently a common designation has been proposed: computer-assisted tissue engineering (Sun & Lal 2002; Sun et al. 2004a,b; Tuan & Hutmacher 2005; Wettergreen et al. 2005).
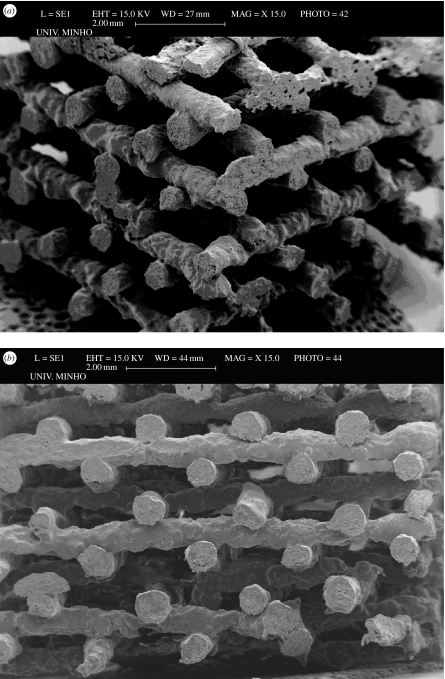
In this area, great emphasis has been given to the development of TE scaffolds by extrusion-based RP. In techniques such as fused deposition modelling (FDM), a filament of material is heated up and melted in an extrusion nozzle and deposited layer by layer, according to a programmable path. The temperature and flow of the material is controlled by an extrusion head, while the scaffold construction in the Z-axis is ensured by the relative movement of the platform to the extrusion head. Hutmacher and co-workers (Hutmacher et al. 2001; Schantz et al. 2002, 2005; Zein et al. 2002) have reported the development of TE scaffolds using the FDM of PCL. In the so-called three-dimensional deposition process, also referred to as three-dimensional bioplotting, the material in the powder or granular form is heated inside a barrel and the molten material is displaced by a plunger or piston, while in precise extrusion, the plasticization of the melt is made by a rotating screw. Our group has been developing TE scaffolds based on thermoplastic blends of starch using this approach. Figure 1 presents, as an example, a scaffold geometry based on starch/PCL blend, featuring an orientation pattern between consecutive layers of 0°/90°, a fibre thickness of 0.5 mm, a spacing between fibres of 2.0 mm (in the same layer) and an offset fibre distance (between consecutive layers) of 1.0 mm. Selective laser sintering has also been used to process natural-based polymer scaffolds. In this technique, each layer of the scaffold is built by scanning and sintering of a powdered substrate by a laser beam.
Figure 1.
Starch/PCL scaffolds produced by three-dimensional bioplotting as observed in (a) the isometric perspective and (b) the XX′ building direction, featuring an orientation pattern between consecutive layers of 0°/90° and 1 mm offset fibre distance between consecutive layers.
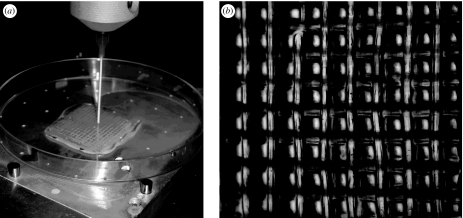
The non-fusible behaviour of many natural-based polymers limits the application of SFF techniques based on melting. Three-dimensional printing is an alternative RP fabrication of scaffolds, which uses starch as a binder (Kim et al. 1998; Lam et al. 2002; Pfister et al. 2004; Seitz et al. 2005). Three-dimensional printing is based on the selective bonding of powder particles via the deposition of a binder resin. Solid freeform fabrication techniques have also focused on the processing of non-fusible natural polymers, such as chitosan and chitosan/hydroxyapatite composites (Ang et al. 2002; Geng et al. 2005). In this approach, the scaffold is built layer by layer through the dispensing of a chitosan/acetic acid solution into sodium hydroxide-based media that causes the neutralization of the acetic acid and the formation of a gel-like chitosan strand. Figure 2 shows chitosan scaffolds being built by three-dimensional bioplotting in our research group, where the reproducibility of the chitosan pattern is evident. The use of low temperature in this processing method enables, in principle, the encapsulation of cells and bioactive molecules, which is not possible in melt-based processes. An example of this is the development of gelatin/alginate scaffolds encapsulating cells (Yan et al. 2005a,b) produced by the consecutive deposition of a mixture of gelatin/alginate and hepatocytes cells, using a microdispenser and subsequent crosslinking by calcium chloride or glutaraldehyde. Other studies (Campbell et al. 2005; Weiss et al. 2005) focused on the scaffold fabrication by inkjet printing of fibrin hydrogen printing incorporating fibroblast growth factor 2 (FGF-2) and bone morphogenetic protein 2 (BMP-2). Hydroxyapatite-based scaffolds have also been developed (Wang et al. 2002b) by the so-called three-dimensional gel lamination system, in which gelling of sodium alginate and calcium chloride was used to gel a hydroxyapatite slurry. An alternative method is to use RP technologies to manufacture a sacrifice mould based on which scaffolds are produced (Sachlos et al. 2003).
Figure 2.
(a) Chitosan scaffold being built by three-dimensional bioplotting by our group and (b) its pattern as observed by stereo light microscopy. The distance between fibre strands is 1 mm.
4. Microsphere-based strategies applied to tissue engineering
The ‘traditional’ drug delivery approach can be applied in the context of TE when strategies include the encapsulation of growth factors or living cells within the scaffolds. In turn, scaffolds can be designed as traditional drug delivery carriers to control a site- and time-specific release profile and also to protect the growth factor (Malafaya et al. 2002). Having this strategy in mind, one can understand the role of microsphere-based technology in tissue engineering applications. Microspheres have been used in the field of tissue engineering mainly to encapsulate growth factors or cells. But microspheres can also be used as injectable scaffolds to support cell growth and proliferation directly or can be aggregated by these cells in order to form living tissue-engineered constructs. Furthermore, the aggregation of the microspheres themselves can be used as a processing technique to produce porous scaffolds. Another approach that has been usually used is to embed loaded microspheres (with growth factors or cells) in hydrogels that are then implanted as an integrated construct. Finally, microspheres can be combined with porous scaffolds by either direct incorporation within the scaffold, injecting together when implanted, or more rarely used as a coating of these scaffolds.
4.1 General considerations on microparticles
Polymer spheres with sizes up to 2 mm are produced by various processes, including precipitation, spray drying or suspension, emulsion and dispersion polymerizations (Tuncel et al. 1996; ODonnell & McGinity 1997). These include microcapsules and vesicular systems in which a cavity is surrounded by a unique polymeric membrane and microspheres, which are matrix-filled systems (Couvreur & Puisieux 1993).
If they are aimed to be used as drug delivery systems and are fabricated as microspheres, they can be injected with a syringe (Eliaz & Kost 2000; Tinsley-Bown et al. 2000) or administrated intranasally as dry powder (Tinsley-Bown et al. 2000), and therefore surgical implant is avoided. In the case of bone and cartilage tissue engineering, they can be injected or combined with the scaffold used for implantation on the repair site, as well as can serve as substrates for cells of interest. In the latter, they can serve either as part of an in vitro strategy or an in vivo vehicle.
4.2 Cell–microparticle constructs
Cell culture techniques have become vital for the study of animal cell structure, function and differentiation and for the production of many important biological materials, such as vaccines, enzymes, hormones, antibodies, interferons and nucleic acids. Microcarrier culture makes possible the practical high-yielding culture of anchorage-dependent cells. In microcarrier culture, cells grow as monolayers on the surface of small spheres, which are usually suspended in culture medium by gentle stirring. Cells cultured on microcarriers are often used as substrates for the production of viruses or cell products and the microcarrier method is compatible with standard production procedures.
These principles were extrapolated to tissue engineering, where these systems might be used both as a strategy for cell expansion and as components of constructs containing materials and cells. An example of such extrapolation is found in Qiu et al. (1998, 2001), where the authors were able to create bone tissue engineering construct using rat marrow stromal cultures and microcarriers in a high aspect rotating vessel (HARV) bioreactor.
This approach holds great potential for tissue engineering applications, and the challenge remains in designing the appropriate microcarriers that suit the needs of the system. Microspheres have also been used as injectable scaffolds for tissue engineering. In this way, it is expected that easy filling of irregularly shaped defects can be done through minimally invasive surgical procedures. One can easily identify two different approaches: one where microspheres can be injected immediately mixed with cells (Kang et al. 2005) and another where microspheres can serve as a carrier for in vitro cell attachment and are injected at a later stage (Senuma et al. 2000; Newman & McBurney 2004). An interesting study (Curran et al. 2005) refers to the use of TGF-β1-loaded microspheres as microcarriers for chondrocytes expansion and ECM formation in a bioreactor that can be applied in cartilage tissue engineering as injectable system or as integral construct, depending on the formation (or not) and size of aggregates of chondrocytes-seeded microspheres.
Another strategy based on microsphere-based technology is the production of porous scaffolds based on the chemical, thermal or physical agglomeration of microspheres. A novel processing route based on chitosan particle aggregation was already described for the production of cartilage and osteochondral tissue engineering scaffolds (Malafaya et al. 2005), where bilayered scaffolds were developed using the particle aggregation (Malafaya et al. 2005). The group of C. T. Laurencin (Devin et al. 1996; Ambrosio et al. 2001; Borden et al. 2002a,b, 2003; Khan et al. 2004) has been applying this technique for the development of microsphere-based matrices for bone repair. The researchers have tried different approaches by developing sintered microsphere-based matrices (Borden et al. 2002b, 2003) or gel microsphere-based matrices (Borden et al. 2002a). Composite microspheres containing hydroxyapatite were also used for the fabrication of polymer–ceramics three-dimensional matrices for bone applications (Devin et al. 1996; Ambrosio et al. 2001; Khan et al. 2004). Heating the pre-fabricated microspheres above the glass transition temperature further processes the three-dimensional structures, as a means of forming contacts between neighbouring microspheres (Borden et al. 2002a). In the gel microsphere-based matrix approach, the gel microspheres are previously obtained by emulsion with poly(vinyl alcohol) (PVA; Borden et al. 2002a). The ensuite aggregation process is based on multiple steps, including air drying, freeze drying, rehydration with salt leaching and new freeze drying. In general, microsphere-based matrices show very interesting properties for a possible application in bone repair. Recently, the same group has been working with blends of chitosan/PLGA (Jiang & Laurencin 2005) to take advantage of chitosan's great flexibility in chemical modifications within its amino and hydroxyl groups.
These three-dimensional structures based on microparticles can also serve as vehicles for the delivery of biologically active factors (e.g. growth, differentiation factors or DNA) in order to manipulate cellular processes occurring within the scaffold microenvironment. Nof et al. (Nof & Shea 2002) have developed DNA-loaded microspheres that were subsequently aggregated into an interconnected open-pore scaffold (above 94% porosity) via a gas-foaming/particulate leaching process.
Another widely used strategy is based on the incorporation of microspheres into hydrogels. Ideally, these combined systems allow greater control over protein activity and stability when compared with proteins incorporated directly into the matrix (which is often associated with a fast release even if covalently linked), and also provide advantages over methods relying on ‘fine tuning’ of the hydrogel structure and diffusivity. A tissue engineering strategy for adipose tissue uses gelatin microspheres incorporating basic fibroblast growth factor (bFGF) that are integrated into an extract of basement membrane protein (Matrigel) (Tabata et al. 2000; Kimura et al. 2002). This strategy aimed at increasing the development of a vascular supply, essential for generation and maintenance of the adipose tissue, as well as for other main living tissues such as bone. Furthermore, this work (Kimura et al. 2002) concludes that gelatin microspheres incorporating bFGF enable Matrigel to efficiently induce de novo adipogenesis at the implanted site in respect of the formation rate and volume of adipose tissue when compared with the controls.
Leach et al. (Leach & Schmidt 2005) have developed a combined system composed of BSA-loaded microspheres incorporated into a photopolymerizable hyaluronic acid-based hydrogel. Another example is the combination of microspheres containing TGF-β1 (DeFail et al. 2006) or TGF-β1 together with microspheres containing IGF-1 (Elisseeff et al. 2001), which have been incorporated into PEO-based hydrogels with or without chondrocytes encapsulated for cartilage tissue engineering applications.
Another strategy presented by the group of Mikos (Payne et al. 2002a–c) is to use gelatin microspheres as microcarriers for marrow stromal cells (Payne et al. 2002c) that are further incorporated into injectable polymeric scaffolds (Payne et al. 2002a,b). The results suggest that temporary encapsulation of cells in crosslinked gelatin microparticles may preserve the viability of cells, creating the possibility of using these as part of an injectable hydrogel scaffold for bone tissue engineering applications aimed at minimally invasive procedures.
Finally, microspheres can be combined with porous scaffolds by either direct incorporation within the scaffold or injecting together when implanted. The study of Isogai et al. (2005) clearly demonstrates that the impregnation of the loaded microspheres into the scaffolds is much more effective when compared with the simultaneous injection of the loaded microspheres.
Other studies may be found in the literature where gelatin microspheres are combined with collagen sponges for different applications, such as periodontal tissue regeneration (Nakahara et al. 2003) or adipose tissue engineering (Kimura et al. 2003).
A common concern for tissue engineering of different living tissues is the vascularization. Site-specific delivery of angiogenic growth factors from tissue-engineered devices should provide an efficient means of stimulating localized vessel recruitment to the cell transplants and would ensure cell survival and function. Obviously, the microsphere-based technology seems to be ideal to induce this complementary vascularization in tissue-engineered constructs. An example is given by Perets et al. (2003) where bFGF-loaded microspheres are incorporated into alginate porous scaffolds to enhance vascularization after implantation in rat peritoneum. The number of penetrating capillaries into the bFGF releasing scaffolds was nearly fourfold higher than that into the control scaffolds (Perets et al. 2003). Furthermore, the released bFGF induced the formation of large and matured blood vessels, as judged by the massive layer of mural cells surrounding the endothelial cells (Perets et al. 2003).
For cartilage tissue engineering, studies that use microsphere-based technologies have mainly focused on the use of chitosan-based systems. Kim et al. (2003) have designed a type of porous chitosan scaffold, containing TGF-β1, to enhance chondrogenesis. It was demonstrated that the scaffold containing the loaded chitosan microspheres significantly increased the cell proliferation and production of ECM. A similar approach using chitosan-based materials was reported by Lee et al. (2004a), where a three-dimensional collagen/chitosan/glycosaminoglycan scaffold was seeded with rabbit chondrocytes and combined with TGF-β1-loaded chitosan microspheres. This set-up allowed for evaluating the effect of released TGF-β1 on the chondrogenic potential of rabbit chondrocytes in such combined systems. It was observed that both the proliferation rate and GAG production were significantly higher in the TGF-β1 microsphere-incorporated scaffolds than in the control scaffolds without microspheres.
To summarize, the described combined systems using microsphere-based technologies seem to be suitable for delivering cells or agents, such as growth factors, in scaffolding for tissue engineering applications of living tissue regeneration such as for bone and cartilage.
5. Membranes as wound dressing
Skin is a very heterogeneous membrane and its primary function is to serve as protective barrier against the external environment. The ability of the skin to repair itself after a minor wound is remarkable, but when the damage is severe or occurs in large amounts of skin area, proper and immediate coverage of the wound surface with an adequate dressing is needed to protect and accelerate wound healing (Bradley et al. 1999; Clark & Singer 2000). Ultimately, the immediate wound coverage, permanent or temporary, is one of the principal goals of wound management (Clark & Singer 2000). Ideally, autologous human skin is considered as the ‘gold standard’ for treatment of skin wounds. However, skin grafts are not always the perfect solution. They are limited with respect to the conditions needed for tissue availability, graft rejections and conformability with the surrounding tissue with respect to thickness and pigmentation (Bradley et al. 1999; Clark & Singer 2000; Young & Parenteau 2002).
Considering tissue engineering and wound repair, several approaches involving the use of growth factors, matrix materials, epidermal sheets, dermal replacements and complex skin substitutes have been described (Clark & Singer 2000; Bello et al. 2001; Bakos & Koller 2002; Jones et al. 2002; Young & Parenteau 2002; Horch et al. 2005). Most of the commercial bioengineered skin devices consist of a combination of sheets of biomaterial matrix (e.g. collagen, hyaluronic acid) containing cultured cells (Bello et al. 2001; Jones et al. 2002; Horch et al. 2005).
Several kinds of temporary dressings have been designed to provide a bacterial barrier, to control pain and contribute to an adequate environment for epithelial regeneration. Based on their nature of action, wound dressings are generally classified as passive, interactive and bioactive products (Bradley et al. 1999; Paul & Sharma 2004). Traditional wound dressings such as gauze, knitted viscose dressings and tulle dressings are passive devices that protect the wound from further injury, while wound healing takes place beneath naturally. However, these wound dressings may adhere to the wound, promoting the patient's pain and trauma. Interactive products include polymeric films, hyaluronic products, hydrocolloids and hydrogels, which can alter the local wound environment.
Even with advancements in wound dressings, there is no single dressing suitable for all types of wounds, and often, different types are needed to be used during the healing of a single wound (Bradley et al. 1999). Currently, a variety of natural polymer-based membranes obtained from chitin (Yusof et al. 2003, 2004; Kumar et al. 2004; Tanodekaew et al. 2004; Muzzarelli et al. 2005), chitosan (Khan et al. 2000; Wang et al. 2002a; Khan & Peh 2003; Azad et al. 2004; Kumar et al. 2004; Marreco et al. 2004; Paul & Sharma 2004; Wu et al. 2004; Campos et al. 2005; Wongpanit et al. 2005), alginate (Wang et al. 2002a), cellulose (Wu et al. 2004; Czaja et al. 2006), hyaluronic acid (RuizCardona et al. 1996), gelatin (Chang et al. 2003b; Tanaka et al. 2005), collagen (Grzybowski et al. 1997; Lee et al. 2002; Wu et al. 2003; Sripriya et al. 2004) and their derivatives have been developed, in an attempt to supply the high demand for new materials for skin repair, wound cover or dressings in the treatment of different wounds.
Researchers have focused on bilayered membranes for wound dressing, with incorporation of antibiotics into these matrices for preventing infections. These types of bilayer wound dressings are composed of dense top layer and underlying porous sponge-like layer. The external layer protects the wound and serves as an artificial epidermis, while the inner layer is designed for the drainage of wound exudates and attachment of wound tissues (Grzybowski et al. 1997; Mi et al. 2001, 2002, 2003; Lee et al. 2002; Sripriya et al. 2004). Mi et al. (2002, 2003) reported the incorporation of silver sulphadiazine (AgSD) into asymmetric chitosan. The results indicate that AgSD-incorporated bilayer chitosan wound dressing may be effective in the treatment of infected wounds.
Current strategies for tissue regenerative wound dressings have also aimed at the development of implantable matrices that mimic the natural tissue. In this context, natural proteins such as collagen (Grzybowski et al. 1997; Lee et al. 2002; Ruszczak 2003; Wu et al. 2003; Sripriya et al. 2004), gelatin (Chang et al. 2003b), soy protein (Silva et al. 2003, 2005b), casein (Silva et al. 2003) and silk fibroin (Santin et al. 1999) have been studied separately or as composite materials in a template to be used in skin tissue engineering due to their similarity with the living tissues. An important issue for the treatment of an infected wound is to sustain sufficient drug concentration at the site of infection. Therefore, different types of medicated collagen dressings with antibiotics were developed (Lee et al. 2002; Sripriya et al. 2004). Lee et al. (2002) have developed an infection-preventing bilayered membrane by combining silver sulphadiazine and a laminin-modified collagen membrane, which was shown to facilitate the dermal wound healing process. A similar bilayer wound dressing system that mimics the natural skin was later reported by Sripriya et al. (2004).
In another approach (Chang et al. 2003b), protein modifications such as crosslinking were used for the improvement of functional properties of gelatin, in order to solve the problem of its high solubility in aqueous environments. It was found that the degree of inflammatory reaction of membranes treated with genipin (crosslinking agent) was significantly less severe than the one observed for the glutaraldehyde-crosslinked dressing. Tanaka et al. (2005) reported the in vivo efficiency of a gelatin film sheet impregnated with epidermal growth factor (EGF) for a novel therapeutic device for cutaneous wound repair.
Despite significant wound care advances, unmet needs remain. Nevertheless, since many wounds can be healed with dressings that do nothing more than cover and protect the wound from infection, biomacromolecule-based bilayer dressings could represent a significant advance in wound management.
6. Encapsulation and injectable systems
6.1 General principles
The principle of bioencapsulation has long been accepted by the scientific community (King & Goosen 1993). This concept arose as a consequence of the use of polymeric microcapsules for the impetus behind the need for immunoisolation of transplanted cells or small clusters of tissue, when treating serious and disabling human conditions. The main goal of encapsulated cell therapy research is not only to develop a confined barrier to entrap living xenogeneic or allogeneic cells to be transplanted, but also to prohibit the entrance of the hosts' antibodies and immune cells, and by this means avoiding immune rejection or the use of immunosuppressive drugs (Gentile et al. 1995; Brissová et al. 1998; Li 1998). Additionally, this barrier must also be selectively permeable to allow the diffusion of oxygen, supply of nutrients and release of toxic metabolites. In this context, a suitable material to accomplish the encapsulation must act as a membrane to provide a protective environment, while supporting cells to maintain their function, differentiation and proliferation (Zielinski & Aebischer 1994). Thus, an important component of this three-dimensional volume is the availability of an ECM that allows the anchor of cells or simply the stabilization of their positioning (Zielinski & Aebischer 1994; Uludag et al. 2000).
Numerous techniques have been developed for the immobilization of cells prior to transplantation (López et al. 1997; Uludag et al. 2000; Muralidhar et al. 2001; Yeo & Park 2004), but this can be achieved by two basic mechanisms: (i) microencapsulation, which typically involves the use of spheres with size ranging from 100 to 300 μm in diameter, and (ii) macroencapsulation, where a higher number of cells can be transplanted inside a chamber, or in one/several relatively large capsules/hollow fibres of 0.5–6 mm in diameter and 0.5–10 cm in length (Gentile et al. 1995). Another recent alternative has been the use of injectable, biodegradable materials, which can be easily formulated with cells and later on, harden in situ. Despite the advantage of using microcapsules due to the optimal geometry for diffusive transport (high surface to volume ratio), the chambers or capsules have attracted a great deal of attention because they present better mechanical and chemical stability and are easier to implant (Li 1998; Uludag et al. 2000; Weber et al. 2004). On the other hand, injectable systems are advantageous when the site of the injury is difficult to access, the material of interest cannot be processed by melt- or solvent-based techniques or because the device is designed to incorporate active agents that do not withstand such processing routes.
The main applications of these systems are as drug delivery carriers, as encapsulation agents for a diversity of cells, proteins and other bioactive agents, as well as tissue engineering scaffolds, mainly of soft tissue (Boesel & Reis 2004; Gomes et al. 2004b; Silva et al. 2005a, submitted).
The current injectable systems may be divided into four main classes: (i) thermoplastic pastes (polymers that can be melted or softened at low temperatures and injected into the body), (ii) in situ crosslinking/polymerizing systems, (iii) in situ precipitation systems (water-insoluble polymers that are dissolved in a physiologically compatible solvent and precipitate upon contact with body fluids), and (iv) injectable hydrogels, which solidify due to a change in their environment (pH, temperature, shear, etc.).
Recently, natural-based materials have become the focus of attention regarding injectable systems. Besides degradability, they offer the additional advantage of easier synthesis/preparation and large availability.
Sections 6.2–6.4 will review the most recent advances in the use of natural-based, degradable materials as encapsulation and injectable formulations.
6.2 Chitosan
One common alternative for preparing injectable materials has been by grafting synthetic polymers with low critical solution temperature (LCST) character to natural-based polymers. Examples include the grafting of poly(N-isopropylacrylamide) (PNIPAAm; Cho et al. 2004; Lee et al. 2004b; Chung et al. 2005a), Pluronics (Chung et al. 2005a,b) and polyethylene glycol (PEG; Bhattarai et al. 2005a,b) to a chitosan backbone.
PNIPAAm is soluble below its LCST, but becomes insoluble (hydrophobic) above this temperature (Cho et al. 2004). In PNIPAAm-g-chitosan, the grafting percentage varied between 9.6 and 712 wt%, and the polymer presented an LCST of 32°C, making it suitable for biomedical applications (Lee et al. 2004b). The system was adequate for supporting the culture of MSCs, allowing their differentiation into chondrocytes both in vitro (Cho et al. 2004; Chung et al. 2005a) and in vivo (Cho et al. 2004), and was shown to be non-toxic and biocompatible (Lee et al. 2004b).
Pluronics is a family of PEO–PPO–PEO triblock copolymers of ethylene oxide (EO) and propylene oxide (PO) (Chung et al. 2005b) and some members are thermoreversible gels. Their graft onto chitosan yielded a thermosensitive hydrogel, with the transition temperature of 30–35°C when the grafting percentage was 460–1100 wt% and the solution concentration was higher than 16% (Chung et al. 2005b).
In the case of PEG, hydrogen bonds between hydrophilic groups of PEG and water predominate at low temperature, while hydrophobic interactions between polymer chains prevail as temperature increases (Bhattarai et al. 2005b).
Another alternative method to prepare thermally gelling materials is by chemical modification of chitosan with anhydrides or aldehydes (Gérentes et al. 2002). The physical hydrogel is obtained when the equilibrium between polymer/polymer interactions and polymer/solvent hydrophilic interactions is achieved. For chitosan, this occurs for a DA of approximately 80% (Gérentes et al. 2002).
Neutralization of acidic chitosan solutions with glycerol phosphate (GP) allowed the production of a thermally gelling solution (C–GP) at approximately neutral pHs (6.8–7.2) (Chenite et al. 2000, 2001). The most important interactions responsible for this thermal sol–gel transition are the chitosan–chitosan hydrophobic interactions, enhanced by the structuring action of glycerol on water. These gels were able to deliver active bone protein in vivo and lead to de novo cartilage and bone formation (Chenite et al. 2000). C–GP gels, when loaded with primary articular chondrocytes, preserved chondrocyte viability and phenotype, resided at least for one week in a mobile osteochondral defect and served as a scaffold to help build new tissue (Hoemann et al. 2005). To decrease the very fast release of low molecular weight hydrophilic compounds, liposomes were added to the C–GP solution, allowing a controlled delivery of at least two weeks while increasing the gelation rate and gel strength (Ruel-Gariépy et al. 2002). Hydrophobic drugs, on the other hand, could be delivered for at least a month without modification of the formulation (Ruel-Gariépy et al. 2004). Intratumoral injection of the drug-loaded gel was as effective as four intravenous injections of the same drug (Molinaro et al. 2002). However, an important drawback was the acute inflammatory response of the gels when injected subcutaneously (Molinaro et al. 2002); the higher the DA, the stronger will be the response.
6.3 Alginates
A rapid curing homogeneous and stable gel was formulated with a solution of alginate (2%) and CaCl2 (Stevens et al. 2004). Variations of the alginate and calcium salt concentration, choice of gelling ion and guluronic units content influence the gelation kinetics (Stevens et al. 2004). The use of poorly water-soluble salts (CaCO3 and CaSO4) influences gelation rate and, consequently, mechanical properties (Kuo & Ma 2001). If tricalciumphosphate is used to promote gelation, it additionally creates an osteoconductive environment that can facilitate cell attachment (Luginbuehl et al. 2005). Such injectable gels were also mixed with insulin-like growth factor, resulting in a sevenfold increase in proliferation rate of osteoblast-like cells (Luginbuehl et al. 2005). When cultured with chondrocytes, Ca-alginate was able to support periosteum-derived chondrogenesis, although the constructs did not form a hyaline-like cartilaginous tissue (Stevens et al. 2004).
A periodate-oxidized sodium alginate has been used to crosslink gelatin in the presence of borax to produce injectable systems (Balakrishnan & Jayakrishnan 2005). Hepatocytes were successfully encapsulated in the gel and remained viable for more than four weeks.
Similarly, Na-alginate and chitosan solutions were mixed to produce an injectable gel (Park et al. 2005a). The gel was used to encapsulate MSCs and BMP-2, and the system was injected subcutaneously, being able to stimulate new trabecular bone formation (Park et al. 2005a).
6.4 Other systems
Hyaluronic acid. It has been shown to be possible to control the degradation and mechanical behaviour of photopolymerized HA networks (Burdick et al. 2005) and encapsulate articular chondrocytes using such type of network in vivo (Nettles et al. 2004). These findings are important since the photopolymerizable systems can be easily used in other applications (Masters et al. 2005) and with minimally invasive procedures (Park et al. 2005b), thus facilitating the filling of irregularly shaped defects.
Cellulose sulphate. Cellulose sulphate (CS) consisting of a sulphate ion substitutes at a carboxyl group at every third position. Cellulose sulphate was shown to have the potential to immunoisolate cells from rejection process through encapsulation (Pelegrin et al. 1998; Schaffellner et al. 2005). This also allowed CS to be used for the designing of multicomponent capsules for maintenance of cells during the cryopreservation processes (Canaple et al. 2001). A pioneering work using a one-step CS/poly(diallyl-dimethyl-ammonium chloride) (CellMAC) assembly on a copolymerized transient alginate scaffold fosters advances for hardware-driven microencapsulation, as well as protein production in animals and bioreactors (Weber et al. 2004).
Collagen. Collagen gels belong to a special kind of injectable hydrogel, which solidify due to changes in shear stress. The gels are shear-thinning, which means that their viscosity decreases as the applied shear increases, and when the shear is removed, they regain their semi-solid aspect (Wallace & Rosenblatt 2003; Boesel & Reis 2004). These gels are available as injectable suspensions of collagen fibres or viscous solutions of non-fibrillar collagen in aqueous media. However, the collagen network is too open to retain most of the active agents of interest, requiring secondary mechanisms to produce gels adequate for encapsulation or tissue engineering purposes (Wallace & Rosenblatt 2003).
One example is the crosslinking of alkali-treated collagen with a PEG-based star polymer under physiological conditions (Taguchi et al. 2005). In situ encapsulated chondrocytes remained viable and were able to express mRNA and aggrecan after three weeks of encapsulation (Taguchi et al. 2005). Another system consisted of denatured single-stranded collagen copolymerized with dextran (Marston et al. 2005).
In addition to the aforementioned polymers, other natural-based materials have been used for preparing encapsulation and injectable systems. Such type of polymers include starch (Ignoffo et al. 1991), agarose (Orive et al. 2003; Sakai et al. 2005), carrageenan (Bartkowiak & Hunkeler 2001; Muralidhar et al. 2001), silk fibroin (Yeo et al. 2003), gelatin (Payne et al. 2002c) and gellan gum (Chilvers & Morris 1987; Moslemy et al. 2002).
7. In vitro and in vivo biological performance
Nowadays, it has been demonstrated that the suitability of natural origin polymers for tissue engineering purposes is highly dependent on the tissue that needs to be engineered. The histological, physiological and biomechanical properties of each tissue determine the success of the regenerative process, therefore restraining the choice of the materials.
7.1 Cartilage regeneration
Cartilage is a tissue with remarkable functions that arise in part from the composition and structure of the highly hydrated ECM. The major challenge in the regeneration of cartilage is to avoid the formation of fibrous cartilage, which lacks the biochemical and mechanical properties necessary to yield a complete and durable repair (Solchaga et al. 2001). This inability to reconstitute the cartilage tissue and the need to maintain the phenotype of chondrocytes have promoted the proposition of various materials (Chang et al. 2003a; Gigante et al. 2003; Iwasaki et al. 2004; Mouw et al. 2005; Wang et al. 2005) to perform as three-dimensional culture systems for cartilage regeneration.
Chondrogenesis from chondrocytic cell lines (Li & Zhang 2005), primary chondrocytes (Gigante et al. 2003; Masuoka et al. 2005; Mouw et al. 2005) or MSCs (Meinel et al. 2004a; Wang et al. 2005) seeded in natural origin scaffolds has been achieved, although not yet fully understood.
Alginate has often been proposed for cartilage applications due to its similarity with GAGs that compose the ECM of chondrocytes. Alginate gels, containing hyaluronan or fibrin, induce higher chondrocyte proliferation when compared with alginate alone, which was also attributed to the presence of matrix-like molecules, from hyaluronan and fibrin, that function as anchor sites for the cells (Lindenhayn et al. 1999). Nonetheless, alginate gels implanted in rabbits after in vitro pre-culture with allogenic chondrocytes induced the formation of a tissue, which was practically indistinguishable from the normal cartilage (Fragonas et al. 2000). The different results might be explained by the fact that transport of soluble factors varies with cell density, cell–cell signalling and, consequently, an alteration in matrix metabolism (Williams et al. 2005; Sakai et al. 2006). In fact, alginate gels with increasing cell density present enhanced mechanical integrity due to extensive matrix deposition.
The hypothesis that cationic chitosan hydrogels would form an ideal environment in which large quantities of newly synthesized anionic proteoglycan could be entrapped has been under examination (Roughley et al. 2006). In fact, chitosan has been shown to be able to retain the majority of the proteoglycan produced by entrapped intervertebral disc cells, independently of the method used to generate the hydrogel. Thus, chitosan is also being used in the field of cartilage regeneration to improve cell adhesion to alginate structures. Chitosan–alginate scaffolds exhibit superior biological and mechanical properties over their chitosan counterpart, showing great potential to accelerate tissue growth and retain the chondrocytic phenotype (Iwasaki et al. 2004; Li & Zhang 2005). The combination of chitosan and gelatine has also been shown to be a promising alternative for auricular cartilage regeneration using an autologous approach (Xia et al. 2004).
Primary porcine chondrocytes seeded on gelatin/chondyoitin-6-sulphate/hyaluronan tri-copolymer, aimed to mimic natural cartilage matrix, after seeding and culture in spinner flasks, which allowed a better distribution of the cells, were able to retain chondrocyte phenotype for five weeks. However, the amount of synthesized matrix was not enough to form cartilage (Chang et al. 2003a). The in vivo study (Chang et al. 2006) of the same materials using a porcine model showed that although a good appearance was seen in the condyles treated with either allogenic or autologous graft, the overall results were not overwhelming, since it was only possible to regenerate hyaline cartilage and/or fibrocartilage.
Silk-based materials were not able, per se, to transmit the necessary signal to trigger the chondrogenic pathway (Wang et al. 2005). The presence of differentiation factors such as dexamethasone and transforming growth factor (TGF-β3) was proved to be essential for the survival and differentiation of the MSCs, at least for up to two weeks of culture, which correspond to the maximum proliferation rate in those scaffolds. In addition, very small differences in the transcript levels of specific cartilage ECM genes were detected when comparing the cells in the silk-based scaffolds with those in the pellet culture under the same conditions (Wang et al. 2005).
Although collagen scaffolds with a premature degradation do not provide the necessary structural integrity for ECM deposition during chondrocyte cultivation (Meinel et al. 2004a), atelocollagen scaffolds supported the formation of cartilage in vitro with enough elasticity and stiffness to be handled in vivo (Masuoka et al. 2005). However, the mechanical strength of the regenerated articular cartilage remains to be investigated. In fact, a comparative study between atelocollagen and alginate gels (Sakai et al. 2006) suggests that although cells proliferate better in atelocollagen, alginate is more appropriate to obtain high levels of proteoglycan synthesis and accumulation.
Despite the many evolving approaches in cartilage tissue engineering, many of the relevant variables involved in the process are yet to be defined. Cells, for instance, range from primary expanded chondrocytes to manipulated progenitor cells and their differentiation, towards a chondrogenic phenotype within the construct environment, are highly dependent on the properties of the material. In the case of progenitor cells in which relatively rapid changes in phenotype are expected, the influence of the material environment on cell behaviour and matrix composition may be more pronounced than in the primary chondrocytes, thus leading to different results.
7.2 Bone regeneration
The major concerns associated with polymeric scaffolds for bone tissue engineering are low mechanical strength and shape retention failure.
Silk-based scaffolds have been shown to support human MSC (hMSC) attachment, spreading and growth in vitro. However, depending on variables such as processing conditions (Jin et al. 2004; Kim et al. 2005), surface properties (Meinel et al. 2004b) and degradability rates (Meinel et al. 2004b), different responses in terms of bone-like tissue formation were observed.
Collagen scaffolds, due to their fast degradation, do not allow isomorphous replacement with a newly formed bone. Presumably, due to the stable macroporous structure and slow degradation, the progression and extent of osteogenesis were markedly and significantly higher for silk and RGD–silk scaffolds when compared with collagen scaffolds (Meinel et al. 2004b). Other studies have nonetheless proved that the crosslinking (Xiao et al. 2003; Meinel et al. 2004c) or the reinforcement of collagen scaffolds with materials having improved mechanical properties (Kose et al. 2004; Li et al. 2005, 2006) renders scaffolds adequate for bone tissue engineering. Some chitosan scaffolds present mechanical weakness and instability due to the high degree of swelling. The increase in mechanical strength, achieved not only by changing the processing methodology (Seol et al. 2004), but also by chemically bonding chitosan with other natural origin polymers such as alginate (Li & Zhang 2005), promoted the in vitro proliferation and differentiation of osteoblastic cells (Seol et al. 2004; Li & Zhang 2005), as well as the rapid vascularization and deposition of connective tissue and calcified matrix in vivo.
Hyaluronic acid has been mainly used as a carrier of bone (recombinant bone morphogenetic protein (rhBMP)-2; Hunt et al. 2001) and vascularization inducing agents (copper ions; Giavaresi et al. 2005) for cancellous bone allografts and not as a scaffolding material. In fact, although the advantages of the hyaluronic acid as natural material and the fact that the cell-signalling functions of this material are dependent on molecular weight are evident (Huang et al. 2003), there are still many uncertainties about how it affects osteoblast activities, thus diminishing its application as a bone tissue engineering scaffold.
The mimicking of ECM by using natural origin materials has been further attempted using complementary approaches, in order to improve the performance of these materials. A nano- and microfibre combined starch-based scaffold (Tuzlakoglu et al. 2005) showed that its unique architecture, being able to support and guide cells, can also provide an ideal structure for cell deposition and organization to be used in bone tissue engineering.
A real situation in the assessment of the suitability of scaffolds to be used in bone tissue engineering has to do with the specific in vitro calcium deposition on the top and bottom of the scaffold, when using static culture conditions, instead of being throughout the structure (Gomes et al. 2003; Meinel et al. 2004b). A better cell distribution and, consequently, the uniformity of mineralization (Gomes et al. 2003; Meinel et al. 2004c) as well as the orientation of bone matrix (Meinel et al. 2004c), although restricted by the interconnectivity degree of the scaffold, can be achieved under a flow environment. Bone marrow cells cultured with starch-based scaffolds also responded to flow perfusion conditions by augmenting the production of several bone-related growth factors, namely TGF-β1, FGF-2, vascular endothelial growth factor (VEGF) and BMP-2 (Gomes et al. 2006).
The success of subsequent transplantation of the in vitro engineered construct is believed to rely not only on the properties of the materials but also on the osteoprogenitor cell sources. It is expected that the seeded cells will secrete specific ECM components in vitro, to induce proliferation and differentiation into osteoblasts and result in the formation of a new bone in vivo (Mizuno et al. 1997). In the majority of the studies, the choice of cell source is not clear, since it is not based on relevant aspects, such as the capacity of the transplanted cells to trigger endogenous cascades for recruitment and differentiation. In fact, the precise role of implanted vital cells in the induction and the mineralization in vivo is not yet determined. Xiao et al. (2003) showed that differentiated osteoblasts and in vitro developed matrix are not involved in direct bone formation. However, the used construct did appear to induce differentiation of host mesenchymal cells during the repair of critical size bone defects due to the bone-related proteins (ALP, osteocalcin, OPN, BMP-2 and BMP-4) deposited along the collagen scaffold or present in the intercellular matrix within the scaffold.
7.3 Skin substitution
Wound repair has been achieved following two main tissue engineering strategies, either using scaffolds or matrices, which can be cellular or acellular (Jimenez & Jimenez 2004). Decellularized matrices have been shown to stimulate angiogenesis and to modulate endogenous growth factor functions (Chandler et al. 2000; Jimenez & Jimenez 2004). So far, scaffolds made of fibrin (Bensaid et al. 2003; Hokugo et al. 2006), hyaluronic acid (Mao et al. 2003; Liu et al. 2004), chitosan (Berthod et al. 1996; Ishihara et al. 2001; Gingras et al. 2003) and collagen (Guerret et al. 2003; Hudon et al. 2003; Ruszczak 2003; Jarman-Smith et al. 2004) from a variety of origins, alone or combined (Berthod et al. 1996; Hafemann et al. 1999, 2001; Klein et al. 2001; Mao et al. 2003), were shown to be promising for wound healing applications.
The use of cells (e.g. keratinocytes and fibroblasts) within a three-dimensional matrix (Jimenez & Jimenez 2004) is also a usual approach in skin tissue engineering. However, wound dressing treatment, especially when the skin substitution is required, is not a problem-free process. That is, it requires the restoration of the continuity of a living tissue and an integrated response of several cells to injury, which are necessary to promote cellular colonization and remodel the matrix. Therefore, inflammation and neovascularization, for example, are required processes for healing, if only controlled (Klein et al. 2001). In fact, animal origin membranes composed of fibrous collagen and elastin as dermal substitute scaffolds were shown to elicit a chronic inflammatory response, leading to the damage of the tissues instead of tissue repair (Klein et al. 2001). Immunogenicity issues, in fact, can be raised when considering protein-based biomaterials. Collagen, as an animal origin protein, would be expected to elicit specific and uncontrolled immune reactions. However, collagen is one of the most popular materials for the majority of dermal substitutes, as it possesses different levels of structural order and is a natural substrate for cellular attachment, growth and differentiation (Ruszczak 2003). Some in vitro studies demonstrated that human dermal fibroblasts and keratinocytes can be cultured with collagen membranes, indicating its suitability for permanent dermis replacement or wound healing situations, after chemical modifications (Hafemann et al. 2001; Hudon et al. 2003; Jarman-Smith et al. 2004). Another study (Keefe et al. 1992) also revealed a normal inflammatory response after the in vivo implantation of collagen-based scaffolds. Thus, the practical use of collagen for wound healing is limited not due to its immunogenicity but due to the problems related to storage stability and the time required to prepare enriched collagen solutions (Ruszczak 2003).
Other natural origin materials are also proposed for skin substitution. Fibrin-based scaffolds with poly(glycolic acid) (PGA) for guided skin regeneration have been tested in vitro and in vivo (Hokugo et al. 2006). In addition, fibrin scaffolds proved to be good matrices for the spreading and proliferation of hMSCs, enhancing their suitability for wound healing and skin substitution (Bensaid et al. 2003). Chitosan alone (Berthod et al. 1996; Ishihara et al. 2001; Gingras et al. 2003), modified with hyaluronic acid (Mao et al. 2003) or collagen (Berthod et al. 1996), has also been reported to be suitable for wound healing and skin substitution. Berthod et al. (1996) developed a collagen–chitosan sponge on which human fibroblasts, cultured for one month, produced differentiated connective tissue. An in vivo study demonstrated that a tissue engineering construct of collagen–chitosan and human skin fibroblasts and keratinocytes, transplanted in the back of nude mice, enhanced nerve regeneration, proving it to be a suitable construct also for skin substitution (Gingras et al. 2003).
7.4 Cardiovascular tissue regeneration
The overall goal of vascular tissue engineering is to obtain the same mechanical and biological properties as a native vessel (Boccafoschi et al. 2005). The use of collagen conjugated with different types of cells in vitro, such as primary rat aortic smooth muscle cells (Weinberg & Bell 1986; Tranquillo et al. 1996; Boccafoschi et al. 2005) and endothelial cells (Weinberg & Bell 1986; Boccafoschi et al. 2005), to mimic the blood vessels is not a new approach. It represents a suitable option for vascular tissue engineering, due to the low in vitro thrombogenicity and the ability of the scaffold to support the spreading of primary smooth muscle and endothelial cells (Boccafoschi et al. 2005). Nowadays, other studies are being carried out to evaluate long-term cytocompatibility and the potential of both elastin and collagen scaffolds to be repopulated by host cells in vivo (Simionescu et al. 2006). Tan & Desai (2003, 2004, 2005) proposed a new strategy for the construction of blood vessels using different types of natural origin matrices, such as collagen, chitosan, Matrigel and fibrin. These materials were used to create a three-dimensional pattern within microchannels composed of poly(dimethylsiloxane). The multilayer structure within the microchannels was built through microfluidic delivery of cells within the biopolymer matrices, layer by layer with a controlled flow rate, achieving a three-dimensional configuration (Tan & Desai 2003, 2004). Three types of cells (human lung fibroblasts, human umbilical vein smooth muscle cells and human umbilical vein endothelial cells) were used to create the multicellular three-dimensional culture system, which was shown to be useful to build up multiple layers of cells in well-defined geometries with controlled ECM composition. This three-dimensional well-controlled micropatterned multilayer system allowed the re-establishment of the in vivo-like cell–cell interaction concept that exists in the vascular system or other laminar-structured tissues (Tan & Desai 2005).
The use of natural origin polymers for heart valve replacement or septal occlusion has also been proposed. In vitro and in vivo (in a lamb model) studies with natural scaffolds based on collagen showed low infiltration with inflammatory cells (Jux et al. 2003).
7.5 Knee ligament
Collagen (Noth et al. 2005) and silk (Altman et al. 2002; Chen et al. 2003) have been proposed as natural origin materials to be used as scaffolds for knee ligament tissue engineering scaffolds.
The ideal knee ligament replacement scaffold should exhibit sufficient mechanical strength, demonstrate mechanical behaviour similar to natural ligament and promote the formation of ligamentous tissue. The scaffold should degrade at a rate that allows the new tissue to receive an appropriate level of load without danger of rupture (Laurencin & Freeman 2005), with concomitant structured matrix deposition (Altman et al. 2002). Silk fibre matrices have been shown to provide a suitable scaffold to support adult stem cell differentiation towards a ligament lineage (Altman et al. 2002). Although promising, silk performance was shown to be improved by coating the surface with RGD sequences (Chen et al. 2003). Ge and co-workers (Ge et al. 2005) concluded that bone marrow is a better source of MSCs for anterior cruciate ligament when compared with fibroblasts from the anterior cruciate and the medial collateral ligaments (Ge et al. 2005).
In another study with bone marrow-derived MSCs seeded on a type I collagen hydrogel, Noth and colleagues (Noth et al. 2005) suggested that these cells repeatedly adopt a defined longitudinal orientation and organize the surrounding matrix in parallel, highly organized fibres in a wavy pattern with bundles of ECM when submitted to cyclic stretching. However, these constructs are still too weak to allow the in vivo implantation.
8. Final remarks
In the field of tissue engineering, a great deal of effort has been put in to prepare different formulations based on natural polymers. However, despite all the extensive literature containing references to the so-called ‘natural-based’ systems, it is the authors' opinion that much work is still needed to obtain clinically successful materials. Many improvements can be made in this context, as such systems offer a great versatility. They may be, for example, chemically modified and combined with ceramics or other polymers (blends, copolymers and inter-penetrated networks). Their interaction with cells and tissues may be improved or tuned, by adequate chemical modifications, such as grafting with peptides (e.g. RGD), or by surface treatments (e.g. surface plasma modification or modification of surface chemistry through grafting with other polymers). Many attempts have been made to produce smart naturally derived systems that could be used in tissue engineering applications, including (i) development of new injectable thermogelling materials that could be used to deliver cells or growth factors through non-invasive routes, (ii) novel matrices that can be degraded by adequate cell signals and actions, (iii) scaffolds or hydrogels that can deliver relevant bioactive agents in specific conditions (temperature, pH, ionic strength or presence of specific enzymes), and (iv) self-assembling systems that can be tuned by external signals. Different processing routes have been proposed to produce scaffolds with a great variety of porous architectures. This is an especially delicate task, as naturally derived macromolecules are typically non-meltable (without degradation) and may be insoluble in many solvents. This compromises the use of many of the most traditional techniques to produce tissue engineering scaffolds. Efforts should, in fact, be made to develop or improve new or combined processing strategies to obtain repeatable porous constructs with controlled porous morphology, preferably at different scale levels (e.g. combination of nano- and microelements or pores), comprising different materials that are spatially organized and having the capability to deliver relevant molecules such as growth factors in a controlled way. Such new scaffolds should be designed for different applications, addressing particular aspects, such as: to induce vascularization; facilitate the deposition of calcium phosphates in predefined regions; guide the regeneration of tissue in certain directions (through gradient delivery of factors or anisotropic porous architecture); permit the development of different tissues (e.g. regeneration of osteochondral defects); or inhibit calcification and cell adhesion (e.g. in cardiovascular application), just to cite some examples. The field is widely open for new creative researchers to make some dreams come true in real clinical applications.
References
- Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Altman G.H, Horan R.L, Lu H.H, Moreau J, Martin I, Richmond J.C, Kaplan D.L. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/S0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- Altman G.H, Diaz F, Jakuba C, Calabro T, Horan R.L, Chen J.S, Lu H, Richmond J, Kaplan D.L. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Ambrosio A.M, Sahota J.S, Khan Y, Laurencin C.T. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J. Biomed. Mater. Res. 2001;58:295–301. doi: 10.1002/1097-4636(2001)58:3%3C295::AID-JBM1020%3E3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ang T.H, Sultana F.S.A, Hutmacher D.W, Wong Y.S, Fuh J.Y.H, Mo X.M, Loh H.T, Burdet E, Teoh S.H. Fabrication of 3D chitosan–hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C. 2002;20:35–42. doi: 10.1016/S0928-4931(02)00010-3. [DOI] [Google Scholar]
- Arrigoni C, Camozzi D, Imberti B, Mantero S, Remuzzi A. The effect of sodium ascorbate on the mechanical properties of hyaluronan-based vascular constructs. Biomaterials. 2006;27:623–630. doi: 10.1016/j.biomaterials.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Awad H.A, Wickham M.Q, Leddy H.A, Gimble J.M, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Azad A.K, Sermsintham N, Chandrkrachang S, Stevens W.F. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. B. 2004;69:216–222. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- Azevedo H.S, Gama F.M, Reis R.L. In vitro assessment of the enzymatic degradation of several starch based biomaterials. Biomacromolecules. 2003;4:1703–1712. doi: 10.1021/bm0300397. [DOI] [PubMed] [Google Scholar]
- Bakos D, Koller J. Membranes and hydrogels in reconstructive surgery. In: Reis R.L, Cohn D, editors. Polymer based systems on tissue engineering, replacement and regeneration. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. [Google Scholar]
- Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26:3941–3951. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Balgude A.P, Yu X, Szymanski A, Bellamkonda R.V. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/S0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- Bartkowiak A, Hunkeler D. Carrageenan–oligochitosan microcapsules: optimization of the formation process. Colloids Surf. Part B Biointer. 2001;21:285–298. doi: 10.1016/S0927-7765(00)00211-3. [DOI] [PubMed] [Google Scholar]
- Bello Y.M, Falabella A.F, Eaglstein W.H. Tissue-engineered skin—current status in wound healing. Am. J. Clin. Dermatol. 2001;2:305–313. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- Bensaid W, Triffitt J.T, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- Berthod F, Sahuc F, Hayek D, Damour O, Collombel C. Deposition of collagen fibril bundles by long-term culture of fibroblasts in a collagen sponge. J. Biomed. Mater. Res. 1996;32:87–93. doi: 10.1002/(SICI)1097-4636(199609)32:1%3C87::AID-JBM10%3E3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Bhattarai N, Matsen F.A, Zhang M. PEG-grafted chitosan as an injectable thermoreversible hydrogel. Macromol. Biosci. 2005a;5:107–111. doi: 10.1002/mabi.200400140. [DOI] [PubMed] [Google Scholar]
- Bhattarai N, Ramay H.R, Gunn J, Matsen F.A, Zhang M. PEG_grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release. 2005b;103:609–624. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Boccafoschi F, Habermehl J, Vesentini S, Mantovani D. Biological performances of collagen-based scaffolds for vascular tissue engineering. Biomaterials. 2005;26:7410–7417. doi: 10.1016/j.biomaterials.2005.05.052. [DOI] [PubMed] [Google Scholar]
- Boesel L.F, Reis R.L. Injectable biodegradable systems. In: Reis R.L, Román J.S, editors. Biodegradable systems in tissue engineering and regenerative medicine. CRC Press; Boca Raton, FL: 2004. pp. 13–28. [Google Scholar]
- Boontheekul T, Kong H.J, Mooney D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Borden M, Attawia M, Khan Y, Laurencin C.T. Tissue engineered microsphere-based matrices for bone repair: design and evaluation. Biomaterials. 2002a;23:551–559. doi: 10.1016/S0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- Borden M, Attawia M, Laurencin C.T. The sintered microsphere matrix for bone tissue engineering: in vitro osteoconductivity studies. J. Biomed. Mater. Res. 2002b;61:421–429. doi: 10.1002/jbm.10201. [DOI] [PubMed] [Google Scholar]
- Borden M, El-Amin S.F, Attawia M, Laurencin C.T. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials. 2003;24:597–609. doi: 10.1016/S0142-9612(02)00374-5. [DOI] [PubMed] [Google Scholar]
- Bouhadir K.H, Lee K.Y, Alsberg E, Damm K.L, Anderson K.W, Mooney D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- Bradley, M., Cullum, N., Nelson, E. A., Petticrew, M., Sheldon, T. & Torgerson, D. 1999 Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds. In Health technology assessment, vol. 3, pp. 1–47. [PubMed]
- Brissová M, Lacík I, Powers A.C, Anilkumar A.V, Wang T. Control and measurement of permeability for design of microcapsule cell delivery system. J. Biomed. Mater. Res. 1998;39:61–70. doi: 10.1002/(sici)1097-4636(199801)39:1<61::aid-jbm8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Burdick J.A, Chung C, Jia X, Randolph M.A, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonder C, Matthew H.W.T, Van Tassel P.R. Adsorbed layers of oriented fibronectin: a strategy to control cell–surface interactions. J. Biomed. Mater. Res. A. 2005;75:316–323. doi: 10.1002/jbm.a.30417. [DOI] [PubMed] [Google Scholar]
- Campbell P.G, Miller E.D, Fisher G.W, Walker L.M, Weiss L.E. Engineered spatial patterns of FGF-2 immobilized on fibrin direct cell organization. Biomaterials. 2005;26:6762–6770. doi: 10.1016/j.biomaterials.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Campos M.G.N, Grosso C.R.F, Cardenas G, Mei L.H.I. Effects of neutralization process on preparation and characterization of chitosan membranes for wound dressing. Macromol. Symp. 2005;229:253–257. doi: 10.1002/masy.200551131. [DOI] [Google Scholar]
- Canaple L, Angelova N, Saugy D, Hunkeler D, Desvergne B. Maintenance of primary murine hepatocyte functions in multicomponent polymer capsules: in vitro cryopreservation studies. J. Hepatol. 2001;34:11–18. doi: 10.1016/S0168-8278(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Cascone M.G, Barbani N, Cristallini C, Giusti P, Ciardelli G, Lazzeri L. Bioartificial polymeric materials based on polysaccharides. J. Biomater. Sci. Polym. Ed. 2001;12:267–281. doi: 10.1163/156856201750180807. [DOI] [PubMed] [Google Scholar]
- Chandler L.A, Gu D.L, Ma C, Gonzalez A.M, Doukas J, Nguyen T, Pierce G.F, Phillips M.L. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair Regen. 2000;8:473–479. doi: 10.1046/j.1524-475x.2000.00473.x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran R, Janaswamy S. Morphology of Western larch arabinogalactan. Carbohydr. Res. 2002;337:2211–2222. doi: 10.1016/S0008-6215(02)00223-9. [DOI] [PubMed] [Google Scholar]
- Chang C.H, Liu H.C, Lin C.C, Chou C.H, Lin F.H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials. 2003a;24:4853–4858. doi: 10.1016/S0142-9612(03)00383-1. [DOI] [PubMed] [Google Scholar]
- Chang W.H, Chang Y, Lai P.H, Sung H.W. A genipin-crosslinked gelatin membrane as wound-dressing material: in vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2003b;14:481–495. doi: 10.1163/156856203766652084. [DOI] [PubMed] [Google Scholar]
- Chang C.H, Kuo T.F, Lin C.C, Chou C.H, Chen K.H, Lin F.H, Liu H.C. Tissue engineering-based cartilage repair with allogenous chondrocytes and gelatin–chondroitin–hyaluronan tri-copolymer scaffold: a porcine model assessed at 18, 24, and 36 weeks. Biomaterials. 2006;27:1876–1888. doi: 10.1016/j.biomaterials.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Chen G.Q, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565–6578. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Chen G.P, Ushida T, Tateishi T. Hybrid biomaterials for tissue engineering: a preparative method for PLA or PLGA-collagen hybrid sponges. Adv. Mater. 2000;12:455–467. doi: 10.1002/(SICI)1521-4095(200003)12:6%3E455::AID-ADMA455%3C3.0.CO;2-C. [DOI] [Google Scholar]
- Chen J, Altman G.H, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan D.L. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J. Biomed. Mater. Res. A. 2003;67:559–570. doi: 10.1002/jbm.a.10120. [DOI] [PubMed] [Google Scholar]
- Cheng L.P, Dwan A.H, Gryte C.C. Membrane formation by isothermal precipitation in polyamide formic-acid water-systems. 1. Description of membrane morphology. J. Polym. Sci. Part B Polym. Phys. 1995;33:211–222. doi: 10.1002/polb.1995.090330206. [DOI] [Google Scholar]
- Chenite A, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–2161. doi: 10.1016/S0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- Chenite A, Buschmann M, Wang D, Chaput C, Kandani N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001;46:39–47. doi: 10.1016/S0144-8617(00)00281-2. [DOI] [Google Scholar]
- Chilvers G.R, Morris V.J. Coacervation of gelatin–gellan gum mixtures and their use in microencapsulation. Carbohydr. Polym. 1987;7:111–120. doi: 10.1016/0144-8617(87)90053-1. [DOI] [Google Scholar]
- Cho J.H, Kim S.-H, Park K.D, Jung M.C, Yang W.I, Han S.W, Noh J.Y, Lee J.W. Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials. 2004;25:5743–5761. doi: 10.1016/j.biomaterials.2004.01.051. [DOI] [PubMed] [Google Scholar]
- Chung H.J, Bae J.W, Park H.D, Lee J.W, Park K.D. Thermosensitive chitosans as novel injectable biomaterials. Macromol. Symp. 2005a;224:275–286. doi: 10.1002/masy.200550624. [DOI] [Google Scholar]
- Chung H.J, Go D.H, Bae J.W, Jung I.K, Lee J.W, Park K.D. Synthesis and characterization of Pluronic (R) grafted chitosan copolymer as a novel injectable biomaterial. Curr. Appl. Phys. 2005b;5:485–488. doi: 10.1016/j.cap.2005.01.015. [DOI] [Google Scholar]
- Ciardelli G, Chiono V, Vozzi G, Barbani N, Giusti P, Ahluwalia A, Cristallini C, Pracella M. Blends of poly-(e-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules. 2005;6:1961–1976. doi: 10.1021/bm0500805. [DOI] [PubMed] [Google Scholar]
- Clark R.A.F, Singer A.J. Wound repair: basic biology to tissue engineering. In: Lanza R, Langer R, Vacanti J.P, editors. Principles of tissue engineering. Academic Press; London, UK: 2000. [Google Scholar]
- Couvreur P, Puisieux F. Nano- and microparticles for the delivery of polypeptides and proteins. Adv. Drug Deliv. Rev. 1993;10:141–162. doi: 10.1016/0169-409X(93)90046-7. [DOI] [Google Scholar]
- Cristino S, et al. Analysis of mesenchymal stem dimensional HYAFF 11 (R)-based cells grown on a prototype ligament scaffold. J. Biomed. Mater. Res. A. 2005;73:275–283. doi: 10.1002/jbm.a.30261. [DOI] [PubMed] [Google Scholar]
- Curran S.J, Chen R, Curran J.M, Hunt J.A. Expansion of human chondrocytes in an intermittent stirred flow bioreactor, using modified biodegradable microspheres. Tissue Eng. 2005;11:1312–1322. doi: 10.1089/ten.2005.11.1312. [DOI] [PubMed] [Google Scholar]
- Czaja W, Krystynowicz A, Bielecki S, Brown R.M. Microbial cellulose—the natural power to heal wounds. Biomaterials. 2006;27:145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- DeFail A.J, Chu C.R, Izzo N, Marra K.G. Controlled release of bioactive TGF-[beta]1 from microspheres embedded within biodegradable hydrogels. Biomaterials. 2006;27:1579–1585. doi: 10.1016/j.biomaterials.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhao K, Zhang X.F, Hu P, Chen G.Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials. 2002;23:4049–4056. doi: 10.1016/S0142-9612(02)00136-9. [DOI] [PubMed] [Google Scholar]
- Devin J.E, Attawia M.A, Laurencin C.T. Three-dimensional degradable porous polymer-ceramic matrices for use in bone repair. J. Biomater. Sci. Polym. Ed. 1996;7:661–669. doi: 10.1163/156856296x00435. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Sittinger M, Risbud M.V. Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–5990. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Drury J.L, Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Ehrenfreund-Kleinman T, Gazit Z, Gazit D, Azzam T, Golenser J, Domb A.J. Synthesis and biodegradation of arabinogalactan sponges prepared by reductive amination. Biomaterials. 2002;23:4621–4631. doi: 10.1016/S0142-9612(02)00209-0. [DOI] [PubMed] [Google Scholar]
- Eliaz R.E, Kost J. Characterization of a polymeric PLGA-injectable implant delivery system for the controlled release of proteins. J. Biomed. Mater. Res. 2000;50:388–396. doi: 10.1002/(SICI)1097-4636(20000605)50:3%3E388::AID-JBM13%3C3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Fu K, Blunk B.T, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J. Orthop. Res. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- Entcheva E, Bien H, Yin L.H, Chung C.Y, Farrell M, Kostov Y. Functional cardiac cell constructs on cellulose-based scaffolding. Biomaterials. 2004;25:5753–5762. doi: 10.1016/j.biomaterials.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Fragonas E, Valente M, Pozzi-Mucelli M, Toffanin R, Rizzo R, Silvestri F, Vittur F. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials. 2000;21:795–801. doi: 10.1016/S0142-9612(99)00241-0. [DOI] [PubMed] [Google Scholar]
- Franz, G. & Blaschek, W. 1990 Cellulose. In Methods in plant biochemistry. Carbohydrates, vol. 2 (ed. P. M. Dey), pp. 291–322. London, UK: Academic Press.
- Freier T, Koh H.S, Kazazian K, Shoichet M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005a;26:5872–5878. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Freier T, Montenegro R, Koh H.S, Shoichet M.S. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials. 2005b;26:4624–4632. doi: 10.1016/j.biomaterials.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Ge Z, Goh J.C, Lee E.H. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573–583. doi: 10.3727/000000005783982819. [DOI] [PubMed] [Google Scholar]
- Geiger M, Li R.H, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Geng L, Feng W, Hutmacher D.W, Wong Y.S, Loh H.T, Fuh J.Y.H. Direct writing of chitosan scaffolds using a robotic system. Rapid Prototyping J. 2005;11:90–97. doi: 10.1108/13552540510589458. [DOI] [Google Scholar]
- Gentile F.T, Doherty E.J, Rein D.H, Shoichet M.S, Winn S.R. Polymer science for macroencapsulation of cells for central nervous system transplantation. React. Polym. 1995;25:207–227. doi: 10.1016/0923-1137(94)00097-O. [DOI] [Google Scholar]
- Gérentes P, Vachoud L, Doury J, Domard A. Study of a chitin-based gel as injectable material in periodontal surgery. Biomaterials. 2002;23:1295–1302. doi: 10.1016/S0142-9612(01)00247-2. [DOI] [PubMed] [Google Scholar]
- Giavaresi G, Torricelli P, Fornasari P.M, Giardino R, Barbucci R, Leone G. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials. 2005;26:3001–3008. doi: 10.1016/j.biomaterials.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Gigante A, Bevilacqua C, Cappella M, Manzotti S, Greco F. Engineered articular cartilage: influence of the scaffold on cell phenotype and proliferation. J. Mater. Sci. Mater. Med. 2003;14:713–716. doi: 10.1023/A:1024915817061. [DOI] [PubMed] [Google Scholar]
- Gingras M, Paradis I, Berthod F. Nerve regeneration in a collagen–chitosan tissue-engineered skin transplanted on nude mice. Biomaterials. 2003;24:1653–1661. doi: 10.1016/S0142-9612(02)00572-0. [DOI] [PubMed] [Google Scholar]
- Glicklis R, Shapiro L, Agbaria R, Merchuk J.C, Cohen S. Hepatocyte behavior within three-dimensional porous alginate scaffolds. Biotechnol. Bioeng. 2000;67:344–353. doi: 10.1002/(SICI)1097-0290(20000205)67:3%3E344::AID-BIT11%3C3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gomes M.E, Ribeiro A.S, Malafaya P.B, Reis R.L, Cunha A.M. A new approach based on injection moulding to produce biodegradable starch-based polymeric scaffolds: morphology, mechanical and degradation behaviour. Biomaterials. 2001;22:883–889. doi: 10.1016/S0142-9612(00)00211-8. [DOI] [PubMed] [Google Scholar]
- Gomes M.E, Godinho J.S, Tchalamov D, Cunha A.M, Reis R.L. Alternative tissue engineering scaffolds based on starch: processing methodologies, morphology, degradation and mechanical properties. Mater. Sci. Eng. C. 2002;20:19–26. doi: 10.1016/S0928-4931(02)00008-5. [DOI] [Google Scholar]
- Gomes M.E, Reis R.L, Behravesh E, Mikos A.G, Sikavitsas V.I. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J. Biomed. Mater. Res. A. 2003;67:87–95. doi: 10.1002/jbm.a.10075. [DOI] [PubMed] [Google Scholar]
- Gomes, M. E., Reis, R. L., Holtorf, H. & Mikos, A. G. 2004a Influence of the porosity of starch-based fiber meshes on the proliferation and osteogenic differentiation of marrow stromal cells cultured under flow perfusion. In Transactions—7th World Biomaterials Congress, pp. 376.
- Gomes M.E, Reis R.L, Mikos A.G. Injectable polymeric scaffolds for bone tissued engineering. In: Reis R.L, Roman J.S, editors. Biodegradable systems in tissue engineering and regenerative medicine. CRC Press; Boca Raton, FL: 2004b. pp. 29–38. [Google Scholar]
- Gomes M.E, Bossano C.M, Johnston C.M, Reis R.L, Mikos A.G. In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng. 2006;12:177–188. doi: 10.1089/ten.2006.12.177. [DOI] [PubMed] [Google Scholar]
- Gruber H.E, Hoelscher G.L, Leslie K, Ingram J.A, Hanley E.N. Three-dimensional culture of human disc cells within agarose or a collagen sponge: assessment of proteoglycan production. Biomaterials. 2006;27:371–376. doi: 10.1016/j.biomaterials.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Grzybowski J, Kolodziej W, Trafny E, Struzna J. A new anti-infective collagen dressing containing antibiotics. J. Biomed. Mater. Res. 1997;36:163–166. doi: 10.1002/(SICI)1097-4636(199708)36:2%3E163::AID-JBM4%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Guerret S, Govignon E, Hartmann D.J, Ronfard V. Long-term remodeling of a bilayered living human skin equivalent (Apligraf) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wound Repair Regen. 2003;11:35–45. doi: 10.1046/j.1524-475X.2003.11107.x. [DOI] [PubMed] [Google Scholar]
- Gupta M, Gupta A.K. In vitro cytotoxicity studies of hydrogel pullulan nanoparticles prepared by aot/n-hexane micellar system. J. Pharm. Pharm. Sci. 2004;7:38–46. [PubMed] [Google Scholar]
- Hafemann B, Ensslen S, Erdmann C, Niedballa R, Zuhlke A, Ghofrani K, Kirkpatrick C.J. Use of a collagen/elastin-membrane for the tissue engineering of dermis. Burns. 1999;25:373–384. doi: 10.1016/S0305-4179(98)00162-4. [DOI] [PubMed] [Google Scholar]
- Hafemann B, Ghofrani K, Gattner H.G, Stieve H, Pallua N. Cross-linking by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) of a collagen/elastin membrane meant to be used as a dermal substitute: effects on physical, biochemical and biological features in vitro. J. Mater. Sci. Mater. Med. 2001;12:437–446. doi: 10.1023/A:1011205221972. [DOI] [PubMed] [Google Scholar]
- Harris, L., Kim, B. -S. & Mooney, D. J. 1997 Open pore matrices formed with gas foaming. In American Society of Mechanical Engineers, Bioengineering Division (Publication) BED, vol. 35, pp. 351.
- Harris L.D, Kim B.S, Mooney D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998;42:396–402. doi: 10.1002/(SICI)1097-4636(19981205)42:3%3E396::AID-JBM7%3C3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hartgerink J.D, Beniash E, Stupp S.I. Self-assembly and mineralization of peptide–amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- Haugen H, Gerhardt L.C, Will J, Wintermantel E. Biostability of polyether-urethane scaffolds: a comparison of two novel processing methods and the effect of higher gamma-irradiation dose. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005;73:229–237. doi: 10.1002/jbm.b.30237. [DOI] [PubMed] [Google Scholar]
- Hayashi T. Biodegradable polymers for biomedical uses. Prog. Polym. Sci. 1994;19:663–702. doi: 10.1016/0079-6700(94)90030-2. [DOI] [Google Scholar]
- Hoemann C.D, Sun J, Legare A, McKee M.D, Buschmann M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr. Cartilage. 2005;13:318–329. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hokugo A, Takamoto T, Tabata Y. Preparation of hybrid scaffold from fibrin and biodegradable polymer fiber. Biomaterials. 2006;27:61–67. doi: 10.1016/j.biomaterials.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Horch R.E, Kopp J, Kneser U, Beier J, Bach A.D. Tissue engineering of cultured skin substitutes. J. Cell. Mol. Med. 2005;9:592–608. doi: 10.1111/j.1582-4934.2005.tb00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottot A, Vessot S, Andrieu J. A direct characterization method of the ice morphology. Relationship between mean crystals size and primary drying times of freeze-drying processes. Dry. Technol. 2004;22:2009–2021. doi: 10.1081/DRT-200032717. [DOI] [Google Scholar]
- Hou Q.P, Grijpma D.W, Feijen J. Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials. 2003;24:1937–1947. doi: 10.1016/S0142-9612(02)00562-8. [DOI] [PubMed] [Google Scholar]
- Huang L, Cheng Y.Y, Koo P.L, Lee K.M, Qin L, Cheng J.C, Kumta S.M. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mater. Res. A. 2003;66:880–884. doi: 10.1002/jbm.a.10535. [DOI] [PubMed] [Google Scholar]
- Hubbell J.A. Materials as morphogenetic guides in tissue engineering. Curr. Opin. Biotechnol. 2003;14:551–558. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Hudon V, Berthod F, Black A.F, Damour O, Germain L, Auger F.A. A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br. J. Dermatol. 2003;148:1094–1104. doi: 10.1046/j.1365-2133.2003.05298.x. [DOI] [PubMed] [Google Scholar]
- Hunt D.R, Jovanovic S.A, Wikesjo U.M, Wozney J.M, Bernard G.W. Hyaluronan supports recombinant human bone morphogenetic protein-2 induced bone reconstruction of advanced alveolar ridge defects in dogs. A pilot study. J. Periodontol. 2001;72:651–658. doi: 10.1902/jop.2001.72.5.651. [DOI] [PubMed] [Google Scholar]
- Hutmacher D.W, Schantz T, Zein I, Ng K.W, Teoh S.H, Tan K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001;55:203–216. doi: 10.1002/1097-4636(200105)55:2%3E203::AID-JBM1007%3C3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ignoffo C.M, Shasha B.S, Shapiro M. Sunlight ultraviolet protection of the heliothis nuclear polyhedrosis virus through starch-encapsulation technology. J. Invertebr. Pathol. 1991;57:134–136. doi: 10.1016/0022-2011(91)90053-S. [DOI] [Google Scholar]
- Iseki T, Takahashi M, Hattori H, Hatakeyama T, Hatakeyama H. Viscoelastic properties of xanthan gum hydrogels annealed in the sol state. Food Hydrocolloid. 2001;15:503–506. doi: 10.1016/S0268-005X(01)00088-1. [DOI] [Google Scholar]
- Ishihara M, et al. Acceleration of wound contraction and healing with a photocrosslinkable chitosan hydrogel. Wound Repair Regen. 2001;9:513–521. doi: 10.1046/j.1524-475x.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- Isogai N, Morotomi T, Hayakawa S, Munakata H, Tabata Y, Ikada Y, Kamiishi H. Combined chondrocyte-copolymer implantation with slow release of basic fibroblast growth factor for tissue engineering an auricular cartilage construct. J. Biomed. Mater. Res. Part A. 2005;74:408–418. doi: 10.1002/jbm.a.30343. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, et al. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules. 2004;5:828–833. doi: 10.1021/bm0400067. [DOI] [PubMed] [Google Scholar]
- Izydorczyk M, Cui S.W, Wang Q. Polysaccharide gums: structures. Functional properties, and applications. In: Cui S.W, editor. Food carbohydrates: chemistry, physical properties, and applications. CRC Press; Taylor & Francis Group; Boca Raton, FL: 2005. pp. 263–307. [Google Scholar]
- Jarman-Smith M.L, Bodamyali T, Stevens C, Howell J.A, Horrocks M, Chaudhuri J.B. Porcine collagen crosslinking, degradation and its capability for fibroblast adhesion and proliferation. J. Mater. Sci. Mater. Med. 2004;15:925–932. doi: 10.1023/B:JMSM.0000036281.47596.cc. [DOI] [PubMed] [Google Scholar]
- Jen A.C, Wake M.C, Mikos A.G. Review: hydrogels for cell immobilization. Biotechnol. Bioeng. 1996;50:357–364. doi: 10.1002/(SICI)1097-0290(19960520)50:4%3C357::AID-BIT2%3E3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jiang, T. & Laurencin, C. T. 2005 Development and evaluation of novel chitosan/PLAGA composite scaffolds for bone tissue engineering. In Society for Biomaterials 30th Annual Meeting Transactions, pp. 151.
- Jiang H.L, Fang D.F, Hsiao B.S, Chu B, Chen W.L. Optimization and characterization of dextran membranes prepared by electrospinning. Biomacromolecules. 2004;5:326–333. doi: 10.1021/bm034345w. [DOI] [PubMed] [Google Scholar]
- Jimenez P.A, Jimenez S.E. Tissue and cellular approaches to wound repair. Am. J. Surg. 2004;187:56S–64S. doi: 10.1016/S0002-9610(03)00305-2. [DOI] [PubMed] [Google Scholar]
- Jin H.J, Chen J, Karageorgiou V, Altman G.H, Kaplan D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials. 2004;25:1039–1047. doi: 10.1016/S0142-9612(03)00609-4. [DOI] [PubMed] [Google Scholar]
- Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br. J. Plast. Surg. 2002;55:185–193. doi: 10.1054/bjps.2002.3800. [DOI] [PubMed] [Google Scholar]
- Jun Jin Y, Park T.G. Degradation behaviors of biodegradable macroporous scaffolds prepared by gas foaming of effervescent salts. J. Biomed. Mater. Res. 2001;55:401–408. doi: 10.1002/1097-4636(20010605)55:3%3C401::AID-JBM1029%3E3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Jux C, Wohlsein P, Bruegmann M, Zutz M, Franzbach B, Bertram H. A new biological matrix for septal occlusion. J. Interv. Cardiol. 2003;16:149–152. doi: 10.1046/j.1540-8183.2003.08027.x. [DOI] [PubMed] [Google Scholar]
- Kampf N. The use of polymers for coating of cells. Polym. Adv. Technol. 2002;13:896–905. doi: 10.1002/pat.277. [DOI] [Google Scholar]
- Kang S.-W, Jeon O, Kim B.-S. Poly(lactic-co-glycolic acid) microspheres as an injectable scaffold for cartilage tissue engineering. Tissue Eng. 2005;11:438–447. doi: 10.1089/ten.2005.11.438. [DOI] [PubMed] [Google Scholar]
- Karageorgiou V, Kaplan D. Porosity of 3D biornaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Keefe J, Wauk L, Chu S, DeLustro F. Clinical use of injectable bovine collagen: a decade of experience. Clin. Mater. 1992;9:155–162. doi: 10.1016/0267-6605(92)90095-B. [DOI] [PubMed] [Google Scholar]
- Khan T.A, Peh K.K. A preliminary investigation of chitosan film as dressing for punch biopsy wounds in rats. J. Pharm. Pharm. Sci. 2003;6:20–26. [PubMed] [Google Scholar]
- Khan T.A, Peh K.K, Ch'ng H.S. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J. Pharm. Pharm. Sci. 2000;3:303–311. [PubMed] [Google Scholar]
- Khan Y.M, Katt D.S, Laurencin C.T. Novel polymer-synthesized ceramic composite-based system for bone repair: an in vitro evaluation. J. Biomed. Mater. Res. Part A. 2004;69:728–737. doi: 10.1002/jbm.a.30051. [DOI] [PubMed] [Google Scholar]
- Khor E, Lim L.Y. Implantable applications of chitin and chitosan. Biomaterials. 2003;24:2339–2349. doi: 10.1016/S0142-9612(03)00026-7. [DOI] [PubMed] [Google Scholar]
- Kim S.S, Utsunomiya H, Koski J.A, Wu B.M, Cima M.J, Sohn J, Mukai K, Griffith L.G, Vacanti J.P. Survival and function of hepatocytes on a novel three-dimensional synthetic biodegradable polymer scaffold with an intrinsic network of channels. Ann. Surg. 1998;228:8–13. doi: 10.1097/00000658-199807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.S, Baez C.E, Atala A. Biomaterials for tissue engineering. World J. Urol. 2000;18:2–9. doi: 10.1007/s003450050002. [DOI] [PubMed] [Google Scholar]
- Kim S.E, Park J.H, Cho Y.W, Chung H, Jeong S.Y, Lee E.B, Kwon I.C. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-[beta]1: implications for cartilage tissue engineering. J. Control. Release. 2003;91:365–374. doi: 10.1016/S0168-3659(03)00274-8. [DOI] [PubMed] [Google Scholar]
- Kim H.J, Kim U.J, Vunjak-Novakovic G, Min B.H, Kaplan D.L. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005;26:4442–4452. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Ozeki M, Inamoto T, Tabata Y. Time course of de novo adipogenesis in matrigel by gelatin microspheres incorporating basic fibroblast growth factor. Tissue Eng. 2002;8:603–613. doi: 10.1089/107632702760240526. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–2521. doi: 10.1016/S0142-9612(03)00049-8. [DOI] [PubMed] [Google Scholar]
- King G.A, Goosen M.F.A. Fundamentals of animal cell encapsulation and immobilization. CRC Press; Boca Raton, FL: 1993. Cell immobilization technology: an overview. [Google Scholar]
- Klein B, Schiffer R, Hafemann B, Klosterhalfen B, Zwadlo-Klarwasser G. Inflammatory response to a porcine membrane composed of fibrous collagen and elastin as dermal substitute. J. Mater. Sci. Mater. Med. 2001;12:419–424. doi: 10.1023/A:1011249020155. [DOI] [PubMed] [Google Scholar]
- Ko F, Gogotsi Y, Ali A, Naguib N, Ye H.H, Yang G.L, Li C, Willis P. Electrospinning of continuons carbon nanotube-filled nanofiber yarns. Adv. Mater. 2003;15:1161–1165. doi: 10.1002/adma.200304955. [DOI] [Google Scholar]
- Kobayashi S, Fujikawa S, Ohmae M. Enzymatic synthesis of chondroitin and its derivatives catalyzed by hyaluronidase. J. Am. Chem. Soc. 2003;125:14 357–14 369. doi: 10.1021/ja036584x. [DOI] [PubMed] [Google Scholar]
- Kose G.T, Korkusuz F, Korkusuz P, Hasirci V. In vivo tissue engineering of bone using poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) and collagen scaffolds. Tissue Eng. 2004;10:1234–1250. doi: 10.1089/ten.2004.10.1234. [DOI] [PubMed] [Google Scholar]
- Kumar M.N.V.R, Muzzarelli R.A.A, Muzzarelli C, Sashiwa H, Domb A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- Kuo C.K, Ma P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/S0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- Kurita K. Controlled functionalization of the polysaccharide chitin. Prog. Polym. Sci. 2001;26:1921–1971. doi: 10.1016/S0079-6700(01)00007-7. [DOI] [Google Scholar]
- Lam C.X.F, Mo X.M, Teoh S.H, Hutmacher D.W. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2002;20:49–56. [Google Scholar]
- Laurencin C.T, Freeman J.W. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530–7536. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- Leach J.B, Schmidt C.E. Characterization of protein release from photocrosslinkable hyaluronic acid–polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Leach J.B, Bivens K.A, Patrick C.W, Schmidt C.E. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- Lee J.E, Park J.C, Lee H.K, Oh S.H, Suh H. Laminin modified infection-preventing collagen membrane containing silver sulfadiazine–hyaluronan microparticles. Artif. Organs. 2002;26:521–528. doi: 10.1046/j.1525-1594.2002.06890.x. [DOI] [PubMed] [Google Scholar]
- Lee S.H, et al. Elastic biodegradable poly(glycolide-co-caprolactone) scaffold for tissue engineering. J. Biomed. Mater. Res. Part A. 2003;66:29–37. doi: 10.1002/jbm.a.10497. [DOI] [PubMed] [Google Scholar]
- Lee J.E, Kim K.E, Kwon I.C, Ahn H.J, Lee S.-H, Cho H, Kim H.J, Seong S.C, Lee M.C. Effects of the controlled-released TGF-[beta]1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials. 2004a;25:4163–4173. doi: 10.1016/j.biomaterials.2003.10.057. [DOI] [PubMed] [Google Scholar]
- Lee J.W, Jung M.C, Park H.D, Park K.D, Ryu G.H. Synthesis and characterization of thermosensitive chitosan copolymer as a novel biomaterial. J. Biomater. Sci. Polym. Ed. 2004b;15:1065–1079. doi: 10.1163/1568562041526496. [DOI] [PubMed] [Google Scholar]
- Lee S.H, Kim B.S, Kim S.H, Kang S.W, Kim Y.H. Thermally produced biodegradable scaffolds for cartilage tissue engineering. Macromol. Biosci. 2004c;4:802–810. doi: 10.1002/mabi.200400021. [DOI] [PubMed] [Google Scholar]
- Leicher S, Will J, Haugen H, Wintermantel E. MuCell® technology for injection molding: a processing method for polyether-urethane scaffolds. J. Mater. Sci. 2005;40:4613–4618. doi: 10.1007/s10853-005-0853-y. [DOI] [Google Scholar]
- Levesque S.G, Lim R.M, Shoichet M.S. Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications. Biomaterials. 2005;26:7436–7446. doi: 10.1016/j.biomaterials.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Lezica R.P, Quesada-Allué L. Chitin. In: Dey P.M, editor. Methods in plant biochemistry. Carbohydrates. vol. 2. Academic Press; London, UK: 1990. pp. 443–481. [Google Scholar]
- Li R.H. Materials for immunoisolated cell transplantation. Adv. Drug Deliv. Rev. 1998;33:87–109. doi: 10.1016/S0169-409X(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Li D, Xia Y.N. Electrospinning of nanofibers: reinventing the wheel? Adv. Mater. 2004;16:1151–1170. doi: 10.1002/adma.200400719. [DOI] [Google Scholar]
- Li Z, Zhang M. Chitosan–alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. A. 2005;75:485–493. doi: 10.1002/jbm.a.30449. [DOI] [PubMed] [Google Scholar]
- Li D, Wang Y.L, Xia Y.N. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004;16:361–366. doi: 10.1002/adma.200306226. [DOI] [Google Scholar]
- Li X, Feng Q, Jiao Y, Cui F. Collagen-based scaffolds reinforced by chitosan fibres for bone tissue engineering. Polym. Int. 2005;54:1034–1040. doi: 10.1002/pi.1804. [DOI] [Google Scholar]
- Li X, Feng Q, Liu X, Dong W, Cui F. Collagen-based implants reinforced by chitin fibres in a goat shank bone defect model. Biomaterials. 2006;27:1917–1923. doi: 10.1016/j.biomaterials.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Liapis A.I, Pikal M.J, Bruttini R. Research and development needs and opportunities in freeze drying. Dry. Technol. 1996;14:1265–1300. [Google Scholar]
- Lin H.R, Yen Y.J. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2004;71:52–65. doi: 10.1002/jbm.b.30065. [DOI] [PubMed] [Google Scholar]
- Lindenhayn K, Perka C, Spitzer R, Heilmann H, Pommerening K, Mennicke J, Sittinger M. Retention of hyaluronic acid in alginate beads: aspects for in vitro cartilage engineering. J. Biomed. Mater. Res. 1999;44:149–155. doi: 10.1002/(SICI)1097-4636(199902)44:2%3C149::AID-JBM4%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Liu H, Mao J, Yao K, Yang G, Cui L, Cao Y. A study on a chitosan–gelatin–hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2004;15:25–40. doi: 10.1163/156856204322752219. [DOI] [PubMed] [Google Scholar]
- López A, Lázaro N, Marqués A.M. The interphase technique: a simple method of cell immobilization in gel-beads. J. Microbiol. Methods. 1997;30:231–234. doi: 10.1016/S0167-7012(97)00071-7. [DOI] [Google Scholar]
- Luginbuehl V, Wenk E, Koch A, Gander B, Merkle H.P, Meinel L. Insulin-like growth factor I-releasing alginate-tricalciumphosphate composites for bone regeneration. Pharm. Res. 2005;22:940–950. doi: 10.1007/s11095-005-4589-9. [DOI] [PubMed] [Google Scholar]
- Lutolf M.P, Lauer-Fields J.L, Schmoekel H.G, Metters A.T, Weber F.E, Fields G.B, Hubbell J.A. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA. 2003a;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M.R, Weber F.E, Schmoekel H.G, Schense J.C, Kohler T, Muller R, Hubbell J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003b;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- Malafaya P.B, Reis R.L. Porous bioactive composites from marine origin based in chitosan and hydroxylapatite particles. Key Eng. Mater. 2003;240-242:39–42. [Google Scholar]
- Malafaya P.B, Silva G.A, Baran E.T, Reis R.L. Drug delivery therapies I—general trends and its importance on bone tissue engineering applications. Curr. Opin. Solid State Mater. Sci. 2002;6:283–295. doi: 10.1016/S1359-0286(02)00075-X. [DOI] [Google Scholar]
- Malafaya P.B, Pedro A, Peterbauer A, Gabriel C, Redl H, Reis R. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J. Mater. Sci. Mater. Med. 2005;16:1077–1085. doi: 10.1007/s10856-005-4709-4. [DOI] [PubMed] [Google Scholar]
- Mann B.K, Gobin A.S, Tsai A.T, Schmedlen R.H, West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045–3051. doi: 10.1016/S0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- Mao J.S, Liu H.F, Yin Y.J, Yao K.D. The properties of chitosan–gelatin membranes and scaffolds modified with hyaluronic acid by different methods. Biomaterials. 2003;24:1621–1629. doi: 10.1016/S0142-9612(02)00549-5. [DOI] [PubMed] [Google Scholar]
- Marreco P.R, da Luz Moreira P, Genari S.C, Moraes A.M. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J. Biomed. Mater. Res. B Appl. Biomater. B. 2004;71:268–277. doi: 10.1002/jbm.b.30081. [DOI] [PubMed] [Google Scholar]
- Marston W.A, Usala A, Hill R.S, Mendes R, Minsley M.-A. Initial report of the use of an injectable porcine collagen-derived matrix to stimulate healing of diabetic foot wounds in humans. Wound Repair Regen. 2005;13:243–247. doi: 10.1111/j.1067-1927.2005.130305.x. [DOI] [PubMed] [Google Scholar]
- Masters K.S, Shah D.N, Leinwand L.A, Anseth K.S. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Masuoka K, et al. Tissue engineering of articular cartilage using an allograft of cultured chondrocytes in a membrane-sealed atelocollagen honeycomb-shaped scaffold ( ACHMS scaffold) J. Biomed. Mater. Res. B Appl. Biomater. 2005;75:174–184. doi: 10.1002/jbm.b.30284. [DOI] [PubMed] [Google Scholar]
- Mathieu L.M, Bourban P.E, Månson J.A.E, Montjovent M.O, Pioletti D.P. Bioresorbable composites prepared by supercritical fluid foaming. J. Biomed. Mater. Res. A. 2005;75:89–97. doi: 10.1002/jbm.a.30385. [DOI] [PubMed] [Google Scholar]
- Mathieu L.M, Bourban P.E, Månson J.A.E, Mueller T.L, Müller R, Pioletti D.P. Architecture and properties of anisotropic polymer composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:905–916. doi: 10.1016/j.biomaterials.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Mauck R.L, Wang C.C.B, Oswald E.S, Ateshian G.A, Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr. Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Mehvar R. Dextrans for targeted and sustained delivery of therapeutic and imaging agents. J. Control. Release. 2000;69:1–25. doi: 10.1016/S0168-3659(00)00302-3. [DOI] [PubMed] [Google Scholar]
- Meinel L, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J. Biomed. Mater. Res. A. 2004a;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- Meinel L, Langer R, Vunjak-Novakovic G, Zichner L, Karageorgiou V, Kaplan D, Fajardo R, Snyder B, Shinde-Patil V. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004b;32:112–122. doi: 10.1023/B:ABME.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Meinel L, Langer R, Vunjak-Novakovic G, Zichner L, Karageorgiou V, Kaplan D, Fajardo R, Snyder B, Shinde-Patil V. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann. Biomed. Eng. 2004c;32:112–122. doi: 10.1023/B:ABME.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- Mi F.L, Shyu S.S, Wu Y.B, Lee S.T, Shyong J.Y, Huang R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials. 2001;22:165–173. doi: 10.1016/S0142-9612(00)00167-8. [DOI] [PubMed] [Google Scholar]
- Mi F.L, Wu Y.B, Shyu S.S, Schoung J.Y, Huang Y.B, Tsai Y.H, Hao J.Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2002;59:438–449. doi: 10.1002/jbm.1260. [DOI] [PubMed] [Google Scholar]
- Mi F.L, Wu Y.B, Shyu S.S, Chao A.C, Lai J.Y, Su C.C. Asymmetric chitosan membranes prepared by dry/wet phase separation: a new type of wound dressing for controlled antibacterial release. J. Memb. Sci. 2003;212:237–254. doi: 10.1016/S0376-7388(02)00505-7. [DOI] [Google Scholar]
- Miyata S, Furukawa K.S, Ushida T, Nitta Y, Tateishi T. Static and dynamic mechanical properties of extracellular matrix synthesized by cultured chondrocytes. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2004;24:425–429. [Google Scholar]
- Mizuno M, Shindo M, Kobayashi D, Tsuruga E, Amemiya A, Kuboki Y. Osteogenesis by bone marrow stromal cells maintained on type I collagen matrix gels in vivo. Bone. 1997;20:101–107. doi: 10.1016/S8756-3282(96)00349-3. [DOI] [PubMed] [Google Scholar]
- Molinaro G, Leroux J.-C, Damas J, Adam A. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials. 2002;23:2717–2722. doi: 10.1016/S0142-9612(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Moore M.J, Jabbari E, Ritman E.L, Lu L.C, Currier B.L, Windebank A.J, Yaszemski M.J. Quantitative analysis of interconnectivity of porous biodegradable scaffolds with micro-computed tomography. J. Biomed. Mater. Res. Part A. 2004;71:258–267. doi: 10.1002/jbm.a.30138. [DOI] [PubMed] [Google Scholar]
- Morrison W.R, Karkalas J. Starch. In: Dey P.M, editor. Methods in plant biochemistry. Carbohydrates. vol. 2. Academic Press; London, UK: 1990. pp. 323–352. [Google Scholar]
- Moslemy P, Guiot S.R, Neufeld R.J. Production of size-controlled gellan gum microbeads encapsulating gasoline-degrading bacteria. Enzyme Microb. Technol. 2002;30:10–18. doi: 10.1016/S0141-0229(01)00440-9. [DOI] [Google Scholar]
- Mouw J.K, Case N.D, Guldberg R.E, Plaas A.H, Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthr. Cartilage. 2005;13:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Muralidhar R.V, Jayachandran G, Singh P. Development of high-density cultivation systems by bioencapsulation. Curr. Sci. 2001;81:263–269. [Google Scholar]
- Muzzarelli R.A.A, Guerrieri M, Goteri G, Muzzarelli C, Armeni T, Ghiselli R, Cornelissen M. The biocompatibility of dibutyryl chitin in the context of wound dressings. Biomaterials. 2005;26:5844–5854. doi: 10.1016/j.biomaterials.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Na K, Shin D, Yun K, Park K.H, Lee K.C. Conjugation of heparin into carboxylated pullulan derivatives as an extracellular matrix for endothelial cell culture. Biotechnol. Lett. 2003;25:381–385. doi: 10.1023/A:1022442129375. [DOI] [PubMed] [Google Scholar]
- Naessens M, Cerdobbel A, Soetaert W, Vandamme E.J. Leuconostoc dextransucrase and dextran: production, properties and applications. J. Chem. Technol. Biotechnol. 2005;80:845–860. doi: 10.1002/jctb.1322. [DOI] [Google Scholar]
- Nakahara T, Nakamura T, Kobayashi E, Inoue M, Shigeno K, Tabata Y, Eto K, Shimizu Y. Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 2003;9:153–162. doi: 10.1089/107632703762687636. [DOI] [PubMed] [Google Scholar]
- Nazarov R, Jin H.J, Kaplan D.L. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- Nettles D.L, Elder S.H, Gilbert J.A. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002;8:1009–1016. doi: 10.1089/107632702320934100. [DOI] [PubMed] [Google Scholar]
- Nettles, D. L., Vail, T. P., Morgan, M. T., W.Grinstaff, M. & Setton, L. A. 2004. Ann. Biomed. Eng 32, 391–397 [DOI] [PubMed]
- Neves N.M, Kouyumdzhiev A, Reis R.L. The morphology, mechanical properties and ageing behavior of porous injection molded starch-based blends for tissue engineering scaffolding. Mater. Sci. Eng. C. 2005;25:195–200. doi: 10.1016/j.msec.2005.01.009. [DOI] [Google Scholar]
- Newman K.D, McBurney M.W.M.W. Poly(lactic-co-glycolic acid) microspheres as biodegradable microcarriers for pluripotent stem cells. Biomaterials. 2004;25:5763–5771. doi: 10.1016/j.biomaterials.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Ng K.W, Wang C.C.B, Mauck R.L, Kelly T.A.N, Chahine N.O, Costa K.D, Ateshian G.A, Hung C.T. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J. Orthop. Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Nof M, Shea L.D. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J. Biomed. Mater. Res. 2002;59:349–356. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]
- Noth U, et al. Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy. 2005;7:447–455. doi: 10.1080/14653240500319093. [DOI] [PubMed] [Google Scholar]
- O'Connor S.M, Stenger D.A, Shaffer K.M, Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 2001;304:189–193. doi: 10.1016/S0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- ODonnell P.B, McGinity J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997;28:25–42. doi: 10.1016/S0169-409X(97)00049-5. [DOI] [PubMed] [Google Scholar]
- Oliveira J.M, Silva S, Mano J.F, Reis R.L. Innovative technique for the preparation of porous bilayered hydroxyapatite/chitosan scaffolds for osteochondral applications. Key Eng. Mater. 2006;309–311:927–930. [Google Scholar]
- Orive G, Hernández R.M, Gascón A.R, Igartua M, Pedraz J.L. Survival of different cell lines in alginate–agarose microcapsules. Eur. J. Pharm. Sci. 2003;18:23–30. doi: 10.1016/S0928-0987(02)00220-8. [DOI] [PubMed] [Google Scholar]
- Park D.-J, Choi B.-H, Zhu S.-J, Huh J.-Y, Kim B.-Y, Lee S.-H. Injectable bone using chitosan–alginate gel/mesenchymal stem cells/BMP-2 composites. J. Craniomaxillofac. Surg. 2005a;33:50–54. doi: 10.1016/j.jcms.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Park S.-H, Park S.R, Chung S.I, Pai K.S, Min B.-H. Tissue-engineered cartilage using fibrin/hyaluronan composite gel and its in vivo implantation. Artif. Organs. 2005b;29:838–845. doi: 10.1111/j.1525-1594.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Paul W, Sharma C.P. Chitosan and alginate wound dressings: a short review. Trends Biomater. Artif. Organs. 2004;18:18–23. [Google Scholar]
- Pavlov M.P, Mano J.F, Neves N.M, Reis R.L. Fibers and 3D mesh scaffolds from biodegradable starch-based blends: production and characterization. Macromol. Biosci. 2004;4:776–784. doi: 10.1002/mabi.200400002. [DOI] [PubMed] [Google Scholar]
- Payne R.G, McGonigle J.S, Yaszemski M.J, Yasko A.W, Mikos A.G. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 2. Viability of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002a;23:4373–4380. doi: 10.1016/S0142-9612(02)00185-0. [DOI] [PubMed] [Google Scholar]
- Payne R.G, McGonigle J.S, Yaszemski M.J, Yasko A.W, Mikos A.G. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 3. Proliferation and differentiation of encapsulated marrow stromal osteoblasts cultured on crosslinking poly(propylene fumarate) Biomaterials. 2002b;23:4381–4387. doi: 10.1016/S0142-9612(02)00186-2. [DOI] [PubMed] [Google Scholar]
- Payne R.G, Yaszemski M.J, Yasko A.W, Mikos A.G. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations. Part 1. Encapsulation of marrow stromal osteoblasts in surface crosslinked gelatin microparticles. Biomaterials. 2002c;23:4359–4371. doi: 10.1016/S0142-9612(02)00184-9. [DOI] [PubMed] [Google Scholar]
- Pelegrin M, Marin M, Noel D, Rio M.D, Saller R, Stange J, Mitzner S, Gunzburg W.H, Piechaczyk M. Systemic long-term delivery of antibodies in immunocompetent animals using cellulose sulphate capsules containing antibody-producing cells. Gene Ther. 1998;5:828–834. doi: 10.1038/sj.gt.3300632. [DOI] [PubMed] [Google Scholar]
- Percival E, McDowell R.H. Algal polysaccharides. In: Dey P.M, editor. Methods in plant biochemistry. Carbohydrates. vol. 2. Academic Press; London, UK: 1990. pp. 523–547. [Google Scholar]
- Perets A, Baruch Y, Weisbuch F, Shoshany G, Neufeld G, Cohen S. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J. Biomed. Mater. Res. A. 2003;65:489–497. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- Perka C, Spitzer R.S, Lindenhayn K, Sittinger M, Schultz O. Matrix-mixed culture: new methodology for chondrocyte culture and preparation of cartilage transplants. J. Biomed. Mater. Res. 2000;49:305–311. doi: 10.1002/(SICI)1097-4636(20000305)49:3%3C305::AID-JBM2%3E3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pfister A, Landers R, Laib A, Hubner U, Schmelzeisen R, Mulhaupt R. Biofunctional rapid prototyping for tissue-engineering applications: 3D bioplotting versus 3D printing. J. Polym. Sci. Part A Polym. Chem. 2004;42:624–638. doi: 10.1002/pola.10807. [DOI] [Google Scholar]
- Prokop A, Hunkeler D, DiMari S, Haralson M.A, Wang T.G. Water soluble polymers for immunoisolation I: complex coacervation and cytotoxicity. Microencapsul. Microgels Iniferters. 1998a;136:1–51. [Google Scholar]
- Prokop A, Hunkeler D, Powers A.C, Whitesell R.R, Wang T.G. Water soluble polymers for immunoisolation II: evaluation of multicomponent microencapsulation systems. Microencapsul. Microgels Iniferters. 1998b;136:53–73. [Google Scholar]
- Qiu Q, Ducheyne P, Gao H, Ayyaswamy P. Formation and differentiation of three-dimensional rat marrow stromal cell culture on microcarriers in a rotating-wall vessel. Tissue Eng. 1998;4:19–34. doi: 10.1089/ten.1998.4.19. [DOI] [PubMed] [Google Scholar]
- Qiu Q.Q, Ducheyne P, Ayyaswamy P.S. 3D Bone tissue engineered with bioactive microspheres in simulated microgravity. In Vitro Cell. Dev. Biol. Anim. 2001;37:157–165. doi: 10.1290/1071-2690(2001)037%3C0157:BTEWBM%3E2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reneker D.H, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7:216–223. doi: 10.1088/0957-4484/7/3/009. [DOI] [Google Scholar]
- Riddle K.W, Mooney D.J. Role of poly(lactide-co-glycolide) particle size on gas-foamed scaffolds. J. Biomater. Sci. Polym. Ed. 2004;15:1561–1570. doi: 10.1163/1568562042459742. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gonzalez F.J, Ramsay B.A, Favis B.D. Rheological and thermal properties of thermoplastic starch with high glycerol content. Carbohydr. Polym. 2004;58:139–147. doi: 10.1016/j.carbpol.2004.06.002. [DOI] [Google Scholar]
- Rosalam S, England R. Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006;39:197–207. doi: 10.1016/j.enzmictec.2005.10.019. [DOI] [Google Scholar]
- Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Rowley J.A, Madlambayan G, Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/S0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- Ruel-Gariépy E, Leclair G, Hildgen P, Gupta A, Leroux J.-C. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J. Control. Release. 2002;82:373–383. doi: 10.1016/S0168-3659(02)00146-3. [DOI] [PubMed] [Google Scholar]
- Ruel-Gariépy E, Shive M, Bichara A, Berrada M, Garrec D.L, Chenite A, Leroux J.-C. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 2004;57:53–63. doi: 10.1016/S0939-6411(03)00095-X. [DOI] [PubMed] [Google Scholar]
- RuizCardona L, Sanzgiri Y.D, Benedetti L.M, Stella V.J, Topp E.M. Application of benzyl hyaluronate membranes as potential wound dressings: evaluation of water vapour and gas permeabilities. Biomaterials. 1996;17:1639–1643. doi: 10.1016/0142-9612(95)00324-X. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher M.D. New perspectives in cell-adhesion—rgd and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Ruszczak Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003;55:1595–1611. doi: 10.1016/j.addr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Sachlos E, Reis N, Czernuszka J.T, Ainsley C, Derby B. Novel collagen scaffolds with predefined internal morphology made by solid freeform fabrication. Biomaterials. 2003;24:1487–1497. doi: 10.1016/S0142-9612(02)00528-8. [DOI] [PubMed] [Google Scholar]
- Sakai S, Kawabata K, Ono T, Ijima H, Kawakami K. Development of mammalian cell-enclosing subsieve-size agarose capsules (<100 mm) for cell therapy. Biomaterials. 2005;26:4786–4792. doi: 10.1016/j.biomaterials.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Sakai D, Mochida J, Iwashina T, Watanabe T, Suyama K, Ando K, Hotta T. Atelocollagen for culture of human nucleus pulposus cells forming nucleus pulposus-like tissue in vitro: influence on the proliferation and proteoglycan production of HNPSV-1 cells. Biomaterials. 2006;27:346–353. doi: 10.1016/j.biomaterials.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Salgado A.J, Gomes M.E, Chou A, Coutinho O.P, Reis R.L, Hutmacher D.W. Preliminary study on the adhesion and proliferation of human osteoblasts on starch-based scaffolds. Mater. Sci. Eng. C. 2002;20:27–33. doi: 10.1016/S0928-4931(02)00009-7. [DOI] [Google Scholar]
- Salgado A.J, Coutinho O.P, Reis R.L. Novel starch-based scaffolds for bone tissue engineering: cytotoxicity, cell culture, and protein expression. Tissue Eng. 2004a;10:465–474. doi: 10.1089/107632704323061825. [DOI] [PubMed] [Google Scholar]
- Salgado A.J, Reis R.L, Coutinho O.P. Novel starch-based scaffolds for bone tissue engineering: cytotoxicity, cell culture, and protein expression. Tissue Eng. 2004b;10:465–474. doi: 10.1089/107632704323061825. [DOI] [PubMed] [Google Scholar]
- Santin M, Motta A, Freddi G, Cannas M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res. 1999;46:382–389. doi: 10.1002/(SICI)1097-4636(19990905)46:3%3C382::AID-JBM11%3C3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Schaffellner S, Stadlbauer V, Stiegler P, Hauser O, Halwachs G, Lackner C, Iberer F, Tscheliessnigg K.H. Porcine islet cells microencapsulated in sodium cellulose sulfate. Transplant. Proc. 2005;37:248–252. doi: 10.1016/j.transproceed.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Schantz J.T, Ng M.M.L, Netto P, Ming J.C.L, Wong K.M, Hutmacher D.W, Teoh S.H. Application of an X-ray microscopy technique to evaluate tissue-engineered bone-scaffold constructs. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2002;20:9–17. [Google Scholar]
- Schantz J.T, Brandwood A, Hutmacher D.W, Khor H.L, Bittner K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J. Mater. Sci. Mater. Med. 2005;16:807–819. doi: 10.1007/s10856-005-3584-3. [DOI] [PubMed] [Google Scholar]
- Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005;74:782–788. doi: 10.1002/jbm.b.30291. [DOI] [PubMed] [Google Scholar]
- Senuma Y, Franceschin S, Hilborn J.G, Tissieres P, Bisson I, Frey P. Bioresorbable microspheres by spinning disk atomization as injectable cell carrier: from preparation to in vitro evaluation. Biomaterials. 2000;21:1135–1144. doi: 10.1016/S0142-9612(99)00276-8. [DOI] [PubMed] [Google Scholar]
- Seol Y.J, Lee J.Y, Park Y.J, Lee Y.M, Young K, Rhyu I.C, Lee S.J, Han S.B, Chung C.P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004;26:1037–1041. doi: 10.1023/B:BILE.0000032962.79531.fd. [DOI] [PubMed] [Google Scholar]
- Shumilina E.V, Shchipunov Y.A. Chitosan–carrageenan gels. Colloid J. 2002;64:372–378. doi: 10.1023/A:1015985229667. [DOI] [Google Scholar]
- Silva G.A, Vaz C.M, Coutinho O.P, Cunha A.M, Reis R.L. In vitro degradation and cytocompatibility evaluation of novel soy and sodium caseinate-based membrane biomaterials. J. Mater. Sci. Mater. Med. 2003;14:1055–1066. doi: 10.1023/B:JMSM.0000004002.11278.30. [DOI] [PubMed] [Google Scholar]
- Silva G.A, Czeisler C, Niece K.L, Beniash E, Harrington D.A, Kessler J.A, Stupp S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- Silva G.A, Costa F.J, Neves N.M, Coutinho O.P, Dias A.C.P, Reis R.L. Entrapment ability and release profile of corticosteroids from starch-based particles. J. Biomed. Mater. Res. A. 2005a;73:234–243. doi: 10.1002/jbm.a.30287. [DOI] [PubMed] [Google Scholar]
- Silva S.S, Santos M.I, Coutinho O.P, Mano J.F, Reis R.L. Physical properties and biocompatibility of chitosan/soy blended membranes. J. Mater. Sci. Mater. Med. 2005b;16:575–579. doi: 10.1007/s10856-005-0534-z. [DOI] [PubMed] [Google Scholar]
- Silva, G. A., Coutinho, O. P., Ducheyne, P., Shapiro, I. M. & Reis, R. L. Submitted. Starch-based microparticles as vehicles for the delivery of active platelet-derived growth factor. [DOI] [PubMed]
- Simionescu D.T, Lu Q, Song Y, Lee J.S, Rosenbalm T.N, Kelley C, Vyavahare N.R. Biocompatibility and remodeling potential of pure arterial elastin and collagen scaffolds. Biomaterials. 2006;27:702–713. doi: 10.1016/j.biomaterials.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Solchaga L.A, Yoo J.U, Lundberg M, Dennis J.E, Huibregtse B.A, Goldberg V.M, Caplan A.I. Hyaluronan-based polymers in the treatment of osteochondral defects. J. Orthop. Res. 2000;18:773–780. doi: 10.1002/jor.1100180515. [DOI] [PubMed] [Google Scholar]
- Solchaga L.A, Goldberg V.M, Caplan A.I. Cartilage regeneration using principles of tissue engineering. Clin. Orthop. Relat. Res. 2001:S161–S170. doi: 10.1097/00003086-200110001-00016. [DOI] [PubMed] [Google Scholar]
- Sripriya R, Kumar M.S, Sehgal P.K. Improved collagen bilayer dressing for the controlled release of drugs. J. Biomed. Mater. Res. Part B Appl. Biomater. B. 2004;70:389–396. doi: 10.1002/jbm.b.30051. [DOI] [PubMed] [Google Scholar]
- Stephen A.M, Churms S.C, Vogt D.C. Exudate gums. In: Dey P.M, editor. Methods in plant biochemistry. Carbohydrates. vol. 2. Academic Press; London, UK: 1990. pp. 483–522. [Google Scholar]
- Stevens M.M, Qanadilo H.F, Langer R, Shastri V.P. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25:887–894. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Sun W, Lal P. Recent development on computer aided tissue engineering—a review. Comput. Methods Programs Biomed. 2002;67:85–103. doi: 10.1016/S0169-2607(01)00116-X. [DOI] [PubMed] [Google Scholar]
- Sun W, Darling A, Starly B, Nam J. Computer-aided tissue engineering: overview, scope and challenges. Biotechnol. Appl. Biochem. 2004a;39:29–47. doi: 10.1042/BA20030108. [DOI] [PubMed] [Google Scholar]
- Sun W, Starly B, Darling A, Gomez C. Computer-aided tissue engineering: application to biomimetic modelling and design of tissue scaffolds. Biotechnol. Appl. Biochem. 2004b;39:49–58. doi: 10.1042/BA20030109. [DOI] [PubMed] [Google Scholar]
- Svensson A, Nicklasson E, Harrah T, Panilaitis B, Kaplan D.L, Brittberg M, Gatenholm P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials. 2005;26:419–431. doi: 10.1016/j.biomaterials.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Tabata Y, Miyao M, Inamoto T, Ishii T, Hirano Y, Yamaoki Y, Ikada Y. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng. 2000;6:279–289. doi: 10.1089/10763270050044452. [DOI] [PubMed] [Google Scholar]
- Tae Gwan P. Perfusion culture of hepatocytes within galactose-derivatized biodegradable poly(lactide-co-glycolide) scaffolds prepared by gas foaming of effervescent salts. J. Biomed. Mater. Res. 2002;59:127–135. doi: 10.1002/jbm.1224. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Xu L, Kobayashi H, Taniguchi A, Kataoka K, Tanaka J. Encapsulation of chondrocytes in injectable alkali-treated collagen gels prepared using poly(ethylene glycol)-based 4-armed star polymer. Biomaterials. 2005;26:1247–1252. doi: 10.1016/j.biomaterials.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Tan W, Desai T.A. Microfluidic patterning of cells in extracellular matrix biopolymers: effects of channel size, cell type, and matrix composition on pattern integrity. Tissue Eng. 2003;9:255–267. doi: 10.1089/107632703764664729. [DOI] [PubMed] [Google Scholar]
- Tan W, Desai T.A. Layer-by-layer microfluidics for biomimetic three-dimensional structures. Biomaterials. 2004;25:1355–1364. doi: 10.1016/j.biomaterials.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Tan W, Desai T.A. Microscale multilayer cocultures for biomimetic blood vessels. J. Biomed. Mater. Res. A. 2005;72:146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Nagate T, Matsuda H. Acceleration of wound healing by gelatin film dressings with epidermal growth factor. J. Vet. Med. Sci. 2005;67:909–913. doi: 10.1292/jvms.67.909. [DOI] [PubMed] [Google Scholar]
- Tanodekaew S, Prasitsilp M, Swasdison S, Thavornyutikarn B, Pothsree T, Pateepasen R. Preparation of acrylic grafted chitin for wound dressing application. Biomaterials. 2004;25:1453–1460. doi: 10.1016/j.biomaterials.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Thanh T.T.T, Yuguchi Y, Mimura M, Yasunaga H, Takano R, Urakawa H, Kajiwara K. Molecular characteristics and gelling properties of the carrageenan family, 1—preparation of novel carrageenans and their dilute solution properties. Macromol. Chem. Phys. 2002;203:15–23. doi: 10.1002/1521-3935(20020101)203:1%3C15::AID-MACP15%3E3.0.CO;2-1. [DOI] [Google Scholar]
- Tinsley-Bown A.M, Fretwell R, Dowsett A.B, Davis S.L, Farrar G.H. Formulation of poly(d,l-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. J. Control. Release. 2000;66:229–241. doi: 10.1016/S0168-3659(99)00275-8. [DOI] [PubMed] [Google Scholar]
- Tranquillo R.T, Girton T.S, Bromberek B.A, Triebes T.G, Mooradian D.L. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials. 1996;17:349–357. doi: 10.1016/0142-9612(96)85573-6. [DOI] [PubMed] [Google Scholar]
- Tuan H.S, Hutmacher D.W. Application of micro CT and computation modeling in bone tissue engineering. CAD Comput. Aided Des. 2005;37:1151–1161. [Google Scholar]
- Tuncel A, Ecevit K, Kesenci K, Piskin E. Nonswellable and swellable ethylene glycol dimethacrylate-acrylic acid copolymer microspheres. J. Polym. Sci. Part A Polym. Chem. 1996;34:45–55. doi: 10.1002/(SICI)1099-0518(19960115)34:1%3C45::AID-POLA4%3E3.0.CO;2-2. [DOI] [Google Scholar]
- Tuzlakoglu K, Alves C.M, Mano J.F, Reis R.L. Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications. Macromol. Biosci. 2004;4:811–819. doi: 10.1002/mabi.200300100. [DOI] [PubMed] [Google Scholar]
- Tuzlakoglu K, Bolgen N, Salgado A.J, Gomes M.E, Piskin E, Reis R.L. Nano- and micro-fiber combined scaffolds: a new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005;16:1099–1104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- Uludag H, Vos P.D, Tresco P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000;42:29–64. doi: 10.1016/S0169-409X(00)00053-3. [DOI] [PubMed] [Google Scholar]
- vandeWitte P, Dijkstra P.J, vandenBerg J.W.A, Feijen J. Phase separation processes in polymer solutions in relation to membrane formation. J. Memb. Sci. 1996;117:1–31. doi: 10.1016/0376-7388(96)00088-9. [DOI] [Google Scholar]
- Vaz C.M, Fossen M, van Tuil R.F, de Graaf L.A, Reis R.L, Cunha A.M. Casein and soybean protein-based thermoplastics and composites as alternative biodegradable polymers for biomedical applications. J. Biomed. Mater. Res. A. 2003;65:60–70. doi: 10.1002/jbm.a.10416. [DOI] [PubMed] [Google Scholar]
- Voet D, Voet J.G, Pratt C.W. Wiley; New York, NY: 1999. Fundamentals of biochemistry. [Google Scholar]
- Wallace D.G, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003;55:1631–1649. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Wang L.H, Khor E, Wee A, Lim L.Y. Chitosan–alginate PEC membrane as a wound dressing: assessment of incisional wound healing. J. Biomed. Mater. Res. 2002a;63:610–618. doi: 10.1002/jbm.10382. [DOI] [PubMed] [Google Scholar]
- Wang X, Tian J, Li X, Zhang Y. Hydroxyapatite artificial bone prepared via a three-dimensional gel-lamination route. Key Eng. Mater. 2002b;224–226:437–440. [Google Scholar]
- Wang Y, Kim U.-J, Blasioli D.J, Kim H.-J, Kaplan D.L. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–7094. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Weber W, Rinderknecht M, Baba M.D.-E, de Glutz F.-N, Aubel D, Fussenegger M. CellMAC: a novel technology for encapsulation of mammalian cells in cellulose sulfate/pDADMAC capsules assembled on a transient alginate/Ca2+ scaffold. J. Biotechnol. 2004;114:315–326. doi: 10.1016/j.jbiotec.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Weinberg C.B, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Weiss L.E, Amon C.H, Finger S, Miller E.D, Romero D, Verdinelli I, Walker L.M, Campbell P.G. Bayesian computer-aided experimental design of heterogeneous scaffolds for tissue engineering. CAD Comput. Aided Des. 2005;37:1127–1139. [Google Scholar]
- Wettergreen M.A, Bucklen B.S, Starly B, Yuksel E, Sun W, Liebschner M.A.K. Creation of a unit block library of architectures for use in assembled scaffold engineering. Comput. Aided Des. 2005;37:1141–1149. doi: 10.1016/j.cad.2005.02.005. [DOI] [Google Scholar]
- Widner B, et al. Hyaluronic acid production in Bacillus subtilis. Appl. Environ. Microbiol. 2005;71:3747–3752. doi: 10.1128/AEM.71.7.3747-3752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.F, Martin D.P, Horowitz D.M, Peoples O.P. PHA applications: addressing the price performance issue I. Tissue engineering. Int. J. Biol. Macromol. 1999;25:111–121. doi: 10.1016/S0141-8130(99)00022-7. [DOI] [PubMed] [Google Scholar]
- Williams G.M, Klein T.J, Sah R.L. Cell density alters matrix accumulation in two distinct fractions and the mechanical integrity of alginate–chondrocyte constructs. Acta Biomaterialia. 2005;1:625–633. doi: 10.1016/j.actbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Wongpanit P, Sanchavanakit N, Pavasant P, Supaphol P, Tokura S, Rujiravani R. Preparation and characterization of microwave-treated carboxymethyl chitin and carboxymethyl chitosan films for potential use in wound care application. Macromol. Biosci. 2005;5:1001–1012. doi: 10.1002/mabi.200500081. [DOI] [PubMed] [Google Scholar]
- Wu Z.G, Sheng Z.Y, Sun T.Z, Geng M, Li J.Y, Yao Y.M, Huang Z.X. Preparation of collagen-based materials for wound dressing. Chin. Med. J. 2003;116:419–423. [PubMed] [Google Scholar]
- Wu Y.B, Yu S.H, Mi F.L, Wu C.W, Shyu S.S, Peng C.K, Chao A.C. Preparation and characterization on mechanical and antibacterial properties of chitosan/cellulose blends. Carbohydr. Polym. 2004;57:435–440. doi: 10.1016/j.carbpol.2004.05.013. [DOI] [Google Scholar]
- Xia W, Liu W, Cui L, Liu Y, Zhong W, Liu D, Wu J, Chua K, Cao Y. Tissue engineering of cartilage with the use of chitosan–gelatin complex scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004;71:373–380. doi: 10.1002/jbm.b.30087. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Qian H, Young W.G, Bartold P.M. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue Eng. 2003;9:1167–1177. doi: 10.1089/10763270360728071. [DOI] [PubMed] [Google Scholar]
- Xie S.X, Liu Q, Cui S.W. Starch modification and applications. In: Cui S.W, editor. Food carbohydrates: chemistry, physical properties, and applications. CRC Press; Taylor & Francis Group; Boca Raton, FL: 2005. pp. 357–405. [Google Scholar]
- Xu C.Y, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanotibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/S0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- Yan Y, et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005a;26:5864–5871. doi: 10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Yan Y, Wang X, Xiong Z, Liu H, Liu F, Lin F, Wu R, Zhang R, Lu Q. Direct construction of a three-dimensional structure with cells and hydrogel. J. Bioactive Compat. Polym. 2005b;20:259–269. doi: 10.1177/0883911505053658. [DOI] [Google Scholar]
- Yang F, Murugan R, Ramakrishna S, Wang X, Ma Y.X, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Yeo Y, Park K. A new microencapsulation method using an ultrasonic atomizer based on interfacial solvent exchange. J. Control. Release. 2004;100:379–388. doi: 10.1016/j.jconrel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Yeo J.-H, Lee K.-G, Lee Y.-W, Kim S.Y. Simple preparation and characteristics of silk fibroin microsphere. Eur. Polym. J. 2003;39:1195–1199. doi: 10.1016/S0014-3057(02)00359-2. [DOI] [Google Scholar]
- Yi H.M, Wu L.Q, Bentley W.E, Ghodssi R, Rubloff G.W, Culver J.N, Payne G.F. Biofabrication with chitosan. Biomacromolecules. 2005;6:2881–2894. doi: 10.1021/bm050410l. [DOI] [PubMed] [Google Scholar]
- Yoon J.J, Kim J.H, Park T.G. Dexamethasone-releasing biodegradable polymer scaffolds fabricated by a gas-foaming/salt-leaching method. Biomaterials. 2003;24:2323–2329. doi: 10.1016/S0142-9612(03)00024-3. [DOI] [PubMed] [Google Scholar]
- Young J.H, Parenteau N.L. Bilayered skin constructs. In: Atala A, Lanza R, editors. Methods of tissue engineering. Academic Press; London, UK: 2002. [Google Scholar]
- Yu J.H, Fridrikh S.V, Rutledge G.C. Production of submicrometer diameter fibers by two-fluid electrospinning. Adv. Mater. 2004;16:1562–1566. doi: 10.1002/adma.200306644. [DOI] [Google Scholar]
- Yusof N.L.B.M, Wee A, Lim L.Y, Khor E. Flexible chitin films as potential wound-dressing materials: wound model studies. J. Biomed. Mater. Res. Part A. 2003;66:224–232. doi: 10.1002/jbm.a.10545. [DOI] [PubMed] [Google Scholar]
- Yusof N.L.B.M, Lee L.Y, Khor E. Flexible chitin films: structural studies. Carbohydr. Res. 2004;339:2701–2711. doi: 10.1016/j.carres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Zein I, Hutmacher D.W, Tan K.C, Teoh S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23:1169–1185. doi: 10.1016/S0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- Zeman L, Fraser T. Formation of air-cast cellulose-acetate membranes. 1. Study of macrovoid formation. J. Memb. Sci. 1993;84:93–106. doi: 10.1016/0376-7388(93)85053-Y. [DOI] [Google Scholar]
- Zhang J.C, Wu L.B, Jing D.Y, Ding J.D. A comparative study of porous scaffolds with cubic and spherical macropores. Polymer. 2005;46:4979–4985. [Google Scholar]
- Zhao F, Yin Y, Lu W.W, Leong J.C, Zhang W, Zhang J, Zhang M, Yao K. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan–gelatin network composite scaffolds. Biomaterials. 2002;23:3227–3234. doi: 10.1016/S0142-9612(02)00162-X. [DOI] [PubMed] [Google Scholar]
- Zielinski B.A, Aebischer P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 1994;15:1049–1056. doi: 10.1016/0142-9612(94)90090-6. [DOI] [PubMed] [Google Scholar]