Abstract
In the year 1994, the Serengeti lion population was decimated by a canine distemper disease outbreak. Retrospective investigations showed that this host population had already been in contact with the pathogen in 1981 without any detected sign of disease. As an alternative to the virus mutation hypothesis to explain this difference in virulences observed in 1981 and 1994, we propose a novel mechanism of disease emergence based on variation in population immunity. We use a stochastic model to show that stochastic fluctuations in pathogen circulation, owing to a low probability of virus transmission from its reservoir to the target host and thereby resulting in variations in the global immunity level of the target host population, can explain the observations made in Serengeti. This mechanism may also be involved in other infectious disease emergences or re-emergences.
Keywords: canine distemper virus, lion Panthera leo, stochastic model, population immunity level, infectious disease re-emergence
1. Introduction
The impact of an infectious agent on a host population, i.e. the number of disease cases and their consequences, depends on the individual host–parasite interaction and the host population structure, which can change both in space and time. These changes can result in an apparent increase in the aggressiveness of a pathogen for its host, and thus in the emergence or re-emergence of the infectious disease caused by the pathogen. When such an emergence happens, it is almost always explained by a first infestation of the host population by the pathogen, for example, owing to the recent appearance of the latter or following an increase in host–pathogen contacts. A mutation in a more pathogenic strain of the parasite is generally assumed when previous infections have been shown.
The appearance of new pathogenic strains has, for instance, been invoked to explain how different mortality rates in hares (Lepus europaeus) are induced by the European brown hare syndrome virus (Scicluna et al. 1994). This scenario seems plausible because significant variations in virulence can exist among different strains of a given pathogen, as observed among myxoma virus strains that infect rabbits (Oryctolagus cuniculus; Fenner 1953; Fenner & Fantini 1999). We propose in this study that such a hypothesis, which is almost consistently invoked but rarely tested, is not necessarily the only explanation for differences in pathogen impact, using the example of the canine distemper in Serengeti lion (Panthera leo).
In early 1994, lions from the Serengeti Reserve in Tanzania were victims of a violent outbreak of canine distemper disease. One-third of the population, i.e. approximately 1000 animals, died following this epizootic (Roelke-Parker et al. 1996). The aetiological agent, canine distemper virus (CDV), has been known as a domestic dog (Canis familiaris) pathogen for numerous decades. Although canine distemper cases and epizootics have been observed in a wide range of terrestrial and marine mammals during the last few decades (Harder & Osterhaus 1997; Swinton et al. 1998; Leisewitz et al. 2001), the lion was not suspected of being sensitive to the virus (Harder et al. 1995), making the Serengeti epidemic very surprising. The initial explanation for this 1994 lethal outbreak was that it was the first time that the lion was infected by CDV which, as a new pathogen for this species, did not encounter host resistance (Osterhaus 2001). The increase in the numbers of dogs in the surrounding area, which were identified as the reservoir for the virus, could have triggered interspecies virus transmission in 1994 (Cleaveland et al. 2000).
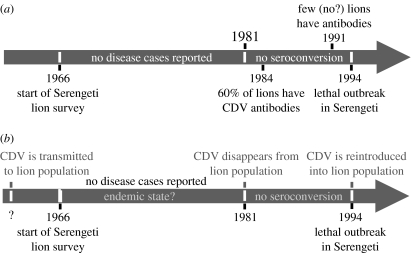
However, further investigations proved that this was not the first contact between CDV and lions. Several cases of fatal diseases in big cats, reported before 1992 in circuses and zoos in Switzerland and North America, have since been identified as canine distemper (Appel et al. 1994; Myers et al. 1997). Moreover, serological evidences showed retrospectively that the Serengeti lion population had already been infected by the pathogen in the early 1980s, without the detection of the disease. Since lions born after 1981 did not have CDV antibodies before the 1994 epidemic, it is assumed that the pathogen was absent from the Serengeti population between 1981 and 1994 (Packer et al. 1999; figure 1a).
Figure 1.
Data about (a) CDV/Serengeti lion interaction available in 2003 and (b) hypothesis invoked to explain these data.
These considerations lead us to a crucial question: why did the virus seem to be non-pathogenic in the early 1980s, while causing huge mortality 10 years later?
Obviously, the interaction between the pathogen and its host changed.
The most common explanation for this type of observation is to infer a change in the host–parasite interaction at the individual level. More explicitly, it is typical to suggest that the mutation of the virus from a non-pathogenic strain to a highly virulent one induces greater mortality in the host population (Carpenter et al. 1998; Packer et al. 1999). Isolated CDV strains that give rise to severe diseases are locally very similar in all the species they infect, but highly different between animals from different countries, even from the same species (Harder & Osterhaus 1997; Carpenter et al. 1998). Assuming mutation as the explanation for the first appearance of canine distemper in a given area implies that several pathogenic strains suddenly appeared in different ecological conditions in distant countries. Moreover, these changes should have occurred in the reservoir species, without leading to any apparent change in the interaction between CDV and those species. A more parsimonious hypothesis, suggesting that higher CDV virulence in 1994 was due to aggravating copathogens, has also been implicated (Harder & Osterhaus 1997), but none of the tested associations with another pathogen has yet been conclusively shown (Roelke-Parker et al. 1996; Packer et al. 1999).
As an alternative, we propose that the CDV-related mortality in 1994 was not induced by a change in CDV virulence, but by a change in the immune state of the lion population. It was observed that the proportion of Serengeti lions that had already been in contact with CDV was very high in 1981 and very low in 1994 (Packer et al. 1999). Assuming that such contact is followed by several years of long-lasting protection of the host against reinfection, as observed in other mammal/CDV systems (Blixenkrone-Moller 1993), the lion population could have been globally less sensitive to the virus in the early 1980s than in 1994, owing to this high level of immunity. Indeed, this population immune state can notably reduce the risk of an epidemic, since the encounter rate between infectious individuals and susceptible ones is very low (Anderson & May 1985). Furthermore, since few individuals are susceptible to the virus in the population, this would result in few cases of disease, possibly few enough to remain undetected. We know that the lion population was highly immune in the early 1980s and lost its immunity between 1981 and 1994. We have to now explain how the CDV could appear non-pathogenic for lions in the early 1980s, but provoked an epidemic in 1994, based only on our population immunity hypothesis: (i) since immunity is attributable to pathogen persistence in the host population, the loss of the high immunity level implies the pathogen's absence followed by the death of immune individuals and (ii) the initial introduction of the pathogen into the host population, before 1980, should have provoked an epidemic prior to pathogen persistence, then the endemic should have pre-dated the host population survey and remained undocumented. In our system, the lion population survey began in 1966 (Packer 1990), meaning that the endemic period would have lasted at least 15 years. This scenario is summarized in figure 1b.
Our aim was to test whether this scenario, which has until now been overlooked, is plausible using a modelling approach. In particular, we wanted to answer the following two fundamental questions.
Could the pathogen circulate over a very long time (several decades), remain absent for more than one decade and provoke an epidemic without any subsequent endemic period in the same host population?
Could the presence of the virus in a widely immune host population remain undetected?
Our hypothesis relies on two unpredictable events: (i) the pathogen appearance in the lion population through interspecies transmission and (ii) its disappearance from the lion population through the death or recovery of the last infectious individual. These two events result from stochasticity in pathogen circulation.
We built a stochastic mathematical model based on the biological data related to the Serengeti lion/CDV system and the available information about the 1994 epidemic to test if a low interspecies transmission probability, resulting in variations in population immunity, is sufficient to explain the events observed in the Serengeti lion population before 1993.
2. Material and methods
2.1 Canine distemper virus and pathology
The CDV, which causes canine distemper disease, belongs to the genus Morbillivirus from the family Paramyxoviridae. This RNA virus with an external envelope, making it highly vulnerable outside its host, is extremely contagious. It is transmitted mainly through aerosols (Appel & Summers 1995).
Little information is available about the disease in lions, except that the clinical signs are quite similar to those observed in dogs, facilitating its diagnosis (Packer 1996). Infection in dogs is followed by a few days of incubation period (from 3 to 7 days), after which clinical signs appear including listlessness, anorexia, vomiting and/or diarrhoea, purulent discharges from the mucous membranes, pneumonia and encephalitis. The infected animal then starts to excrete the virus. Infection ends after 2–4 weeks with either death or recovery of the host, implying several years of long-lasting protection against the disease (Blixenkrone-Moller 1993).
2.2 Mathematical model
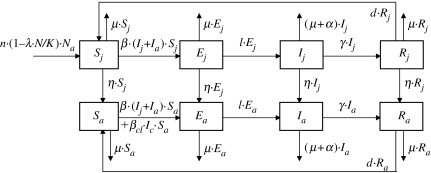
Our basic model divided individuals from the host population into different serological and demographical classes. In accordance with epidemiological knowledge about the lion/CDV system, which is assumed to be similar to the dog/CDV system, we considered four different epidemiological states: susceptible (S), latent infected (E), infectious (I) and recovered with immunity against reinfection (R) (Blixenkrone-Moller 1993; Appel & Summers 1995; Leisewitz et al. 2001). Since the age of sexual maturity in lions (3–4 years old) is not negligible compared with its life expectancy (approx. 15 years in natural conditions; Packer & Pusey 1993; Packer et al. 1998), we considered two age classes: young individuals (j) and breeders (a). The four epidemiological classes combined with the two age classes generated eight subdivisions in the population, as represented in figure 2. The times spent in each of these eight states, in particular, the latency period, the infectious period and also the two age stages, are exponentially distributed.
Figure 2.
Model with four serological (S, E, I, R) and two age (j, a) classes. Na=Sa+Ea+Ia+Ra and N=S+E+I+R.
Even though lions live in prides, we considered the population to be homogeneous for the sake of simplicity. This approximation relied on strong viral contagiousness and frequent contact between prides and nomadic male lions (Grinnell et al. 1995). Moreover, such contacts increase with lion density and the virus is transmitted via aerosols (Appel & Summers 1995; Harder & Osterhaus 1997); thus, the more numerous the infectious animals the higher the contamination risk. These considerations led us to assume transmission to be density dependent. Thus, we chose a mass-action transmission β·S·I with a constant value β.
Since the course of the disease lasts some weeks, time was measured in weeks. Life expectancy (μ−1) and the age of sexual maturation (η−1) in lions are 780 and 156–208 weeks, respectively. Population size K at disease-free equilibrium is approximately 3000 individuals. Some parameters were not available from the literature and were estimated through deterministic studies. Demographic parameters (birth rate n and adjustment coefficient λ, where K/λ is the carrying capacity and for which the population size reaches K at disease-free equilibrium) were estimated from the lion population demographic recovery after the 1994 epidemic, since in 1997, the population reached a size almost equivalent to that in 1993 before the epidemic (Packer et al. 1999). Epidemiological parameters (intraspecies transmission rate β, rate l at which lions quit the latent state, disease-induced mortality rate α and recovery rate γ) were chosen to model the 1994 epidemic pattern. The rate of immunity loss d represented the reduction of seroprevalence from 60% in 1984 to almost 0% in 1991, as observed by Packer et al. (1999) in the Serengeti lion population. Since the virus was not detected in the lion population for over a decade (Packer et al. 1999), we considered interspecies virus transmission as relatively rare. We assumed that limited variation in the number of infectious dogs (Ic) had little effect on interspecies transmission. Thus, for the sake of simplicity, we assumed that Ic was constant and positive. Therefore, βcl·Ic represents the low probability of interspecies transmission, with a mean frequency of successful virus transmission from dog to lion of less than one event per year.
The values of all parameters obtained were biologically realistic. Parameter names, meanings and values are summarized in table 1.
Table 1.
Parameter values. (Time is measured in weeks and ‘ind’ refers to ‘individuals’.)
| name | meaning | value |
|---|---|---|
| n | birth rate | 0.02 (week−1) |
| λ | adjustment coefficient | 0.9188 |
| K | maximal population | 3000 (ind) |
| μ | natural mortality rate | 0.0013 (week−1) |
| α | mortality due to disease rate | 0.1058 (week−1) |
| η | sexual maturation rate | 0.0048 (week−1) |
| β | intra species transmission rate | ∈[2.6×10−4, 8×10−4] (week−1 ind−1) |
| βcl | inter species transmission rate | ∈[10−9, 10−8] (week−1 ind−1) |
| Ic | number of infectious dogs | 260 (ind) |
| l | rate at which lions quit the latent state | 0.5 (week−1) |
| γ | recovery rate | 0.1799 (week−1) |
| d | immunity loss rate | 0.0011 (week−1) |
The deterministic system was controlled by nonlinear differential equations (see appendix 1 in the electronic supplementary material). A continuous-time Markov chain generated the stochastic version of the model, for which the events and corresponding rates are reported in table 2.
Table 2.
Transition rates of the continuous-time Markov chain, where N=Sj+Ej+Ij+Rj+Sa+Ea+Ia+Ra, Na=Sa+Ea+Ia+Ra and Ic is the number of infectious dogs from Serengeti District.
| event | transition | transition rate |
|---|---|---|
| birth | Sj→Sj+1 | n(1−λ(N/K))Na |
| death of Hh, where H∈{S,E,R}, h∈{j,a} | Hh→Hh−1 | μ·Hh |
| death of Ih, where h∈{j,a} | Ih→Ih−1 | (μ+α)Ih |
| maturation of Hj, where H∈{S,E,I,R} | Hj→Hj−1, Ha→Ha+1 | η·Hj |
| infection of young | Sj→Sj−1, Ej→Ej+1 | β(Ij+Ia)Sj |
| infection of adult | Sa→Sa−1, Ea→Ea+1 | [β(Ij+Ia)+βcl·Ic]Sa |
| end of latence state of Eh, where h∈{j,a} | Eh→Eh−1, Ih→Ih+1 | l·Eh |
| recovery of Ih, where h∈{j,a} | Ih→Ih−1, Rh→Rh+1 | γ·Ih |
| end of immunity of Rh, where h∈{j,a} | Rh→Rh−1, Sh→Sh+1 | d·Rh |
3. Results
3.1 Endemicity and periods of pathogen absence
We endeavoured to explain the emergence of canine distemper in the Serengeti and thus the differences in CDV impact in relation to variations in lions' immunity level, which arises from a succession of endemic and long virus absence periods in a unique host population. It is known that after an epidemic, which might follow the (re)introduction of a pathogen from its reservoir, either pathogen disappearance or endemic states can be obtained, and that endemic states are themselves subject to stochastic fadeout (Swinton 1998; Andersson & Britton 2000). However, the length of the endemic periods can be highly related to the population characteristics, whereas the length of the pathogen absence periods depends not only on the probability of the pathogen reintroduction, but also on the immune state of the host population at this time. The first question we explored was then whether the two patterns of virus circulation can be obtained using our biological data, derived from the knowledge of the Serengeti lions after 1994. In particular, we asked if the endemic period could have been long enough to pre-date the beginning of the lion survey in 1966.
We assumed a constant rate of interspecies transmission between the infectious dogs and the lion population, for which all individuals but one infected (I) were initially considered as susceptible (S). Simulations were carried out for different values of intra- and interspecies transmission rates, since these are crucial parameters for pathogen circulation. For each pair of values {β, βcl}, we generated 150 replicates of a 50-year-long simulation. Replicates represent different patterns of pathogen circulation (see appendix 2 in the electronic supplementary material).
Unsurprisingly, an increase in transmission rates β or βcl induces an increase in the frequency of long endemic periods and a decrease in the frequency of long pathogen absence periods. However, most replicates included at least 15-year-long endemic or pathogen absence periods. These 15-year periods allow, on the one hand, the endemic to pre-date the lion survey and, on the other hand, a virus absence long enough to lead to a significant decrease in immunity level. We report this result in table 3.
Table 3.
For each couple of parameters, percentage of replicates (out of 150) which generate at least a 15-year-long endemic period (E), pathogen absence period (A) or both (B), and 95% confidence intervals.
| βcl=10−9 | βcl=5×10−9 | βcl=10−8 | |
|---|---|---|---|
| β=5×10−4 | E: 26% [19,33] | E: 33% [25,41] | E: 54% [46,62] |
| A: 86% [80,92] | A: 54% [46,62] | A: 15% [9,21] | |
| B: 14% [8,20] | B: 8% [4,12] | B: 3% [0,6] | |
| β=8×10−4 | E: 76% [69,83] | E: 93% [89,97] | E: 97% [94,100] |
| A: 39% [31,47] | A: 15% [9,21] | A: 5% [2,8] | |
| B: 15% [9,21] | B: 9% [4,14] | B: 2% [0,4] |
This result shows that we can observe both endemic and long pathogen absence periods in the same host population. Here, we simulated 50-year-long replicates, since we supposed that the initial CDV introduction into the lion population occurred after the 1940s, but it might in fact have occurred much earlier. It is also worth noting that the longer the time frame, the greater the probability of observing sequences of endemic and long pathogen absence and thus variation in population immunity.
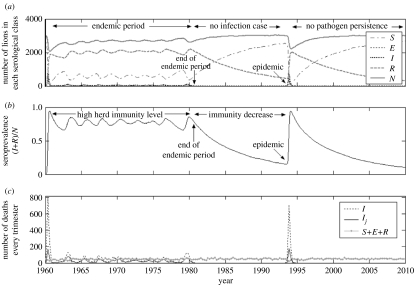
Such variation in immunity level can explain how the pathogen presence can either cause huge mortality or no apparent disease cases. To illustrate this, we present in figure 3 a typical replicate, which accurately fits the lion/pathogen biological data, in terms of the size of each serological class (S, E, I and R) and of the global population (N=S+E+I+R) (figure 3a), and the proportion ((I+R)/N) of seroprevalent animals, which present CDV antibodies (figure 3b), both in relation to time. Replicates also generated a date for an initial epidemic, as a consequence of contact between the practically entirely susceptible host population and an infected lion, introduced at the beginning of the replicate or infected later through interspecies transmission, resulting in rapid intraspecies disease transmission. This epidemic is dated to 1960 in figure 3.
Figure 3.
Example of pathogen circulation pattern. (a) Number of individuals S (dash-dot line), E (dotted line), I (dotted bold line), R (dashed line) and total population N (=S+E+I+R; solid line) with time. (b) Seroprevalence with time. (c) Number of deaths in infectious state (young and adults I, dotted line; only young Ij, solid line) and in non-infectious state (S+E+R, solid grey line) every trimester. For this simulation, βcl=10−9 and β=5×10−4.
Following this initial epidemic, we postulate a long endemic period from 1960 to 1981, during which the number of infected individuals (E+I) was rather low, the number of individuals in the global population (N) was relatively large and the proportion of immunized individuals (R) was high (approx. 0.75). Subsequently, the pathogen disappeared and remained absent from the lion population between 1981 and 1994. We also observe an increase in the global number of individuals in the population and the expected decrease in the seroprevalence from high (80% in 1980) to low (less than 15% in 1994) values, consistent with the data observed in the biological system between 1981 and 1994 (Packer et al. 1999).
In accordance with the biological data from 1994, we observe a fatal epidemic in 1994, with almost 1000 deaths and a very high proportion of seropositive individuals (more than 85%) at the end of the epidemic, which, as expected, is consistent with biological observations made about the real 1994 epidemic (Roelke-Parker et al. 1996). The pathogen did not persist after the epidemic, and the population recovered very quickly, as reported by Packer et al. (1999).
3.2 Could endemicity remain undetected?
The second pertinent question was to determine if undetectable endemicity is plausible. In order to estimate what proportion of the Serengeti lion deaths could be attributed to CDV, we also report in figure 3c the number of lions dead in an infectious state I, the number of young dead in an infectious state Ij and the number of lions dead in non-infectious states S, E or R every trimester, for the same replicate as described above.
Since the lion population survey began in 1966, we are interested in what happened after this date in our simulation. We show in figure 3c that the 1994 epidemic implied a very large number of dead animals in the infectious state I (several hundreds in the same trimester). During the endemic period (1966–1981), the number of actual animals that were infectious when they died was in fact low (between 2 and 130 dead animals in an infectious state every trimester, average 50). Moreover, we noted that a large proportion of these dead lions were young animals, and that deaths in an infectious state represented approximately 50% of total deaths during the endemic period.
4. Discussion
Our scenario for the emergence of canine distemper in lions in the Serengeti, i.e. host population protection due to pathogen circulation until 1981, then latency over a long period without any infection, resulting in the 1994 lethal outbreak, is of course not necessarily the actual course of events that took place. Since the hypothesis of increased virulence due to genetic changes in the infectious agent is difficult to test, we can hardly conclude to neither its relevance nor its irrelevance. Moreover, the coinfection hypothesis is currently being reassessed with a newly considered copathogen (C. Packer 2007, personal communication). Further information should permit discrimination of the most pertinent hypothesis. In particular, the forthcoming serological survey of the Serengeti lion population should be very informative in terms of verifying our scenario. Non-pathogenic exposure of lions to CDV has been observed in 1996 (C. Packer 2007, personal communication) and 2000 (Cleaveland et al. 2007). However, knowledge on the population immunity levels at these times, the propagation of these infections and even the dates of exposure do not seem precise enough to confirm or reject our hypothesis.
Actually, we have shown that our hypothesis is realistic and that the mechanism of variation in population immunity level due to endemic and pathogen absence periods is highly plausible. As a consequence of population immunity, the presence of the pathogen could either give rise to only a few cases of disease or huge fatal outbreaks, without requiring any other change in environmental conditions or virus characteristics. This preliminary result could only be obtained through stochastic modelling, since unpredictable biological events, such as appearance and disappearance of a pathogen in its host population, are due to stochastic fluctuations, which cannot be incorporated into deterministic models.
We also verified that the number of deaths due to the disease was clearly lower during an endemic state than during an epidemic outbreak like the one observed in lions in 1994, and most of these deaths concerned young animals. We investigated whether deaths due to canine distemper during the endemic period could remain undetected over decades as we hypothesized. It is possible that very few carcasses of diseased animals were found outside epidemic outbreaks. Roelke-Parker et al. (1996) reported that 11 carcasses found between January and March 1994 indicated ‘a dramatic increase in mortality from previous years and that a serious epidemic was emerging’. Moreover, during the whole epidemic, only 39 deaths were actually documented. We suggest that during endemics or periods of pathogen absence, in which the global number of deaths is significantly lower than during epidemics, very few carcasses are observed. This may partly be explained by very quick decomposition or scavenging of lion carcasses (Packer 1996), which is probably quicker for young animals. As a consequence, without a detailed survey, detection of the pathogen presence in the host population outside epidemic outbreaks may be difficult. If the increase in mortality was apparent during the 1994 epidemic, the additional mortality due to the disease during the postulated endemic period (1966–1981) may not be detectable compared to the regular mortality observed during periods without any infection (1981–1994).
Nonetheless, it seems relevant to wonder whether a huge mortality induced by a first introduction of CDV into the lion population before 1966 could have remained undetected. The fact that the number of lion carcasses observed during the 1994 lethal epidemic was pretty low in spite of a close survey leads us to assume that this hypothesis is not unlikely.
It is worth noting that we do not consider in our model potential cub protection by maternal antibodies, thereby limiting the risk of severe disease if contact with the virus occurs at an early age. Information about this phenomenon, which is not rare in epidemiology (Zinkernagel 2001; Fouchet et al. 2006), is not available for all carnivore/CDV systems, but passive immunity to CDV in the case of lions seems biologically plausible. This additional mechanism would strengthen population immunity, thereby further reducing the number of disease cases during the endemic period and, as a consequence, lowering the likelihood of detecting the pathogen circulating in the lion population.
Furthermore, in relation to the observation of lion carcasses, the facts that (i) infection is sometimes inapparent (Roelke-Parker et al. 1996), (ii) death of a lion in an infectious state is not always disease related, and (iii) CDV was not considered to infect lions (Harder et al. 1995) could have made canine distemper diagnosis difficult. Thus, the paucity of lion carcasses attributed to canine distemper disease in the period prior to 1981 does not necessarily mean that CDV was not present in the population.
5. Conclusion
This study has focused on one plausible explanation for the apparent emergence of canine distemper that decimated a large part of Serengeti lion population in 1994. Other hypotheses have been invoked, but until now none are as consistent with all of our knowledge about the biological system.
Although we cannot confirm that our scenario (variations in population immunity level due to stochastic fluctuations in pathogen circulation) entirely explains events in the Serengeti lion population, we suggest here a new and more intuitive, but until now overlooked, interpretation of the serological data and demonstrate its coherence with epidemiological data.
Furthermore, this scenario suggests a general mechanism of infectious disease emergence (or more precisely, re-emergence), which could be valuable for several other host–pathogen systems. It could, for instance, explain why rabbit haemorrhagic disease virus has apparently variable impacts in different rabbit populations (Lavazza & Capucci 1990; Le Gall-Reculé 2002). Since population immunity in this system is due to passive immunity of young animals, mortality due to a disease is reduced rapidly over time (Lavazza & Capucci 1990; Le Gall-Reculé 2002). In populations where virus disappearance occurs, the level of immunity also declines and reintroduction of the virus will lead to lethal outbreaks.
Acknowledgments
We thank the ANR ‘Santé-Environnement et Santé-Travail’, programme ‘Pathocénose et émergence des maladies transmissibles: un concept unificateur mis à l'épreuve sur des pathologies exemplaires’ for financial support, J. O'Brien and two anonymous reviewers for their helpful comments, Dr Craig Packer for detailed information about canine distemper in Serengeti lions, and Etienne Rajon, Manuela Royer, Jean-Michel Gaillard, Robin Buckland, Robert Eymard and Sophie Mercier for their discussions on different aspects of this paper.
Supplementary Material
The eight non-linear differential equations that control the deterministic system and distribution of different length periods observed in 150 replicates
References
- Andersson H, Britton T. Stochastic epidemics in dynamic populations: quasi-stationarity and extinction. J. Math. Biol. 2000;41:559–580. doi: 10.1007/s002850000060. [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Vaccination and herd immunity to infectious diseases. Nature. 1985;318:323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- Appel M.J.G, Summers B.A. Pathogenicity of morbilliviruses for terrestrial carnivores. Vet. Microbiol. 1995;44:187–191. doi: 10.1016/0378-1135(95)00011-X. [DOI] [PubMed] [Google Scholar]
- Appel M.J.G, et al. Canine distemper epizootic in lions, tigers, and leopards in North America. J. Vet. Diag. Invest. 1994;6:277–288. doi: 10.1177/104063879400600301. [DOI] [PubMed] [Google Scholar]
- Blixenkrone-Moller M. Biological properties of phocine distemper virus and canine distemper virus. Acta Pathol. Microbiol. Immunol. Scand. Suppl. 1993;34:5–51. [PubMed] [Google Scholar]
- Carpenter M.A, Appel M.J.G, Roelke-Parker M.E, Munson L, Hofer H, East M, O' Brien S.J. Genetic characterization of canine distemper virus in Serengeti carnivores. Vet. Immunol. Immunopathol. 1998;65:259–266. doi: 10.1016/S0165-2427(98)00159-7. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Appel M.G.J, Chalmers W.S.K, Chillingworth C, Kaare M, Dye C. Serological and demographic evidence for domestic dogs as a source of canine distemper virus infection for Serengeti wildlife. Vet. Microbiol. 2000;72:217–227. doi: 10.1016/S0378-1135(99)00207-2. [DOI] [PubMed] [Google Scholar]
- Cleaveland, S., Packer, C., Hampson, K., Kaare, M., Kock, R., Mlengeya, T. & Dobson, A. 2007 The multiple roles of infectious diseases in the Serengeti ecosystem. In Serengeti III (eds A. R. E. Sinclair & C. Packer). Chicago, IL: Chicago University Press.
- Fenner F. Changes in the mortality rate due to myxomatosis in the Australian wild rabbit. Nature. 1953;171:172–228. doi: 10.1038/171562a0. [DOI] [PubMed] [Google Scholar]
- Fenner F, Fantini B. CABI Publishing; Oxon, UK: 1999. Biological control of vertebrate pests. The history of myxomatosis, an experiment in evolution. [Google Scholar]
- Fouchet D, Marchandeau S, Langlais M, Pontier D. Waning of maternal immunity and the impact of diseases: the example of myxomatosis in natural rabbit populations. J. Theor. Biol. 2006;242:81–89. doi: 10.1016/j.jtbi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Grinnell J, Packer C, Pusey A.E. Cooperation in male lions: kinship, reciprocity or mutualism? Anim. Behav. 1995;49:95–105. doi: 10.1016/0003-3472(95)80157-X. [DOI] [Google Scholar]
- Harder T.C, Osterhaus A.D.M.E. Canine distemper virus—a morbillivirus in search of new hosts? Trends Microbiol. 1997;5:120–125. doi: 10.1016/S0966-842X(97)01010-X. [DOI] [PubMed] [Google Scholar]
- Harder T.C, Kenter M, Appel M.J.G, Roelke-Parker M.E, Barrett T, Osterhaus A.D.M.E. Phylogenetic evidence of canine distemper virus in Serengeti's lions. Vaccine. 1995;13:521–523. doi: 10.1016/0264-410X(95)00024-U. [DOI] [PubMed] [Google Scholar]
- Lavazza, A. & Capucci, L. 1990 Viral haemorrhagic disease of rabbits and European brown hare syndrome: an update. Technical report, 14ème Conférence de la Commission Régionale de l'OIE pour l'Europe, Sofia, Bulgaria, 2–5 October 1990.
- Le Gall-Reculé, G. 2002 Les Hépatites virales des lagomorphes: la maladie hémorragique virale du lapin (VHD ou RHD)—Le syndrome du lièvre brun européen (EBHS). Technical report, Agence Française de la Sécurité Sanitaire des Aliments (AFSSA).
- Leisewitz A.L, Carter A, van Vuuren M, van Blerk L. Canine distemper infections, with special reference to South Africa, with a review of the literature. J. S. Afr. Vet. Assoc. 2001;72:127–136. doi: 10.4102/jsava.v72i3.635. [DOI] [PubMed] [Google Scholar]
- Myers D.L, Zurbriggen A, Lutz H, Pospischil A. Distemper: not a new disease in lions and tigers. Clin. Diag. Lab. Immunol. 1997;4:180–184. doi: 10.1128/cdli.4.2.180-184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhaus A. Catastrophes after crossing species barriers. Phil. Trans. R. Soc. B. 2001;356:791–793. doi: 10.1098/rstb.2001.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer, C. 1990 Serengeti lion survey. Technical report, TAPANA, SWRI, MWEKA and the Wildlife Division, 15 November 1990.
- Packer C. Coping with a lion killer. Nat. Hist. 1996;6:14–17. [Google Scholar]
- Packer C, Pusey A.E. Dispersal, kinship, and inbreeding in African lions. In: Thornhill N.W, editor. The natural history of inbreeding and outbreeding. University of Chicago Press; Chicago, IL: 1993. pp. 375–391. [Google Scholar]
- Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–811. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- Packer C, Altizer S, Appel M, Brown E, Martenson J, O' Brien S.J, Roelke-Parker M, Hofmann-Lehmann R, Lutz H. Viruses of the Serengeti: patterns of infection and mortality in African lions. J. Anim. Ecol. 1999;68:1161–1178. doi: 10.1046/j.1365-2656.1999.00360.x. [DOI] [Google Scholar]
- Roelke-Parker M.E, et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicluna M.T, Lavazza A, Capucci L. European brown hare syndrome in northern Italy: results of a virological and serological survey. Rev. Off. Int. Epizoot. 1994;13:893–904. doi: 10.20506/rst.13.3.801. [DOI] [PubMed] [Google Scholar]
- Swinton J. Extinction times and phase transitions for spatially structured closed epidemics. Bull. Math. Biol. 1998;60:215–230. doi: 10.1006/bulm.1997.0014. [DOI] [PubMed] [Google Scholar]
- Swinton J, Harwood J, Grenfell B.T, Gilligan C.A. Persistence thresholds for phocine distemper virus infection in harbour seal (Phoca vitulina) metapopulations. J. Anim. Ecol. 1998;67:54–68. doi: 10.1046/j.1365-2656.1998.00176.x. [DOI] [Google Scholar]
- Zinkernagel R.M. Maternal antibodies, childhood infections, and autoimmune diseases. New Engl. J. Med. 2001;345:1331–1335. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The eight non-linear differential equations that control the deterministic system and distribution of different length periods observed in 150 replicates