Abstract
Recently, with the development of smart polymers, research has looked to using thermoresponsive polymers as cell culture substrates. These novel surfaces allow the cultivation of cells without enzymes using the thermoresponsive phase transition property of poly(N-isopropylacrylamide) (PNIPAAm). However, this requires expensive techniques to generate a sufficiently thin film that allows cell adhesion. In this study, we looked at simple solvent cast films which normally show poor cell adhesion, but here the films are coated with cell adhesion promoters (CAPs) to improve cell growth without altering the copolymer thermoresponsive behaviour.
A copolymer of PNIPAAm and N-tert-butylacrylamide (NtBAm) with a ratio of 85 : 15, respectively, was synthesized and solvent cast. The copolymer films were coated with CAPs, such as collagen, fibronectin and laminin, to increase their cell adhesion and growth properties. Cell activity measured by the alamarBlue assay showed similar results for coated copolymer films and standard tissue culture plastic controls. Deposition of CAPs on to the copolymer films was characterized by scanning electron microscopy and atomic force microscopy. Cell detachment from the copolymer films is not affected by the surface coatings of CAPs, and endothelial cells are recovered as an intact sheet, which has great potential for uses in tissue engineering applications. The results demonstrate a versatile method for the cultivation of cells while eliminating the need for the use of digestive enzymes such as trypsin. This study shows that cultivation on physically bonded PNIPAAm copolymers is viable and achievable by relatively simple methods.
Keywords: poly(N-isopropylacrylamide), cell detachment, atomic force microscopy, cell adhesion promoters
1. Introduction
New methodologies for the recovery of cells and cell sheets from in vitro culture are continually being developed to aid the advancement of tissue engineering. Conventional methods which use proteolytic enzymes, such as trypsin or dispase, have been shown to irreparably damage cells and their functions (Waymouth 1974). Current research has looked at methods for cell recovery without the use of proteolytic enzymes. A recent study developed ultrasound-induced cell detachment which caused up to 95% detachment; however, this resulted in a drop of cell viability to approximately 30% (Lu & Zhong 2005). One well-studied and promising method for non-enzymatic cell recovery is based on thermoresponsive polymers.
Thermoresponsive polymers based on poly-N-isopropylacrylamide (PNIPAAm) allow cell growth and proliferation, and then the consequent harvesting of intact cell sheets by a simple change in temperature (Okano et al. 1993). Several papers have argued a hypothetical mechanism for this cell sheet lift-off from thermoresponsive polymers (Okano et al. 1995; Cheng et al. 2004).
The underlying principle is that PNIPAAm demonstrates a lower critical solution temperature (LCST) of approximately 32°C in water (Heskins et al. 1968; Bae et al. 1990). Above the LCST in aqueous environments, PNIPAAm chains are aggregated and substantially dehydrated as a result of increased intra- and intermolecular hydrophobic interactions, but below the LCST, these interactions are decreased and the PNIPAAm chains become hydrated and extended. This sharp abrupt physical change over a mild temperature change has sparked a lot of research interest into these polymers and their possible applications. PNIPAAm and various copolymers have been developed for a wide variety of uses including chemical/pH sensors, drug delivery, bioseparation, actuators and chemical valves in microfluidics (Bromberg & Ron 1998; Kohori et al. 1999; Garret-Flaudy & Freitag 2000; Saitoh et al. 2002; Harmon et al. 2003; Kuckling et al. 2003; Pan & Chien 2003; Gerlach et al. 2004).
Surface modification with PNIPAAm generates a surface wettability which can change in response to temperature change. PNIPAAm modification can be achieved through several methods. As previously reported, the solvent casting method used in this study generates PNIPAAm copolymer films approximately 4 μm in thickness (Rochev et al. 2004). The molecular composition of this copolymer system has previously been reported (Moran et al. in press). PNIPAAm can also be grafted to polystyrene (PS) by adding an IPAAm solution in isopropyl alcohol to a PS dish and then irradiating the dish with an electron beam generating films with a controlled thickness between 15 and 5000 nm (Kikuchi & Okano 2005). PNIPAAm films may also be prepared by adsorption onto glass. Solutions of PNIPAAm in water can be added to glass substrata and, after incubation at 50°C for a given time, films of 2–3 nm thickness are formed (Callewaert et al. 2004). PNIPAAm surfaces can also be prepared by plasma polymerizing NIPAAm (Canavan et al. 2005) and by grafting onto PS and poly(ethylene terephthalate) (Curti et al. 2002).
Cells have previously been cultured on pure PNIPAAm films with a thickness of less than 15 nm, and these surfaces have shown great adhesion and reversible cell detachment properties (Takezawa et al. 1990; Kwon et al. 2003; Akiyama et al. 2004). Though these films are expensive and complicated to produce, a film created by simple solvent casting methods would be more desirable. However, the ability of adherent cells to grow on these modified surfaces with thicknesses of greater than 15–20 nm is poor in comparison with conventional cell culture grade PS (Piskareva et al. 1999; Kikuchi & Okano 2005). The aim of this study was to show that solvent cast films of thermoresponsive copolymers can be used as substrata for the temperature-induced cell detachment, and that by coating these copolymers with cell adhesion promoters (CAPs), cells would readily adhere and grow and the substrate would still retain the cell detachment properties.
The use of solvent cast copolymer films as opposed to chemical attachment of PNIPAAm to a surface greatly simplifies the protocol for a temperature-induced cell detachment. In previous work, we showed that these CAP-coated PNIPAAm copolymers can support cell growth for basic fibroblastic cell lines (Moran et al. in press). In this study, we show that primary endothelial cells can also be cultured on the copolymer, and one of the main aims was to detach endothelial cells from the copolymer surface using a cold treatment instead of enzymatic means which would ensure that their cell–cell junctions were maintained and an intact cell sheet could be harvested.
2. Material and methods
2.1 Materials
N-Isopropylacrylamide (NIPAAm; Aldrich) and NtBAm (Fluka, Switzerland) were recrystallized from hexane and acetone, respectively, and dried at room temperature under vacuum. 2,2′-Azobisisobutyronitrile (AIBN; Phase separation Ltd, UK) was recrystallized from methanol. Benzene was dried under sodium and distilled before use. All other solvents were reagent grade and were purified by conventional methods before use.
2.2 Copolymer synthesis
Poly(NIPAAm-co-NtBAm) (15 mol% NtBAm) was prepared by free radical polymerization, using AIBN (0.5 mol% of AIBN) as an initiator in benzene (10%, w/w) under argon. After polymerization at 60°C for 24 h, the mixture was precipitated in hexane. Precipitation was repeated three times using acetone as a solvent and hexane as a non-solvent, and the product was dried at 45°C in a vacuum oven.
2.3 Film preparation
For cell culture experiments, copolymer films were cast in 24-well PS tissue culture grade dishes from a 4% (w/v) solution of copolymer in dry ethanol (20 μl per well), and for microscopy experiments Thermanox coverslips were used. Films were allowed to dry slowly in an ethanol atmosphere overnight and then transferred to a vacuum oven and dried at 40°C for 18 h resulting in copolymer films with a thickness of 4–5 μm. Films were sterilized under mild UV light for 3 h prior to cell culture experiments.
2.4 Cell culture
Human umbilical vein endothelial cells (HUVEC) were supplied by Cambrex. Cells were cultured in endothelial basal medium (EBM-2) with supplements, also supplied by Cambrex. Cells were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Medium was changed every 2 days until the monolayer reached 90% confluency, at which point the cells were harvested for reseeding or for experiments.
2.5 Cell adhesion promoter (CAP) deposition
The adhesion promoters were physically bound to the copolymer surface as previously described. Briefly, rat tail collagen, type 1 (3 mg ml−1 in 0.1 M acetic acid) was diluted to 200 μg ml−1 with phosphate-buffered saline (PBS) and 150 μl was added to each well and allowed to dry thoroughly in a laminar flow hood for several hours. Wells were rinsed with Hanks' buffered salt solution (HBSS; Sigma) containing phenol red, preheated to 37°C, until surface was neutralized and then seeded with cells in appropriate media.
An amount of 100 μl of mouse laminin (Ln-1) (Sigma; 100 μg ml−1 in PBS) was added to separate wells and spread carefully over the entire surface. Plates were then allowed to dry thoroughly in a laminar flow hood for 3 h and then rinsed with HBSS and seeded with cells.
An amount of 500 μl of fibronectin (Fn) solution (16 μg ml−1 in HBSS) was added to each well and incubated for 2 h at 37°C. Fn solution was then removed and wells were rinsed with HBSS and seeded with cells.
2.6 Cell activity assays
HUVEC metabolic activity was assessed by the alamarBlue assay at 24 h (Voytik-Harbin et al. 1998). Total DNA was also quantified at 24 h by the Quant-iT PicoGreen dsDNA assay kit. Cells were seeded in wells containing EBM-2 onto copolymer films already coated with CAPs (50 000 cells per cm2). Plates were incubated for 24 h at 37°C in a humidified atmosphere containing 5% CO2 for both assays. Assays were carried out as previously described (Moran et al. in press).
2.7 Cell detachment
Plates containing copolymer films already coated with CAPs were seeded with HUVECs in EBM-2 (50 000 cells per cm2). Plates were incubated at 37°C in a humidified atmosphere containing 5% CO2 until they reached 90–95% confluency. At this stage, plates were rinsed with HBSS at 37°C to remove all serum and then HBSS at 4°C was added. Micrographs of the plates were frequently captured on a phase contrast microscope until all cells had completely detached from the plate.
2.8 Surface characterization
The copolymer surfaces were examined both before and after protein deposition. Samples were examined by atomic force microscopy (AFM) and scanning electron microscopy (SEM).
AFM images were obtained in tapping mode in air using a Dimension 3100 AFM (Digital Instruments, Santa Barbara, CA, USA). First-order flattening was carried out on all height images to remove any bowing phenomenon present. Roughness is reported as root mean square (r.m.s.) values, where r.m.s. denotes the standard deviation of the Z-values within a given area.
The SEM analysis samples were gold coated and then examined using a Hitachi S-4700 SEM in low magnification mode at an accelerating voltage of 5 kV.
3. Results and discussion
3.1 Surface analysis
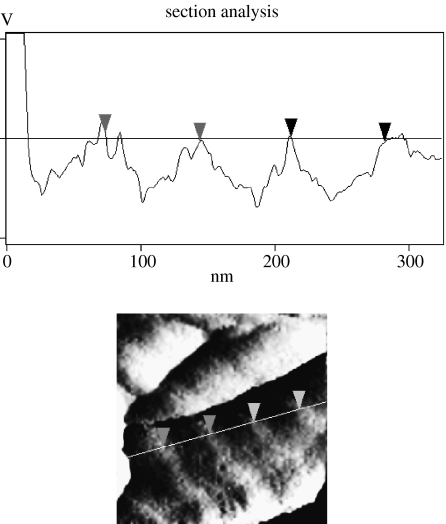
As previously reported (Moran et al. in press), AFM and SEM images show that collagen and laminin appear to generate a homogenous coverage of the copolymer films. The collagen fibrils typical of type 1 collagen fibrillogenesis are clearly visible. Cross-section analysis of the collagen fibril under AFM (figure 1) clearly shows the repeating monomer units of collagen every 69 nm. The laminin structure is similar to that already reported for laminin observed under AFM (Chen et al. 1998).
Figure 1.
Cross-section analysis of a collagen fibril using AFM. The horizontal distance between markers is approximately 69 nm. This corresponds to the distance between repeating monomer units in the type I collagen molecule and confirms that collagen coating on the copolymer is in its fibrillar form.
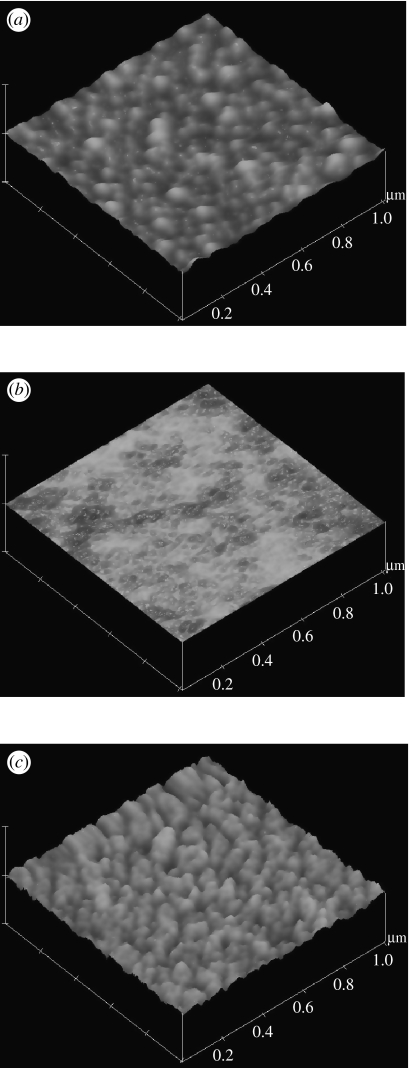
In the current study, we also investigated fibronectin as a possible CAP for the copolymer. Figure 2 shows the surface during the different steps involved in preparing a homogenous coverage of fibronectin on the copolymer obtained using AFM. Roughness values measured by AFM show that the bare PS has an r.m.s. value of 1.059 nm, and polymer casting creates a smoother surface with an r.m.s. value of 0.214 nm, but the roughness is then increased again after fibronectin coating as would be expected for a protein-covered surface, r.m.s. of 2.047 nm.
Figure 2.
AFM images of steps during preparation of 85 : 15 copolymer coated with fibronectin. (a) PS surface prior to copolymer casting, (b) dried copolymer film cast over the PS and (c) fibronectin coating on the copolymer. Z-Range for all images is 50 nm. The fibronectin forms a highly uniform coating over the copolymer surface which provides a vast amount of anchorage sites for adhering cells. This is confirmed by the micrographic and cell assay data, as cell adhesion is greatly improved by fibronectin coating.
3.2 Cell adhesion and growth
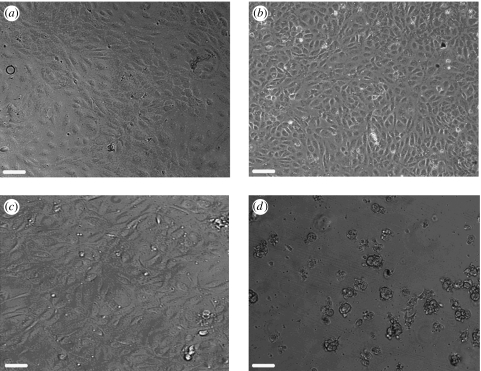
HUVECs were grown on three differing copolymers with three separate CAPs. Figure 3 shows HUVECs grown on 85 : 15 copolymer with the separate CAPs and on the uncoated copolymer alone.
Figure 3.
Phase contrast images of HUVEC cells on 85 : 15 copolymer coated with (a) collagen, (b) laminin, (c) fibronectin and (d) no CAP coating. The images clearly show the level of improvement of cell adhesion after CAP coating. Images (a), (b) and (c) show confluent monolayers of attached HUVECs while (d) shows rounded, clumped and unattached cells after seeding on the uncoated copolymer. Scale bar, 120 μm.
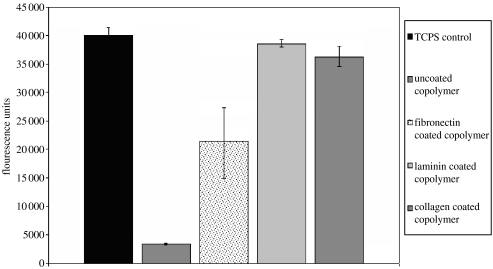
Metabolic activity of the cells was measured by the alamarBlue assay (figure 4), and it clearly shows that the HUVECs have increased cell activity, due to increased cell numbers, on the copolymer after it is coated with CAPs and this activity is equivalent to that observed with HUVECs grown on standard tissue culture grade PS. The total DNA count (figure 5) shows that cell numbers are less on the uncoated copolymer compared with the CAP-coated copolymers. The levels are not as different as those seen for the metabolic activity. This is due to the cells having poor adhesion, and thus likely to be in a state of acquiescence and are not metabolically active.
Figure 4.
Metabolic activity of HUVECs on CAP-coated copolymer as measured by the alamarBlue assay. The cells on uncoated copolymer show very low metabolic activity, and this is due to the cells not having any anchorage sites; therefore, they are in a state of acquiescence and are not metabolically active. Cells on the CAP-coated copolymer show a significant increase in cell activity, and the levels shown on the laminin- and collagen-coated copolymer are up to 90% of that shown on TCPS. Cells on the fibronectin-coated copolymer show activity levels of approximately 50–60% compared with TCPS.
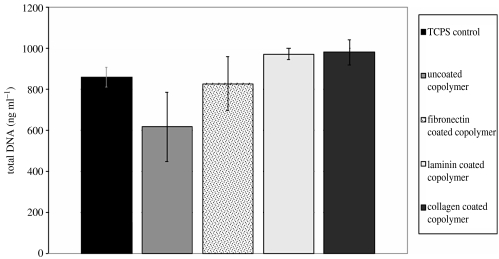
Figure 5.
Total DNA content of HUVECs on CAP-coated copolymer as measured by the QuantiT PicoGreen dsDNA assay. The level of DNA is a direct measure for cell number, and it is clear that more cells adhere to the copolymer after it is CAP coated. There is no significant difference for the number of cells, but levels for TCPS are similar to CAP-coated copolymer while the numbers on uncoated copolymer are lower than the rest.
Using both microscopic and activity assay evidence, it is clearly shown that the HUVECs adhere much better to the copolymer after CAP coating, as there are much more cells present and they have adopted the typical cobblestone morphology of endothelial cells in culture. The HUVECs also exhibit cellular activity levels similar to normal culture techniques. These experimental data show that the method used in this study is as effective for HUVECs in vitro culture as standard cell culture techniques.
3.3 Cell detachment
HBSS at 4°C was added to confluent monolayers to reduce the temperature below the LCST and cause the surface to change from hydrophobic to hydrophilic. Images were captured during the detachment process which showed complete detachment from the copolymer after 20 min. As shown in figure 6, the cells detached from the outside in towards the centre. We hypothesize that the HBSS cannot penetrate the tight cell–cell junctions that are maintained after detachment, and that the detachment occurs as the surface dissolves into the solution, along the edge of the cell sheet, thereby removing the cell anchorage sites. During the current detachment process, the cell sheets tend to fold and overlay upon themselves. This is also evident in the SEM images of completely detached cell sheets. In future, a more careful method will have to be devised to ensure the cell sheet remains flat. After the cell sheet has detached, the surface returns to its original PS state because once the copolymer has dissolved, the adhered cells detach along with all the ECM components deposited as their sites of anchorage are no longer present (figure 7).
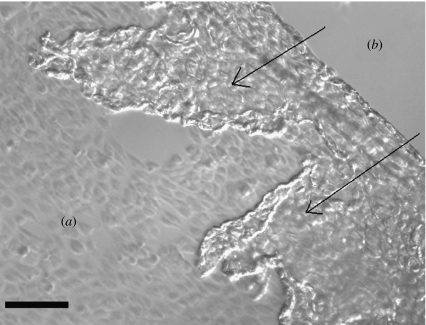
Figure 6.
Phase contrast microscope image of the HUVEC cell sheet detaching from copolymer after 8 min at 4°C. (a) The area where cells are still attached in their typical cobblestone morphology and (b) the area where the cell sheet has already detached. The arrows indicate the direction of detachment and the detached sheet is seen to be folding over upon itself. Detachment occurs from the outer edge of the sheet in towards the centre as a result of the copolymer dissolving, as it becomes exposed to the aqueous media. Detachment does not occur initially at the centre because the very tight intercellular junctions do not allow a sufficient volume of media to pass through to cause complete dissolution of the copolymer. Scale bar, 130 μm.
Figure 7.
SEM image of a cell sheet detached from the copolymer. It is visible here how the sheet folds and overlaps upon itself during the detachment. The overlaps are caused by currents in the media which gently move the detached cell sheet around and over onto the cells which are still attached. Future work will look at devising a method to prevent the cell movement during the detachment process. Scale bar, 500 μm.
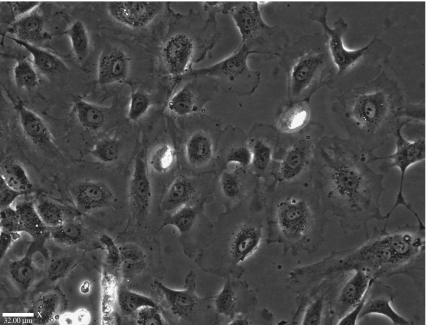
The detached HUVECs were then reseeded on to tissue culture PS to assess viability after detachment at 4°C. As can be clearly seen in figure 8, taken after 72 h, the HUVECs have attached to the PS and grown to confluency, adopting the typical cobblestone morphology of endothelial cells in culture. The growth rate of confluency of HUVECs within 72 h is normal for this cell line, and this suggests that the copolymer growth and subsequent detachment do not cause any adverse effects on cell adhesion and growth.
Figure 8.
HUVECs on TCPS, 4 days after non-enzymatic detachment from copolymer. The cells have reattached, and this shows that cells are not damaged by the low-temperature detachment and they are still able to attach and behave as normal endothelial cells in culture. Scale bar, 32 μm.
4. Conclusions
This study has shown that despite previous research, cells can be grown on PNIPAAm-based copolymer films that are solvent cast with a thickness of approximately 4 μm, by simply adding CAPs which do not affect the thermoresponsive nature of the copolymer. Cells can be recovered as an intact cell sheet and then used for tissue engineering applications. Further development is required to ensure that cell sheets do not fold during the detachment process.
Acknowledgments
We would like to thank the Higher Education Authority, Ireland for funding this project through the Programme for Research in Third Level Institutes (PRTLI-3).
References
- Akiyama Y, Kikuchi A, Yamato M, Okano T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20:5506–5511. doi: 10.1021/la036139f. [DOI] [PubMed] [Google Scholar]
- Bae Y.H, Okano T, Kim S.W. Temperature-dependence of swelling of cross-linked poly(N,N′-alkyl substituted acrylamides) in water. J. Polym. Sci. Part B Polym. Phys. 1990;28:923–936. doi: 10.1002/polb.1990.090280609. [DOI] [Google Scholar]
- Bromberg L.E, Ron E.S. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug Deliv. Rev. 1998;31:197–221. doi: 10.1016/S0169-409X(97)00121-X. [DOI] [PubMed] [Google Scholar]
- Callewaert M, Grandfils C, Boulange-Petermann L, Rouxhet P.G. Adsorption of poly (N-isopropylacrylamide) on glass substrata. J. Colloid Interface Sci. 2004;276:299–305. doi: 10.1016/j.jcis.2004.03.063. [DOI] [PubMed] [Google Scholar]
- Canavan H.E, Cheng X.H, Graham D.J, Ratner B.D, Castner D.G. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir. 2005;21:1949–1955. doi: 10.1021/la048546c. [DOI] [PubMed] [Google Scholar]
- Chen C.H, Clegg D.O, Hansma H.G. Structures and dynamic motion of laminin-1 as observed by atomic force microscopy. Biochemistry. 1998;37:8262–8267. doi: 10.1021/bi973097j. [DOI] [PubMed] [Google Scholar]
- Cheng X.H, Wang Y.B, Hanein Y, Bohringer K.F, Ratner B.D. Novel cell patterning using microheater-controlled thermoresponsive plasma films. J. Biomed. Mater. Res. A. 2004;70:159–168. doi: 10.1002/jbm.a.30053. [DOI] [PubMed] [Google Scholar]
- Curti P.S, De Moura M.R, Radovanovic E, Rubira A.F, Muniz E.C. Surface modification of polystyrene and poly(ethylene terephthalate) by grafting poly(N-isopropylacrylamide) J. Mater. Sci. Mater. Med. 2002;13:1175–1180. doi: 10.1023/A:1021154424189. [DOI] [PubMed] [Google Scholar]
- Garret-Flaudy F, Freitag R. Use of the avidin (imino)biotin system as a general approach to affinity precipitation. Biotechnol. Bioeng. 2000;71:223–234. doi: 10.1002/1097-0290(2000)71:3%3C223::AID-BIT1012%3E3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Gerlach G, Guenther M, Suchaneck G, Sorber J, Arndt K.F, Richter A. Application of sensitive hydrogels in chemical and pH sensors. Macromol. Symp. 2004;210:403–410. doi: 10.1002/masy.200450645. [DOI] [Google Scholar]
- Harmon M.E, Tang M, Frank C.W. A microfluidic actuator based on thermoresponsive hydrogels. Polymer. 2003;44:4547–4556. doi: 10.1016/S0032-3861(03)00463-4. [DOI] [Google Scholar]
- Heskins M, Guillet J.E, James E.J. Solution properties of poly(N-isopropylacrylamide) J. Macromol. Sci. 1968;A2:1441–1445. [Google Scholar]
- Kikuchi A, Okano T. Nanostructured designs of biomedical materials: applications of cell sheet engineering to functional regenerative tissues and organs. J. Control. Release. 2005;101:69–84. doi: 10.1016/j.jconrel.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Kohori F, Sakai K, Aoyagi T, Yokoyama M, Yamato M, Sakurai Y, Okano T. Control of adriamycin cytotoxic activity using thermally responsive polymeric micelles composed of poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(d,l-lactide) Colloids Surf. B Biointerfaces. 1999;16:195–205. doi: 10.1016/S0927-7765(99)00070-3. [DOI] [Google Scholar]
- Kuckling D, Richter A, Arndt K.F. Temperature and pH-dependent swelling behavior of poly(N-isopropylacrylamide) copolymer hydrogels and their use in flow control. Macromol. Mater. Eng. 2003;288:144–151. doi: 10.1002/mame.200390007. [DOI] [Google Scholar]
- Kwon O.H, Kikuchi A, Yamato M, Okano T. Accelerated cell sheet recovery by co-grafting of PEG with PIPAAm onto porous cell culture membranes. Biomaterials. 2003;24:1223–1232. doi: 10.1016/S0142-9612(02)00469-6. [DOI] [PubMed] [Google Scholar]
- Lu X.C, Zhong P. Ultrasound-induced cell detachment and gene transfection in adherent cells. Acoust. Res. Lett. Online Arlo. 2005;6:195–200. doi: 10.1121/1.1909243. [DOI] [Google Scholar]
- Moran, M. T., Carroll, W. M., Selezneva, I., Gorelov, A. & Rochev, Y. In press. Cell growth and detachment from protein-coated PNIPAAm-based copolymers J. Biomed. Mater. Res. A 9999. [DOI] [PubMed]
- Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery-system for cultured-cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J. Biomed. Mater. Res. 1993;27:1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomatererials. 1995;16:297–303. doi: 10.1016/0142-9612(95)93257-E. [DOI] [PubMed] [Google Scholar]
- Pan L.C, Chien C.C. A novel application of thermo-responsive polymer to affinity precipitation of polysaccharide. J. Biochem. Biophys. Methods. 2003;55:87–94. doi: 10.1016/S0165-022X(02)00180-X. [DOI] [PubMed] [Google Scholar]
- Piskareva O.A, Rochev Y.A, Gavrilyuk B.K, Gorelov A.V, Golubeva T.A, Dawson K.A. Effect of a matrix based on thermoreverse polymers and collagen on-the growth of human fibroblasts. Biofizika. 1999;44:281–283. [PubMed] [Google Scholar]
- Rochev Y, O'Halloran D, Gorelova T, Gilcreest V, Selezneva I, Gavrilyuk B, Gorelov A. Rationalising the design of polymeric thermoresponsive biomaterials. J. Mater. Sci. Mater. Med. 2004;15:513–517. doi: 10.1023/B:JMSM.0000021130.11711.16. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Suzuki Y, Hiraide M. Preparation of poly(N-isopropylacrylamide)-modified glass surface for flow control in microfluidics. Anal. Sci. 2002;18:203–205. doi: 10.2116/analsci.18.203. [DOI] [PubMed] [Google Scholar]
- Takezawa T, Mori Y, Yoshizato K. Cell culture on a thermo-responsive polymer surface. Biotechnology. 1990;8:854–856. doi: 10.1038/nbt0990-854. [DOI] [PubMed] [Google Scholar]
- Voytik-Harbin S.L, Brightman A.O, Waisner B, Lamar C.H, Badylak S.F. Application and evaluation of the alamarBlue assay for cell growth and survival of fibroblasts. In Vitro Cell. Dev. Biol. Anim. 1998;34:239–246. doi: 10.1007/s11626-998-0130-x. [DOI] [PubMed] [Google Scholar]
- Waymouth C. To disaggregate or not to disaggregate—injury and cell disaggregation, transient or permanent. In Vitro J. Tissue Culture Assoc. 1974;10:97–111. doi: 10.1007/BF02615343. [DOI] [PubMed] [Google Scholar]