Abstract
Sepsis is characterized by an inappropriate host immune-inflammatory response and sustained oxidative damage. Nrf2, a bZIP oxidant-responsive transcription factor, regulates a battery of cytoprotective genes including antioxidants and maintains cellular redox homeostasis. Mouse studies have demonstrated a critical role of Nrf2 in improving survival during sepsis. This preclinical ex vivo study using neutrophils and peripheral blood mononuclear cells (PBMCs) as a surrogate cells evaluates the efficacy of CDDO-Im and CDDO-Me [imidazole and methyl ester derivative of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO)] to activate the Nrf2 pathway and protect from lipopolysaccharide (LPS)-induced inflammatory response in humans. CDDO-Im treatment significantly induced Nrf2–dependent antioxidative genes (HO-1, GCLC, GCLM, and NQO1) in PBMCs isolated from six normal subjects. CDDO-Im increased nuclear accumulation of Nrf2 protein. Pretreatment of PBMC by CDDO-Im significantly attenuated LPS-induced cytokine expression. Similar increases in levels of antioxidant genes and suppression of LPS-induced cytokine expression was observed after CDDO-Me pretreatment. CDDO-Im also greatly inhibited LPS, fMLP, TNF-α, and TPA-induced ROS generation in neutrophils. In conclusion, these results demonstrate that activation of the Nrf2-dependent antioxidative pathway by CDDO-Im or CDDO-Me protects against the LPS-induced inflammatory response and suggest that they can be potential therapeutic candidates for intervening sepsis syndrome.
INTRODUCTION
Sepsis is a complex syndrome that is characterized by systemic inflammatory response to infection, with its severe form associated with multiorgan failure (9). Around 750,000 patients have sepsis each year in the United States (2), and a recent study reported a 70% increase in the number of severe sepsis cases from 1993 to 2003 (8). Despite prompt treatment with antibiotics and advancement in the critical care of patients in the intensive care units, the case-fatality rate remains extremely high (35–50%). Early diagnosis and therapy are critical to improve the prognosis (8). In sepsis, infection (predominantly bacterial) stimulates immune cells (neutrophils, macrophages, and lymphocytes) and nonimmune cells (endothelial and epithelial cells) to produce exaggerated amounts of proinflammatory mediators (TNF-α, IL-6, and IL-1), reactive oxygen species (ROS), and proteases. Greater activation of NF-κB, the primary transcription factor regulating the expression of proinflammatory mediators, is associated with higher mortality and a worse clinical outcome in sepsis (1). To counteract the early amplified proinflammatory response, the host mounts a compensatory antiinflammatory response (secretion of 1L-10, 1L-13), leading to a hyporeactive defense system. However, newer data indicate that this concept of a simple, linear progression does not fully address the complex nature of sepsis (24). Therapeutic strategies that can aid in maintaining an adequate immune–inflammatory response as well as reduce oxidative stress may have the potential to improve survival during sepsis (9).
Reactive oxygen species (ROS) play a key role in the pathogenesis of sepsis. ROS modulates TLR4 signaling (23, 25) and regulates priming of immune cells (20). ROS also causes oxidative damage to endothelial and epithelial cells, leading to tissue damage and organ failure (9). Nuclear factor-erythroid 2–related factor 2 (Nrf2) is a bZIP redox-sensitive transcription factor that regulates a cytoprotective transcriptional program that includes antioxidants, xenobiotic conjugating enzymes, ubiquitin/proteasomes, chaperone, and heat-shock proteins in response to cellular stresses including ROS (16, 31, 34). Disruption of Nrf2 causes a decrease in the constitutive expression of some cytoprotective genes and greatly impairs the capacity to mount an adaptive stress response (16, 34). Studies from our laboratory with murine models suggest that the Nrf2-dependent transcriptional program determines the host response to inflammatory and oxidative stress and that Nrf2 acts as a critical determinant of susceptibility to several inflammatory diseases, including cigarette smoke–induced emphysema (27), hyperoxia-induced acute lung injury (4), allergen-induced asthma (28), bleomycin-induced lung fibrosis (5), and cancer (26). We reported that Nrf2 functions as a critical host factor for survival from sepsis with using murine sepsis models (33). Disruption of Nrf2 caused enhanced sensitivity to both endotoxin-induced shock and cecal ligation and puncture (CLP)-induced septic shock. Nrf2 mediated its protective response by regulating the innate immune response by attenuating oxidative stress (35). Gene-expression studies demonstrated amplified expression of proinflammatory mediators, including cytokines, chemokines, and adhesion molecules in the lungs, as well as innate immune cells (macrophages and neutrophils) of Nrf2–deficient mice when challenged with lipopolysaccharide (LPS), a major ligand for TLR4 signaling (33). Moreover, polymorphism in the Nrf2 gene has been associated with increased risk of development of acute lung injury in trauma and septic patients (19).
We have hypothesized that activators of the Nrf2-mediated cytoprotective pathways protect against sepsis syndrome by the regulating innate immune response as well as attenuating oxidative pathologic organ damage (14, 35). Triterpenoid analogues such as CDDO-Im {1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole}, an imidazole derivative of CDDO, are exceptionally potent activators of Nrf2 in cell culture and in vivo (37, 38). We demonstrated recently in mice that activation of the Nrf2 pathway by CDDO-Im attenuated LPS-induced ROS generation and protected from exaggerated expression of proinflammatory mediators in macrophages and neutrophils (35). More important, CDDO-Im decreased mortality in wild-type mice exposed to LPS, while failing to protect Nrf2–deficient mice from exaggerated inflammation and greater mortality (35). However, the capacity of CDDO-Im to activate the Nrf2 pathway in humans is not known. In the current study, we investigated ex vivo by using PBMCs and neutrophils of normal subjects: (a) the efficacy of triterpenoid analogues (CDDO-Im and CDDO-Me) to activate Nrf2 signaling, (b) the interindividual variation in the activation of the Nrf2 pathway by triterpenoid analogues, and (c) the ability of CDDO-Im and CDDO-Me to suppress LPS-induced ROS generation and inflammation.
METHODS
Subjects
Peripheral blood mononuclear cells (PBMCs) and neutrophils were isolated from six healthy human subjects. None of the subjects had any relevant acute or chronic disease and were not taking any medication that might affect the immune response. The purpose, potential risks, and benefits of the study were explained, and written informed consent was obtained from each participant. The Johns Hopkins University Institutional Review Board approved the study design.
Isolation of PMBCs and neutrophils
Normal human PBMCs and neutrophils were isolated from the EDTA-anticoagulated venous blood by using density-gradient centrifugation over Ficoll-Histopaque plus (Pharmacia, Uppsala, Sweden). In brief, blood samples were diluted 1:2 with Hank's balanced salt solution (HBSS), layered over Ficoll-Histopaque plus, and centrifuged at 600 g for 20 min. PBMC-rich plasma above the Ficoll-Histopaque was collected by a plastic-tip pipette. Neutrophils were prepared after Ficoll-Histopaque separation of PBMCs and sedimentation of the erythrocyte–granulocyte pellet in 1% dextran. Neutrophils pellets were resuspended in NH4Cl lysis buffer to eliminate remaining erythrocytes, and the neutrophils were pelleted and washed twice with HBSS. The purity and viability of the neutrophils preparation was more than 95%, as assessed by Giemsa staining and the trypan blue dye exclusion test, respectively. The number of PBMCs and neutrophils was counted by using a hemocytometer and they were resuspended at a density of 2 × 106 /ml in RPMI 1640 with 5% fetal calf serum, 1% Penn-strep, and cultured at 37°C in a 5% CO2 atmosphere.
Treatment
PBMCs and neutrophils were plated at a density of 2 × 106 cells/ml in tissue-culture plates and were treated with vehicle or CDDO-Im or CDDO-Me for 20 h. After 20-h pretreatment, cells were incubated with LPS (100 ng/ml; Escherichia coli, serotype 055.B5; Sigma, St. Louis, MO) for an additional 4 or 24 h. Culture media and cells were harvested. Cells were processed for RNA and protein extraction. For measurement of ROS, neutrophils were harvested immediately after 20-h pretreatment with vehicle or CDDO-Im and suspended in complete PBS.
Measurement of ROS
Generation of ROS was assessed by luminol-derived horse-radish peroxidase–dependent chemiluminescence (35). Neutrophils (1 × 105) were incubated in a test tube containing 10 μM luminol and 0.5 unit of horseradish peroxidase at 37°C for 10 min, after which, cells were placed in the luminometer (Biolumat LB 9505, Berthold Co., Wildbad, Germany) and activated with chemotactic peptide fMLP (10 μM), TNF-α (10 ng/ml), LPS (100 ng/ml) or phorbol 12-myristate 13-acetate (TPA, 50 nM) at time 0. Chemiluminescence was recorded immediately for 30 min.
Measurement of gene expression with real-time PCR
Nrf2–regulated antioxidative genes and inflammatory cytokines were measured in PBMCs and neutrophils with real-time PCR (33). Total RNA was extracted from the cells by using TriZol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Total RNA was used for cDNA synthesis with random hexamers and MultiScribe reverse transcriptase, according to the manufacturer's recommendations (Applied Biosystems). cDNA (100 ng) was used for quantitative PCR analyses of selected genes heme oxygenase-1 (HO-1), glutamate cysteine ligase catalytic (GCLC), and modifier subunits (GCLM), NADPH-quinone oxidoreductase (NQO1), TNF-α, and IL-6 by using primers and probe sets commercially available from Applied Biosystems. Assays were performed by using the ABI 7000 Taqman system (Applied Biosystems). β-Actin was used for normalization. Relative fold change for each gene was calculated as described elsewhere (35).
Measurement of Nrf2 activation by immunoblot
Nuclear and cytoplasmic extracts from PBMCs and neutrophils were prepared by using NE-PER Nuclear Extraction Reagents (Pierce). Protein concentrations were measured by the BCA method (Pierce). Immunoblot analysis was performed according to previously published procedures (30), by using antibodies specific for Nrf2 and Lamin B1 (Santa Cruz Biotechnology, Inc.).
Statistics
The nonparametric Wilcoxon signed-rank test was used to compare the levels of Nrf2 and expression of target genes and inflammatory markers between the drug-treated and control cultures at baseline and after inflammatory stimuli. Statistical significance was accepted at p < 0.05.
RESULTS
CDDO-Im significantly upregulated Nrf2–dependent antioxidative genes in human PBMC ex vivo
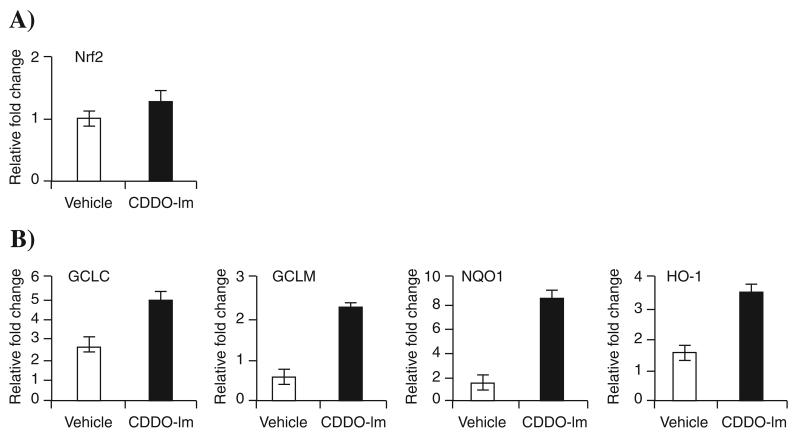
To evaluate the efficacy of CDDO-Im to activate the Nrf2 pathway, we measured the expression of Nrf2 and several antioxidant genes (NQO1, GCLM, GCLC, and HO-1) in PBMCs of six subjects. The constitutive expression of the Nrf2 gene in PBMC ranged threefold among the six subjects, and CDDO-Im treatment had no effect (Fig. 1). However, expression of several Nrf2-dependent antioxidative genes was significantly elevated in treated PBMCs of all subjects (Fig. 1B). The mean fold increase in transcript levels by CDDO-Im compared with vehicle was 16-fold for NQO1 and threefold to fourfold for the other antioxidative genes (GCLM, GCLC, and HO-1), suggesting that NQO1 might be a suitable biomarker for assessing Nrf2 activation in human PBMCs.
FIG. 1. CDDO-Im pretreatment up-regulates expression of Nrf2-dependent antioxidative genes in human PBMCs.
(A) mRNA expression of Nrf2 in PBMCs isolated from six normal subjects. (B) mRNA expression of Nrf2-dependent antioxidants NQO1, GCLM, HO-1, and GCLC in PBMCs isolated from six normal subjects. Isolated PBMCs were treated either with vehicle or CDDO-Im (20 nM) for 20 h, and gene-expression analysis was done by real-time PCR. Data are expressed as fold changes relative to the lowest value in the vehicle-treated groups. Horizontal line represents the mean fold change. *Distribution differs from vehicle-treated group (p < 0.05).
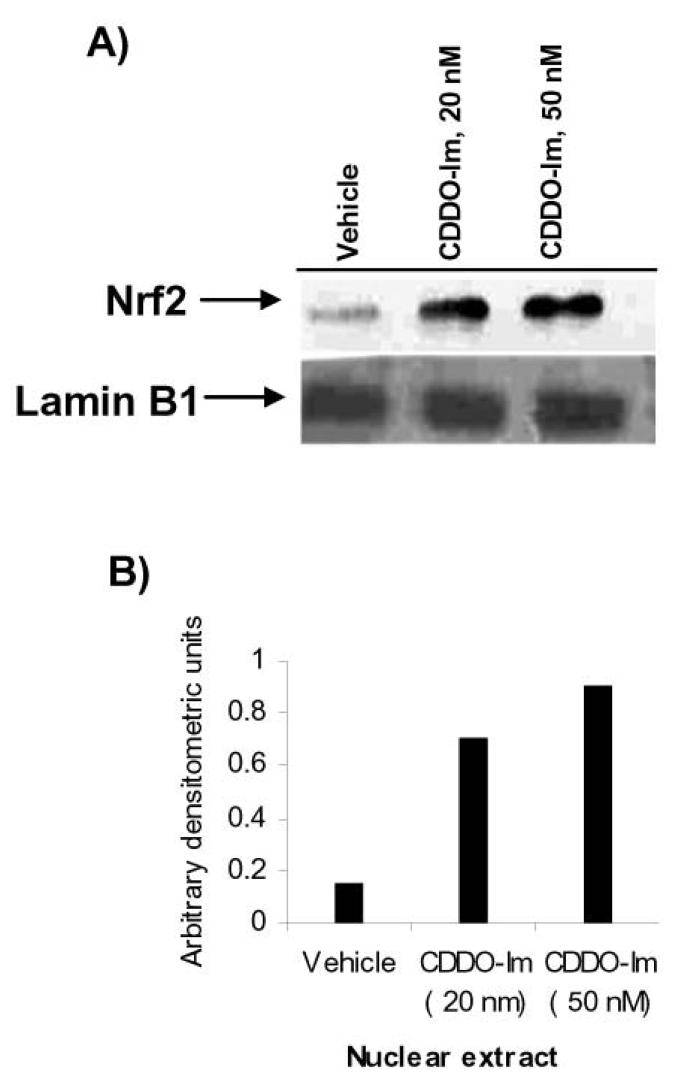
CDDO-Im treatment increased nuclear Nrf2 protein levels
CDDO-Im pretreatment showed no effect on the mRNA expression of the Nrf2 gene in PBMC (see Fig. 1A). Under normal condition, cellular levels of Nrf2 protein are very low, as Keap1 binds to Nrf2 and constantly targets it for proteosomal degradation. However, in conditions of stress or treatment with inducers, Nrf2 escapes degradation and translocates to the nucleus. Nuclear levels of Nrf2 protein increase, and it binds to antioxidant response elements to transactivate the expression of target genes (17). To probe the underlying mechanism of induction of Nrf2 targets by CDDO-Im, we measured the nuclear distribution of Nrf2 in PBMCs isolated from one subject. CDDO-Im at 20 nM and 50 nM concentrations increased the nuclear levels of Nrf2 protein fourfold to fivefold; however, no significant difference in nuclear levels was found between the two concentrations of CDDO-Im (Fig. 2).
FIG. 2. CDDO-Im induces nuclear accumulation of Nrf2 protein.
(A) Immunoblot demonstrating increase levels of Nrf2 protein in nuclear extracts of PBMCs treated with CDDO-Im. PBMCs were treated with either vehicle or CDDO-Im (20 nM or 50 nM) for 6 h. Cells were then harvested and processed for nuclear extraction. Nuclear proteins (20 μg) were resolved on a 10% PAGE, and immunoblotting was performed with an Nrf2 antibody, as described in Methods. The blot was reprobed with anti-Lamin B1 antibody as a marker of nuclear proteins. (B) Quantification of Nrf2 protein. For band densitometry, bands in nuclear extract blot were normalized to Lamin B1.
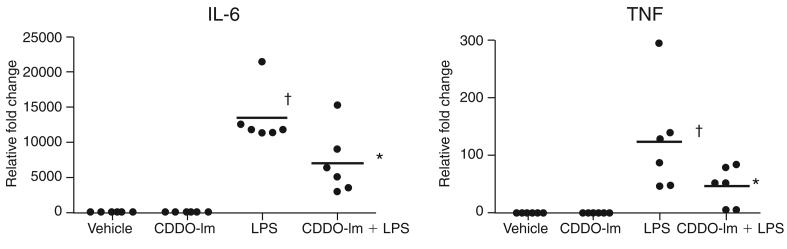
CDDO-Im treatment inhibited LPS-induced inflammatory response in PBMCs
Previously we demonstrated in mice that CDDO-Im significantly suppresses LPS-induced inflammation, primarily by activating the Nrf2 pathway (35). To test whether CDDO-Im inhibits LPS-induced inflammation in human PBMCs, PBMCs isolated from all six subjects were challenged with LPS after 20 h of CDDO-Im pretreatment. Vehicle or CDDO-Im alone did not show any significant induction of IL-6; however, TNF-α showed a small but significant increase. After LPS stimulation for 4 h, a dramatic increase was noted in the expression of IL-6 and TNF-α in PBMCs of all subjects. CDDO-Im pretreatment substantially blunted the expression of IL-6 and TNF-α in PBMCs of all the subjects (Fig. 3) when compared with LPS alone.
FIG. 3. CDDO-Im pretreatment attenuated LPS-induced cytokine expression in PBMCs isolated from normal subjects.
CDDO-Im pretreatment suppressed LPS-induced mRNA expression of IL-6 and TNF-α in PBMCs of six subjects measured 4 h after LPS treatment. Ex vivo PBMCs were pretreated with DMSO or CDDO-Im for 20 h followed by LPS (100 ng/ml) stimulation. Horizontal line represents the mean fold change. †Differs from vehicle control. *Differs from only LPS-treated group (p < 0.05).
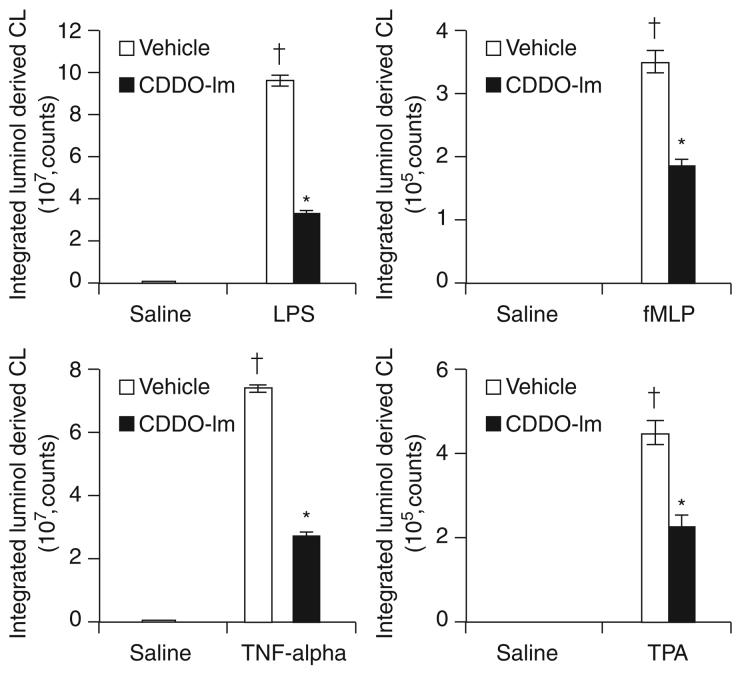
CDDO-Im upregulated Nrf2 pathway and attenuated ROS generation induced by different inflammatory stimuli in neutrophils ex vivo
Excessive production ROS along with proinflammatory mediators by neutrophils contributes to pathologic damage and multiorgan failure (10). ROS greatly affect neutrophil function by modulating TLR4-dependent NF-κB activation (3). We first assessed the efficacy of CDDO-Im to activate the Nrf2 pathway in peripheral blood neutrophils. Activation of Nrf2 pathway was measured by real-time RT-PCR for antioxidative target genes after CDDO-Im treatment in blood neutrophils pooled from two subjects. As in PBMCs, CDDO-Im did not affect basal expression of Nrf2 mRNA in neutrophils (Fig. 4). CDDO-Im significantly induced expression of glutathione-biosynthesizing enzymes (GCLC and GCLM), NQO1, and HO-1 (Fig. 4B). NQO1 transcripts showed the highest induction. Next we assessed whether CDDO-Im suppressed ROS generation in neutrophils after stimulation with LPS and other soluble stimuli, such as fMLP, TNF-α, and TPA. All of the inflammatory agents rapidly induced ROS generation, as measured by luminol-based chemiluminescence (Fig. 5). TPA induced the highest levels of ROS (4.4 × 109 counts) followed by fMLP (3.5 × 108 counts), LPS (9.6 × 107 counts), and TNF-α (7.3 × 107 counts). Regardless of inflammatory stimulus, CDDO-Im pretreatment suppressed ROS generation more than twofold, suggesting that it can potentially be used to blunt the response of neutrophils in septic patients (see Fig. 5).
FIG. 4. Treatment of peripheral blood neutrophils with CDDO-Im upregulated Nrf2–dependent antioxidative genes.
(A) mRNA expression of Nrf2 in neutrophils after CDDO-Im. (B) CDDO-Im induced expression of Nrf2–dependent antioxidative genes (GCLC, GCLM, NQO1, and HO-1). Ex vivo blood neutrophils pooled from two subjects were treated with either vehicle or CDDO-Im (20 nM) for 20 h and processed immediately for measurement of antioxidant gene expression. Experiments were performed in triplicate.
FIG. 5. CDDO-Im treatment attenuated ROS generation in peripheral blood neutrophils induced by LPS, fMLP, TNF-α, and TPA.
Ex vivo pooled peripheral blood neutrophils isolated from two subjects were treated with DMSO or CDDO-Im (20 nM) for 20 h. Immediately after this, cells were harvested and stimulated with different inflammatory agents (LPS, 100 ng/ml; fMLP, 10 μM; TNF-α, 10 ng/ml; TPA, 50 nM). Each bar is the mean ± SD (n = 3) of values presenting the integration of the area under curve for 30 min, expressed in counts. *Differs from LPS alone. δ, Differs from saline (p < 0.05).
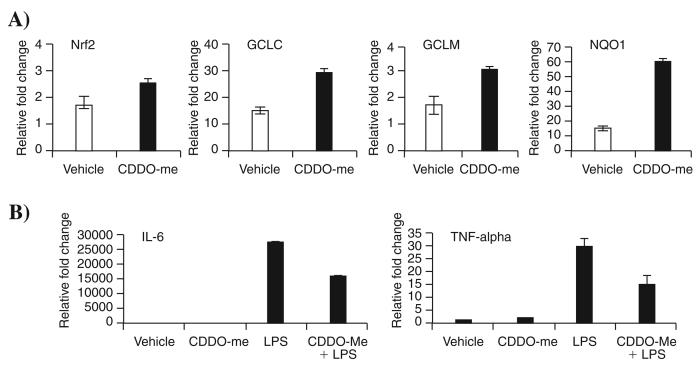
CDDO-Me, a methyl ester derivate of CDDO, also activated Nrf2 pathway and attenuated LPS-induced inflammation in PBMCs
Along with CDDO-Im, we also evaluated the efficacy of CDDO-Me to activate gene expression in PBMCs isolated from a normal subject. CDDO-Me is already in phase I clinical trials. Like CDDO-Im, CDDO-Me significantly activated the Nrf2 pathway, as assessed by measuring expression of NQO1, GCLC, GCLM, and HO-1 transcripts and also suppressed LPS-induced expression of IL-6 and TNF-α (Fig. 6).
FIG. 6. CDDO-Me, a methyl ester derivative of CDDO, significantly upregulated Nrf2–dependent antioxidants and suppressed LPS-induced cytokine expression in PBMCs isolated from a normal subject.
(A) mRNA levels of Nrf2 and antioxidative genes (GCLC, GCLM, and NQO1) in PBMCs after CDDO-Me treatment. (B) IL-6 and TNF-α expression in PBMCs pretreated with CDDO-Me after LPS challenge. Ex vivo PBMCs isolated from normal subjects were treated with either vehicle or CDDO-Me (20 nM) for 20 h and processed immediately for measurement of antioxidant gene expression. For assessing LPS-induced inflammation, cells were incubated with LPS for additional 4 h, and expression of IL-6 and TNF-α was assessed by real-time PCR. Experiments were performed in triplicate.
DISCUSSION
We established that Nrf2 is a critical host factor in protecting against sepsis-induced mortality in mice (33, 35). Absence of effective therapies for sepsis syndrome has led to an urgent need to explore novel targets for intervention. Based on the animal studies of efficacy of the Nrf2 activator CDDO-Im in protecting against LPS-mediated mortality (14) and association of Nrf2 polymorphism in septic patients (15), we performed a preclinical evaluation of triterpenoid analogues as Nrf2 activators to inhibit the oxidative stress and inflammatory response elicited by LPS in human peripheral blood cells. Major findings of the study are severalfold. First, CDDO-Im and CDDO-Me upregulate the expression of Nrf2 transcriptional targets NQO1, GCLC, GCLM, and HO-1. Second, CDDO-Im induced nuclear translocation of Nrf2 but did not affect Nrf2 transcript levels. Third, CDDO-Im and CDDO-Me suppressed LPS-induced inflammatory response in PBMCs. Fourth, CDDO-Im attenuated LPS-induced ROS generation and the expression of inflammatory cytokines in neutrophils.
Among the different analogues of triterpenoids, CDDO-Im and CDDO-Me have been reported to exhibit proapoptotic activity that was mainly mediated by inhibition of NF-κB activation (29, 39). Interestingly, at low nanomolar concentrations, CDDO-Im and CDDO-Me upregulated cytoprotective genes mediated by activation of Nrf2 (7). Yates et al. (38) extensively characterized the pharmacodynamics of CDDO-Im in activating the Nrf2 pathway in different organs (lung, liver, kidney, heart, intestine, brain) of mice (38). Our study reports the ability of CDDO-Im and CDDO-Me to activate Nrf2 signaling in peripheral immune cells of normal subjects ex vivo. Although a considerable degree of interindividual variation was noted in the magnitude of induction, CDDO-Im upregulated gene expression in each of the subject's PBMCs. In line with the results of the murine study reported by Yates et al., the present study also found that expression of NQO1 exhibits a large dynamic range, rendering it suitable as a marker for assessment of Nrf2 activation. Although the study focused on the expression of antioxidative genes, Nrf2 is known to induce a host of other cytoprotective genes, including xenobiotic conjugating enzymes, ubiquitin/proteosomes, chaperones, and heat-shock proteins (16).
Sepsis syndrome is characterized by an inappropriate response of immune cells, particularly neutrophils. A growing body of evidence relates neutrophil dysfunction with severity of sepsis and is linked with end-organ failure and mortality (9). Excessive release of proinflammatory mediators, ROS, and proteases by activated neutrophils exacerbates sepsis by increasing inflammation, oxidative tissue damage, vascular permeability, and organ injury (9). With animal models of sepsis, depletion of neutrophils before CLP or administration of Staphylococcus aureus has been shown to increase bacteremia and mortality (10, 36). Interestingly, depletion of neutrophils after CLP in the mice model has been shown to reduce bacteremia significantly, to reduce liver and renal dysfunction, as well as to decrease serum levels of proinflammatory cytokines (10). Similarly, in a rat model of peritonitis, neutrophils have been associated with liver failure (21). Recently, Kaufmann et al. (15) reported neutrophil dysfunction in patients with severe sepsis (15). Neutrophils from severely septic patients exhibited a compromised phagocytic function; however, they produced higher amounts of ROS on activation by soluble stimuli (like fMLP, TNF-α, and TPA) compared with healthy subjects (15). Our study illustrates the effectiveness of CDDO-Im to attenuate ROS production by neutrophils stimulated with LPS or soluble stimuli like, fMLP, TNF-α, and TPA, or both. We previously showed that CDDO-Im mediates its antioxidant activity by inducing the Nrf2 pathway (35). CDDO-Im failed to inhibit LPS-induced ROS production in peritoneal neutrophils of Nrf2–deficient, but not wild-type mice. In another study, CDDO-Im was reported to inhibit IFN-γ–induced nitric oxide production by macrophages (7). The inhibition of nitric oxide was correlated with Nrf2–mediated inhibition of iNOS expression. Taken together, these results support a potential therapeutic role of CDDO-Im in modulating neutrophil function during sepsis. This effect may be beneficial for preventing organ failure.
Enhanced apoptosis of lymphocytes is another characteristic feature of septic patients that leads to immunosuppression and increases susceptibility to further infection (12). In animal models of sepsis, overexpression of antiapoptotic protein such as Bcl2 and inhibition of caspases resulted in reduction in lymphocyte apoptosis as well as improved survival (11, 13). Activation of the Nrf2 pathway protects cells from oxidative and inflammatory stimulus–induced apoptosis by maintaining cellular redox homeostasis. Nrf2–deficient splenocytes are more susceptible to hydrogen peroxide–induced apoptosis (18). Interestingly, Nrf2 also protected from FasL- and TNF-α–induced apoptosis by maintaining GSH levels (22). The ability of CDDO-Im and CDDO-Me to upregulate the Nrf2 pathway in PBMCs, which encompasses lymphocytes as a major population, might protect them from proapoptotic ligands such as FasL and TNF-α during sepsis.
As the sepsis syndrome was characterized by an amplified innate immune response, most of the clinical trials were directed toward suppressing the immune response by using antiinflammatory agents such as anti-endotoxin, anti-CD14, anti-LBP, anti–platelet-activating factor, anti-TNF, anti–IL-1, and glucocorticoid therapies. Unfortunately, most of these trials showed poor or negative outcomes. Death in septic patients is due to multiorgan failures that are caused mainly by inflammation as well as sustained oxidative tissue damage. Patients with higher levels of oxidative damage byproducts and low antioxidants levels have a higher incidence of multiorgan failure and poorer prognosis (6). Because Nrf2–regulated cytoprotective genes protect from oxidative damage as well as suppress inflammation by modulating immune cell function, we speculate that the activation of the Nrf2 pathway might prove to be a suitable therapeutic strategy for intervention in sepsis as well as in systemic inflammatory response and other inflammatory disorders such as acute lung injury and acute respiratory distress syndrome. This view is reinforced by recent reports that other classes of Nrf2 activators also protect against sepsis in rodents (14), the association of an Nrf2 polymorphism with increased risk of developing acute lung injury in trauma and septic patients (19), and a significant decline in the Nrf2 pathway with age in animal models (32).
ACKNOWLEDGMENTS
We thank all the subjects for their time and cooperation. We acknowledge the help of Juliana M Cuervo-Rojas, Nicolas Epie, and Melinda Yates during the study. This work was supported by NIH grants GM079239 and HL081205 (S.B.), and P50 CA058184 and CA94076 (T.W.K.), NIEHS center grant P30 ES 03819, Young Clinical Scientist award from Flight Attendant Research Institute (S.B.), CA78814 (M.B.S.), and Reata Pharmaceuticals (M.B.S.).
ABBREVIATIONS
- CDDO-Im
1-2-cyano-3-12-dioxooleana-1,9(11)-dien-28–oyl]-imidazole
- CDDO-Me
methyl ester derivative of [2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid
- LPS
lipopolysaccharide
- Nrf2
nuclear factor-erythroid 2–related factor 2
- PBMCs
peripheral blood mononuclear cells
- ROS
reactive oxygen species
REFERENCES
- 1.Abraham E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis. 2003;187(suppl 2):S364–S369. doi: 10.1086/374750. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NF-kappaB. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 4.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 5.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, Napoli C. The role of oxidative stress in adult critical care. Free Radic Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 7.Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102:4584–4589. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007 doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 9.Hoesel LM, Gao H, Ward PA. New insights into cellular mechanisms during sepsis. Immunol Res. 2006;34:133–141. doi: 10.1385/IR:34:2:133. [DOI] [PubMed] [Google Scholar]
- 10.Hoesel LM, Neff TA, Neff SB, Younger JG, Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV, Ward PA. Harmful and protective roles of neutrophils in sepsis. Shock. 2005;24:40–47. doi: 10.1097/01.shk.0000170353.80318.d5. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 14.Karuri AR, Huang Y, Bodreddigari S, Sutter CH, Roebuck BD, Kensler TW, Sutter TR. 3H-1,2–Dithiole-3–thione targets nuclear factor kappaB to block expression of inducible nitric-oxide synthase, prevents hypotension, and improves survival in endotoxemic rats. J Pharmacol Exp Ther. 2006;317:61–67. doi: 10.1124/jpet.105.096396. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann I, Hoelzl A, Schliephake F, Hummel T, Chouker A, Peter K, Thiel M. Polymorphonuclear leukocyte dysfunction syndrome in patients with increasing sepsis severity. Shock. 2006;26:254–261. doi: 10.1097/01.shk.0000223131.64512.7a. [DOI] [PubMed] [Google Scholar]
- 16.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2006 doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JM, Chan K, Kan YW, Johnson JA. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proc Natl Acad Sci U S A. 2004;101:9751–9756. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, Kleeberger SR. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007 doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 20.Mitra S, Abraham E. Participation of superoxide in neutrophil activation and cytokine production. Biochim Biophys Acta. 2006;1762:732–741. doi: 10.1016/j.bbadis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Molnar RG, Wang P, Ayala A, Ganey PE, Roth RA, Chaudry IH. The role of neutrophils in producing hepatocellular dysfunction during the hyperdynamic stage of sepsis in rats. J Surg Res. 1997;73:117–122. doi: 10.1006/jsre.1997.5216. [DOI] [PubMed] [Google Scholar]
- 22.Morito N, Yoh K, Itoh K, Hirayama A, Koyama A, Yamamoto M, Takahashi S. Nrf2 regulates the sensitivity of death receptor signals by affecting intracellular glutathione levels. Oncogene. 2003;22:9275–9281. doi: 10.1038/sj.onc.1207024. [DOI] [PubMed] [Google Scholar]
- 23.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, Sasidhar M, Nabel EG, Takahashi T, Lukacs NW, Ryter SW, Morita K, Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 25.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A, Rotstein OD. Oxidative stress generated by hemorrhagic shock recruits toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med. 2006;203:1951–1961. doi: 10.1084/jem.20060943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. A synthetic triterpenoid, CDDO-Me, inhibits IkappaBalpha kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of nuclear factor kappaB-regulated gene products in human leukemic cells. Clin Cancer Res. 2006;12:1828–1838. doi: 10.1158/1078-0432.CCR-05-2044. [DOI] [PubMed] [Google Scholar]
- 30.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohe R, Kensler TW, Yamamoto M, Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006 doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 35.Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, Honda T, Gribble GW, Sporn MB, Kensler TW. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2–regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- 38.Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2–regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- 39.Yore MM, Liby KT, Honda T, Gribble GW, Sporn MB. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232–3239. doi: 10.1158/1535-7163.MCT-06-0444. [DOI] [PubMed] [Google Scholar]