Abstract
Because most naturally occurring toxins taste bitter to humans, any mechanism that reduces the rate at which bitter substances are ingested and digested should be adaptive. Based on the recent discovery of T2R bitter taste receptors in the gastrointestinal tract of rodents, we asked whether intragastric (IG) infusion of denatonium (a ligand for T2R receptors) would condition a flavor aversion and/or delay gastric emptying. Four experiments tested for post-oral responses to denatonium in rodents. First, Sprague-Dawley rats were trained to associate intake of a flavored solution (the CS+) with IG denatonium infusions, and intake of a different-flavored solution (the CS−) with IG water infusions during 30 min/day sessions. The rats acquired an aversion to the CS+ flavor when it was paired with IG infusions of 10 mM (but not 2.5 mM) denatonium. Intragastric infusions of 10 mM denatonium also delayed gastric emptying of food in the same rats. Second, we asked how long it took for rats to suppress their drinking while being infused IG with 10 mM denatonium. Rats drinking a palatable solution paired with IG infusions of 10 mM denatonium suppressed their licking within 6 min, as compared to rats infused IG with water. Third, we trained C57BL/6J (B6) mice 24 h/day to associate a CS+ flavor paired with IG infusions of 12 mM denatonium (diluted to 6 mM by orally consumed CS+). Like rats, the mice acquired a robust aversion to the CS+ flavor when it was paired with IG infusions of denatonium. A final experiment assessed the potential toxicity of denatonium. To this end, we gave B6 mice a 6 mM denatonium solution as their only source of water for 3 weeks. The mice grew normally and did not display any clinical signs of denatonium toxicosis. This study provides the first evidence that rodents respond to the presence of “bitter” substances in their gastrointestinal tract by generating both behavioral and physiological responses.
Keywords: bitter, gastrointestinal tract, rat, mouse, flavor aversion learning, gastric emptying
1. Introduction
Many naturally occurring foods contain toxins [7,21,22]. The orosensory systems (taste, smell and somatosensation) constitute an important mechanism for detecting these dangerous foods because most toxins elicit aversive oral sensations (e.g., bitterness or astringency) in humans [2,6,7,14,35] and in other animals [16]. This oral protective mechanism is not foolproof, however. For example, the aversive oral sensations elicited by many bitter compounds can be masked (partially or completely) by the presence of sugars [27,31,51] or sodium [4,5]. Further, once animals adapt to the taste of some harmless bitter compounds [23,33,56,59], their tolerance to the taste of other toxic bitter compounds would increase [28,34,37]. There is evidence that leeches possess a “bitter taste” system in their gastrointestinal tract, which might function in situations where the oral mechanism failed [30]. We asked whether an analogous system exists in rodents.
Several observations provide support for the notion that mammals possess an extensive, but diffuse, network of chemosensory cells in their gut (i.e., stomach, small intestine, pancreatic duct and colon), which senses bitter substances. First, some of the chemosensory cells in the gut express the same proteins that mediate bitter taste in the oral cavity, including T2R bitter taste receptors [40,57,58] and three downstream signaling proteins, α-gustducin, PLC β2 and Trpm5 [3,12,24,25,50,52]. Second, two T2R ligands (denatonium and phenylthiocarbamide [38,43]) induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells [9]. Third, gastric infusion of denatonium elicits a strong excitatory response in the rat's vagus nerve [53], which relays input from chemosensory cells in the gut to the brainstem [45]. Fourth, bitter stimuli elicit a concentration-dependent decrease in stomach contractions when infused intragastrically in dogs [8,36].
Because rodents lack the vomiting reflex, they cannot eject poisons once they have been swallowed. For this reason, it has been suggested that rodents should have evolved multiple mechanisms for protecting them against toxic foods [10]. Here, we tested the hypothesis that rodents respond to the presence of “bitter” substances in their gastrointestinal tract by reducing the rate at which foods are ingested and digested. To evaluate this hypothesis, we conducted four experiments. First, we asked whether intragastric (IG) infusion of a bitter taste stimulus (denatonium) would generate negative feedback in rats, leading to a conditioned flavor aversion or delayed gastric emptying. Second, we determined how long it took for IG infusions of denatonium to inhibit licking for a palatable substance. Third, we ascertained whether IG infusions of denatonium would condition a flavor aversion in mice. Fourth, we explored the possibility that denatonium was conditioning an aversion in the mice through a toxicity mechanism.
2. Do IG infusions of denatonium elicit behavioral or physiological responses in rats? (Experiment 1)
Prior studies reported that denatonium and other T2R ligands can both inhibit feeding [1,27] and delay gastric emptying [13] in rats. However, because the T2R ligands were ingested, it is impossible to determine the relative contribution of oral versus post-oral stimulation to the responses. In this experiment, we asked whether IG stimulation by denatonium alone is sufficient to condition a feeding aversion and delay gastric emptying in rats.
2.1. Methods
2.1.1. Animals
We used 15 male Sprague-Dawley rats born in our laboratory from stock purchased from Charles River Laboratories (Wilmington, MA). The rats were 100 days old at the start of testing. We housed each rat individually in a standard wire-mesh cage in a vivarium maintained at 21°C with a 12:12 h light/dark cycle (lights on 0800 h). We provided powdered chow (No. 5001, PMI Nutrition International, Brentwood, MO) and tap water ad libitum. We subjected the same 15 rats to Experiments 1A-D.
2.1.2. Surgery
We anesthetized the rats with ketamine (63 mg/kg) and xylazine (9.4 mg/kg) and then fitted them with an IG catheter based on a technique adapted from Davis and Campbell [11]. In brief, a silastic tube (i.d.: 1.02 mm; o.d.: 2.16 mm) was inserted into the fundic region of the stomach. We routed the silastic tube under the skin to the back of the neck, and then connected it to a Luer-lock assembly, which was fixed to the skull with dental cement and stainless steel screws.
2.1.3. Apparatus
We trained and tested the rats in plastic infusion cages. For the IG infusions, we ran plastic tubing from a syringe pump to the input port of a swivel on a counterbalanced lever. We connected the swivel's output port to the rat's IG catheter via additional plastic tubing, which was protected by a stainless-steel spring.
We offered the conditioning stimuli (i.e., two different fruit-flavored solutions; see below) in two stainless steel drinking spouts. The spouts could be accessed through slots in the front wall of the plastic infusion cage, centered and 32 mm apart. We attached each drinking spout to a separate fluid reservoir, each of which was mounted on a motorized holder. These holders positioned the spouts in front of the slots (so that they could be accessed by the rats) at the start of the 30-min test session, and retracted them at the end of the session. Trays below the sipper tubes collected spillage. We monitored licking with an electronic lickometer (Med Electronics, St. Albans, VT) and a microcomputer, which controlled the syringe pumps.
During each training or testing session, the computer software accumulated licks and turned the infusion pumps on or off, as required, every 3 sec. The infusion pump delivered the test solution directly into the stomach at a rate of 1.3 ml/min; the oral intake/infusion ratio was maintained at ∼ 1:1 by computer software. With this system, the animal controlled the infusion volume by its licking behavior, and the concentration of infused denatonium was diluted by the orally consumed fluid; e.g., an infusion of 2.5 mM denatonium was diluted to 1.25 mM denatonium in the stomach. We recorded CS intakes to the nearest 0.1 g and IG infusions to the nearest 0.5 ml
2.1.4 Test solutions
Each conditioning stimulus (CS) contained a 0.05% Kool-Aid flavor (General Foods, White Plains, NY) and 0.2% sodium saccharin (Sigma-Aldrich, St. Louis, MO) dissolved in water. We used three different flavor pairs: cherry and grape in Experiment 1A; orange and lemon-lime in Experiment 1B; and raspberry and arctic green apple in Experiment 1C. The unconditioned stimulus (US) contained one of three concentrations of denatonium benzoate (Sigma-Aldrich) dissolved in water: 2.5 mM in Experiment 1A; 10 mM in Experiment 1B; and 1.25 mM in Experiment 1C. In this and all subsequent experiments, the CS and US solutions were presented at room temperature.
Because 1.25 mM is the lowest concentration of denatonium that rats avoid reliably in oral taste tests [49], we used concentrations that were equal to or greater than 1.25 mM. To this end, we infused the rats IG (in Experiment 1A-B) with 2.5 and 10 mM denatonium, which would be diluted in the stomach to 1.25 and 5 mM denatonium, respectively, by the ingested CS solution. Further, we used an oral US that contained 1.25 mM denatonium in Experiment 1C.
In each test, we offered the rats one flavor paired with denatonium (the CS+) and another flavor paired with water (the CS−). To control for potential flavor biases, we counter-balanced the flavors in the CS+ and CS− (e.g., cherry and grape) across subjects, separately for each experiment.
2.1.5 Adaptation to test solutions and IG infusions
Prior to surgery, we familiarized the rats with unflavored 0.2% saccharin solution by giving them ad libitum access to the saccharin solution together with water and powdered chow in their home cages for two days. Then, we adapted the rats to drink saccharin in the test cages overnight with food ad libitum; the saccharin bottle was automatically positioned to the front of the cages for 30 min every hour. Following recovery from surgery (∼10 days), we placed the rats on a food-restriction schedule, maintaining them at 85% of their ad libitum body weight. We adapted them to drink the saccharin solution in the test cages during daily 30-min sessions; intake of the saccharin solution was paired with matched IG infusions of water.
2.1.6 Do IG infusions of 2.5 mM denatonium condition flavor aversions? (Experiment 1A)
Each rat was run through six one-bottle training sessions (30 min/day). Half of the rats were given the CS+ flavor paired with IG infusions of 2.5 mM denatonium during training days 1, 3, and 5, and the CS− flavor paired with IG infusions of water during training days 2, 4, and 6. The remaining rats were trained in the reverse order.
Two steps were implemented to minimize the possibility that denatonium might spill onto the head connector and be tasted by the rats during training. First, at the start of the session, the infusion tubing connected to the head cap was filled with water (∼ 0.8 ml) before connecting it to the syringe containing denatonium. Second, at the end of the session, the tubing was flushed with 2.5 ml of water before it was disconnected from the head cap.
After 6 days of training, a two-bottle preference test was conducted, in which the rats had a choice between the CS+ and CS− solutions across two 30 min sessions (each on separate days); the left-right position of the CS solutions was alternated from the first to the second session. The rats were not infused on two-bottle test days.
Upon completion of the eight training and testing sessions, we ran a second cycle of training and testing using the same procedures. We performed this replicate because we failed to obtain evidence for avoidance conditioning, and wanted to ensure that this negative finding was repeatable.
2.1.7 Do IG infusions of 10 mM denatonium condition flavor aversions? (Experiment 1B)
After one session with unflavored saccharin paired with IG water, the rats were given another six one-bottle training sessions as in Experiment 1A, except that we infused 10 mM denatonium and used new flavors in the CS solutions (i.e., orange and lemon-lime). Following training, the rats were given two-bottle preference tests with the CS+ versus CS− for four 30 min/day sessions without IG infusions.
2.1.8 Does oral intake of 1.25 mM denatonium condition flavor aversions? (Experiment 1C)
After one 30 min test session with unflavored saccharin, the rats were given six one-bottle training sessions with no IG infusions. During three of the training sessions, they received the CS+ solution, which contained 1.25 mM denatonium, a 0.05% Kool-Aid flavor, and 0.2% saccharin in deionized water. During the other three training sessions, the rats received the CS− solution, which contained a different 0.05% Kool-Aid flavor, and 0.2% saccharin. The CS flavors were raspberry and arctic apple. After training, a two-bottle test was conducted (two 30 min/day sessions) with the CS+ and CS− flavors presented in solutions containing only saccharin.
2.1.9 Does IG infusion of 10 mM denatonium delay gastric emptying? (Experiment 1D)
The food-restricted rats from Experiments 1A-C were acclimated to eating 8 g of wet mash (4 g powdered chow, 4 g water) during two 30 min/day sessions; as they ate the mash they were infused IG with 8 ml of water (1.3 ml/min). (Two rats that developed problems with their gastric catheter were excluded from this experiment.) During a third session, seven rats were infused with 8 ml of 10 mM denatonium and six rats were infused with 8 ml of water as they ate the mash. Because the mash diet contained 4 g of water, the concentration of the denatonium in the stomach was diluted to about ∼ 6.6 mM, which approximated the net 5 mM concentration experienced by the rats in Experiment 1B.
Two hours after the end of the third 30-min test session, the rats were sacrificed and their stomach contents were removed and dried overnight. Although food spillage during the feeding test was minimal (∼ 0.06 g), we nevertheless corrected each stomach content measure to accommodate this spillage.
2.1.10 Data analysis
We used the same analysis approach for results from Experiments 1A-C. First, we calculated mean intake/session across each of the three 30-min training sessions, separately for the CS+ and CS− solutions. Then, we compared the mean intakes from the CS+ and CS−, using paired t-tests. Second, we calculated mean intake from the CS+ and CS−solutions across two successive preference test sessions. Then, we compared intake from both solutions, using the paired t-test. For Experiment 1D, we compared stomach content weight between rats infused with water and those infused with 10 mM denatonium, using the unpaired t-test. We set the alpha level at 0.05 in this and all subsequent experiments.
2.2. Results
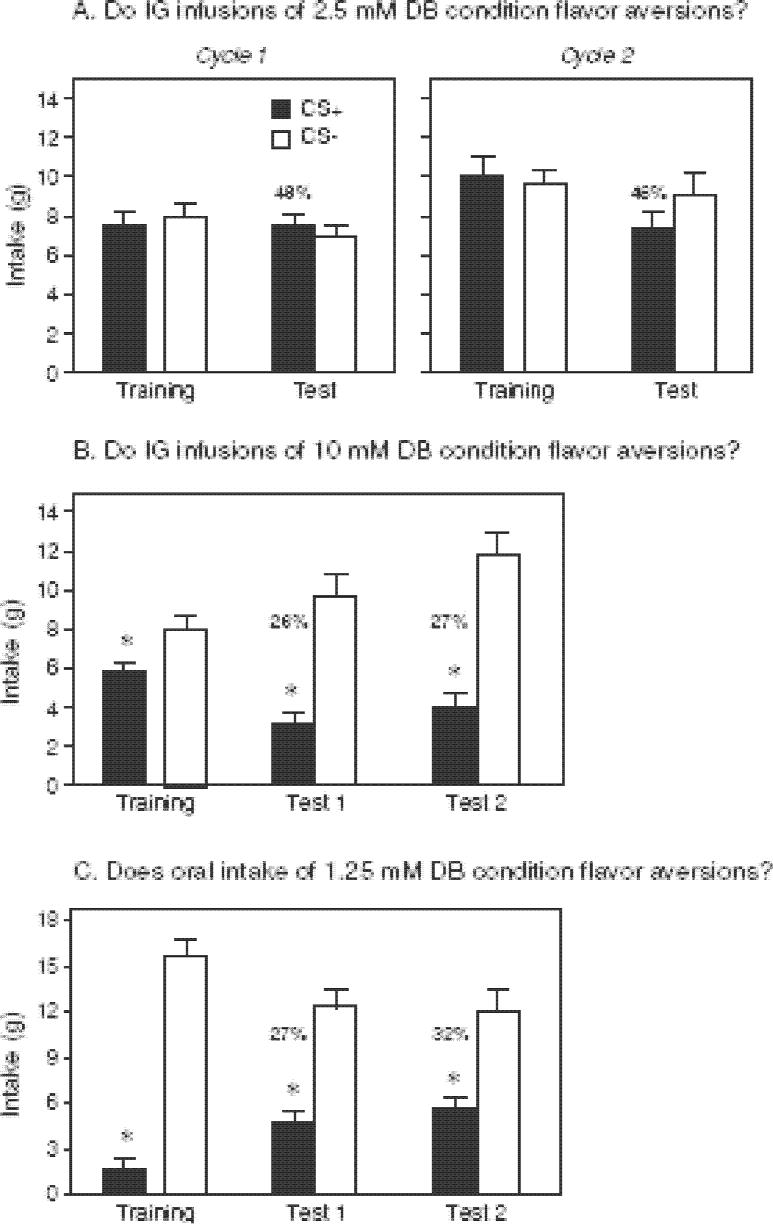
In Experiment 1A, the rats were trained with the CS+ flavor paired with IG infusions of 2.5 mM denatonium. Their intakes of the CS+ and CS− solutions did not differ in the one-bottle training and two-bottle tests sessions over the two cycles (Fig. 1A). This indicates that IG infusions of 2.5 mM denatonium (1.25 mM net concentration when diluted with orally consumed CS+ solution) is ineffective in producing a conditioned flavor aversion.
Figure 1.
Intake of CS+ and CS− solutions (mean ± S.E.) during one-bottle training and two-bottle preference tests with Sprague-Dawley rats. Numbers atop bars represent mean percentage of CS+ consumed during the preference test, calculated separately for each rat. A. Rats were given two cycles of training (six 30-min sessions each) with the CS+ paired with IG infusions of 2.5 mM denatonium and CS− paired with IG water. In the two-bottle tests (2 sessions each) with CS+ vs. CS− there were no IG infusions (see Experiment 1A). B. Intake of the CS+ was paired with IG infusions of 10 mM denatonium during one-bottle training. Following 6 training sessions, two-bottle tests (four 30-min sessions) were conducted without infusions (see Experiment 1B). C. Rats were not infused, but were given a CS+ solution containing 1.25 mM denatonium during one-bottle training. Following six training sessions, two-bottle CS+ vs. CS− tests (four 30-min sessions) were conducted; the CS+ did not contain denatonium (see Experiment 1C). The same 15 rats were used in all three panels. * P < 0.05 (paired t-test).
In Experiment 1B, the rats were retrained with new CS flavors paired with IG infusions of 10 mM denatonium and water, respectively. As indicated in Fig. 1B, the rats consumed significantly less CS+ than CS− during the one-bottle training sessions and substantially less CS+ during the two-bottle preference tests. The average denatonium dosage that the rats experienced across each of the three 30-min training sessions with the CS+ was 67 mg/kg. It is notable that the CS+ aversion was expressed in the absence of reinforcement (i.e., associated infusions of 10 mM denatonium) and remained stable at ∼27% over Tests 1 and 2 (each a mean of two sessions). This finding shows that IG infusions of 10 mM denatonium conditioned a robust flavor aversion.
Experiment 1C determined whether 1.25 mM denatonium could condition a flavor aversion when consumed orally. This concentration was selected because it is avoided in drinking tests [49], but failed to condition an aversion in Experiment 1A. The rats consumed very little of the CS+ solution containing 1.25 mM denatonium during one-bottle training, and displayed a significant aversion to the CS+ flavor (in the absence of denatonium) over the four test sessions; percent CS+ intakes ranged between 27 and 32% (Fig. 1C).
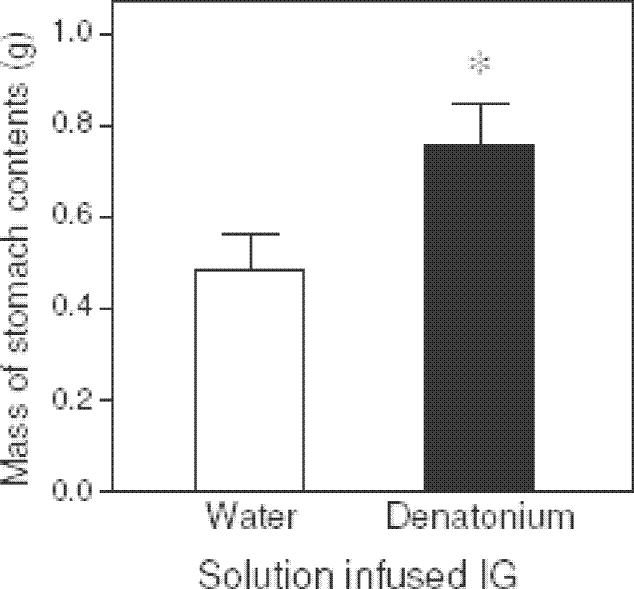
Experiment 1D revealed that the weight of stomach contents in rats infused with 10mM denatonium was significantly greater than that of rats infused with water (Fig. 2). Because the rats in the two groups consumed the same quantity of mash during the session (∼8 g), this result indicates that the gastric emptying rate was slower in the denatonium-infused rats than the water-infused rats.
Figure 2.
Weight of stomach contents (mean ± S.E.) following the mash-diet intake test with Sprague-Dawley rats (see Experiment 1D). Seven rats were infused with 10 mM denatonium during the 30-min mash diet test and 6 rats were infused IG with water. Two hours after the end of the feeding test, the rats were sacrificed, stomach contents removed and dried. N = 15 rats; * P < 0.05 (unpaired t-test).
3. How long does it take for IG denatonium infusions to reduce intake by rats? (Experiment 2)
A 5 mM concentration of denatonium can completely suppress licking in rats within 5 s when it is sampled orally [49]. Here, we asked how long it takes for denatonium to suppress licking when infused IG. To this end, rats were infused IG with 10 mM denatonium or water as they ingested a familiar, palatable solution; note that the infused denatonium solution would be diluted to 5 mM by the orally consumed palatable solution. To determine when the IG denatonium had an intake-inhibiting post-oral action, we compared minute-by-minute lick rates of the rats receiving IG denatonium or water. We inferred that the unconditioned inhibitory action of the bitter tastant had begun when the denatonium-infused rats licked significantly less vigorously than the water-infused rats.
3.1. Methods
3.1.1. Animals
We used 24 female Sprague-Dawley rats born in our laboratory from stock purchased from Charles River Laboratories (Wilmington, MA). All husbandry and maintenance details were as in Experiment 1. The rats had been used in prior experiment comparing the IG preference conditioning and satiating effects of different carbohydrate and fats (Ackroff and Sclafani, unpublished experiment).
3.1.2 Experimental procedures
The procedural details for anesthesia, surgery, IG infusions and food restriction schedule were similar to those of Experiment 1. As part of a prior study, the rats were adapted to drink a palatable solution containing 2% maltodextrin (Maltrin QD 580, Grain Processing, Muscatine, IA) and 0.2% sodium saccharin solution that was paired with matched IG infusions of water during 30 min/day sessions. In the present experiment we gave the rats an additional 30-min session with the maltodextrin/saccharin solution paired with IG water infusions. We then divided the rats into two groups (n = 12 each) equated for their 30-min maltodextrin/saccharin solution intake (19.0 g each) and total licks (3802.7 vs. 3839.2 licks). We conducted a single 30-min test in which the maltodextrin/saccharin solution was paired with matched IG infusion of 10 mM denatonium in one group and IG water infusion in the second group.
3.1.3. Data analysis
We compared 30-min intake of the maltodextrin/saccharin solution across the denatonium- and water-infused groups with an unpaired t-test. Further, we evaluated licking rates (expressed as licks/min) over the 30-min test with a mixed-model 2-way ANOVA. We treated time (30 successive 1-min time bins) as a within factor, and infusion group (denatonium vs. water) as a between factor. We determined the earliest time-bin during which the denatonium-infused rats licked less than the water-infused rats with the unpaired two-tailed t-test. Because we ran only a single t-test, we did not require a Bonferroni correction. We excluded one water-infused rat from the lick rate analysis because of a problem with its lickometer circuit.
3.2 Results
The denatonium-infused rats consumed significantly less of the maltodextrin/saccharin solution than did the water-infused rats (mean = 15.9 vs. 19.7 g) during the 30-min test session (t22 = 3.4). The analysis of the lick rate data revealed significant main effects of infusion group (F1, 609 = 9.0) and time (F29, 609 = 19.0), and a significant interaction of infusion group x time (F29, 609 = 1.7). This result reflects the fact that the denatonium- and water-infused rats licked equally vigorously over the initial five minutes of the test session, but at minute 6 the denatonium-infused rats began to lick more slowly (Fig. 3). The suppressed licking in the denatonium-infused rats persisted for about 11 min, after which both groups of rats licked relatively infrequently.
Figure 3.
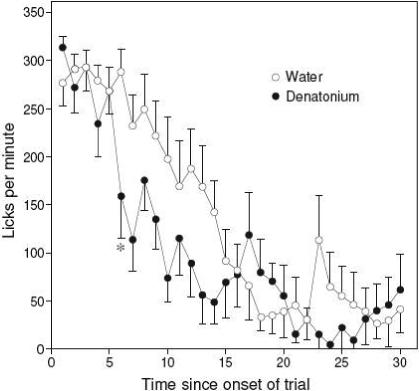
Impact of IG infusions of 10 mM denatonium (denatonium) versus water on licking (mean ± S.E. licks) for a palatable solution (2% maltodextrin + 0.2% saccharin) in rats (see Experiment 2). We present number of licks taken across successive 1-min time bins from the palatable solution as the rats received matched IG infusions of denatonium or water. Each rat was tested only once. We indicate the first time-bin (i.e., 6 min) during which the denatonium rats took significantly fewer licks than the water rats (see asterisk; * P < 0.05, unpaired t-test). N = 11−12 rats per infusion treatment.
4. Do IG denatonium infusions condition flavor aversions in mice? (Experiment 3)
Like rats, mice learn prefe rences for flavors that are associated with intragastric infusions of sugars [46,47]. Here, we attempted to condition an aversion to a flavored solution by associating its intake with IG infusions of denatonium. This experiment was designed to complement Experiment 1, and determine whether a similar phenomenon occurs in mice. In this case, the mice were trained and tested 24 h/day with ad libitum access of food and fluid.
4.1. Methods
4.1.1. Animals
We used 9 male C57BL/6J (B6) mice (Jackson Laboratory, Bar Harbor ME), which were 10 weeks old at the time of surgery. Prior to testing, we housed all mice individually in standard tub cages (27.5 × 17 × 12.5 cm) in a vivarium maintained at 23°C with a 12 h:12 h light:dark cycle. Eight days prior to surgery, we transferred the mice to the infusion cages so that they could acclimate to them. Throughout the study, we provided the mice with Purina Chow (5001, PMI Nutrition International, Brentwood, MO) and tap water ad libitum.
4.1.2. Surgery
The surgical details are provided in Sclafani and Glendinning [47]. In brief, we anesthetized the mice with isoflurane (2−4%) inhalation and fitted them with a gastric catheter constructed of micro-renathane tubing. We passed the distal end of the catheter through an incision in the abdominal muscle, routed it under the skin to the back of the neck, and then passed it through a hole in the skin. We closed the tip of the catheter with a stainless-steel stylet. The abdominal incision was closed using Nexaband adhesive (Veterinary Products Laboratories, Phoenix, AZ) and the skin incision was sutured closed (5−0 silk) and treated with triple antibiotic ointment. In a second procedure performed 3−7 days later, we anesthetized each mouse for 5 min with 2% isoflurane inhalation and extended the gastric catheter with a 27-cm length of micro-renathane tubing. We passed the tubing through an infusion harness and spring tether (CIH62, Instech Laboratories, Plymouth Meeting, PA); we secured the infusion harness to the mouse with belly-bands. Following each procedure, we allowed the mice to recover in small, heated tub cages before returning them to the infusion cages.
Prior to the surgery, we adapted the mice to consume 3 ml of chocolate Ensure™, a palatable nutritive solution, for several days. To facilitate post-operative recovery, the mice were given Ensure (3 ml/day), in addition to their lab chow, for several days after surgery.
4.1.3. Testing apparatus
We ran the conditioning procedure in custom-made infusion cages (15 × 15 × 32 cm high), which were constructed of clear plastic with a stainless-steel perforated floor. We offered fluid with one or two stainless steel sipper spouts, which were attached to 50-ml plastic centrifuge tubes. The mice licked the spouts through two slots (5 × 20 mm, 32 mm apart) in a stainless steel plate at the front of the cages. Chow pellets were available continuously from a stainless steel wire mesh food hopper that entered the back wall of the cage
After fitting a mouse with the infusion harness, we connected the spring tether to a swivel on a counterbalanced lever (Instech Laboratories) positioned at the top of the cage. Then, we inserted the output port of the swivel into the mouse's gastric catheter tubing, and connected the input port of the swivel to a 30-ml plastic syringe, which was in turn secured in a syringe pump (A-99, Razel Scientific, Stamford, CT). We monitored licking with an electronic lickometer (Med Electronics, St. Albans, VT) and a microcomputer, which controlled the syringe pumps. The computer software accumulated licks and turned the infusion pumps on or off, as required, every 3 sec. We set the pump rate to 0.5 ml/min, and maintained oral intake/infusion ratio at approximately 1:1 by adjusting a lick/pump activation parameter. In two-bottle tests, we attached two infusion pumps via a 26 g Y-connector to the input port of the swivel. We recorded intakes to the nearest 0.1 g and IG infusions to the nearest 0.5 ml.
4.1.4. Training and testing
Each mouse was run through six training sessions, each of which lasted 24 hr. Intake of the CS+ solution was paired with matched IG infusions of 12 mM denatonium on training days 1, 3, and 5, and intake of the CS− solution was paired with matched IG infusions of water during training days 2, 4, and 6. Following training, we subjected the mice to 4 consecutive two-bottle preference sessions between the CS+ and CS−. During the first 2 days, the mice received matched infusions of denatonium or water when they drank the CS+ or CS−, respectively (reinforced test). During the next two days, intake of both CS+ and CS− solutions was paired with matched IG infusions of water (non-reinforced test). The left-right position of the CS solutions was alternated daily.
4.1.5. Test Solutions
The CS solutions contained 0.05% (w/w) cherry or grape Kool-Aid Mix (General Foods, White Plains, NY) and 0.2% saccharin. For half the mice, cherry was the CS+ flavor paired with IG infusion of 12 mM denatonium, and grape was the CS− flavor paired with IG infusion of water; for the other half of the mice, the flavor-infusate pairs were reversed. Because the orally consumed CS+ solution was mixed with equal volumes of gastrically infused 12 mM denatonium solution, the final denatonium concentration in the gut was 6 mM. Both of these concentrations of denatonium are aversive to B6 mice in short-term lick [18] and long-term preference [42] tests.
4.1.6. Data analysis
During training, we calculate d mean intake per day (across each of the 3 days), separately for the CS+ and CS− solutions. Then, we compared mean training intake/day from the CS+ and CS−, using the paired t-test. For each preference test, we calculated mean intake/session from the CS+ and CS− solutions. Then, we compared intake from both solutions, using the paired t-test.
4.2. Results
Gastric infusions of 12 mM denatonium conditioned a strong aversion to the CS+ in the B6 mice (Fig. 3). The mice consumed significantly less CS+ than CS− during one-bottle training, and showed a significant aversion to the CS+ (i.e., = 32%) in the two-bottle tests. The average denatonium dosage that the mice experienced across each of the three 24-hr training sessions with the CS+ was 977.0 mg/kg; this translates to a denatonium dosage of 20.4 mg/kg per 30 min.
The aversion to the CS+ solution was expressed both when its consumption was paired with IG denatonium (reinforced test) and with IG water (non-reinforced test). The latter finding confirms that the mice had acquired a specific aversion to the CS+ flavor, and were not simply avoiding the IG denatonium infusions.
5. Does prolonged intake of denatonium produce toxic effects? (Experiment 4)
Oral toxicity studies indicate that denatonium has low acute and chronic toxicity in adult rats [15,20,29], but little is known about its toxicity to mice. For this reason, we assessed the toxicity of chronic intake of 6 mM denatonium in B6 mice. We selected the 6 mM concentration because it is orally aversive to B6 mice [18] and because it is what the mice experienced in Experiment 3 (i.e., the 12 mM infusate was diluted ∼ 50% by the matched intake of CS+ solution).
5.1 Methods
5.1.1. Procedure
We randomly assigned 24 male B6 mice (obtained from Jackson Laboratories; Bar Harbor, ME) to one of two fluid treatments: control or experimental. All mice were 7 weeks old at the onset of the experiment. The control mice were offered water ad libitum over the 3-week experiment, whereas the experimental mice were offered 6 mM denatonium ad libitum. Based on prior work with mice and guinea pigs [23,56], we expected the mice would adapt to the aversive taste of the denatonium solution, and thereby obtain sufficient water. The mice were maintained in standard tub cages with laboratory chow ad libitum.
We weighed mice on a daily basis during the initial week, and on a weekly basis during the two subsequent weeks. We focused on body weight because it has been found to be a sensitive measure of toxicity in previous studies with rodents [32,41]. We also observed each mouse daily for clinical signs of denatonium toxicosis—i.e., decreased activity, ataxia, ocular porphyrin discharge, diarrhea, corneal opacity, tremors, hypothermia, hypersalivation, respiratory congestion [15,20,29].
5.1.2. Data analysis
To test for toxic effects of the denatonium, we analyzed body weight changes over the 3-week exposure period. To this end, we ran a two-way ANOVA, with time (i.e., day 1, 7, 14 and 21) as a within factor and fluid treatment (i.e., water or denatonium) as a between factor.
5.2. Results
Despite having the 6 mM denatonium solution as their only source of water for three weeks, the B6 mice showed no ill effects. We did not observe any clinical signs of denatonium toxicosis in the denatonium-exposed mice. Further, there was a significant effect of time on body weight (F3, 66 = 36.1; P < 0.05), but no significant effect of test solution (F1, 12 = 0.3; P > 0.05) or interaction of time × test solution (F3, 66 = 1,3; P > 0.05) (Fig. 5). In fact, there was a trend for the denatonium-exposed mice to gain more weight than the water-exposed mice. These results indicate that 6 mM denatonium is not toxic to the mice when ingested chronically.
Figure 5.
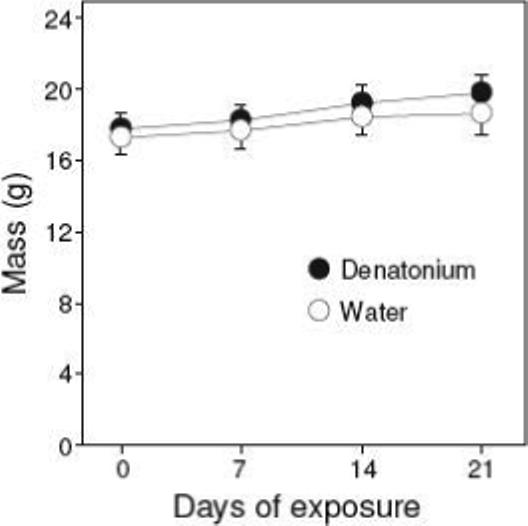
Body weight of B6 mice (mean ± S.E.) given 6 mM denatonium or water as their only source of fluid across a three-week period (see Experiment 4). N = 12 mice/treatment group.
6. Discussion
Because most naturally occurring toxins have a bitter taste, free-ranging animals should benefit, on average, by limiting intake of foods that elicit this taste quality [2,6,7,14,35]. Although the oral bitter taste system can perform this function [16], it is possible that the gastrointestinal bitter taste system contributes as well. The present study provides evidence consistent with this hypothesis. We found that mice and rats learn to avoid a flavor stimulus when its intake is paired with IG infusions of denatonium. This Pavlovian conditioning process would limit subsequent intake of bitter (and potentially toxic) substances, assuming the animal survived the initial ingestion event. In addition, we found that rats delay gastric emptying in response to IG infusions of denatonium. This physiological response would slow the rate at which bitter (and potentially toxic) substances enter the small intestine, thereby providing detoxification enzymes in the gut and liver with more time to break them down prior to entering the systemic circulation.
Three lines of evidence suggest that the IG infusions of denatonium produced their effects by stimulating a chemosensory (i.e., “bitter taste”) rather than a toxicity mechanism. First, as noted earlier, rodents express the chemosensory “machinery” in their gastrointestinal tract for responding to denatonium. Second, if a flavor aversion to 6 mM denatonium was produced in the mice through a toxicity mechanism, then we would have expected 3 weeks of exposure to this solution (without an alternative source of water) to have produced toxicosis. Contrary to this expectation, the mice grew normally and failed to exhibit any clinical signs of denatonium toxicosis. Third, we conditioned a flavor aversion to the CS+ in rats by associating its intake with IG infusions of 10 mM denatonium, during three relatively short (i.e., 30-min) training sessions. The rats received a mean IG dosage of only 67 mg/kg of denatonium training session, which is a fraction of the acute oral LD50 dosage for denatonium in adult rats (i.e., 584−640 mg/kg) [15,20,29].
Additional work is needed to answer two questions raised by this study. First, why was there a 6-min delay before IG denatonium suppressed licking? We suspect that the 6-min delay is an underestimate because the rats were consuming a familiar and highly palatable solution. Even with a novel, less palatable solution, however, it is unlikely that the response latency for IG denatonium would reach to that for oral denatonium: 5 s [49]. The second question is how did IG denatonium produce its aversive chemosensory effects? We have preliminary data indicating that the flavor conditioning process is not affected by genetic ablation of α-gustducin in mice, or transection of the vagus nerve in rats [19]. Based on this latter observation, and the fact that IG denatonium took 6-min to suppress licking, it is more likely the post-oral response to denatonium is mediated by a humoral than a neural mechanism.
6.1. Is the aversive flavor-conditioning process more responsive to oral or post-oral input?
In Experiment 1, we paired intake of the CS+ with IG infusions of 2.5 or 10 mM denatonium, respectively, but only the 10 mM concentration produced a flavor aversion. Because these IG-infused solutions were each diluted ∼ 50% by the associated CS+ intake, it follows that the presence of 5 mM, but not 1.25 mM, denatonium in the gastrointestinal tract was sufficient to condition a flavor aversion to the CS+. We also paired intake of the CS+ with the flavor of 1.25 mM denatonium, and successfully conditioned a flavor aversion to the CS+. Taken together, these results show that 1.25 mM denatonium can condition a flavor aversion when it is ingested, but not when it is infused IG. A key distinction between these two routes of administration is that when the denatonium is ingested, it would stimulate both the oral and post-oral chemosensory systems. Accordingly, the higher responsiveness of rats to ingested 1.25 mM denatonium could reflect a greater impact of oral input and/or the fact that oral and post-oral input were acting in an additive manner.
6.2. Functional implications
The present study shows that IG infusions of denatonium can condition a flavor aversion and delay gastric emptying in rodents. Given that the IG infusions suppressed licking within 6 min, our results substantiate the existence of a secondary “bitter taste” system in the gastrointestinal tract, which could help rodents limit intake of potentially toxic foods.
Future studies should determine whether IG infusions of other bitter taste stimuli produce similar effects. If so, then this would indicate that many of the bitter compounds in foods [39] could delay gastric emptying and condition flavor aversions. While this would be adaptive for toxic bitter foods [14], it would be maladaptive for bitter foods that are harmless [6,16] or medicinally active [6,26,48,54,55]. At this point, however, we do not have a clear sense about the relative abundance of toxic, harmless and medicinally active foods in nature [17]. One study assayed the entire plant fauna at four sites on the Caribbean island of Aruba for the presence of poisons (i.e., phenolics, saponins, alkaloids and cyanogenic compounds) [44]. Among the plant species that were of a size and texture to be eaten by the local herbivore (a whiptail lizard, Cnemidophorus arubensis), 63% contained poisons. This observation illustrates that bitter and potentially toxic plants can constitute a substantial proportion of the available foods in a habitat.
Figure 4.
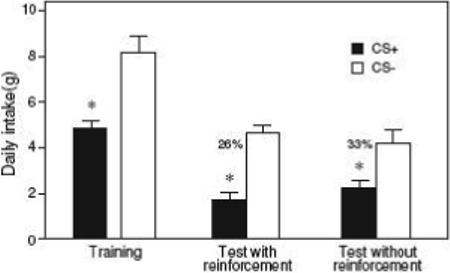
Intake of CS+ and CS− solutions (mean ± S.E.) during one-bottle training and two-bottle preference tests (24 h) with B6 mice (Experiment 3). Numbers atop bars represent mean percentage of CS+ consumed during the preference test, calculated separately for each mouse. During one-bottle training, intakes of the CS+ and CS− were paired with IG infusions of 12 mM denatonium and water, respectively (6 sessions total). In the first 2 days of two-bottle testing intake of the CS+ was paired with IG infusions of 12 mM denatonium (reinforced test) and in the last two days CS+ intake was paired with IG infusions of water (non-reinforced test). N = 9 mice; * P < 0.05 (paired t-test).
Acknowledgements
All procedures were approved by the Institutional Animal Care and Use Committees at Brooklyn College (Experiments 1−3) and Columbia University (Experiment 4). The research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-31135) to AS, and the National Institute on Deafness and Other Communication Disorders Grant (DC007475) to JIG.
References
- 1.Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–50. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- 2.Bate-Smith EC. In: Phytochemical ecology: Proceedings of the phytochemical society symposium. Harborne JB, editor. Academic Press; London: 1972. pp. 45–56. [Google Scholar]
- 3.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses. 2007;32:41–49. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 4.Breslin PAS, Beauchamp GK. Suppression of bitterness by sodium: variation among bitter taste stimuli. Chem. Senses. 1995;20:609–23. doi: 10.1093/chemse/20.6.609. [DOI] [PubMed] [Google Scholar]
- 5.Breslin PAS, Beauchamp GK. Salt enhances flavour by suppressing bitterness. Nature. 1997;387:563. doi: 10.1038/42388. [DOI] [PubMed] [Google Scholar]
- 6.Brieskorn CH. In: Bitterness in foods and beverages. Rouseff RL, editor. Elsevier; New York: 1990. pp. 15–33. [Google Scholar]
- 7.Brower LP. In: The biology of butterflies. Vane-Wright RI, Ackery PR, editors. Academic Press; London: 1984. pp. 109–34. [Google Scholar]
- 8.Carlson AJ, Van de Erve J, Lewis JH, Orr SJ. Contributions to the physiology of the stomach. XVI. The action of the so-called stomachics or bitters on the hunger mechanism. J. Pharmacol. Expmtl. Therap. 1914−15;6:209–18. [Google Scholar]
- 9.Chen MC, Wu SV, Reeve JR, Jr., Rozengurt E. Bitter stimuli induce Ca2 signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2 channels. Am. J. Physiol. 2006;291:C726–C39. doi: 10.1152/ajpcell.00003.2006. [DOI] [PubMed] [Google Scholar]
- 10.Davis CJ, Harding RK, Leslie RA, Andrews PLR. In: Nausea and vomiting: Mechanisms and treatment. Davis CJ, Lake-Bakaar GV, Grahame-Smith DG, editors. Springer-Verlag; New York: 1986. pp. 65–75. [Google Scholar]
- 11.Davis JD, Campbell CS. In: Physiological techniques in behavioral research. Singh D, Avery DD, editors. Brooks Cole; Monterey, CA: 1975. pp. 163–77. [Google Scholar]
- 12.Dyer J, Salmon KSH, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem. Soc. Trans. 2005;33:302–05. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 13.Furudono Y, Ando C, Kobashi M, Yamamoto C, Yamamoto T. The role of orexigenic neuropeptides in the ingestion of sweet-tasting substances in rats. Chem. Senses. 2005;30(suppl 1):i186–i87. doi: 10.1093/chemse/bjh176. [DOI] [PubMed] [Google Scholar]
- 14.Garcia J, Hankins WG. Olfaction and taste. V.. In: Denton DA, Coghlan JP, editors. Proceedings of the 5th international symposium in Melbourne, Australia.; New York: Academic Press. 1975. pp. 39–45. [Google Scholar]
- 15.Geil RG, Dean WP. 1976.
- 16.Glendinning JI. Is the bitter rejection response always adaptive? Physiol. Behav. 1994;56:1217–27. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 17.Glendinning JI. How do predators cope with chemically defended foods? Biol. Bull. 2007 doi: 10.2307/25066643. In press. [DOI] [PubMed] [Google Scholar]
- 18.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem. Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 19.Glendinning JI, Yiin Y-M, Ackroff K, Schwartz GJ, Margolskee RM, Sclafani A. “Bitter taste“ in the gut? Flavor avoidance conditioned by intragastric denatonium in rodents. Chem. Senses. 2007;32:A18. [Google Scholar]
- 20.Hansen SR, Janssen C, Beasley VR. Denatonium benzoate as a deterrent to ingestion of toxic substances: toxicity and efficacy. Vet. Hum. Toxicol. 1993;35:234–36. [PubMed] [Google Scholar]
- 21.Harborne JB. Introduction to ecological biochemistry. Second Edition Academic Press; New York: 1988. [Google Scholar]
- 22.Harborne JB, Baxter H. Phytochemical dictionary: A handbook of bioactive compounds from plants. Taylor & Francis; Washington, D.C.: 1993. [Google Scholar]
- 23.Harder DB, Maggio JC, Whitney G. Assessing gustatory detection capabilities using preference procedures. Chem. Senses. 1989;14:547–64. [Google Scholar]
- 24.Höfer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem. Cell Biology. 1998;110:303–09. doi: 10.1007/s004180050292. [DOI] [PubMed] [Google Scholar]
- 25.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. 1996;93:6631–34. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huffman MA, H. O, Kawanaka M, Page JE, Kirby GC, Gasquet M, Murakami A, Koshimizu K. In: Towards Natural Medicine Research in the 21st Century. Ebizuka Y, editor. Elsevier Science B.V.; Amsterdam: 1998. pp. 113–23. [Google Scholar]
- 27.Kappauf WE, Burright RG, DeMarco W. Sucrose-quinine mixtures which are isohedonic for the rat. J. Comp. Physiol. Psych. 1963;56:138–43. [Google Scholar]
- 28.Keast RSJ, Breslin PA. Cross-adaptation and bitterness inhibition of L-tryptophan, L-phenylalanine and urea: further support for shared peripheral physiology. Chem. Senses. 2002;27:123–31. doi: 10.1093/chemse/27.2.123. [DOI] [PubMed] [Google Scholar]
- 29.Klein-Schwartz W. Denatonium benzoate: review of efficacy and safety. Vet. Hum. Toxicol. 1991;33:545–47. [PubMed] [Google Scholar]
- 30.Kornreich L, Kleinhaus AL. Postingestive chemosensation and feeding by leeches. Phyiol. Behav. 1999;67:635–41. doi: 10.1016/s0031-9384(99)00121-3. [DOI] [PubMed] [Google Scholar]
- 31.Lawless HT. Evidence for neural inhibition in bittersweet taste mixtures. J. Comp. Physiol. Psychol. 1979;93:538–47. doi: 10.1037/h0077582. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y-L, Malik N, Sanger GJ, Friedman MI, Andrews PLR. Pica—A model of nausea? Species differences in response to cisplatin. Physiol. Behav. 2005;85:271–77. doi: 10.1016/j.physbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 33.London RM, Snowdon CT, Smithana JM. Early experience with sour and bitter solutions increases subsequent ingestion. Physiol. Behav. 1979;22:1149–55. doi: 10.1016/0031-9384(79)90270-1. [DOI] [PubMed] [Google Scholar]
- 34.McBurney DH, Smith DV, Shick TR. Gustatory cross adaptation: sourness and bitterness. Percep. Psychophy. 1972;11:228–32. [Google Scholar]
- 35.Molyneux RJ, Ralphs MH. Plant toxins and palatability. J. Range Manag. 1992;45:13–18. [Google Scholar]
- 36.Moorhead LD. Contributions to the physiology of t he stomach. XXVII. Further studies on the action of bitter tonics on the secretion of gastric juice. J. Pharmacol. Expmtl. Therap. 1915;7:577–89. [Google Scholar]
- 37.Moskowitz HW, Kumaraiah V, Sharma KN, Jacobs HL, Sharma SD. Cross-cultural differences in simple taste preferences. Science. 1975;190:1217–18. doi: 10.1126/science.1198109. [DOI] [PubMed] [Google Scholar]
- 38.Pronin AN, Tang H, Connor J, Keung W. Identification of ligands for two human bitter T2R receptors. Chem. Senses. 2004;29:583–93. doi: 10.1093/chemse/bjh064. [DOI] [PubMed] [Google Scholar]
- 39.Rouseff RL. Bitterness in foods and beverages. Elsevier Science Publishers; New York: 1990. [Google Scholar]
- 40.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G77. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 41.Rudd JA, Yamamoto K, Yamatodani A, Takeda N. Differential action of ondansetron and dexamethasone to modify cisplatin-induced acute and delayed kaolin consumption (“pica”) in rats. Eur. J. Pharmacol. 2002;454:47–52. doi: 10.1016/s0014-2999(02)02472-x. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Avila L, Wong G, Damak S, Margolskee RF. Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in a-gustducin. Proc. Natl. Acad. Sci., USA. 2001;98:8868–73. doi: 10.1073/pnas.151235798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sainz E, Cavenagh MM, Gutierrez J, Battey JF, Northup JK, Sullivan SL. Functional characterization of human bitter taste receptors. Biochem. J. 2007;403:537–43. doi: 10.1042/BJ20061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schall JJ, Ressel S. Toxic plant compounds and the diet of the predominantly herbivorous whiptail lizard, Cnemidophorus arubensis. Copeia. 1991;1991:111–19. [Google Scholar]
- 45.Schwartz GJ, Moran TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am. J. Physiol. 1998;274:R1236–R42. doi: 10.1152/ajpregu.1998.274.5.R1236. [DOI] [PubMed] [Google Scholar]
- 46.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol. Behav. 2003;79:783–88. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 47.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am. J. Physiol. 2005;289:R712–R20. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 48.Siegel RK. Intoxication: life in pursuit of artificial paradise. E.P. Dutton; New York: 1989. [DOI] [PubMed] [Google Scholar]
- 49.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J. Neurosci. 2002;22:1937–41. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am. J. Physiol. 2007;292:G457–G61. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 51.Stevens JC. Detection of taste in mixtures with other tastes: issues of masking and aging. Chem. Senses. 1996;21:211–21. doi: 10.1093/chemse/21.2.211. [DOI] [PubMed] [Google Scholar]
- 52.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am. J. Physiol. 2007;292:G1420–28. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- 53.Uneyama H, Tanaka T, Torii K. Gut nutrient sensing by the abdominal vagus. Folia Pharmacol. Jpn. 2004;124:210–18. doi: 10.1254/fpj.124.210. [DOI] [PubMed] [Google Scholar]
- 54.Villalba JJ, Provenza FD, Shaw R. Sheep self-medicate when challenged with illness-inducing foods. Anim. Behav. 2006;71:1131–39. [Google Scholar]
- 55.Vitazkova SK, Long E, Paul A, Glendinning JI. Mice suppress malaria infection by sampling a ”bitter” chemotherapy agent. Anim. Behav. 2001;61:887–94. [Google Scholar]
- 56.Warren RP, Pfaffman C. Early experience and taste aversion. J. Comp. Physiol. Psych. 1959;52:263–66. doi: 10.1037/h0047655. [DOI] [PubMed] [Google Scholar]
- 57.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression and function of bitter taste receptors (T2R) in mouse and rat. Physiol. Genomics. 2005;22:139–49. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 58.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc. Natl. Acad. Sci. USA. 2002;99:2392–97. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zellner DA, Berridge KC, Grill HJ, Ternes JW. Rats learn to like the taste of morphine. Behav. Neurosci. 1985;99:290–300. doi: 10.1037//0735-7044.99.2.290. [DOI] [PubMed] [Google Scholar]