Abstract
Endotoxins represent one of the most potent classes of microbial immunoactive components that can cause pulmonary inflammation. The aim of this study was to compare the inflammatory potency of two types of Neisseria meningitidis endotoxins (lipooligosaccharides) in lungs: wild type (hexaacylated, LOSwt) and mutant type (pentaacylated, LOSmsbB), and to determine the importance of MD-2 in endotoxin responses in lungs in vivo. Endotoxin-normoresponsive mice (BALB/c) were exposed to selected doses of penta- and hexaacylated lipooligosaccharides (LOS) by nasal aspiration. Cellular and cytokine/chemokine inflammatory responses in bronchoalveolar lavage were measured at 1-, 4-, 8-, 16-, 24-, and 48-hour time points. MD-2–null mice were exposed to one dose of hexaacylated LOS and inflammatory responses were measured after 4 and 24 hours. Inhalation of hexaacylated LOS resulted in strong inflammatory responses, while pentaacylated LOS was much less potent in inducing increases of neutrophils, TNF-α, macrophage inflammatory protein-1α, IL-6, granulocyte colony-stimulating factor, and IL-1β concentration in bronchoalveolar lavage. Similar kinetics of inflammatory responses in lungs were found in both types of endotoxin exposures. Inhalation of hexaacylated LOS in MD-2–null mice resulted in significantly lower numbers of neutrophils in bronchoalveolar lavage than in normoresponsive mice. Markedly lower inflammatory potency of pentaacylated LOS was observed compared with hexaacylated LOS. Hyporesponsiveness in MD-2–null mice after nasal aspiration of wild-type LOS indicate its essential role in airway responsiveness to endotoxin.
Keywords: endotoxin, lipooligosaccharide, inhalation, pulmonary inflammation
CLINICAL RELEVANCE
Alteration of fatty acyl chains within lipid A markedly affects inflammatory potency in vivo by impairing Toll-like receptor 4 activation by endotoxin:MD-2 complexes. Blunted airway responses to inhaled endotoxin in MD-2– null mice demonstrates the essential role of MD-2.
Innate immunity represents the first line of defense against infection. When bacteria and bacterial components invade the host, they stimulate a variety of specialized host cells, including epithelial cells, monocytes, macrophages, neutrophils, and endothelial cells, as part of the acute inflammatory response (1). These responses are crucial for host defense but, in excess, can be very harmful causing local and multi-system derangements, including pulmonary inflammation (2), shock, and occasionally death (3).
Lipopolysaccharides (LPS) or endotoxins represent one of the most potent classes of microbial immunoactive components. Endotoxins are composed of a lipid A region, responsible for proinflammatory activity, covalently linked to an oligo- or polysaccharide chain. Lipopolysaccharides lacking the distal O-specific carbohydrate are termed lipooligosaccharides (LOS) (4), and are characteristic of the endotoxins produced by many gram-negative bacterial inhabitants of the oropharyngeal flora. One such LOS derived from Neisseria meningitidis has been studied as a model compound for immune recognition (5–9).
Endotoxins are highly water insoluble, existing either as integral components of the gram-negative bacterial outer membrane or, after extraction, as aggregates shielding the lipid A region from water (4). Maximum host cell responsiveness to endotoxin requires at least four extracellular and cell surface host proteins (LPS binding protein [LBP], cluster determinant 14 [CD-14], lymphocyte antigen 96 [MD-2], and Toll-like receptor [TLR]4). These proteins engage endotoxin in a series of sequential protein–endotoxin and protein–protein interactions (8). LBP catalyzes the transfer of LPS from bacterial membranes (10) or aggregates of purified LPS to CD-14 either in soluble form or membrane-bound via a glycosyl-phosphatidylinositol (GPI)-linked membrane anchor (11). Monomeric endotoxin:CD-14 complexes that are released are the preferred substrate form of endotoxin for MD-2, presented either in extracellular form (6, 7) or membrane bound via association with TLR4 (9). Simultaneous engagement of MD-2 with endotoxin and TLR4 results in receptor and cell activation, initiating a signal transduction cascade leading to the release of proinflammatory mediators (1, 12).
The chemistry and geometry of the isolated endotoxin molecule and of supramolecular assemblies of endotoxin can greatly affect the proinflammatory activity of endotoxin (13–15). Among the most important structural determinants of endotoxin activity are the number, chain length, and location of fatty acyl chains within lipid A (13–17). In general, the most potent endotoxin species are hexaacylated, whereas either less or more highly acylated endotoxins are substantially less potent inducers of host proinflammatory responses (15, 18). Differences in the potency of hexa-, tetra-, and pentaacylated endotoxins reflect differences in the ability of endotoxin-bound MD-2 to induce activation of TLR4 (17, 19). On the basis of these observations, it has been suggested that the elaboration of underacylated endotoxins by Pseudomonas aeruginosa early in the evolution of pulmonary infection in cystic fibrosis (15, 20, 21) and by Yersinia pestis in pneumonic plague (22) contributes greatly to the virulence of these airway bacterial pathogens by blunting early host inflammatory responses needed for efficient mobilization of host defenses. However, the ability of the mammalian airway to mount graded responses to administered endotoxin, depending on endotoxin lipid A properties, and the role of MD-2 in airway responses to endotoxin have not yet been directly tested.
The first aim of this study was to determine the ability of Neisseria meningitidis serogroup B (NMB) LOS to induce airway inflammation in vivo and to compare the inflammatory potency of wild-type hexaacylated NMB LOS (LOSwt) with that of pentaacylated LOS (LOSmsbB) derived from the msbB mutant strain of NMB. The second aim was to determine the role of MD-2 in airway responsiveness to LOS. Some of the results have been previously reported in the form of abstracts (23–25).
MATERIALS AND METHODS
Lipooligosaccharide
Metabolically 14C-labeled LOS was isolated from an acetate auxotroph of wild-type NMB and from a msbB mutant derived from NMB as previously described (5, 6, 17) (see the online supplement). Concentrations of LOS (endotoxin activity [units]; EU) were measured using the kinetic chromogenic Limulus Amebocyte Lysate assay as previously described (26) and indicated an activity of LOSwt of 18,000,000 EU/ml (1,200 μg/ml) and for LOSmsbB 33,800,000 (2,250 μg/ml) (see the online supplement). Stocks of LOS were diluted fresh before use with sterile, pyrogen-free saline.
Animals
Six-week-old male BALB/c mice from Jackson Laboratories (Bar Harbor, ME) were used. After a 10-day quarantine and before exposures, two mice were necropsied and evaluated for inflammation to ensure the health of the shipment. MD-2–null mice on C57BL/6 background were bred and genotyped in our vivarium. Exposed and control BALB/c and MD-2–null mice were housed separately and supplied with HEPA-filtered air, filter-sterilized water, and ovalbumin-free mouse food (sterile Teklad 5% stock diet; Harlan, Madison, WI) ad libitum. Experimental protocols were approved by the Institutional Animal Care and Use Committee and adhered to NIH Guidelines.
Experimental Protocol
Dose–response and time course studies were performed using LOSwt and LOSmsbB isolated from the wild-type and mutant type of NMB serogroup B. In the dose–response study, groups of 6- to 10-week-old endotoxin-normoresponsive (BALB/c) mice were exposed to one of five LOSwt doses (5, 10, 30, 300, and 3,000 EU/mouse) or one of three LOSmsbB doses (30, 300, and 3,000 EU) as shown in Figure E1 in the online supplement. Control groups inhaled sterile, pyrogen-free saline. LOS was inhaled by nasal aspiration under short-term Halothane anesthesia. Four hours after exposure mice were killed by injection with Nembutal (150 mg/kg, intraperitoneally) and processed for necropsy, bronchoalveolar lavage (BAL), and histopathology. In the time-course studies, groups of six to nine mice were exposed to sterile, pyrogen-free saline (controls), LOSwt, or LOSmsbB (30 and 300 EU/mouse). At 1, 4, 8, 16, 24, and 48 hours after exposure to LOSwt and 4 and 24 hours after exposure to LOSmsbB, BAL fluid and lungs were processed. MD-2–null mice were exposed to 300 EU LOSwt (n = 17) and necropsied 4 or 24 hours after exposure.
BAL and Processing of Lungs
At the appropriate time points mice were killed and BAL fluid was collected as previously described (26) (see the online supplement). After lavage, lungs were perfused through the heart with sterile saline, cannulated, and fixed in zinc formalin. Cassettes with the lungs were processed and lungs were embedded in paraffin and cut in 5-μm sections. Slides were stained with hematoxylin and eosin and evaluated for inflammation.
Cytokine/Chemokine Assays
Supernatants from BAL fluid were assayed to determine cytokine/chemokine concentrations using a multiplexed fluorescent bead-based immunoassay (BioRad Laboratories, Inc., Hercules, CA). The Bio-Plex mouse cytokine system was used to measure concentrations of 18 cytokines/chemokines (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 [p40], IL-12 [p70], IL-17, granulocyte colony-stimulating factor [G-CSF], granulocyte macrophage colony-stimulating factor [GM-CSF], murine CXCL1 (KC), TNF-α, macrophage inflammatory protein [MIP]-1α, IL-6, IFN-γ, and regulated on activation, normal T cell expressed and secreted [RANTES]) or, for some groups, 6 cytokines (TNF-α, MIP-1α, G-CSF, IL-6, IL-1β, and IL-17). Cytokine concentrations below the valid range of the standard curve were assigned the limit of detection divided by √2 (27).
Statistical Analysis
Log transformation of BAL cells and cytokine concentrations yielded normally distributed data. Data were represented by their geometric mean and SEM. Statistical analyses were performed comparing LOSwt- and LOSmsbB-exposed mice to controls using two-sided Dunnett t for multiple comparisons (Ver. 15; SPSS, Inc., Chicago, IL). Comparisons of LOS treatment versus control across time points were performed using ANOVA. Comparisons of dose–response curves between treatments used generalized linear models. P values ⩽ 0.05 were considered significant and ⩽ 0.01 highly significant.
RESULTS
Comparison of Dose-Dependent Effects of Nasally Instilled LOSwt and LOSmsbB
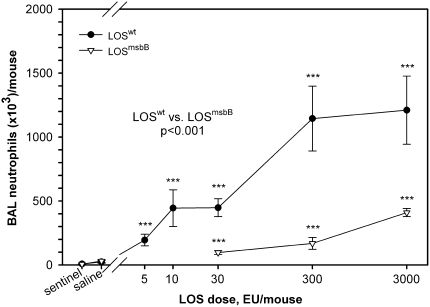
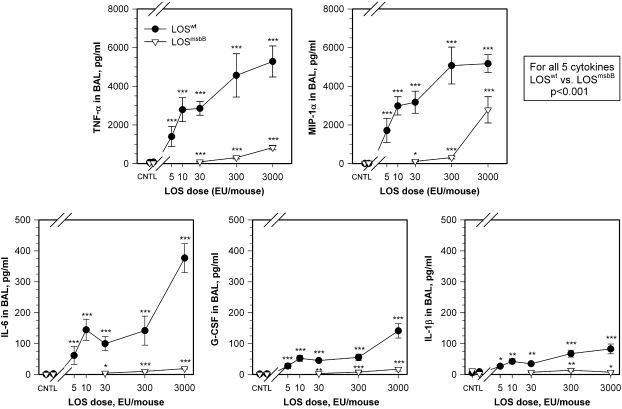
Endotoxin-responsive BALB/c mice were used to establish dose–response, time-course, and histopathologic changes in the lungs induced by exposure to purified LOS aggregates. Early inflammatory responses in the lungs were manifest in BAL fluid primarily by recruitment of neutrophils and increased concentrations of inflammatory cytokines and chemokines. Experiments showed that increasing LOSwt doses from 0 (controls) to 5, 10, 30, 300, and 3,000 EU/mouse induced significant increases of total cell counts (not shown) and neutrophils (Figure 1) in the BAL fluid compared with controls (P < 0.01). Exposure to LOSmsbB also induced a significant increase in neutrophils recovered in the BAL fluid in comparison with controls (P = 0.05), but required 10- to 100-fold higher concentrations of LOS to induce a response comparable to that induced by LOSwt (Figure 1). Both LOSwt and LOSmsbB induced accumulation of TNF-α, MIP-1α, IL-6, G-CSF, and IL-1β in BAL fluid; however, LOSwt was approximately 100 times more potent (Figure 2). In mice exposed to LOSwt, little change was observed in BAL fluid concentrations of IL-1α, IL-2, IL-4, IL-5, IL-10, IL-12 (p70), IL-17, GM-CSF, KC, IFN-γ, and RANTES (data not shown).
Figure 1.
Dose–response data showing the number of neutrophils in bronchoalveolar lavage (BAL) after nasal aspiration of LOSwt and LOSmsbB in comparison with controls (***P < 0.001). There was a significantly higher neutrophilic response in LOSwt- than in LOSmsbB-exposed mice 4 hours after exposure (P < 0.001).
Figure 2.
Cytokine dose–response curves for TNF-α, macrophage inflammatory protein (MIP)-1α, IL-6, granulocyte colony-stimulating factor (G-CSF), and IL-1β in BAL fluid of LOSwt- in comparison with LOSmsbB-exposed mice. Data show a dose–response relationship where increasing doses of endotoxin produced significant increases of TNF-α, MIP-1α, G-CSF, IL-6, and IL-1β in BAL fluid of LOSwt-exposed mice in comparison with those exposed to LOSmsbB (P < 0.001 for each cytokine). Each data point compared with respective control: *P < 0.05, **P < 0.01, and ***P < 0.001.
Kinetics of Airway Inflammation Induced by Nasally Instilled LOSwt and LOSmsbB
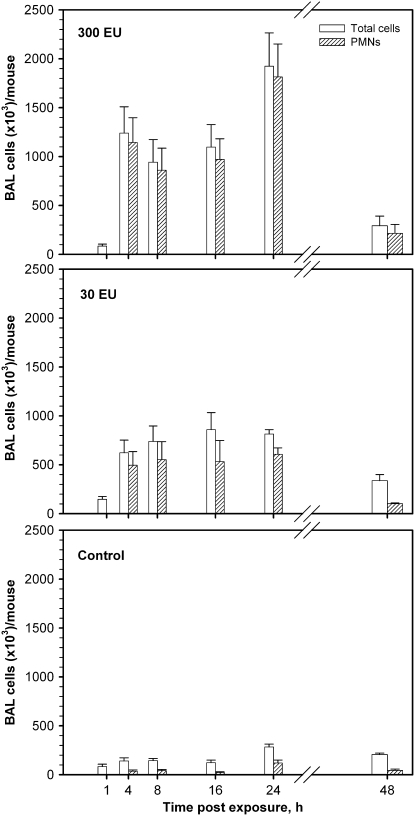
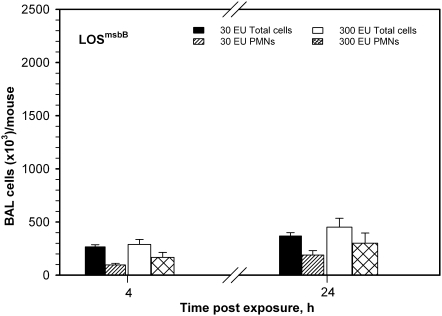
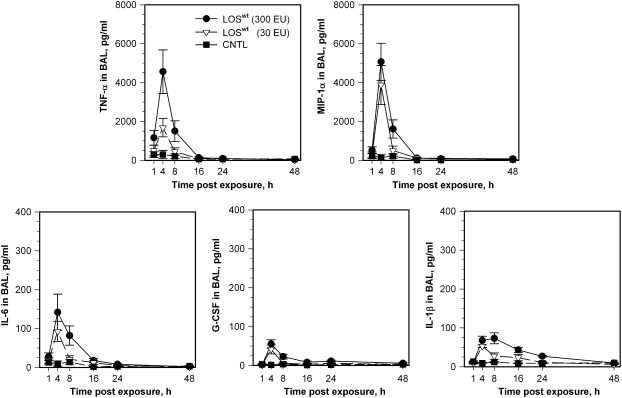
To determine if markedly reduced airway inflammatory responses to LOSmsbB versus LOSwt manifest at 4 hours were due to an altered magnitude of host responses and/or altered kinetics of the inflammatory responses, two doses of LOSwt and LOSmsbB (30 and 300 EU/mouse) were selected for a time-course study. Pulmonary inflammatory cell responses to inhaled LOSwt at 1, 4, 8, 16, 24, and 48 hours are shown in Figure 3. A similar time course of total cell and neutrophilic response was found in both LOSwt dose-exposure groups of mice. Total cell counts and neutrophils were greatly increased within 4 hours of exposure and remained very high for 24 hours. Total and neutrophil concentrations in BAL were still above baseline levels at 48 hours. In mice treated with 30 or 300 EU LOSwt/mouse (Figure 3, middle and top panels, respectively), we observed a 14- to 32-fold increase in neutrophils at the 4-hour time point compared with controls (Figure 3, bottom panel). Neutrophils continued to increase for the first 24 hours after initial exposure. Nearly all the increase in total BAL cells was attributable to neutrophils. Intra-nasal instillation of LOSmsbB induced much less accumulation of neutrophils in BAL at all time points examined (compare Figures 3 and 4), indicating that the magnitude but not the kinetics of airway inflammatory responses is affected by the dose or potency of the LOS administered. A similar pattern was observed for the cytokines/chemokines (TNF-α, MIP-1α, IL-6, G-CSF, and IL-1β) induced by instillation of LOSwt, except that the levels of these proteins in BAL returned close to baseline within 16 to 48 hours (Figure 5). At the doses tested, LOSmsbB induced only very modest increases in BAL of the same cytokines and chemokines induced more robustly by LOSwt (see Figure 2) with apparently similar kinetics (data not shown).
Figure 3.
Time-course for pulmonary inflammatory responses (total cells and neutrophils) in BAL after the nasal aspiration of LOSwt in the dose of (top panel) 300 EU/mouse and (middle panel) 30 EU/mouse in comparison with (bottom panel) saline (control). The higher endotoxin dose showed maximal total cells and neutrophils in BAL at 24 hours, with a small elevation of neutrophil counts persisting at 48 hours.
Figure 4.
Pulmonary inflammatory responses after nasal aspiration of LOSmsbB in the dose of 30 EU and 300 EU/mouse at 4- and 24-hour time points shown on the same scale as in Figure 3. Responses at both doses and time points were much lower than observed in LOSwt-exposed mice.
Figure 5.
Time course for the concentration of TNF-α, MIP-1α, IL-6, G-CSF, and IL-1β in lavage after inhalation of LOSwt in the dose of 300 and 30 EU/mouse in comparison with controls.
Dose- and Time-Dependent Histopathologic Changes in the Lungs of Mice Exposed to Nasally Instilled LOSwt and LOSmsbB
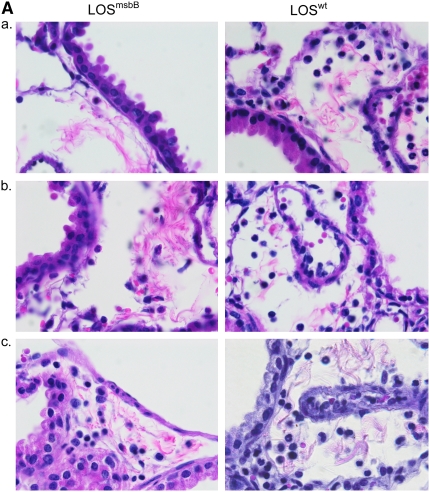
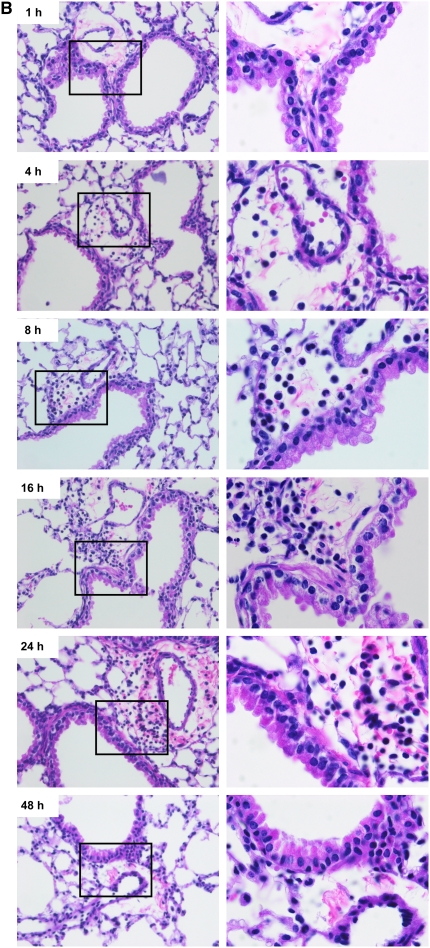
In addition to lung lavage cellular and cytokine responses, we also examined the lungs for histopathologic changes. Four hours after exposure to LOSwt or LOSmsbB at 30, 300, or 3,000 EU/mouse, the tissues exhibited peribronchiolar neutrophilia that was not evident in sentinels or saline and increased with increasing dose. The abundance of neutrophils was far greater in the lungs of the LOSwt exposure group compared with the LOSmsbB group of mice (Figure 6A), with increasing numbers of neutrophils in the peribronchiolar and perivascular tissue up to 24 hours. The accumulation of neutrophils seen microscopically corresponded closely to the dose- and time-dependent accumulation of these cells in BAL fluid in response to LOS exposure (Figure 6B).
Figure 6.
Representative photomicrographs of lungs stained with hematoxylin and eosin. (A) Hexa- and pentaacylated LOS (LOSwt and LOSmsbB)-induced accumulation of neutrophils in the peribronchiolar region 4 hours after nasal aspiration of (a) 30 EU, (b) 300, and (c) 3,000 EU/mouse. Magnification: ×100. (B) Neutrophil recruitment in the peribronchiolar region 1, 4, 8, 16, 24, and 48 hours after exposure of 300 EU of LOSwt. Magnifications: ×40 and ×100.
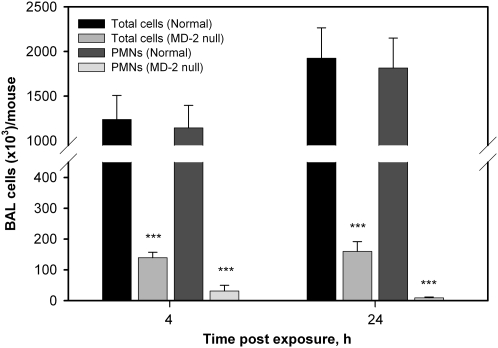
Airway Responses to Nasally Instilled LOS Is MD-2 Dependent
Previous studies in mice have demonstrated the prominent roles of LBP, CD14, and TLR4 in airway/lung responses to inhaled endotoxin (28–30), but the role of MD-2 has not yet been directly examined. Previous in vitro studies have demonstrated the critical role of MD-2 in determining the different potencies of hexaacylated versus underacylated endotoxins, including that of LOSwt versus LOSmsbB. The findings above demonstrating the marked differences in proinflammatory potency of nasally instilled LOSwt versus LOSmsbB are thus consistent with an important role of MD-2 in determining airway responses to instilled LOS. To test the role of MD-2 more directly, we bred and back-crossed MD-2–null mice (31) onto the C57BL/6 background for more than 7 generations and then compared the responsiveness of wild-type and MD-2–null mice to nasally instilled LOSwt. In contrast to the wild-type mice, the MD-2–null mice did not display appreciable cellular airway inflammatory changes (Figure 7) in response to instilled LOSwt 4 or 24 hours after exposure. Lavage cellularity in exposed MD-2–null mice were no different than in saline-exposed controls. These findings show clearly the importance of MD-2 in sensitive airway inflammatory responses to nasally instilled endotoxin.
Figure 7.
Number of total cells and neutrophils in bronchoalveolar lavage fluid from normoresponsive mice in comparison with MD-2−/− mice at 4 and 24 hours after exposure (***P < 0.001).
DISCUSSION
The ability of the immune system to recognize endotoxin (lipopolysaccharides and lipooligosaccharides) is crucial in host defense against many gram-negative bacteria but can also lead to inflammation-driven host pathology. Accordingly, the LPS response system is highly complex and controlled, providing many levels of regulation. Factors regulating the expression and function of LPS-binding protein, CD-14, MD-2, and TLR-4 likely affect the host response to environmental endotoxin exposure and constitute an important gene–environment interaction affecting the incidence and severity of diseases such as asthma, asthma-like syndrome, organic dust toxic syndrome, and some forms of bioaerosols-induced alveolitis.
Inflammatory responses to inhaled endotoxin have been studied in both humans (2, 32–34) and mice (35–38). In general, inhalation or nasal aspiration of endotoxin by mice induces similar host responses and changes (35, 39), and these reflect human airway responsiveness (33).
The data presented in this study demonstrate that the ability of mice to mount rapid and robust airway inflammatory responses to nanogram amounts of nasally instilled endotoxin depends on the acylation state but not, apparently, specific features of the oligo(poly)saccharide chain of endotoxin. The magnitude and kinetics of airway inflammatory responses to meningococcal LOS observed in this study closely resembled those previously described for other administered purified hexaacylated LPS species (33, 37, 40). Thus, meningococcal LOS or Escherichia coli or P. aeruginosa LPS each induced neutrophil recruitment to the airway that was manifest within 4 hours and persisted for at least 48 hours. The kinetics of cytokine responses induced by airway exposure to purified LOS aggregates or by purified P. aeruginosa LPS (40) was also similar and resembled that investigated by exposure of humans to inhaled grain dust containing LPS (33). Taken together, these findings suggest a similar capacity of the airways/lungs of mice and humans to respond to a broad array of inhaled or instilled endotoxin species of varied bacterial origin.
In contrast, our studies revealed substantial differences in the magnitude of airway responses to endotoxin species that differ in the lipid A region by only one fatty acid/endotoxin molecule. Approximately 100-fold higher amounts of pentaacylated LOSmsbB, in comparison to hexaacylated LOSwt, were needed to induce comparable airway inflammatory responses. The potency of tetraacylated endotoxin species is likely to be even lower (18). If such differences are also manifest during infections of the airways and lungs, this would result in delayed mobilization of airway host defenses, giving invading bacteria more time to further multiply, adapt, and disseminate. Our findings thus support earlier speculations that the elaboration of pentaacylated LPS by P. aeruginosa initially colonizing individuals with cystic fibrosis and tetracylated LPS by Y. pestis greatly increases host susceptibility to these airway pathogens.
The differences in potency we observed between LOSwt and LOSmsbB in inducing airway inflammatory responses in vivo were strikingly similar to differences previously observed in LBP-dependent in vitro activation by these LOS species of cells expressing mCD14, MD-2, and TLR4 (17). Our findings thus strongly suggest that the molecular requirements for airway responsiveness to endotoxin correspond closely to the requirements defined in vitro for LBP/CD-14/MD-2/TLR4-dependent cell activation by endotoxin. Previous studies in mice have demonstrated the prominent role of LBP, CD14, and TLR4 in airway/lung responses to endotoxin (28–30) and our findings in this study now definitively establish an essential role for MD-2 as well (Figure 7). MD-2–null mice do not mobilize airway inflammatory responses to nasally instilled LOS aggregates but are responsive to purified monomeric LOS:MD-2 (unpublished observation), confirming that the loss of responsiveness of MD-2–null mice to endotoxin is due to the absence of MD-2. In vitro, differences in potency of hexaacylated versus underacylated endotoxin species in inducing TLR4-dependent cell activation reflect differences in the functional properties of endotoxin bound to MD-2 (17, 19). Thus, the differences we observed in this study in the ability of LOSwt versus LOSmsbB to induce airway inflammation most likely reflect differences in the functional properties of hexacylated versus pentacylated LOS bound to MD-2 in the airway.
In vivo studies in mice have suggested that TLR4-dependent responses to endotoxin in the resting airway are mediated mainly by bone marrow–derived cells (e.g., alveolar macrophages), with airway epithelia less directly involved (29). We have shown that primary cultures of well-differentiated human and murine airway epithelia are hyporesponsive to endotoxin, despite expression of CD14 and TLR4, because of limited expression of MD-2 (7) (unpublished observations). The demonstration in this study that MD-2 is required for airway responses to nasally instilled endotoxin is consistent with the speculation that it is the absence of MD-2 expression by airway epithelia in the resting airway that normally limits their direct responses to inhaled or aspirated endotoxin. The production of MD-2 by alveolar macrophages but not by airway epithelia may help to promote host responses to deeply invading bacteria while minimizing inflammatory reactions to more incidental exposure to endotoxin-bearing environmental pollutants.
Supplementary Material
Acknowledgments
The authors thank Dr. Andrea Adamcakova-Dodd and Dr. Nervana Metwali for their assistance with the experiments. They thank Dr. Douglas T. Golenbock of the University of Massachusetts Medical School, Worcester, MA, for supplying the MD-2–null mice.
This study was supported by NIH P30ES05605, NIH R01AI059372, NIH P01AI044642, the Roy J. Carver Charitable Trust (PBM), and the State of Iowa through the U.I. Centers for Enterprise.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0418OC on January 18, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta 2002;323:59–72. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz DA, Thorne PS, Yagla SJ, Burmeister LF, Olenchock SA, Watt JL, Quinn TJ. The role of endotoxin in grain dust-induced lung disease. Am J Respir Crit Care Med 1995;152:603–608. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol 2000;12:20–26. [DOI] [PubMed] [Google Scholar]
- 4.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res 2001;7:167–202. [PubMed] [Google Scholar]
- 5.Giardina PC, Gioannini T, Buscher BA, Zaleski A, Zheng DS, Stoll L, Teghanemt A, Apicella MA, Weiss J. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J Biol Chem 2001;276:5883–5891. [DOI] [PubMed] [Google Scholar]
- 6.Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA 2004;101:4186–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB Jr. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol 2004;287:L428–L437. [DOI] [PubMed] [Google Scholar]
- 8.Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res 2005;11:117–123. [DOI] [PubMed] [Google Scholar]
- 9.Prohinar P, Re F, Widstrom R, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. Specific high affinity interactions of monomeric endotoxin.protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem 2007;282:1010–1017. [DOI] [PubMed] [Google Scholar]
- 10.Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem 2005;280:38383–38394. [DOI] [PubMed] [Google Scholar]
- 11.Weiss J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans 2003;31:785–790. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol 2006;24:353–389. [DOI] [PubMed] [Google Scholar]
- 13.Brandenburg K, Wiese A. Endotoxins: relationships between structure, function, and activity. Curr Top Med Chem 2004;4:1127–1146. [DOI] [PubMed] [Google Scholar]
- 14.Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dent Res 2005;84:584–595. [DOI] [PubMed] [Google Scholar]
- 15.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 2005;3:36–46. [DOI] [PubMed] [Google Scholar]
- 16.Munford RS. Detoxifying endotoxin: time, place and person. J Endotoxin Res 2005;11:69–84. [DOI] [PubMed] [Google Scholar]
- 17.Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. J Immunol 2005;175:4669–4676. [DOI] [PubMed] [Google Scholar]
- 18.Munford RS, Varley AW. Shield as signal: lipopolysaccharides and the evolution of immunity to gram-negative bacteria. PLoS Pathog 2006;2:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coats SR, Pham TT, Bainbridge BW, Reife RA, Darveau RP. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol 2005;175:4490–4498. [DOI] [PubMed] [Google Scholar]
- 20.Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 1999;286:1561–1565. [DOI] [PubMed] [Google Scholar]
- 21.Ernst RK, Adams KN, Moskowitz SM, Kraig GM, Kawasaki K, Stead CM, Trent MS, Miller SI. The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J Bacteriol 2006;188:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 2006;7:1066–1073. [DOI] [PubMed] [Google Scholar]
- 23.Hađina S, Thorne PS, Kulhankova K, McCray PBJ, Weiss JP. Lipooligosaccharide inhalation produces dose- and time-dependant lung inflammation and cytokine release in mice [abstract]. J Allergy Clin Immunol 2006;117:S149. [Google Scholar]
- 24.Hađina S, Thorne PS, McCray PBJ, Weiss JP. Lung inflammation depends on the acylation of lipooligosaccharide (LOS) [abstract]. Eur Respir J 2006;S227.
- 25.Hađina S, Weiss JP, McCray PBJ, Widstrom R, Thorne PS. The importance of MD-2 in the induction of TLR-4 signaling and release of inflammatory cytokines in lungs [abstract]. Am J Respir Crit Care Med 2007;175:A311. [Google Scholar]
- 26.Thorne PS. Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology 2000;152:13–23. [DOI] [PubMed] [Google Scholar]
- 27.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5:46–51. [Google Scholar]
- 28.Brass DM, Savov JD, Whitehead GS, Maxwell AB, Schwartz DA. LPS binding protein is important in the airway response to inhaled endotoxin. J Allergy Clin Immunol 2004;114:586–592. [DOI] [PubMed] [Google Scholar]
- 29.Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med 2005;171:806–813. [DOI] [PubMed] [Google Scholar]
- 30.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun 2005;73:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol 2002;3:667–672. [DOI] [PubMed] [Google Scholar]
- 32.Jagielo PJ, Thorne PS, Watt JL, Frees KL, Quinn TJ, Schwartz DA. Grain dust and endotoxin inhalation challenges produce similar inflammatory responses in normal subjects. Chest 1996;110:263–270. [DOI] [PubMed] [Google Scholar]
- 33.Deetz DC, Jagielo PJ, Quinn TJ, Thorne PS, Bleuer SA, Schwartz DA. The kinetics of grain dust-induced inflammation of the lower respiratory tract. Am J Respir Crit Care Med 1997;155:254–259. [DOI] [PubMed] [Google Scholar]
- 34.Kline JN, Cowden JD, Hunninghake GW, Schutte BC, Watt JL, Wohlford-Lenane CL, Powers LS, Jones MP, Schwartz DA. Variable airway responsiveness to inhaled lipopolysaccharide. Am J Respir Crit Care Med 1999;160:297–303. [DOI] [PubMed] [Google Scholar]
- 35.Jagielo PJ, Thorne PS, Kern JA, Quinn TJ, Schwartz DA. Role of endotoxin in grain dust-induced lung inflammation in mice. Am J Physiol 1996;270:L1052–L1059. [DOI] [PubMed] [Google Scholar]
- 36.Thorne PS, DeKoster JA. Pulmonary effects of machining fluids in guinea pigs and mice. Am Ind Hyg Assoc J 1996;57:1168–1172. [DOI] [PubMed] [Google Scholar]
- 37.Thorne PS, McCray PB, Howe TS, O'Neill MA. Early-onset inflammatory responses in vivo to adenoviral vectors in the presence or absence of lipopolysaccharide-induced inflammation. Am J Respir Cell Mol Biol 1999;20:1155–1164. [DOI] [PubMed] [Google Scholar]
- 38.Thorne PS, Adamcakova-Dodd A, Kelly KM, O'Neill ME, Duchaine C. Metalworking fluid with mycobacteria and endotoxin induces hypersensitivity pneumonitis in mice. Am J Respir Crit Care Med 2006;173:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz E, Jones M, Wohlford-Lenane C, Meyer N, Frees KL, Arbour NC, Schwartz DA. Genes other than TLR4 are involved in the response to inhaled LPS. Am J Physiol Lung Cell Mol Physiol 2001;281:L1106–L1114. [DOI] [PubMed] [Google Scholar]
- 40.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2007;292:L312–L322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.