Abstract
While signal transducer and activator of transcription (STAT) 3 signaling has been linked to multiple pathways influencing immune function and cell survival, the direct influence of this transcription factor on innate immunity and tissue homeostasis during pneumonia is unknown. Human patients with dominant-negative mutations in the Stat3 gene develop recurrent pneumonias, suggesting a role for STAT3 in pulmonary host defense. We hypothesized that alveolar epithelial STAT3 is activated by IL-6 family cytokines and is required for effective responses during gram-negative bacterial pneumonia. STAT3 phosphorylation was increased in pneumonic mouse lungs and in murine lung epithelial (MLE)-15 cells stimulated with pneumonic bronchoalveolar lavage fluid (BALF) through 48 hours of Escherichia coli pneumonia. Mice lacking active STAT3 in alveolar epithelial cells (Stat3Δ/Δ) had fewer alveolar neutrophils and more viable bacteria than control mice early after intratracheal E. coli. By 48 hours after E. coli infection, however, lung injury was increased in Stat3Δ/Δ mice. Bacteria were cleared from lungs of both genotypes, albeit more slowly in Stat3Δ/Δ mice. Of the IL-6 family cytokines measured in lungs from infected C57BL/6 mice, IL-6, oncostatin M, leukemia inhibitory factor (LIF), and IL-11 were significantly elevated. Neutralization studies demonstrated that LIF and IL-6 mediated BALF-induced STAT3 activation in MLE-15 cells. Together, these results indicate that during E. coli pneumonia, select IL-6 family members activate alveolar epithelial STAT3, which functions to promote neutrophil recruitment and to limit both infection and lung injury.
Keywords: lung, neutrophils, STAT3, pneumonia, cytokines
CLINICAL RELEVANCE
Our results indicate that alveolar epithelial STAT3 activation requires IL-6 family cytokines and mediates inflammation during pneumonia. This pathway is a plausible therapeutic target for improving host defense and/or preventing lung injury.
Lung infections account for a tremendous burden of disease worldwide and are a leading cause of acute lung injury (1, 2). While Streptococcus pneumoniae is the most common agent in patients with community-acquired pneumonia (3), gram-negative rods such as Escherichia coli are a frequent cause of nosocomial pneumonia (4). Elimination of these and other pathogens from the lower respiratory tract is made possible by an effective innate immune response (5), which is necessary yet potentially dangerous to the infected host. For this reason, cytokine networks, neutrophil emigration, plasma extravasation, and other characteristics of acute inflammation must be precisely regulated to maintain tissue homeostasis.
The STAT3 transcription factor influences both immunity and inflammatory injury, but the importance of STAT3 signaling during pneumonia is unknown. STAT3 activity has been attributed both inflammatory (6–9) and anti-inflammatory (10–12) roles. Likewise, the cytokine interleukin (IL)-6, which largely signals through STAT3 (13, 14), has also been described as both pro- (15–19) and anti-inflammatory (16, 20–22), depending on the biological context. During E. coli pneumonia, neutrophil recruitment and bacterial clearance are impaired in IL-6–deficient mice (15). While the mechanisms through which IL-6 functions during this infection were not determined, tyrosine 705-phosphorylated STAT3 (pSTAT3) content was reduced in the lungs of IL-6–deficient mice, suggesting that this pathway may be required for activation of innate host defense during gram-negative pneumonia. A role for STAT3 in pulmonary host defenses is also suggested by human patients with hyper-IgE syndrome, in which defective STAT3 activity results in recurrent lung infections (23–25).
IL-6 is but one member of a family of cytokines bearing its name, all of which signal through STAT3 (14). In the current study we hypothesized that alveolar epithelial STAT3 is activated by IL-6 family cytokines and is required for host defense and the prevention of lung injury during gram-negative pneumonia. We focused on alveolar epithelial cells for several reasons: (1) STAT3 is rapidly activated in this cell type during inflammatory responses to LPS (26), the major inflammatory stimulus of gram-negative bacteria; (2) alveolar epithelial cells produce cytokines and other inflammatory mediators required for host defense (27, 28); (3) activated STAT3 in alveolar epithelial cells is required to prevent lung injury in response to hyperoxia (10) and adenovirus exposure (12); and (4) STAT3 overexpression in alveolar epithelial cells is sufficient to induce pulmonary inflammation (9). To address our hypothesis, we identified factors required for alveolar epithelial STAT3 activation, and determined the outcome of bacterial pneumonia in mice lacking functional STAT3 in alveolar epithelial cells.
MATERIALS AND METHODS
Mice
Triple transgenic mice were bred as previously described (10) to generate colonies of control mice (SP-CrtTA−/−/(tetO)7CMV-Cretg/tg/Stat3flx/flx) or Stat3Δ/Δ mice (SP-CrtTAtg/-/(tetO)7CMV-Cretg/tg/Stat3flx/flx), which cannot express the Y705-phosphorylated, active form of STAT3 in their alveolar epithelial cells. For select experiments, SP-CrtTA−/−/(tetO)7CMV-Cretg/tg (rtTA−) mice were bred with SP-CrtTAtg/-/(tetO)7CMV-Cretg/tg (rtTA+) mice to generate colonies containing rtTA and Cre-recombinase mutations without a mutation in either Stat3 allele. Results obtained from transgenic mice were compared with littermate controls. Food for breeders contained doxycycline (625 mg/kg) to induce Cre-recombinase–mediated STAT3 deletion. The differentiation pattern of cells bearing surfactant protein C promoter activity during lung development results in gene rearrangement within virtually all alveolar epithelial cells (both types I and II) using this doxycycline regimen (29). Progeny were not exposed to the doxycycline diet once weaned from their mothers at 3 weeks of age, reducing the possibility that doxycycline might be present during experiments. At the time of experimentation, mice were 6 to 9 weeks of age. Experiments with nontransgenic mice were performed using C57BL/6 mice. All experimental protocols were approved by the Harvard Medical Area Standing Committee on Animals.
Pneumonia
Mice were anesthetized by an intraperitoneal injection of ketamine (50 mg/kg)/xylazine (5 mg/kg). An angiocatheter was placed down the left bronchus, and mice received intratracheal administrations of 50 μl saline containing approximately 106 colony-forming units (CFU) E. coli (American Type Culture Collection # 19138; ATCC, Manassas, VA). The concentration of viable bacteria was estimated by optical density and subsequently verified by enumerating CFU from serial dilutions grown on 5% sheep blood agar plates. For histologic experiments, the instillate contained 1% colloidal carbon to visualize pulmonary deposition.
Lung Histology and Morphometry
After 24 or 48 hours of infection, mice were killed by halothane overdose and the heart was ligated to maintain pulmonary blood volume. Lungs were removed and instilled with 6% gluteraldehyde at 23 cm H2O pressure for fixation. The percentage of alveolar airspace occupied by neutrophils or edema fluid was quantified by blinded morphometric analysis on hematoxylin/eosin-stained lung sections as previously described (30). In rare cases in which lung sections from Stat3Δ/Δ or rtTA+ mice contained areas of emphysema-like airspace enlargement, these regions were excluded from morphometric analyses.
Bacteriology
Lungs were collected 24 and 48 hours after intratracheal E. coli, homogenized in 10 ml sterile H2O, serially diluted, and grown overnight at 37°C on 5% sheep blood agar plates. Viable bacteria were determined by colony counts and expressed as total CFU per lung.
Cytokine Protein Measurement
Lungs were homogenized in H2O containing the Roche Complete protease inhibitor cocktail (Roche, Indianapolis, IN), resuspended in lysis buffer (0.5% Triton X-100, 150 mM NaCl, 15 mM Tris-HCl, 1 mM CaCl2, 1 mM MgCl2), and incubated on ice for 30 minutes. After the incubation, lysates were cleared by centrifugation, and supernatants were collected for protein analyses. Cytokine concentrations were determined using enzyme-linked immunosorbent assay according to the protocols provided by the manufacturer (R&D Systems, Minneapolis, MN).
Real-Time RT-PCR
Cytokine and S100 mRNA levels were quantified in lung tissue using real-time RT-PCR. Left lung lobes were removed from mice at the indicated times after i.t. E. coli and preserved in RNAlater solution (Qiagen, Valencia, CA). Total RNA was extracted and purified using the RNeasy Mini Kit and RNAse-free DNase set, respectively (Qiagen). Real-time RT-PCR was performed on 10 ng purified RNA using the iScript One-Step RT-PCR Kit for Probes (Bio-Rad, Hercules, CA) and the iCycler iQ Real-Time PCR detection system (Bio-Rad). The Beacon Designer software (Premier Biosoft International, Palo Alto, CA) was used to design primers and Taqman probes (Table 1). Probes were modified with 6-FAM (reporter dye) and Black Hole Quencher-1 (quencher dye) at the 5′ and 3′ ends, respectively. Values for each sample were normalized to the content of 18S rRNA and expressed as the fold induction compared with uninfected mice (15).
TABLE 1.
PRIMER AND PROBE SEQUENCES FOR REAL-TIME RT-PCR
| Gene | Forward Primer | Reverse Primer | Taqman Probe |
|---|---|---|---|
| S100A8: | CTTGTTCAGAGAATTGGACATC | TGCCACACCCACTTTTATC | CCATCGCAAGGAACTCCTCGAAGT |
| S100A9: | AGGCTGTGGGAAGTAATTAAGAGG | CCAGAACAAAGGCCATTGAGTAAG | AGCCATGTGACTGCTGCCCAACCA |
| IL-6: | AGTTGCCTTCTTGGGACTGATG | CAGGTCTGTTGGGAGTGGTATC | AACCACGGCCTTCCCTACTTCACA |
| OSM: | CCCTATATCCGCCTCCAAAACC | GACTCTGTCCAGTGTGGTGTAC | CCTGACCTGAGAGCTGCCTGCACC |
| LIF: | TCTTCCCATCACCCCTGTAAATG | CTTGATCTGGTTCATGAGGTTGC | CCTGTGCCATACGCCACCCATGCC |
| IL-11: | CCCCAGACCAACCTGTGATC | AGTCACAGTCGAGTCTTTAACAAC | CGCACGGCCCAGTCCAAGGTCAG |
| CT-1: | GAGGCCAAGATCCGCCAGAC | TGCACGTATTCCTCCAGAAGTTG | ACAACCTTGCCCGCCTCCTGACCA |
| CNTF: | TGACTTCCATCAGGCAATACATAC | TTTCAGGGACCTTCTGTTCCAG | ACGCTCCAAGTTTCTGCCTTCGCC |
| NP: | TTCAGTGACCCCGGCTTCTC | GGACTGCCAGGATTCAGATAACTC | AGCTCCAGCTCAGCACCCTGCCTT |
| CLC: | TCAACGAGCCTGACTTCAATCC | GTAACACAGGAGGTGACTGTACG | TCTGGGTCAGCCGCAGCCTGTCAT |
| IL-27: | CCTACCAGCTCCTTCACTCC | TTAGGAATCCCAGGCTGAGC | TCCTGTCTCGGGCTGTTCGGGACC |
Definition of abbreviations: CLC, cardiotrophin-like cytokine; CNTF, ciliary neurotrophic factor; CT-1, cardiotrophin-1; LIF, leukemia inhibitory factor; NP, neuropoietin; OSM; oncostatin M.
Primer and probes were designed (listed 5′–3′) to amplify an 80– to 200–base pair region within the open reading frame of the following transcripts: S100A8, S100A9, IL-6, OSM, LIF, IL-11, CT-1, CNTF, NP, CLC, and IL-11.
Lung Wet:Dry Weight Ratios
Mice were killed as above 48 hours after intratracheal E. coli and exsanguinated via the abdominal aorta. The left bronchus was ligated, and left lung lobes were removed and weighed immediately. After desiccation at 60°C, lungs were weighed again to determine wet:dry ratios.
Bronchoalveolar Lavage
At the indicated times after intratracheal E. coli, lungs were removed from killed mice and tracheas were cannulated with a 20-gauge, blunted stainless steel needle. Antibiotic-free DMEM supplemented with 10% FBS (1 ml) was instilled and withdrawn. Bronchoalveolar lavage fluid (BALF) was then centrifuged at 300 × g to remove cells followed by 16,100 × g to remove bacteria and other remaining particulate matter. Samples were stored at −20°C until use.
Cell Culture and Immunoblots
Murine lung epithelial (MLE)-15 cells were maintained as described previously (31). Cells were seeded in 24-well tissue culture plates (150,000 cells/well) and incubated overnight (37°C in a humidified atmosphere containing 5% CO2), resulting in approximately 80% confluence. Where indicated, BALF was supplemented with neutralizing antibodies for IL-6, oncostatin M (OSM), and/or leukemia inhibitory factor (LIF) (10 μg/ml each; R&D Systems) and incubated for 1 hour at 37°C. Preliminary experiments were performed using recombinant cytokines to verify antibody efficacy at the selected concentrations. The same concentrations of isotype-matched nonspecific antibody were used for controls, such that the total immunoglobulin concentration (30 μg/ml) was equivalent in each sample. MLE-15 cells were washed once with PBS and stimulated for 10 minutes with 300 μl BALF. At the end of the incubation, cells were washed once with ice-cold PBS and lysed with 50 μl ice-cold lysis buffer (2% NP-40, 25 mM Tris pH 7.4, 50 mM NaCl, 0.5% Na deoxycholate, and 0.2% SDS) containing the Roche Complete protease inhibitor cocktail (Roche). Lysates were incubated on ice for 15 minutes and then cleared by centrifugation. Protein quantification and immunoblots were performed on supernatants as previously described (31, 32). Primary antibodies were directed against pSTAT3 or STAT3 irrespective of phosphorylation (Cell Signaling, Danvers, MA) and developed with a horseradish peroxidase–conjugated anti-rabbit polyclonal Ab (Cell Signaling). Relative densitometric units were determined using the Image J software (National Institutes of Health, Bethesda, MD). pSTAT3 densitometric units were normalized to that of total STAT3, and data were expressed as the percent pSTAT3 immunoreactivity obtained from control (0 h) BALF-stimulated MLE-15 cells.
For in vivo experiments, lungs were collected from killed mice at the indicated times after intratracheal E. coli, and left lobes were homogenized in 1 ml ice-cold lysis buffer (see above) using a rotor-stator homogenizer. Protein extractions and immunoblots were performed as described above.
Statistics
Statistical analyses were performed using GraphPad Prizm (GraphPad Software, San Diego, CA) or Statistica (StatSoft, Tulsa, OK). Data were presented as means ± SE for the number of samples identified in each figure. Real-time RT-PCR data were calculated as fold-induction and thus were presented as geometric means ± geometric SE. Comparisons were performed with a Student's t test, Mann-Whitney U test, or a one-way ANOVA followed by a Bonferroni post hoc analysis. When data did not pass Levene's test for homogeneity of variance they were log-transformed to fit requirements for ANOVA. Differences were considered statistically significant when P < 0.05.
RESULTS
Lung and Alveolar Epithelial STAT3 Are Activated during E. coli Pneumonia
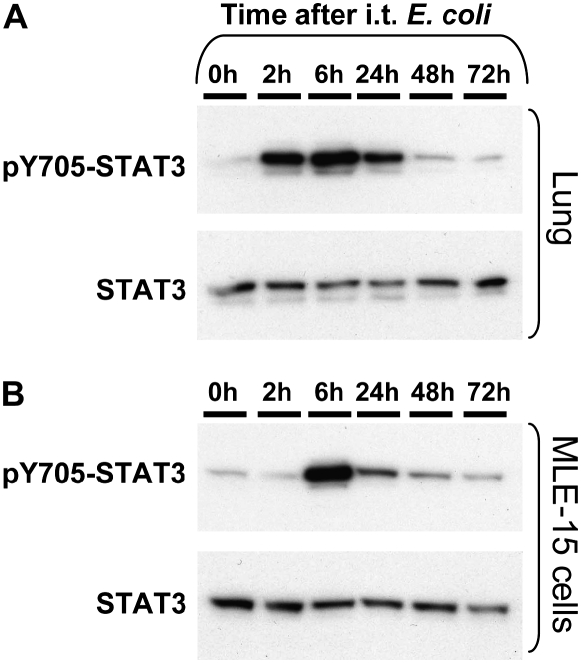
pSTAT3 levels were measured in total lung protein extractions as a determinant of STAT3 activity. After infection of mouse lungs with E. coli, pSTAT3 was detectable by 2 hours, peaked at 6 hours, and remained elevated through 48 hours (Figure 1A). To determine whether an E. coli exudate was capable of activating STAT3 in epithelial cells, we harvested cell/bacteria-free BALF from pneumonic mice 0 to 72 hours after intratracheal E. coli and stimulated the alveolar epithelial cell line MLE-15 (33) for 10 minutes. pSTAT3 increased in MLE-15 cells in response to pneumonic BALF compared with nonpneumonic BALF, peaking with 6 hours of infection and evident through 48 hours. MLE-15 cell pSTAT3 was not increased in response to direct E. coli stimulation (data not shown), suggesting that host-derived factors rather than trace amounts of bacteria were the cause of BALF-induced STAT3 activation. Thus, alveolar lining fluid contains mediators that activate STAT3 phosphorylation during E. coli pneumonia.
Figure 1.
Signal transducer and activator of transcription (STAT)3 activation during pneumonia. STAT3 activation (pSTAT3 content) was determined in (A) lungs and (B) MLE-15 cells 0 to 72 hours after intratracheal E. coli. C57BL/6 mice were instilled with 106 colony-forming units (CFU) E. coli, and left lung lobes or bronchoalveolar lavage fluid (BALF) was collected at the indicated times. Representative images are shown for pSTAT3 and total STAT3 in (A) lungs or (B) MLE-15 cells stimulated with BALF for 10 minutes. Each lane represents results from lung tissue or BALF collected from a single mouse. Immunoreactive bands shown were approximately 90 kD in size. The two rows in each panel show the same membrane labeled first for pSTAT3 and the second, after stripping, for total STAT3. Data were generated with three mice at each time point.
Alveolar Epithelial STAT3 Contributes to Early Inflammation and Host Defense
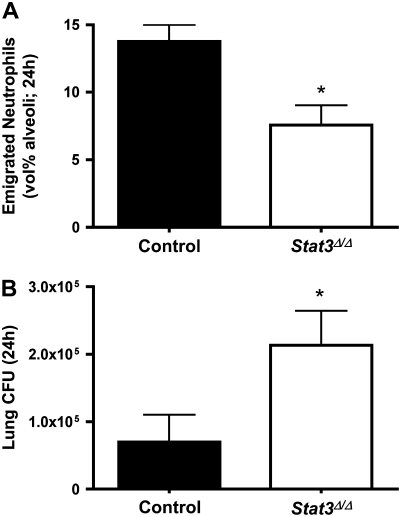
To determine the function of alveolar epithelial STAT3 activation during E. coli pneumonia, we instilled bacteria into the lungs of Stat3Δ/Δ and control mice. Stat3Δ/Δ mice express a truncated form of STAT3 that lacks exon 21, the region encoding the tyrosine residue (Y705) requisite for STAT3 activation, in their alveolar epithelial cells (10). After 24 hours of E. coli pneumonia, Stat3Δ/Δ mice had significantly fewer emigrated neutrophils in their airspaces compared with control mice (Figure 2A). Circulating neutrophil counts did not significantly differ between control (7.95 × 105 ± 0.98 × 105 cells/ml) and Stat3Δ/Δ mice (6.35 × 105 ± 1.11 × 105 cells/ml), and hence did not likely contribute to the differences observed in the airspaces. Decreased neutrophil recruitment in the lungs was associated with and perhaps a cause of increased lung bacterial burdens in Stat3Δ/Δ mice (Figure 2B). These data indicate that the inability to activate STAT3 in alveolar epithelial cells compromised innate immune pulmonary host defense during this early phase of pneumonia.
Figure 2.
Alveolar epithelial STAT3 and host defense at 24 hours. (A) Alveolar neutrophils and (B) lung bacterial clearance were measured 24 hours after intratracheal E. coli in the presence and absence of functional alveolar epithelial STAT3. Alveolar neutrophil counts (A) were determined in control or Stat3Δ/Δ mice by morphometric analysis of histologic lung sections. Viable lung E. coli (B) were quantified by colony counts on 5% sheep blood agar plates. Neutrophil data are presented as means ± SE (n = 9–11) of the percentage of alveolar airspace occupied by neutrophils. Bacteriology data are expressed as means ± SE (n = 5–10) of total lung CFU. *Statistically significant compared with control mice (P < 0.05).
Expression of Multiple Inflammatory Mediators Is Not Dependent on Alveolar Epithelial STAT3 Activation
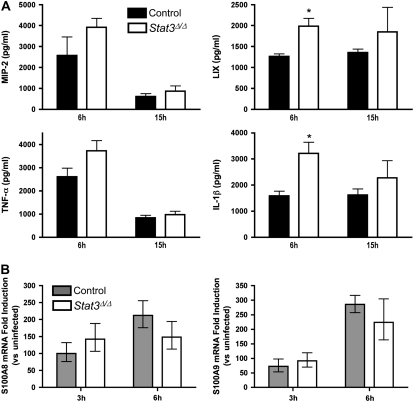
Since neutrophil emigration was significantly impaired in Stat3Δ/Δ mice during pneumonia, we determined whether the expression of early response cytokines and neutrophil chemoattractants is dependent on alveolar epithelial STAT3 activation. CXC ELR+ chemokines and S100 proteins mediate neutrophil migration (34–36), are expressed by alveolar epithelial cells (28, 37), and are regulated at least in part by STAT3 (7, 38). In addition, the early response cytokines TNF-α and IL-1β are required for maximal pulmonary inflammation during E. coli pneumonia (39). The CXC chemokines macrophage inflammatory protein-2 and LPS-induced CXC chemokine (LIX) were not diminished by alveolar epithelial STAT3 deficiency, nor were levels of TNF-α and IL-1β (Figure 3A). In fact, LIX and IL-1β levels were significantly increased at 6 hours in Stat3Δ/Δ mice. Similarly, S100A8/9 mRNA expression was strongly induced in response to E. coli in both Stat3Δ/Δ and control mice, with no significant effect of genotype (Figure 3B). Therefore, neutrophil recruitment was decreased by the deficiency of STAT3 activation in alveolar epithelial cells by a mechanism other than the induction of the inflammatory mediators measured.
Figure 3.
Alveolar epithelial STAT3 and lung inflammatory mediators. Lung ELR+ CXC chemokines, early response cytokines, and S100 expression were quantified after intratracheal E. coli in the presence and absence of alveolar epithelial STAT3. Macrophage inflammatory protein-2, LPS-induced CXC chemokine, TNF-α, and IL-1β protein levels (A) were measured in lung homogenate by enzyme-linked immunosorbent assay 6 and 15 hours after intratracheal E. coli, and expressed as means ± SE pg/ml (n = 4–8). S100A8/9 mRNA (B) was determined by real-time RT-PCR 3 and 6 hours after intratracheal E. coli. Fold-induction values (versus uninfected mice) are expressed as geometric means ± geometric SE (n = 5–10). *Statistically significant compared with control mice (P < 0.05).
Alveolar Epithelial STAT3 Prevents Lung Injury
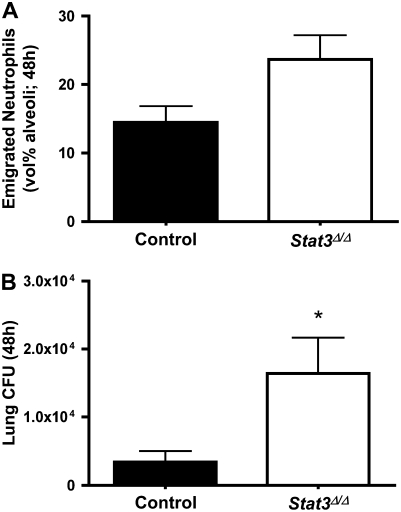
Neutrophil recruitment and bacterial counts were examined 48 hours after infection. Contrasting with the findings at 24 hours, neutrophil recruitment was unaffected or perhaps slightly increased (P = 0.055) by epithelial STAT3 deficiency at this later time point (Figure 4A). Numbers of viable E. coli remained elevated in Stat3Δ/Δ mice 48 hours after E. coli infection (Figure 4B). While bacteria were less effectively cleared from the lungs of Stat3Δ/Δ mice at both 24 and 48 hours, neutrophilic inflammation was restored at the later time point.
Figure 4.
Alveolar epithelial STAT3 and host defense at 48 hours. Alveolar neutrophils (A) and lung bacterial clearance (B) were measured 48 hours after intratracheal E. coli in the presence and absence of alveolar epithelial STAT3. Alveolar neutrophil counts (A) were determined in control or Stat3Δ/Δ mice by morphometric analysis of histologic lung sections. Neutrophil data are presented as means ± SE (n = 9–11) of the percentage of alveolar airspace occupied by neutrophils. Bacteriology data are expressed as means ± SE (n = 5–10) of total lung CFU. *Statistically significant compared with control mice (P < 0.05).
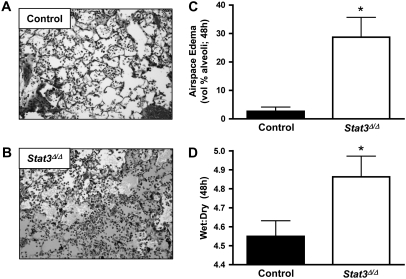
Lung histology at 48 hours demonstrated alveolar flooding that was particularly pronounced in the Stat3Δ/Δ mice (Figures 5A and 5B). Morphometric quantification confirmed that a significantly greater amount of the alveolar airspace volume was filled with edema fluid in lungs collected from STAT3-deficient mice compared with controls at 48 hours (Figure 5C). In separate experiments, wet:dry weight ratios were determined as an independent measure of pulmonary edema. Although the difference between genotypes was relatively modest, wet:dry ratios were significantly increased in Stat3Δ/Δ mice compared with controls 48 hours after intratracheal E. coli (Figure 5D). While we did not perform a formal survival study using Stat3Δ/Δ mice, only one of five lived through 72 hours of infection in a pilot study, whereas seven of nine control mice survived. Together, these data demonstrate that STAT3 in the alveolar epithelium is required for prevention of lung injury during E. coli pneumonia.
Figure 5.
Alveolar epithelial STAT3 and lung injury. Pulmonary edema was examined 48 hours after intratracheal E. coli in the presence and absence of alveolar epithelial STAT3. Representative images are shown of pneumonic lung sections from (A) control or (B) Stat3Δ/Δ mice. Alveolar edema was quantified by morphometric analysis of lung sections (C) and data expressed as means ± SE (n = 7–8) of the percentage of alveolar space occupied by edema fluid. Pulmonary edema was also measured and expressed as means ± SE (n = 8–11) wet:dry (D). *Statistically significant compared with control mice (P < 0.05).
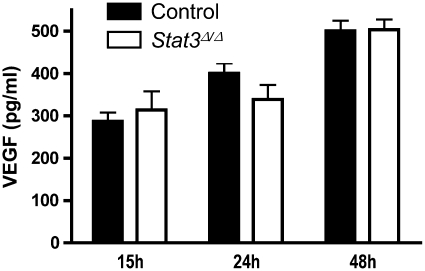
Since vascular endothelial growth factor (VEGF) can be both STAT3 dependent (40) and cytoprotective in the lung (41, 42), we determined its expression in control and Stat3Δ/Δ mice during pneumonia. Lung VEGF content was unaffected by STAT3 deficiency at the time points analyzed (Figure 6), and, therefore, does not likely contribute to the lung injury present in Stat3Δ/Δ mice.
Figure 6.
Alveolar epithelial STAT3 and vascular endothelial growth factor (VEGF). Lung VEGF levels were determined 15, 24, and 48 hours after intratracheal E. coli in the presence and absence of functional alveolar epithelial STAT3. Protein concentrations were measured in lung homogenate by enzyme-linked immunosorbent assay and expressed as means ± SE pg/ml (n = 3–7).
Changes in Host Defense and Lung Injury Are a Direct Result of STAT3 Deficiency
Rearrangement of the Stat3 gene in Stat3Δ/Δ mice is mediated by expression of reverse tetracycline-transactivator (rtTA) and Cre-recombinase in lung epithelial cells. To control for the potential detrimental effects of these two products (43, 44), experiments were performed in mice with or without rtTA transgenes (rtTA+ or rtTA−, respectively) but lacking loxP insertions in Stat3 alleles. The rtTA+ mice expressed both rtTA and Cre-recombinase, whereas rtTA− mice expressed little (if promoter leakage) or none of either transgene. During E. coli pneumonia, differences between rtTA− and rtTA+ mice were not observed in neutrophil emigration (24 h; 14 ± 1 versus 15 ± 1 [vol% alveoli]), bacterial killing (24 h; 56 ± 80 versus 120 ± 76 CFU/lung [× 106]), or lung liquid content (48 h; 4.7 ± 0.1 versus 4.5 ± 0.3 wet:dry ratio). Because the expression of rtTA and Cre-recombinase failed to impact these parameters, we conclude that the phenotypes identified above in Stat3Δ/Δ mice result specifically from the deficiency of STAT3 in alveolar epithelial cells rather than an indirect response to upstream transgene expression.
Alveolar Epithelial STAT3 Is Activated by IL-6 Family Cytokines during E. coli Pneumonia
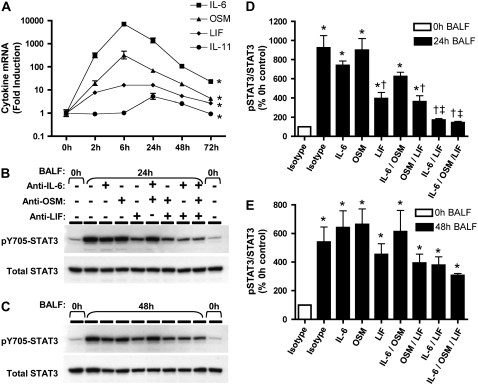
The STAT3 signaling pathway is elicited by diverse cytokines, including all members of the IL-6 family (14). With the exception of IL-6 itself, which has been identified as a necessary component of pulmonary host defense (15, 18), the expression patterns of other IL-6 family cytokines have not been determined during bacterial pneumonia. We measured mRNA for the known IL-6 family cytokines to identify candidate STAT3-activating cytokines during E. coli pneumonia. Lungs were collected for RNA analysis between 0 and 72 hours of infection to associate changes in STAT3 activation (Figure 1) with changes in STAT3-signaling cytokines. Of the cytokines analyzed, IL-6, OSM, LIF, and IL-11 mRNAs were induced in response to intratracheal E. coli (Figure 7A), with no significant changes detectable in the other five cytokines analyzed (CT-1, CNTF, NP, CLC, and IL-27; data not shown). IL-6 expression was highest among the four cytokines (> 7,000-fold), followed in order by OSM (> 300-fold), LIF (> 15-fold), and IL-11 (> 5-fold). Since the kinetics of STAT3 activation (Figure 1) correlated with cytokine mRNA induction, these data implicate IL-6, OSM, LIF, and IL-11 as potential mediators of lung STAT3 signaling during pneumonia.
Figure 7.
IL-6 family cytokines and pneumonia. IL-6 family mRNA in the lungs (A) was measured using real-time RT-PCR for IL-6, oncostatin M (OSM), leukemia inhibitory factor (LIF), and IL-11 mRNA. Pneumonic lung lobes were harvested from C57BL/6 mice 0 to 72 hours after intratracheal E. coli, and fold-induction values (versus uninfected mice) are expressed as geometric means ± geometric SE (n = 5–6). *Statistically significant effect of infection on mRNA levels for all shown (P < 0.05). IL-6 family-dependent STAT3 activation in MLE-15 cells (B–E) was measured using immunoblots. MLE-15 cells were stimulated for 10 minutes with BALF collected 0, 24 (B/D), or 48 hours (C/E) after intratracheal E. coli, in the absence and presence of neutralizing antibodies for IL-6, OSM, and/or LIF. Representative images from one of three separate experiments are shown for pSTAT3 and total STAT3 immunoreactivity in MLE-15 cell protein extracts in response to pooled BALF collected at (A) 24 and (B) 48 hours. Immunoreactive bands shown were approximately 90 kD in size. The two rows in panels B and C show the same membrane labeled first for pSTAT3 and the second, after stripping, for total STAT3. pSTAT3 immunoreactivity was quantified by densitometry (D–E) and normalized to that of total STAT3. pSTAT3/STAT3 ratios are expressed as a percentage of the value determined for MLE-15 cells stimulated with control BALF (0 h BALF) and expressed as means ± SE (n = 3). *Statistically significant compared with cells stimulated with 0 hours of BALF and isotype control IgG (P < 0.05). †Statistically significant compared with cells stimulated with pneumonic BALF and isotype control IgG. ‡Statistically significant compared with cells stimulated with pneumonic BALF and anti-LIF (P < 0.05).
To determine contributions of IL-6 family members to alveolar epithelial STAT3 activation, MLE-15 cells were stimulated with BALF in the absence or presence of neutralizing antibodies for IL-6, OSM, and LIF, the three most highly expressed cytokines identified above. Consistent with our findings in Figure 1B, BALF from mice exposed to E. coli for 24 hours strongly stimulated STAT3 phosphorylation in MLE-15 cells (Figures 7B and 7D). Neutralization of IL-6 or OSM alone did not significantly affect BALF-induced STAT3 phosphorylation. LIF neutralization significantly diminished STAT3 activation in response to 24-hour BALF. Antibody combinations revealed an additional contribution of IL-6 but not OSM to MLE-15 cell STAT3 signaling, such that neutralization of both LIF and IL-6 resulted in significantly less pSTAT3 than did LIF blockade alone. In fact, STAT3 activation induced by 24-hour BALF was virtually abolished by the combined inhibition of both IL-6 and LIF, with densitometric values not significantly different from those measured in response to 0 hours control BALF. After stimulation with 48-hour BALF (Figures 7C and 7E), pSTAT3 levels were modestly affected by LIF and IL-6 neutralization, but changes in densitometric values did not reach statistical significance despite consistent patterns observed over three separate experiments. Considerable pSTAT3 immunoreactivity was detected in MLE-15 cells after stimulation with 48-hour BALF even in the presence of all three blocking antibodies. Taken together, LIF and IL-6 in alveolar lining fluid are necessary for early alveolar epithelial STAT3 activation. However, additional STAT3-activating cytokines are important for this process at later time points.
DISCUSSION
Alveolar epithelial STAT3 was required early (24 h) for maximal neutrophil recruitment and bacterial killing during E. coli pneumonia. Later in the infection (48 h), STAT3 prevented alveolar edema and lung injury. In addition, our in vitro findings suggest that IL-6 family cytokines, particularly LIF, in alveolar lining fluid are necessary for STAT3 activation. We conclude from these data that STAT3 activity in alveolar epithelial cells is downstream of IL-6 family cytokine expression and functions to both promote innate host defense and limit inflammatory injury during pneumonia.
The phenotype of Stat3Δ/Δ mice at 24 hours of infection was similar to that of IL-6–deficient mice with E. coli pneumonia, including decreased neutrophil recruitment and bacterial clearance (15). IL-6 deficiency transiently decreased total lung pSTAT3 content (15), and the present data demonstrate a transient role for IL-6 in activation of STAT3 in BALF-stimulated MLE-15 cells. Together, these studies implicate alveolar epithelial STAT3 as one avenue through which IL-6 promotes innate immunity. It is likely, however, that IL-6 signals through other cell types as well, such that deletion of STAT3 in only alveolar epithelial cells understates the full contribution of IL-6 to innate immunity in the lungs. The mechanisms, however, through which STAT3 signaling promotes inflammation at this stage of infection remain unknown. STAT3 has been shown to influence the expression of multiple inflammatory mediators (7, 9), yet our current results indicate no contribution of STAT3 on the expression of CXC chemokines, early response cytokines, or S100 proteins during E. coli pneumonia. Future research will be necessary to elucidate the factors that link IL-6 family-induced STAT3 activity to alveolar neutrophil emigration.
At 48 hours, the deficiency of STAT3 in alveolar epithelial cells resulted in exacerbated lung injury as measured by alveolar flooding and lung liquid accumulation. Although bacterial burdens were increased in Stat3Δ/Δ mice compared with controls at this later time point, the CFU values observed at 48 hours represent less than 2% of the original E. coli inoculum. Therefore, exaggerated lung injury at 48 hours likely resulted from dysregulated inflammation or epithelial integrity rather than overwhelming infection. The results at 48 hours also indicate the possibility that STAT3 influences host defense independent of its early contribution to neutrophil recruitment (at 24 h), since bacterial burdens were higher in Stat3Δ/Δ mice despite normal or perhaps increased emigrated neutrophils. While neutrophil recruitment is a critical determinant of bacterial clearance in the lower respiratory tract, it is also possible that other bactericidal factors are downstream of STAT3 activation in the epithelium.
STAT3 activity has diverse biological consequences, but perhaps the most consistent role is tissue protection (10, 12, 45). Many mechanisms are possible, including but by no means limited to regulation of apoptosis (46), surfactant production (47), VEGF expression (40), and heme oxygenase-1 expression (48). STAT3 in alveolar epithelial cells helps prevent lung injury after hyperoxia or adenovirus administration via mechanisms that include the expression of surfactant protein-B and possibly the anti-apoptotic protein Bcl-xL (10, 12). The overexpression of a constitutively active form of STAT3 in the airway epithelium protects mice from hyperoxic lung injury, in part due to decreased expression of matrix metalloproteinases 9 and 12 (11). VEGF is also expressed in response to STAT3 activation (40) and can be cytoprotective in the lungs (41, 42), but our current data suggest that this growth factor was not responsible for the phenotype observed in Stat3Δ/Δ mice. STAT3 target genes important during pneumonia may include those identified above as well as others. The net effects of STAT3 activity in the alveolar epithelium of infected lungs are to increase bacterial clearance and limit lung injury.
It was recently shown that dominant-negative mutations in the Stat3 gene in humans result in the hyper-IgE syndrome (24, 25). Recurrent pneumonias are a hallmark of this disease, and lung infection is either directly or indirectly the cause of death in these patients (23). The present results establish a causal link between STAT3 and host responses to bacteria in the lungs. Our current data indicate that STAT3 deficiency in the alveolar epithelial cells reduces both innate immune responsiveness and tissue protection during pneumonia, which suggests that defective functions in these cells may contribute to the pathogenesis of hyper-IgE syndrome.
Because alveolar epithelial STAT3 has such important functions during pneumonia, we sought to determine upstream factors contributing to its activation. Multiple cytokines and growth factors stimulate STAT3 signaling. We focused on IL-6 family members due to the importance of IL-6 during pneumonia (15, 18) and the shared requisite use of the gp130/STAT signaling pathway by this family of cytokines (49). Alveolar epithelial cells express gp130 and are responsive to IL-6 family cytokines (26, 47, 50). Little is known about IL-6 family members other than IL-6 during pneumonia, but OSM and LIF are increased in serum and BALF of patients with pneumonia and acute lung injury (51, 52). We observed increased lung mRNA for IL-6, OSM, LIF, and IL-11 after intratracheal E. coli. The combined blockade of both IL-6 and LIF completely inhibited STAT3 phosphorylation induced by BALF from mice infected 24 hours previously, suggesting these two cytokines as essential to activating alveolar epithelial STAT3 early during pneumonia. Despite its comparatively lower level of mRNA induction, LIF was the most essential of the IL-6 family cytokines in BALF for activating STAT3 phosphorylation in MLE-15 cells, supporting a role for LIF in particular during pneumonia.
As with STAT3, LIF has protective effects during inflammation. Hyperoxia-induced lung injury, which is exacerbated in the absence of alveolar epithelial STAT3 (10), is ameliorated when LIF is overexpressed in the lungs (53). Similarly, intratracheal administration of recombinant LIF protects rats from hyperoxia-induced lung injury (54) and inhibits LPS-induced pulmonary inflammation (55). The roles of endogenous LIF are less clear. However, increased susceptibility to endotoxemia-induced shock in LIF-deficient mice again suggests a protective effect of this IL-6 family member during inflammation (56). The regulation and function of LIF in pneumonic lungs is an important direction for future research.
Together these results indicate a critical role for STAT3 in alveolar epithelial cells during pneumonia. Early during infection, epithelial STAT3 contributes to neutrophil recruitment, whereas later it serves a role in protection from lung injury. During E. coli pneumonia, both IL-6 and LIF activate STAT3 phosphorylation in alveolar epithelial cells. Variation among patients in the LIF/IL-6:STAT3 signaling pathway may influence both infection and lung injury during pneumonia.
Acknowledgments
The authors thank Satoshi Uematsu and Shizuo Akira for generating the floxed STAT3 mice. The authors also thank Mariya Kogan for technical assistance.
This work was supported by National Institutes of Health Grants HL079392 (to J.P.M.), and HL68153, HL07118, and ES00002 (to L.J.Q.). L.J.Q. was supported by an American Lung Association Senior Research Fellowship. M.R.J. was supported by an American Physiological Society postdoctoral fellowship in physiological genomics.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0365OC on January 10, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA Jr, Hoffman E, Hubmayr RD, et al. Future research directions in acute lung injury: summary of a national heart, lung, and blood institute working group. Am J Respir Crit Care Med 2003;167:1027–1035. [DOI] [PubMed] [Google Scholar]
- 2.Mizgerd JP. Lung infection–a public health priority. PLoS Med 2006;3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, Sanchez M, Martinez JA. Severe community-acquired pneumonia: risk factors and follow-up epidemiology. Am J Respir Crit Care Med 1999;160:923–929. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed QA, Niederman MS. Respiratory infection in the chronically critically ill patient. Ventilator-associated pneumonia and tracheobronchitis. Clin Chest Med 2001;22:71–85. [DOI] [PubMed] [Google Scholar]
- 5.Mehrad B, Standiford TJ. Role of cytokines in pulmonary antimicrobial host defense. Immunol Res 1999;20:15–27. [DOI] [PubMed] [Google Scholar]
- 6.Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, Liu F, Goldsmith MA, Link DC, Murray PJ, et al. Stat3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood 2006;108:3682–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB. Stat3 regulates genes common to both wound healing and cancer. Oncogene 2005;24:3397–3408. [DOI] [PubMed] [Google Scholar]
- 8.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. Stat3 regulates cytokine-mediated generation of inflammatory helper t cells. J Biol Chem 2007;282:9358–9363. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Du H, Qin Y, Roberts J, Cummings OW, Yan C. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res 2007;67:8494–8503. [DOI] [PubMed] [Google Scholar]
- 10.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 2004;113:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, et al. Overexpression of stat3c in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 2005;174:7250–7256. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, Hull WM, Wert SE, Whitsett JA. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol 2006;177:527–537. [DOI] [PubMed] [Google Scholar]
- 13.Alonzi T, Fattori E, Cappelletti M, Ciliberto G, Poli V. Impaired stat3 activation following localized inflammatory stimulus in il-6-deficient mice. Cytokine 1998;10:13–18. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (il)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of stat proteins and recruitment of neutrophils during escherichia coli pneumonia. J Infect Dis 2006;193:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med 2002;165:1445–1450. [DOI] [PubMed] [Google Scholar]
- 17.Rijneveld AW, van den Dobbelsteen GP, Florquin S, Standiford TJ, Speelman P, van Alphen L, van der Poll T. Roles of interleukin-6 and macrophage inflammatory protein-2 in pneumolysin-induced lung inflammation in mice. J Infect Dis 2002;185:123–126. [DOI] [PubMed] [Google Scholar]
- 18.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 1997;176:439–444. [DOI] [PubMed] [Google Scholar]
- 19.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, et al. Role of il-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997;6:315–325. [DOI] [PubMed] [Google Scholar]
- 20.McLoughlin RM, Hurst SM, Nowell MA, Harris DA, Horiuchi S, Morgan LW, Wilkinson TS, Yamamoto N, Topley N, Jones SA. Differential regulation of neutrophil-activating chemokines by il-6 and its soluble receptor isoforms. J Immunol 2004;172:5676–5683. [DOI] [PubMed] [Google Scholar]
- 21.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 2000;22:535–542. [DOI] [PubMed] [Google Scholar]
- 22.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. Il-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest 1998;101:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman AF, Kleiner DE, Nadiminti H, Davis J, Quezado M, Anderson V, Puck JM, Holland SM. Causes of death in hyper-ige syndrome. J Allergy Clin Immunol 2007;119:1234–1240. [DOI] [PubMed] [Google Scholar]
- 24.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. Stat3 mutations in the hyper-ige syndrome. N Engl J Med 2007;357:1608–1619. [DOI] [PubMed] [Google Scholar]
- 25.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of stat3 cause hyper-ige syndrome. Nature 2007;448:1058–1062. [DOI] [PubMed] [Google Scholar]
- 26.Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, et al. Activation of the stat pathway in acute lung injury. Am J Physiol 2004;286:L1282–L1292. [DOI] [PubMed] [Google Scholar]
- 27.Thorley AJ, Ford PA, Giembycz MA, Goldstraw P, Young A, Tetley TD. Differential regulation of cytokine release and leukocyte migration by lipopolysaccharide-stimulated primary human lung alveolar type ii epithelial cells and macrophages. J Immunol 2007;178:463–473. [DOI] [PubMed] [Google Scholar]
- 28.Vanderbilt JN, Mager EM, Allen L, Sawa T, Wiener-Kronish J, Gonzalez R, Dobbs LG. Cxc chemokines and their receptors are expressed in type ii cells and upregulated following lung injury. Am J Respir Cell Mol Biol 2003;29:661–668. [DOI] [PubMed] [Google Scholar]
- 29.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 2002;99:10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, Doerschuk CM. Neutrophil emigration in the skin, lungs, and peritoneum: Different requirements for cd11/cd18 revealed by cd18-deficient mice. J Exp Med 1997;186:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of nf-kappab rela during pneumococcal pneumonia. J Immunol 2007;178:1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung nf-kappab activation and neutrophil recruitment require il-1 and tnf receptor signaling during pneumococcal pneumonia. J Immunol 2005;175:7530–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein c/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 1993;90:11029–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine klebsiella pneumonia. J Infect Dis 1996;173:159–165. [DOI] [PubMed] [Google Scholar]
- 35.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of s100: Proteins s100a8, s100a9, and s100a8/a9 induce neutrophil chemotaxis and adhesion. J Immunol 2003;170:3233–3242. [DOI] [PubMed] [Google Scholar]
- 36.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. Cxc chemokine receptor cxcr2 is essential for protective innate host response in murine pseudomonas aeruginosa pneumonia. Infect Immun 2000;68:4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henke MO, Renner A, Rubin BK, Gyves JI, Lorenz E, Koo JS. Up-regulation of s100a8 and s100a9 protein in bronchial epithelial cells by lipopolysaccharide. Exp Lung Res 2006;32:331–347. [DOI] [PubMed] [Google Scholar]
- 38.Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, Garcia M, Venereau E, Preisser L, Guignouard E, et al. Oncostatin m secreted by skin infiltrating t lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol 2007;178:4615–4622. [DOI] [PubMed] [Google Scholar]
- 39.Mizgerd JP, Lupa MM, Hjoberg J, Vallone JC, Warren HB, Butler JP, Silverman ES. Roles for early response cytokines during escherichia coli pneumonia revealed by mice with combined deficiencies of all signaling receptors for tnf and il-1. Am J Physiol 2004;286:L1302–L1310. [DOI] [PubMed] [Google Scholar]
- 40.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al. Targeting stat3 blocks both hif-1 and vegf expression induced by multiple oncogenic growth signaling pathways. Oncogene 2005;24:5552–5560. [DOI] [PubMed] [Google Scholar]
- 41.Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, Chen Q, Ma B, Du Y, Roux F, McArdle J, et al. Il-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 2000;106:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siner JM, Jiang G, Cohen ZI, Shan P, Zhang X, Lee CG, Elias JA, Lee PJ. Vegf-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J 2007;21:1422–1432. [DOI] [PubMed] [Google Scholar]
- 43.Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, Berns A, Jonkers J. Growth inhibition and DNA damage induced by cre recombinase in mammalian cells. Proc Natl Acad Sci USA 2001;98:9209–9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisson TH, Hansen JM, Shah M, Hanson KE, Du M, Ling T, Simon RH, Christensen PJ. Expression of the reverse tetracycline-transactivator gene causes emphysema-like changes in mice. Am J Respir Cell Mol Biol 2006;34:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy DE, Darnell JE, Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3:651–662. [DOI] [PubMed] [Google Scholar]
- 46.Battle TE, Frank DA. The role of stats in apoptosis. Curr Mol Med 2002;2:381–392. [DOI] [PubMed] [Google Scholar]
- 47.Yan C, Naltner A, Martin M, Naltner M, Fangman JM, Gurel O. Transcriptional stimulation of the surfactant protein b gene by stat3 in respiratory epithelial cells. J Biol Chem 2002;277:10967–10972. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Shan P, Jiang G, Zhang SS, Otterbein LE, Fu XY, Lee PJ. Endothelial stat3 is essential for the protective effects of ho-1 in oxidant-induced lung injury. FASEB J 2006;20:2156–2158. [DOI] [PubMed] [Google Scholar]
- 49.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/jak/stat pathway. Biochem J 1998;334:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chattopadhyay S, Tracy E, Liang P, Robledo O, Rose-John S, Baumann H. Interleukin-31 and oncostatin-m mediate distinct signaling reactions and response patterns in lung epithelial cells. J Biol Chem 2007;282:3014–3026. [DOI] [PubMed] [Google Scholar]
- 51.Grenier A, Combaux D, Chastre J, Gougerot-Pocidalo MA, Gibert C, Dehoux M, Chollet-Martin S. Oncostatin m production by blood and alveolar neutrophils during acute lung injury. Lab Invest 2001;81:133–141. [DOI] [PubMed] [Google Scholar]
- 52.Ren SG, Seliktar J, Li X, Braunstein GD, Melmed S. Measurement of leukemia inhibitory factor in biological fluids by radioimmunoassay. J Clin Endocrinol Metab 1998;83:1275–1283. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Chen Q, Corne J, Zhu Z, Lee CG, Bhandari V, Homer RJ, Elias JA. Pulmonary expression of leukemia inhibitory factor induces b cell hyperplasia and confers protection in hyperoxia. J Biol Chem 2003;278:31226–31232. [DOI] [PubMed] [Google Scholar]
- 54.Tsan MF, White JE, Wong GH. D-factor and growth hormone enhance tumor necrosis factor-induced increase of mn superoxide dismutase mrna and oxygen tolerance. Cytokine 1992;4:101–105. [DOI] [PubMed] [Google Scholar]
- 55.Ulich TR, Fann MJ, Patterson PH, Williams JH, Samal B, Del Castillo J, Yin S, Guo K, Remick DG. Intratracheal injection of lps and cytokines. V. Lps induces expression of lif and lif inhibits acute inflammation. Am J Physiol 1994;267:L442–L446. [DOI] [PubMed] [Google Scholar]
- 56.Weber MA, Schnyder-Candrian S, Schnyder B, Quesniaux V, Poli V, Stewart CL, Ryffel B. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab Invest 2005;85:276–284. [DOI] [PubMed] [Google Scholar]