Abstract
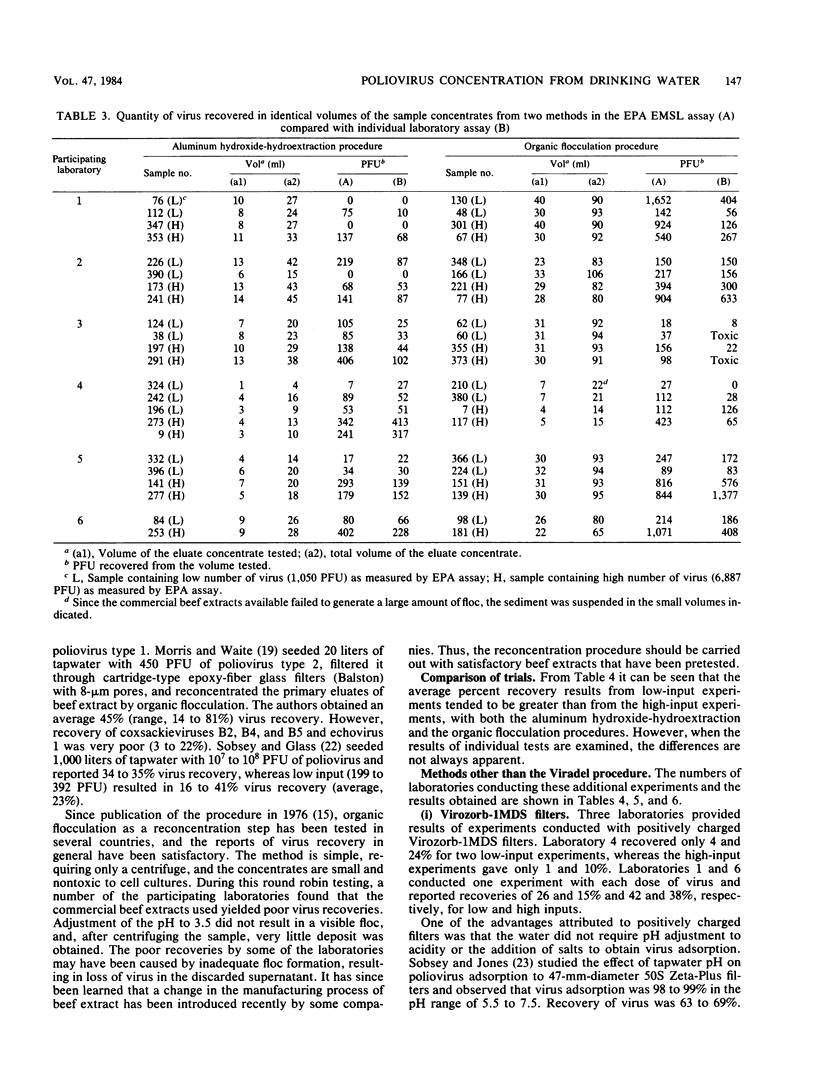
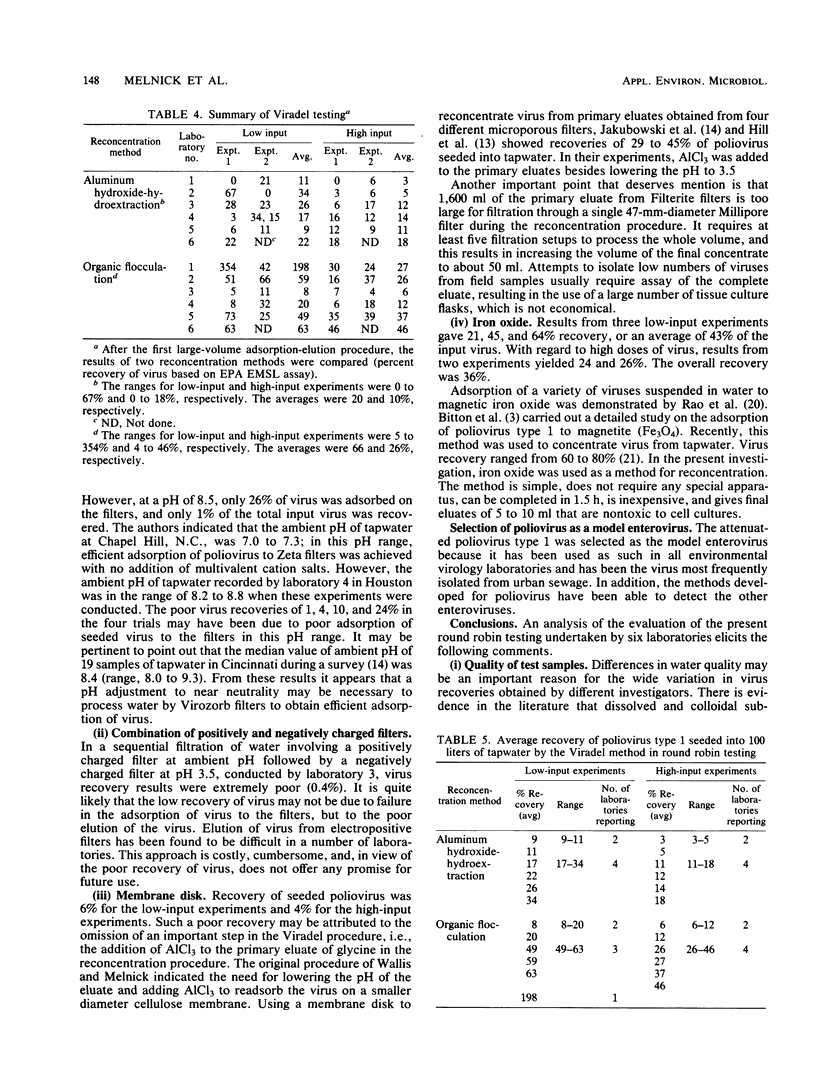
Six laboratories actively involved in water virology research participated in a methods evaluation study, conducted under the auspices of the American Society for Testing and Materials Committee on Viruses in the Aquatic Environment, Task Force on Drinking Water. Each participant was asked to examine the Viradel (virus adsorption-elution) method with cartridge-type Filterite filters for virus adsorption and organic flocculation and aluminum hydroxide-hydroextraction for reconcentration. Virus was adsorbed to filter media at pH 3.5 and eluted with either glycine buffer (pH 10.5) or beef extract-glycine (pHG 9.0). Considerable variation was noted in the quantity of virus recovered from four 100-liter samples of dechlorinated tapwater seeded with low (350 to 860 PFU) and high (1,837 to 4,689 PFU) doses of poliovirus type 1. To have a more uniform standard of comparison, all the test samples were reassayed in one laboratory, where titers were also determined for the virus seed. Test results of the Viradel-organic flocculation method indicated that the average percentage of virus recovery for low-input experiments was 66%, with a range of 8 to 20% in two laboratories, 49 to 63% in three laboratories, and 198% in one laboratory. For the high-input experiments, two laboratories reported recoveries of 6 to 12%, and four laboratories reported recoveries of 26 to 46%. For the Viradel aluminum hydroxide-hydroextraction procedure, two laboratories recovered 9 to 11%, whereas four obtained 17 to 34% for low-input experiments. For the high-input tests, two laboratories reported a recovery of 3 to 5%, and four recovered 11 to 18% of the seeded virus.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Farrah S. R., Gerba C. P., Wallis C., Melnick J. L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976 Feb;31(2):221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Wallis C., Melnick J. L., DuPont H. L. A simple method for concentration of enteroviruses and rotaviruses from cell culture harvests using membrane filters. Intervirology. 1978;9(1):56–59. doi: 10.1159/000148921. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Wallis C., Melnick J. L. Concentration of enteroviruses from estuarine water. Appl Environ Microbiol. 1977 May;33(5):1192–1196. doi: 10.1128/aem.33.5.1192-1196.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Wallis C., Melnick J. L. Concentration of poliovirus from tap water onto membrane filters with aluminum chloride at ambient pH levels. Appl Environ Microbiol. 1978 Mar;35(3):624–626. doi: 10.1128/aem.35.3.624-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrah S., Wallis C., Shaffer P. T., Melnick J. L. Reconcentration of poliovirus from sewage. Appl Environ Microbiol. 1976 Nov;32(5):653–658. doi: 10.1128/aem.32.5.653-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M., Wallis C., Melnick J. L. Concentration and purification of enteroviruses by membrane chromatography. Appl Environ Microbiol. 1976 Nov;32(5):689–693. doi: 10.1128/aem.32.5.689-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F., Jr, Jakubowski W., Akin E. W., Clarke N. A. Detection of virus in water: sensitivity of the tentative standard method for drinking water. Appl Environ Microbiol. 1976 Feb;31(2):254–261. doi: 10.1128/aem.31.2.254-261.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski W., Hill W. F., Jr, Clarke N. A. Comparative study of four microporous filters for concentrating viruses from drinking water. Appl Microbiol. 1975 Jul;30(1):58–65. doi: 10.1128/am.30.1.58-65.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Gerba C. P. Viruses in water and soil. Public Health Rev. 1980 Jul-Dec;9(3-4):185–213. [PubMed] [Google Scholar]
- Melnick J. L., Gerba C. P., Wallis C. Viruses in water. Bull World Health Organ. 1978;56(4):499–508. [PMC free article] [PubMed] [Google Scholar]
- Rao V. C., Waghmare S. V., Lakhe S. B. Detection of viruses in drinking water by concentration on magnetic iron oxide. Appl Environ Microbiol. 1981 Sep;42(3):421–426. doi: 10.1128/aem.42.3.421-426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Glass J. S. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980 Aug;40(2):201–210. doi: 10.1128/aem.40.2.201-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Jones B. L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl Environ Microbiol. 1979 Mar;37(3):588–595. doi: 10.1128/aem.37.3.588-595.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Wallis C., Henderson M., Melnick J. L. Concentration of enteroviruses from large volumes of water. Appl Microbiol. 1973 Oct;26(4):529–534. doi: 10.1128/am.26.4.529-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Henderson M., Melnick J. L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972 Mar;23(3):476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J Virol. 1967 Jun;1(3):478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W. Demonstration of solids-associated virus in wastewater and sludge. Appl Environ Microbiol. 1976 Mar;31(3):354–358. doi: 10.1128/aem.31.3.354-358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


