Abstract
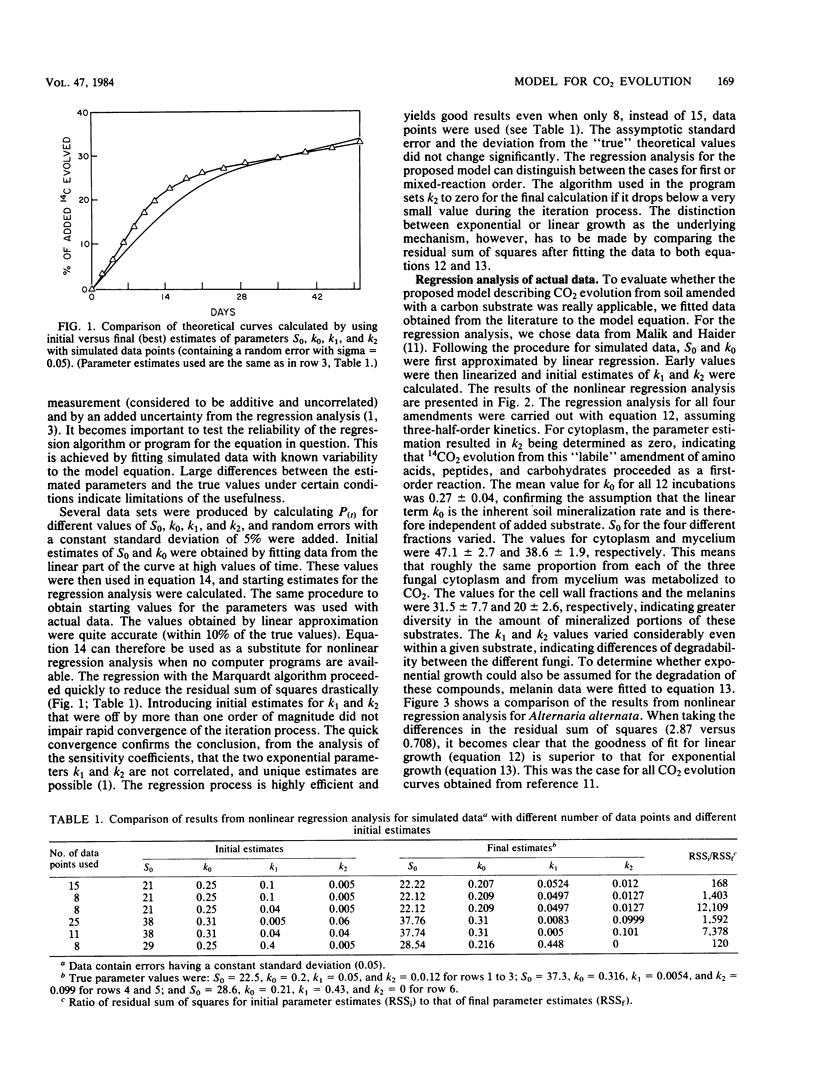
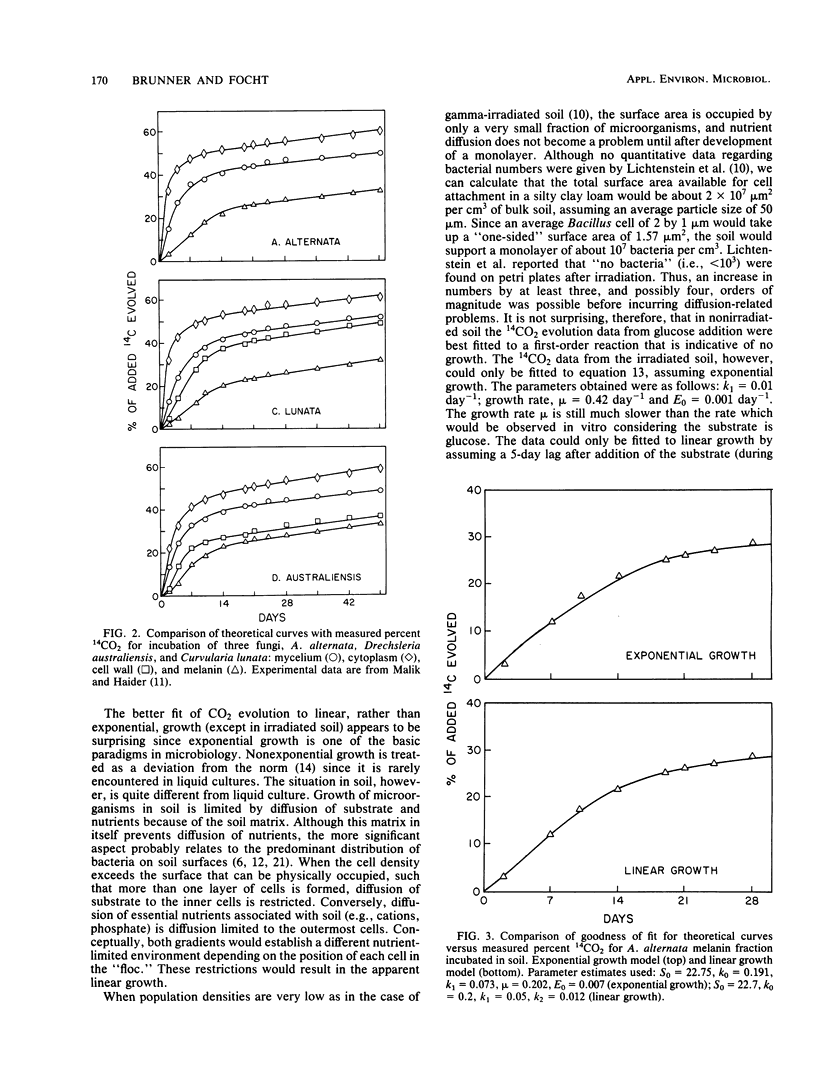
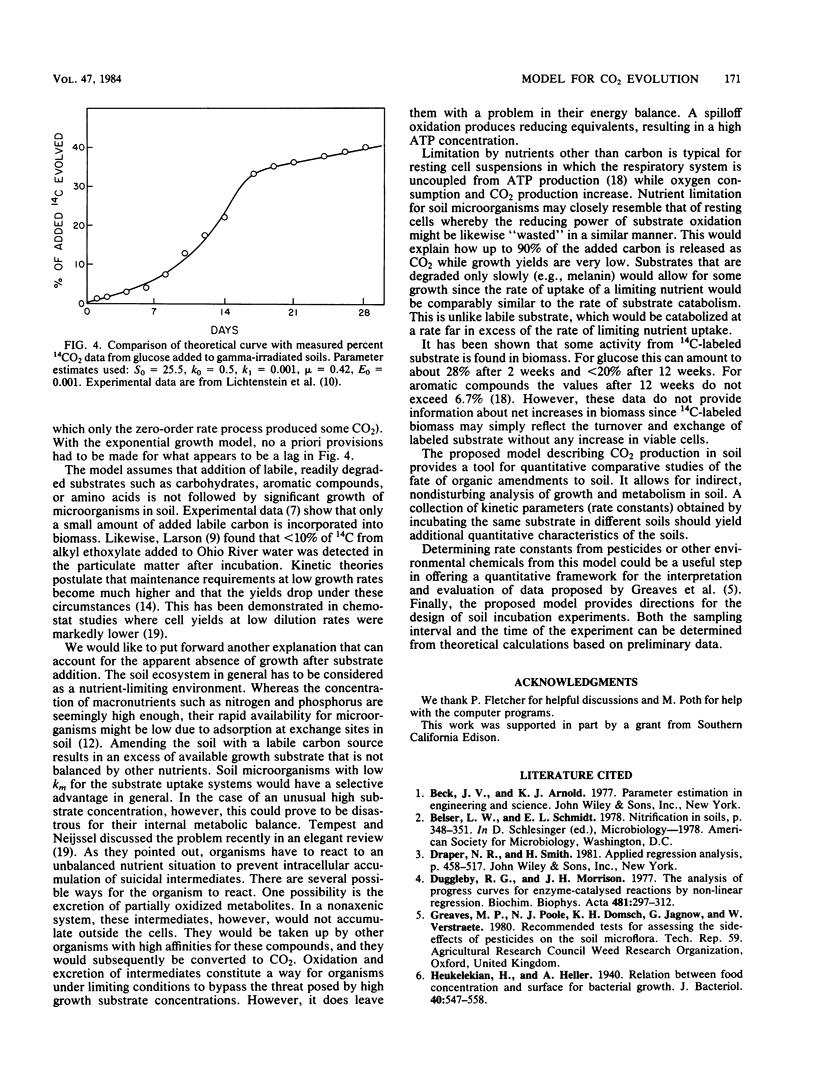
The kinetics of mineralization of carbonaceous substrates has been explained by a deterministic model which is applicable to either growth or nongrowth conditions in soil. The mixed-order nature of the model does not require a priori decisions about reaction order, discontinuity period of lag or stationary phase, or correction for endogenous mineralization rates. The integrated equation is simpler than the integrated form of the Monod equation because of the following: (i) only two, rather than four, interdependent constants have to be determined by nonlinear regression analysis, (ii) substrate or product formation can be expressed explicitly as a function of time, (iii) biomass concentration does not have to be known, and (iv) the required initial estimate for the nonlinear regression analysis can be easily obtained from a linearized form rather than from an interval estimate of a differential equation. 14CO2 evolution data from soil have been fitted to the model equation. All data except those from irradiated soil gave better fits by residual sum of squares (RSS) by assuming growth in soil was linear (RSS = 0.71) as opposed to exponential (RSS = 2.87). The underlying reasons for growth (exponential versus linear), no growth, and relative degradation rates of substrates are consistent with the basic mechanisms from which the model is derived.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duggleby R. G., Morrison J. F. The analysis of progress curves for enzyme-catalysed reactions by non-linear regression. Biochim Biophys Acta. 1977 Apr 12;481(2):297–312. doi: 10.1016/0005-2744(77)90264-9. [DOI] [PubMed] [Google Scholar]
- Heukelekian H., Heller A. Relation between Food Concentration and Surface for Bacterial Growth. J Bacteriol. 1940 Oct;40(4):547–558. doi: 10.1128/jb.40.4.547-558.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Favre J. S., Focht D. D. Conservation in soil of h(2) liberated from n(2) fixation by hup nodules. Appl Environ Microbiol. 1983 Aug;46(2):304–311. doi: 10.1128/aem.46.2.304-311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Nonlinear estimation of Monod growth kinetic parameters from a single substrate depletion curve. Appl Environ Microbiol. 1983 May;45(5):1453–1458. doi: 10.1128/aem.45.5.1453-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. J. Degradation of herbicides by soil micro-organisms. Soc Appl Bacteriol Symp Ser. 1971;1:233–254. doi: 10.1016/b978-0-12-648050-4.50017-1. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


