Abstract
The need for ECG gating presents many difficulties in cardiac magnetic resonance imaging (CMRI). Real-time imaging techniques eliminate the need for ECG gating in cine CMRI, but they cannot offer the spatial and temporal resolution provided by segmented acquisition techniques. Previous MR signal-based techniques have demonstrated an ability to provide cardiac gating information; however, these techniques result in decreased imaging efficiency. The purpose of this work was to develop a new “self-gated” (SG) acquisition technique that eliminates these efficiency deficits by extracting the motion synchronization signal directly from the same MR signals used for image reconstruction. Three separate strategies are proposed for deriving the SG signal from data acquired using radial k-space sampling: echo peak magnitude, kymogram, and 2D correlation. The SG techniques were performed on seven normal volunteers. A comparison of the results showed that they provided cine image series with no significant differences in image quality compared to that obtained with conventional ECG gating techniques. SG techniques represent an important practical advance in clinical MRI because they enable the acquisition of high temporal and spatial resolution cardiac cine images without the need for ECG gating and with no loss in imaging efficiency.
Keywords: CMRI, gating, triggering, cine, cardiac
Cardiac magnetic resonance imaging (CMRI) generally requires that data acquisition be synchronized to the periodic motion of the heart. Except in the case of real-time imaging, CMRI images are reconstructed from data acquired over a number of cardiac cycles. Thus, it is necessary to accurately time the acquisition of data within each heartbeat to consistently capture the same phase of the cardiac cycle from one heartbeat to the next. This precise synchronization is necessary whether a single cardiac phase is imaged or multiphase cine imaging is performed.
CMRI typically relies on the use of an electrocardiogram (ECG) signal to synchronize the acquisition of imaging data with cardiac motion. The need for ECG gating increases the cost and complexity of CMRI exams, and the difficulties associated with ECG acquisition can result in a decrease in overall clinical productivity. Rapid switching of magnetic field gradients and RF pulsing induces significant artifacts in the acquired ECG signal (1-4). The magnetohydrodynamic effect may also superimpose artifacts onto the ECG signal when blood flowing through the static magnetic field generates electrical fields that corrupt the measurement of cardiac tissue depolarization (5). Often patients must be removed from the bore of the magnet in order to reposition the electrodes to obtain a viable signal. Abnormalities of the patient's overall body habitus (e.g., scoliosis), chest geometry (e.g., expanded chest due to chronic obstructive pulmonary disease), or cardiovascular structures (e.g., large pericardial effusion) may lead to ECG signals with low amplitudes, or morphologies that make it difficult to obtain accurate ECG referencing. Additionally, in cases of arrhythmia, the correlation between ECG events and cardiac motion may be degraded. Gating based on the intrinsic motion of the heart may provide a more specific rejection of those beats that would lead to image artifacts.
Several novel methods have been proposed to manage some of the difficulties associated with ECG acquisition within the scanner and subsequent R-wave detection (1,3,4,6). However, these new techniques still require the use of ECG recording and lead systems, as well as additional set-up time with each examination. While ECG monitoring devices have been specifically designed for compatibility with the MR environment, cases of patient injury due to faulty or damaged equipment continue to be reported (7). Additionally, there are situations, such as fetal CMRI, in which acquiring a useful ECG gating signal may not be feasible.
One method of acquiring CMR images without ECG gating involves the use of real-time image acquisition strategies. For these techniques, k-space data for the reconstruction of an image are acquired within a temporally distinct acquisition window inside of one heartbeat, rather than from multiple acquisition windows from multiple cardiac cycles. While several approaches to real-time imaging have made great strides toward achieving good imaging efficiency and quality, segmented acquisitions will always provide a better combination of spatial resolution, temporal resolution, and signal-to-noise ratio (SNR).
In addition to real-time imaging, other CMRI techniques that eliminate the need for ECG synchronization have been described by several groups (8-10). These so-called “wireless” gating techniques involve the acquisition and processing of additional MR signals to derive cardiac cycle timing information. The acquisition of MR signals with little or no spatial phase encoding is interleaved with the acquisition of image data. Localized projection integrals or the real component of the complex echo peaks, which are both provided by the repeatedly acquired gating echoes, are used to derive a signal representative of the cardiac and/or respiratory cycle. This wireless gating signal allows images to be reconstructed at multiple cardiac phases in a manner analogous to retrospectively ECG-gated techniques. Prospective wireless gating approaches have also been proposed in which volume selective excitation along with velocity-encoded acquisition is used to acquire a cardiac gating signal for coronary MRA applications (11). The use of these wireless gating strategies for cine CMRI results in decreased imaging efficiency relative to ECG-gated techniques because the acquisition of image data must be interleaved with the acquisition of gating signals. However, in this work we demonstrate that the wireless gating technique described by Spraggins (8) and Hinks (9) can be directly applied to radial k-space sampling trajectories, with no loss of imaging efficiency.
Radial k-space acquisition (projection reconstruction (PR)) techniques were originally utilized by Lauterbur (12) in some of the very first MRI experiments in 1973. They were recently demonstrated to have several useful advantages over Cartesian sampling techniques, and to improve imaging efficiency for temporally constrained cardiovascular imaging applications (13-20). While PR techniques have the potential to improve imaging efficiency because of their salient undersampling properties (13,21), the current work focuses instead on the cardiac gating information that is provided when radial k-space sampling is used for segmented PR image acquisitions.
The purpose of this study was to develop a new “self-gated” (SG) acquisition technique that eliminates the need to acquire additional gating data by extracting the motion synchronization signal directly from the image data. It will be shown that during the performance of radial k-space sampling techniques, a signal that is synchronous with the cardiac cycle can be extracted directly from the echoes acquired for imaging. We propose three methods to derive the necessary gating information directly from the acquired imaging data for retrospective reconstruction of multiphase cardiac cine images. These techniques include 1) variations on the previously described echo peak technique, 2) a 2D center of mass (COM) kymogram, and 3) 2D low-resolution image correlation. These methods offer the potential to acquire SG image series in the same amount of time required for a corresponding ECG retrospectively gated acquisition. We investigated the feasibility of these techniques in a study of normal volunteer subjects, and the results obtained with SG imaging were compared with those from conventional ECG gating.
THEORY
Retrospective Cardiac Gating
Retrospectively gated cardiac cine imaging involves repeated acquisitions of each line or segment of k-space for a duration spanning the maximum expected cardiac cycle length. The time relative to the last R-wave is recorded with each acquired view for the subsequent retrospectively gated reconstruction (22) of images at each desired cardiac phase.
Retrospectively gated techniques can require a slightly longer overall acquisition time than prospectively triggered techniques, depending upon the degree of temporal oversampling of each cardiac cycle. For example, an acquisition window duration of 1.1 s with a constant heart rate of 60 b.p.m. would result in a 10% longer overall acquisition time compared to that required in prospectively triggered techniques. However, retrospectively gated techniques are not as susceptible to heart rate fluctuations and can provide images throughout the cardiac cycle, which is important for assessing systolic and/or diastolic disease processes. Prospectively triggered techniques struggle to provide late-diastolic images because the inevitable heart rate fluctuations require that the prospective acquisition window be set to a duration that does not exceed the minimum expected cardiac cycle length during the examination.
Motion-Induced Echo Peak Modulation
The complex echo peak recorded during a readout with no phase encoding (central line of k-space) is representative of the complex sum of the transverse magnetization across the entire image volume. If the image volume contains moving or changing structures, a series of consecutively acquired echoes will exhibit peak changes corresponding to proportional changes in the overall transverse magnetization due to motion. While consecutively acquired echo peak values could also exhibit fluctuations due to magnetization recovery after preparation schemes (saturation or inversion), or to a transient approach to the steady-state condition, for the remainder of this work it will be assumed that such schemes were not used and that the acquisitions were performed under steady-state conditions.
Spraggins (8), White et al. (23), and Hinks (9) previously used such echo peak fluctuations to derive wireless cardiac gating signals during retrospectively gated cine acquisitions. Acquisitions of echoes acquired with no phase encoding were interleaved with conventional segmented cine acquisitions, such that every other readout provided echo peak information. The series of real and/or imaginary components of the echo peak values were band-pass-filtered prior to the use of a peak detection algorithm to create an ECG surrogate signal for retrospective image reconstruction at each cardiac phase.
In most cardiac imaging orientations, changes at the echo peak will correspond to changes in the blood volume within the slice, as well as to tissue movement into or out of the excited tissue volume. In typical short- or long-axis orientations, fluctuations in the echo peak will result from changes in the ventricular blood pool volume. With bright blood acquisition techniques (e.g., fast low-angle shot (FLASH) or fast imaging with steady-state free precession (FISP)), this will result in the largest magnitude corresponding to end-diastole and the smallest magnitude corresponding to end-systole.
Both the amplitude and phase of the echo peak can be representative of the cardiac cycle phase. Depending upon the imaging orientation, TE, and the particular gradients applied following RF excitation, the phase of the signal at the echo peak will be modulated by the changing velocities of the circulating blood pool.
The first method we propose for deriving a cardiac gating signal involves the use of the same echo peak monitoring strategy described by Spraggins et al. (8) and White et al. (23). The key advantage of our technique is that it uses an interleaved radial k-space sampling trajectory such that each readout samples the k-space center, thereby making the acquisition of additional gating echoes unnecessary. At the conclusion of a breath-hold acquisition, a gating signal for retrospective reconstruction at each cardiac phase is derived from the series of echo peak magnitudes or phases.
COM Kymogram
Image COM coordinates, xc and yc, can be estimated by solving a system of equations describing their sinusoidal relationship to the 1D COM, ξc(θ), of image projections at multiple angles, p(η,θ). Equation [1] describes the relationship between the object function ρ(x,y) and a 1D projection p(η, θ), while Eq. [2] describes the 1D projection COM ξc(θ). ξc(θ) is related to the image COM coordinates according to Eq. [3]. Knowledge of ξc(θ) at multiple angles should allow the computation of xc and yc. However, because measured ξc(θ) values will typically include some level of noise, the solution of an overdetermined system with N > 2, Eq. [4], should provide a better estimate of xc and yc. A graphical depiction of the relationship described in Eq. [3] is shown in Fig. 1. The 1D projections, p(η, θ), can be derived from the 1D FT of the views acquired while using radial k-space sampling trajectories for projection reconstruction acquisition techniques.
FIG. 1.

Relationship between 2D object COM (xc,yc) and the 1D projection COM (ξc) at five projection orientations (ψ). Note the incremental change in ξc relative to the projection angle (θ), in accordance with Eq. [3].
| [1] |
| [2] |
| [3] |
| [4] |
Gai and Axel (24) demonstrated the utilization of COM projection computations for 2D MRI motion correction, and Wieben et al. (25) extended these techniques to the 3D case. Kachelriess et al. (26) recently described techniques for spiral CT of the heart that derive a gating signal from the temporally evolving 2D COM, which they described as a “kymogram.” This ECG surrogate is derived by computing the 2D COM time series using subsets of sequentially acquired CT image projections before projecting this 2D signal onto a principal axis (that exhibiting maximum displacement) to produce the 1D gating signal. Given that in a chosen imaging orientation the COM changes correspond to the cardiac cycle, the kymogram would be used for retrospective reconstruction of multiple image slices at the same cardiac phase.
The second method we propose for deriving a cardiac gating signal extends the use of the kymogram technique to MRI. An interleaved radial acquisition is performed while the gating signal is derived from the temporally evolving xc and yc COM coordinates that are calculated using a sliding window encompassing sequentially acquired projections. The MR kymogram signal is used to retrospectively synchronize the reconstruction of images at each cardiac phase.
Low-Resolution Region-of-Interest (ROI) Correlation
Hardy et al. (27,28) recently described an adaptive free-breathing coronary MRA technique in which multiple image frames acquired in real time are averaged using an interleaved-spiral pulse sequence to produce a final image with improved SNR and vessel depiction. ROI cross correlations between images acquired in real time are used to select the frames containing the coronary vessel and determine in-plane positional offsets. Only highly correlating image frames are included in the final summation. The technique requires no breath-holding or ECG triggering. In one variation of this technique, real-time images are acquired with one of four separate subtrajectories that separately produce low-resolution images for ROI cross-correlation calculations. The cross-correlation values effectively provide information as to whether an interleave was acquired at the same temporal position within the cardiac and respiratory cycles as a reference image containing the desired vessel. The separate interleaves with corresponding images exhibiting sufficient correlation to a reference image are combined for a high-resolution image reconstruction.
The third SG method we propose again involves the use of an interleaved radial k-space sampling technique followed by the reconstruction of a sliding-window, low-resolution, real-time image series. An ROI including the myocardial wall in a single reference image is selected as a mask kernel, h(x,y), for a correlation-coefficient computation (Eq. [5]) along an ROI-masked representation of the low-resolution image series, f(x,y,t), to produce a cardiac gating signal, c(t). In Eq. [5], f̄ and h̄ represent the temporal means of the respective 2D image functions. The correlation coefficient cardiac signal is used to retrogate each view for the subsequent high-resolution image reconstructions at each cardiac phase. This SG method differs from the techniques described by Hardy et al. (27,28) in that it uses the correlation information to derive a cardiac gating signal, rather than to reject image data that do not meet the correlation criterion at a single given phase.
| [5] |
MATERIALS AND METHODS
Radial TrueFISP Sequence and Retrospectively Gated Image Reconstruction
A schematic of the 2D PR TrueFISP sequence is depicted in Fig. 2a. The radial k-space sampling trajectory is depicted in Fig. 2b. The view acquisitions covered an azimuthal k-space sampling range of 0–180°, with symmetric echo sampling. All studies were performed using 12 views per segment acquisition, and each segment was repeatedly acquired in an interleaved fashion throughout an 1100-ms acquisition window before the next segment was acquired. To better facilitate the derivation of gating signals, the segments were independently interleaved such that of the 12 views acquired for each segment, a subset of six sequentially acquired views spanned roughly 180°. Image acquisition was preceded by the application of an α/2 RF pulse (29), followed by a time interval of TR/2, before the subsequent application of 300 additional dummy ± α RF pulses (for approximately 1000 ms) to bring the magnetization to steady state prior to readout of the imaging data.
FIG. 2.
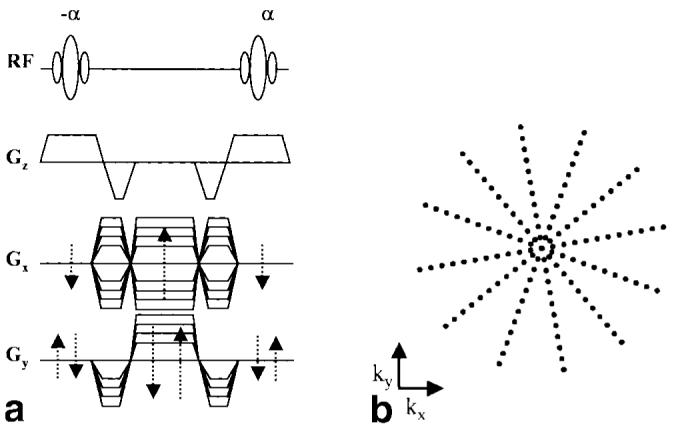
2D PR TrueFISP sequence (a) and the associated k-space sampling trajectory (b).
The raw data and corresponding ECG trigger timestamps for each experiment (as provided by the physiological monitoring system of the Siemens Sonata scanner) were saved and exported offline to a PC with MATLAB software (The Mathworks, Inc., Natick, MA) for derivation of the SG signals. Each recorded time-stamp represented the time interval between the acquisition of a k-space view and an immediately previous R-wave detection. The physiological monitoring system of the scanner used a variable threshold first-difference algorithm for R-wave detection (Siemens NUMARIS 2002B software). Once the SG signals were computed, each one was used to create a corresponding cardiac cycle timestamp series that was derived from the SG signal, rather than from the original ECG signal.
For each gating technique, the set of views necessary for image reconstruction at each cardiac phase was derived using the time-stamp information and a complex linear interpolation algorithm. A simple linear regression algorithm was used to separately calculate the real and imaginary signal components for each sampled k-space position at each of 20 cardiac phases spaced equally through a mean cardiac cycle determined from the time-stamp data (Siemens NUMARIS 2002B software).
After the acquired k-space data were resampled for each cardiac phase, a convolution regridding reconstruction algorithm incorporating a 3 × 3 Kaisser-Bessel kernel function (30) and Ram-Lak density compensation weighting (31) was used for image reconstruction. Reconstruction time was minimized by precomputing the kernel and density compensation values needed for all sampled k-space data points, and storing this information in a look-up table for repeated reference while regridding (32). Magnitude images reconstructed from each coil were combined using a self-weighting root sum of squares technique (33) to produce a single image at each cardiac phase, for each of the gating strategies.
Derivation of Signals for Self-Gating
Echo-Peak Signal
For this initial investigation, the magnitude of the complex echo peak was used for the echo-peak cardiac gating signal.
COM Kymogram Signal
The 2D COM signal was derived using a sliding temporal window encompassing subsets of six sequentially acquired views. The views were Fourier-transformed to produce 1D projections, p(η,θ), at six different angles, with each subset in total spanning roughly 150°. For each view, the projection COM, ζc(θ), was derived using Eq. [2]. The 2D COM coordinates, xc and yc, were then determined by calculating the least-squares solution to the system in Eq. [4].
A complex signal, vc(t), Eq. [6], was then generated from a time series of shifted xc and yc values, Δxc and Δyc, in order to determine the axis of maximum deviation and allow computation of a 1D kymogram gating signal. The Δxc and Δyc signals are generated from xc and yc by simply subtracting the corresponding mean value of each signal. From vc(t), an axis of maximum deviation was determined by calculating the mean of the projection angle of the signal at the maximum magnitude of vc(t) for each acquisition window. This principal axis projection angle, θp, was used to generate a 1D kymogram cardiac gating signal, kym(t), from xc(t) and yc(t) according to Eq. [7].
| [6] |
| [7] |
ROI Correlation Coefficient Signal
A view-sharing technique was first used to reconstruct a low-resolution image series at a frame rate equal to a single-sequence TR. Each low-resolution image was reconstructed using 12 views azimuthally covering roughly 180° of k-space. Only the central 64 of the 256 sampled readout data points were included in the low-resolution reconstruction, resulting in a 4.7 × 4.7 mm2 voxel size with a 300-mm2 FOV. To improve the SNR for these highly undersampled (by a factor of 10) image low-resolution reconstructions, the signals from all of the imaging coils were included.
Of the low-resolution images from the first 2.2 s of the acquisition, the one that exhibited the lowest mean intensity was chosen as the mask image. Because the experiments were all performed using a bright-blood TrueFISP technique, this choice of image for ROI mask kernel selection typically corresponded to end-systole. This image was displayed, and a polygonal ROI tool allowed user selection of h(x,y). For the purposes of this study, h(x,y) was chosen to encompass only the atrial and/or ventricular myocardium and blood pool.
Following selection of h(x,y), computation of the correlation SG signal c(t) was accomplished using Eq. [5]. Accordingly, the images that showed the greatest correlation in the region of the h(x,y) resulted in gating-signal local maxima, whereas those with the least correlation resulted in local minima.
Gating Signal Peak Detection
For this initial investigation, we chose to suppress high-frequency noise components with a very simple low-pass filter incorporating a Hamming function roll-off at 3Hz. Peak detection was performed by locating consecutive signal positions with opposite first derivative polarity. Additionally, detected peaks that were not above a local threshold (typically 70% of the maximum signal amplitude within a window of 3 s) were rejected. The detected peak series was then used to generate a time stamp for each corresponding k-space readout. This time stamp was representative of the time interval between acquisition of that particular k-space view and the immediately previous peak in the gating signal.
Volunteer Studies
Seven healthy volunteers with no history of cardiovascular disease were imaged in apical, mid, and basal cardiac short- and long-axis (four-chamber) orientations.
The imaging parameters included a 780-Hz/pixel bandwidth, 55° flip angle, 256 readout samples, 6-mm slice thickness, 300-mm isotropic in-plane FOV (1.2 × 1.2 mm2 in-plane voxel size), 144 total views per image, symmetric echo sampling (TR/TE = 3.66/1.83 ms), and a breath-hold duration of 13 s at the end of expiration. A single raw data set was acquired at each orientation along with the corresponding ECG time-stamp series.
After the three SG signals and corresponding time-stamp series were generated offline, the images were reconstructed at each of 20 cardiac phases for each acquisition and each gating methodology, resulting in 16 cine image series for each volunteer.
All of the imaging experiments were performed on a Siemens 1.5T Magnetom Sonata scanner (Siemens Medical Solutions, Erlangen, Germany), a two-channel quadrature array coil, a six-channel spine array (typically only two of these channels were selected for use, based on orientation), and a small 4-cm-diameter loop coil. The loop coil was placed directly over the heart in order to acquire a localized MR signal, which tended to attenuate background signals unrelated to cardiac motion. However, SG signals were generated for all coils used in image acquisition. The coil signal used for time-stamp computation was selected based on the coil position relative to the particular imaging orientation and apparent quality of the resulting SG signal.
SG Temporal Variability Analysis
To assess the temporal reproducibility of the SG signal trigger position over multiple cardiac cycles, we measured the heartbeat-to-heartbeat trigger variation for each acquisition and each SG strategy. The delay between the trigger time from the ECG (ECG(i)) and the SG trigger times (EPM(i), KYM(i), and 2DCOR(i)) was recorded for each heartbeat (Figs. 4 and 5). The variability in this delay is a measure of the reproducibility of the SG triggers. The ECG(i) trigger times were provided by the ECG monitoring system of the MR scanner, which has a known systematic variation of up to ±2.5 ms (400 Hz sampling rate) relative to the R-wave position. The mean value of the difference between the echo-peak amplitude SG trigger and the ECG trigger over N heartbeats, μ(EPM – ECG), was calculated using Eq. [8]. The standard deviation (SD) of the trigger delay times over N heartbeats for each acquisition was calculated using Eq. [9]. The same computations were performed for the KYM(i) and 2DCOR(i) trigger delay times to provide a metric with which to compare the trigger position variability of the three SG strategies.
| [8] |
| [9] |
FIG. 4.
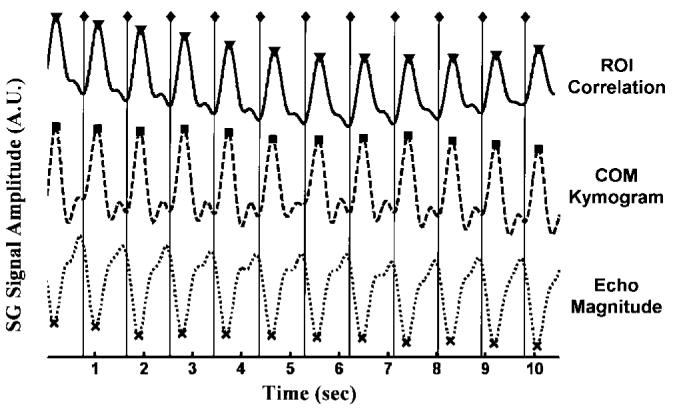
Representative SG signals used for gating the retrospective reconstruction while imaging in a cardiac short-axis orientation (◇ = R-wave position as recorded by the scanner ECG monitor ECG(i), dotted = echo peak magnitude, dashed = COM kymogram, solid = ROI correlation) along with the SG trigger positions (x = EPM(i), ■ = KYM(i), ▼ = 2DCOR(i)). Note the similar morphology of these SG signals, which is typical in the short-axis orientation.
FIG. 5.

Representative SG signals used for gating the retrospective reconstruction while imaging in a cardiac long-axis orientation (◇ = R-wave position as recorded by the scanner ECG monitor ECG(i), dotted = echo peak magnitude, dashed = COM kymogram, solid = ROI correlation) along with the SG trigger positions (x = EPM(i), ■ = KYM(i), ▼ = 2DCOR(i)). Note the different morphology of these SG signals, which is typical in the long-axis orientation.
Cine Image Series Analysis
Each cine image series was recorded as an image stack such that each could be replayed as a cine loop (15 frames per second) using the Scion Image (Scion Corp., Frederick, MD) software package. For each imaged slice, the cine loops reconstructed using each of the four gating techniques were combined into a 4-on-1 display. Slice order and quadrant placement were randomized, and all four quadrants were simultaneously and synchronously displayed dynamically as cine loops. Two independent reviewers, both cardiac MRI specialists with over 30 years of combined experience, assigned an absolute image quality score, as well as a comparative rank, to each of the cine series in each 4-on-1 display. Each cine loop was assigned an absolute quality score from 1 to 4. The absolute image scoring system used was identical to that described by White et al. (23) in previous work involving wireless ECG gating techniques. Images were scored as follows: 4 points = excellent image quality and definition of fine anatomic structures; 3 points = good image quality, permitting identification of fine anatomic structures; 2 points = image quality adequate at best for interpretation; and 1 point = poor image quality, precluding adequate visualization of structures for interpretation. Additionally, to assess subtle differences between the gating techniques, each cine loop was assigned a rank (1 = best, 4 = worst) relative to the others within each 4-on-1 display.
RESULTS
SG Signals
The end-systolic cardiac images in Fig. 3 were each reconstructed using an ECG retrogating signal and raw data acquired with the parameter set described above. The images in Fig. 3a were reconstructed using only the signal from the small loop coil placed on the chest wall, directly over the heart. In both the short- and long-axis orientations, the relatively small sensitivity profile of this coil served to restrict the acquisition of signal to predominantly those spatial positions that exhibited cardiac cycle-related tissue motion. It was from this spatially localized signal that the echo-peak and COM kymogram SG signals were derived, with the exception of cases in which an apical short-axis orientation fell too far from the loop coil position. In these cases, instead of repositioning the loop coil, we used the quadrature array coil for SG signal derivation. The images in Fig. 3b are representative of the low-resolution images acquired in real time and used for ROI correlation mask selection and subsequent time-series ROI correlation computations. These images exhibit considerable streak artifact because of azimuthal k-space undersampling (factor of ∼8), but when observed in a temporal series they clearly depict cardiac motion cycles. Images in Fig. 3c are the corresponding full-resolution reconstructions, including signals acquired with the loop coil, quadrature array coils, and spine array coil elements.
FIG. 3.
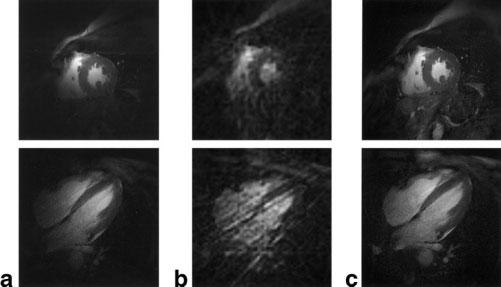
End-systolic images in mid short-axis (top row) and four-chamber long-axis (bottom row) orientations. The images in column a were reconstructed using only the signal acquired with the small loop coil. The low-resolution images (12 views) in column b are representative of those used for ROI correlation mask h(x,y) selection, and the images in column c are the corresponding full-resolution images reconstructed using 144 total views and the signals from five coils.
Representative 2D ROI correlation, COM kymogram, and echo-peak magnitude SG signals, along with corresponding R-wave trigger positions as recorded by the scanner ECG monitor, are shown in Figs. 4 and 5. The signals in Fig. 4 correspond to a mid short-axis orientation acquisition, whereas those in Fig. 5 correspond to a long-axis orientation acquisition. Each of these signals clearly demonstrated a correlation to the cardiac cycle, while the particular morphology of these signals varied among image orientations and volunteers. For these initial studies, the principally monophasic SG signals in the short-axis orientation tended to exhibit morphologies that were less dependent upon the method of SG signal derivation. This was not the case in the long-axis orientations, where the choice of SG signal derivation method resulted in radical changes in SG signal morphology and associated peak position relative to the cardiac cycle, as can be observed in Fig. 5. Such results probably relate to the relatively simple gross motion patterns observed in the short-axis orientation relative to the more complicated motion patterns in the long-axis orientation that includes atrial tissue in addition to ventricular myocardium.
SG Cine Images
Representative end-diastolic and end-systolic short- and long-axis images reconstructed using ECG gating and each of the proposed SG techniques are shown in Figs. 6 and 7, respectively. Notice the relatively equivalent image quality provided by the different gating strategies in each of these examples, as well as the good depiction of papillary musculature and myocardial trabeculations.
FIG. 6.

Representative end-diastole (top row) and end-systole (bottom row) short-axis images from a single volunteer reconstructed using ECG gating (a), and echo-peak magnitude (b), COM kymogram (c), and ROI correlation (d) SG techniques. Note the relatively equivalent image quality among the techniques.
FIG. 7.
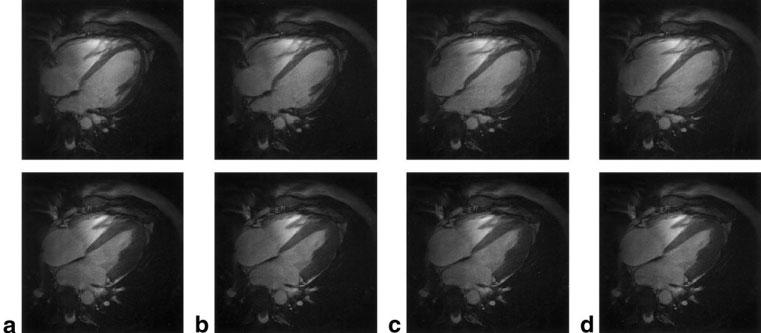
Representative end-diastole (top row) and end-systole (bottom row) long-axis images from a single volunteer reconstructed using ECG gating (a), and echo-peak magnitude (b), COM kymogram (c), and ROI correlation (d) SG techniques. Note the relatively equivalent image quality among the techniques.
SG Temporal Variability Analysis
The mean trigger position variability metrics for the echo-peak magnitude (RMS(EPM – ECG)), COM kymogram (RMS(KYM – ECG)), and 2D ROI correlation (RMS(2DCOR – ECG)) techniques were 7.32 ± 3.41 ms, 8.75 ± 3.23 ms, and 17.98 ± 10.11 ms, respectively (mean ± SD, 28 acquisitions per technique). The only statistically significant differences were between the mean score of the 2D ROI correlation technique and each of the two other SG techniques (P < 0.05). The difference between the mean scores of the echo-peak magnitude and the COM kymogram techniques was not statistically significant.
Reviewer Image Series Scoring
The mean image series comparison rank and mean absolute image quality scores for each gating technique and each reviewer are shown in Tables 1 and 2 (N = 28 (seven volunteers and four orientations)). For reviewer 1, a Friedman nonparametric comparison of ranks revealed no significant difference between the gating techniques relative to the mean ranking scores. However, for reviewer 2, the Friedman nonparametric comparison provided sufficient evidence to suggest a difference between the mean ranking scores (P < 0.05). Subsequently, six Wilcoxon pairwise comparisons of these ranks (echo-peak to 2D correlation, etc.) revealed trends that indicated lower ranks for the 2D correlation gating technique compared to ECG gating and echo-peak self-gating. However, the sample size was too small to reach statistical significance (P = 0.125). For both reviewers, one-way ANOVA revealed no significant difference between the mean absolute quality scores for the gating techniques (P < 0.05 not satisfied). Reviewer 1 scored the image series for each gating technique as equivalent in overall image quality, resulting in identical mean scores. Overall, each gating technique resulted in a mean absolute quality score between good and excellent for both reviewers.
Table 1.
Mean Image Series Comparative Rank
| ECG | Echo peak | Kymogram | 2D correlation |
|
|---|---|---|---|---|
| Reviewer 1 | 1.07 ± 0.26 | 1.0 ± 0.00 | 1.04 ± 0.19 | 1.21 ± 0.57 |
| Reviewer 2 | 1.04 ± 0.19 | 1.04 ± 0.19 | 1.11 ± 0.42 | 1.18 ± 0.39 |
Table 2.
Mean Image Series Absolute Quality Scores
| ECG | Echo peak | Kymogram | 2D correlation |
|
|---|---|---|---|---|
| Reviewer 1 | 3.54 ± 0.51 | 3.54 ± 0.51 | 3.54 ± 0.51 | 3.54 ± 0.51 |
| Reviewer 2 | 3.36 ± 0.56 | 3.36 ± 0.56 | 3.29 ± 0.53 | 3.18 ± 0.72 |
DISCUSSION
The SG technique, which provides cardiac gating information directly from the same data used for image reconstruction, overcomes the efficiency deficits of previous MR signal-based gating techniques. The results from this initial feasibility study demonstrate the potential of SG techniques to eliminate the need for ECG gating in cine CMRI.
The echo-peak magnitude and kymogram SG techniques clearly provide cardiac cycle synchronization with the necessary temporal resolution for segmented cine CMRI. The cardiac gating trigger signal provided by the scanner ECG monitoring system had a temporal variation relative to the R-wave position due to both systematic variation (up to ±2.5 ms) and an unknown level of artifact/noise-induced temporal variation. Given such variation, a more rigorous evaluation using high-fidelity ECG recording and R-wave detection strategies may reveal that the SG techniques provide better synchronization to the cardiac cycle than is appreciable with the trigger position temporal variability measures presented in this work.
The ECG gating and the SG techniques resulted in statistically equivalent absolute image quality scores. Had we used a larger sample size, the ranking scores for the 2D correlation SG technique may have been significantly different from those for the echo-peak and kymogram SG techniques. The 2D correlation SG technique also resulted in significantly greater heartbeat-to-heartbeat temporal variability than the echo-peak magnitude and kymogram SG techniques. However, the gating signal provided by the 2D correlation technique is strongly dependent upon the h(x,y) ROI choice and the level of undersampling streak artifact. Further investigations regarding optimal ROI selection and real-time gating image acquisition parameters may significantly improve the 2D correlation SG technique.
On the basis of this initial feasibility study, the echo-peak SG technique appears to be the most practical choice of the three techniques proposed. The echo-peak SG technique provided similar image quality and temporal trigger variability scores, and required less computational complexity to derive the SG signal. The 2D correlation technique additionally required operator interaction for specification of the h(x,y) ROI, which the echo-peak and kymogram SG techniques did not require. However, each of these SG techniques can potentially derive a gating signal that is sensitive to different types of tissue motion, and further studies may reveal conditions under which one technique succeeds while another fails.
Because each of the SG signals are retrospectively derived from the same set of raw data, it may also be important to determine whether an optimal combination of multiple SG signal derivation techniques can provide improved gating performance. Furthermore, while we chose to use a local threshold technique to account for DC trends present in the SG signals in the process of peak detection, band-pass temporal filtering techniques could alternately be utilized to remove these DC trends.
As currently implemented, the SG techniques have some important intrinsic limitations. The initial SG implementation was a purely retrospective gating technique, and the gating signal could not be used to modify the acquisition. Such an implementation does not allow prospective applications of magnetization preparation schemes. However, it is technically feasible to derive a SG signal in real time and affect the acquisition. Such a strategy would allow, for example, prospective triggering or more efficient cardiac phase to order reconstruction (CAPTOR) retrogating strategies (34). Another potential limitation of self-gating is that the heart rate must be estimated to determine the appropriate cardiac acquisition window duration. Without peripheral monitoring or an ECG, this duration must be estimated with an additional SG scout scan. Additionally, changes in the SG signal morphology will result in different trigger times (relative to the cardiac cycle) for different slice positions. Thus, the first image of a short-axis cine series may correspond to a different cardiac phase than the first image of a long-axis cine series. Such a condition could be problematic when using automated image analysis software or when simultaneously viewing multiple slice levels in a cine loop. Furthermore, as implemented, the SG technique assumes utilization of radial k-space sampling techniques. If the same number of views is sampled, as is typically acquired for rectangular FOV Cartesian cine imaging, the radial acquisitions will remain undersampled, potentially giving rise to streak artifacts. Depending on the level of streak artifact that is tolerable, the improved efficiency of the SG techniques as compared to the Cartesian techniques described by Spraggins (8), White et al. (23), and Hinks (9) may be diminished. Finally, because the quality of the SG signals can potentially vary with coil element position relative to slice orientation, some operator interaction may be necessary to optimize gating channel choice when imaging with coil arrays, or to optimize coil placement.
Because the SG signals are derived from MR signal changes resulting from tissue motion, future clinical validation studies must evaluate the ability of SG techniques to provide cardiac cycle synchronization in cases of severely depressed cardiac function with little ventricular wall motion. Future investigations will also evaluate the use of SG techniques for fetal cine CMRI, respiratory motion tracking (to enable free-breathing cine CMRI), and cardiac applications other than cine imaging. Aberrant cardiac motion patterns reflected directly in the SG signals may also provide improved arrhythmia rejection capabilities. In addition, the SG techniques should be readily applicable to imaging applications that are peripheral to the heart, especially those that require ECG gating to eliminate pulsatile motion artifacts.
CONCLUSIONS
In this work, SG cine CMRI techniques were described, and in vivo experiments demonstrated the feasibility of using these techniques to acquire high-temporal-resolution and high-spatial-resolution cine images without ECG gating. In this initial study, the SG technique produced cine images that were not significantly different from images produced by conventional ECG retrogating techniques. Because of the common difficulties associated with ECG gating, the new SG technique, which provides wireless cardiac cycle synchronization with no loss in imaging efficiency, represents an important practical advance for clinical CMRI.
ACKNOWLEDGMENT
Andrew Larson is supported by a pre-IRTA fellowship grant from the Laboratory of Cardiac Energetics, NHLBI, National Institutes of Health, DHHS, Bethesda, MD.
Grant sponsor: Laboratory of Cardiac Energetics, NHLBI, National Institutes of Health.
REFERENCES
- 1.Rokey R, Wendt R, Johnston D. Monitoring of acutely ill patients during nuclear magnetic resonance imaging: use of a time-varying filter electorcardiographic gating device to reduce gradient artifacts. Magn Reson Med. 1988;6:240–245. doi: 10.1002/mrm.1910060213. [DOI] [PubMed] [Google Scholar]
- 2.Polson M, Barker A, Gardiner S. The effect of rapid rise-time magnetic fields on the ECG of the rat. Clin Phys Physiol Meas. 1982;3:231–234. doi: 10.1088/0143-0815/3/3/008. [DOI] [PubMed] [Google Scholar]
- 3.Shetty A. Suppression of radiofrequency interference in cardiac gated MRI: a simple design. Magn Reson Med. 1988;8:84–88. doi: 10.1002/mrm.1910080110. [DOI] [PubMed] [Google Scholar]
- 4.Damji A, Snyder R, Ellinger D, Witkowski F, Allen P. RF Interference suppression in a cardiac synchronization system operating in a high magnetic field NMR imaging system. Magn Reson Imaging. 1988;6:637–640. doi: 10.1016/0730-725x(88)90086-0. [DOI] [PubMed] [Google Scholar]
- 5.Dimick R, Hedlund L, Herfkens R, Fram E, Utz J. Optimizing ECG electrode placement for cardiac-gated magnetic resonance imaging. Invest Radiol. 1986;22:17–22. doi: 10.1097/00004424-198701000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fischer S, Wickline S, Lorenz C. Novel real-time r-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance applications. Magn Reson Med. 1999;47:361–370. doi: 10.1002/(sici)1522-2594(199908)42:2<361::aid-mrm18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Kugel H, Bremer C, Puschel M, Fischbach R, Lenzen H, Tombach B, Van Aken H, Heindel W. Hazardous situation in the MR bore: induction in ECG leads causes fire. Eur Radiol. 2003;13:690–694. doi: 10.1007/s00330-003-1841-8. [DOI] [PubMed] [Google Scholar]
- 8.Spraggins T. Wireless retrospective gating: application to cine cardiac imaging. Magn Reson Imaging. 1990;8:675–681. doi: 10.1016/0730-725x(90)90001-i. [DOI] [PubMed] [Google Scholar]
- 9.Hinks R. Monitored echo gating (MEGA) for the reduction of data errors and image artifacts. Society of Magnetic Resonance in Medicine Annual Meeting. 1988:744. [Google Scholar]
- 10.Kim W, Min C, Kim D, Cho Z. Extraction of cardiac and respiratory motion cycles by use of projection data and its applications to NMR imaging. Magn Reson Med. 1990;13:25–37. doi: 10.1002/mrm.1910130105. [DOI] [PubMed] [Google Scholar]
- 11.Vasanawala S, Sachs T, Brittain J, Meyer C, Nishimura D. Prospective MR signal-based cardiac triggering. Magn Reson Med. 1999;42:82–86. doi: 10.1002/(sici)1522-2594(199907)42:1<82::aid-mrm12>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Lauterbur P. Image formation by induced local interactions—example employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 13.Peters D, Korosec F, Grist T, Block W, Holden J, Vigen K, Mistretta C. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43:91–101. doi: 10.1002/(sici)1522-2594(200001)43:1<91::aid-mrm11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Barger A, Peters D, Block W, Vigen K, Korosec F, Grist T, Mistretta C. Phase-contrast with interleaved undersampled projections. Magn Reson Med. 2000;43:503–509. doi: 10.1002/(sici)1522-2594(200004)43:4<503::aid-mrm3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Barger A, Grist T, Hany T, Mistretta C. 3D multiphase coronary artery imaging in a single breath-hold using undersampled projection reconstruction; Proceedings of the 8th Annual Meeting of ISMRM; Denver. 2000. p. 1513. [Google Scholar]
- 16.Larson A, Simonetti O, Li D. Coronary MRA with 3D undersampled projection reconstruction trueFISP. Magn Reson Med. 2002;48:594–601. doi: 10.1002/mrm.10262. [DOI] [PubMed] [Google Scholar]
- 17.Peters D, Epstein F, McVeigh E. Myocardial wall tagging with undersampled projection reconstruction. Magn Reson Med. 2000;45:562–567. doi: 10.1002/mrm.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barger A, Grist T, Block W. Single breath-hold 3D contrast enhanced method for assessment of cardiac function. Magn Reson Med. 2000;44:821–824. doi: 10.1002/1522-2594(200012)44:6<821::aid-mrm1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Peters D, Ennis D, McVeigh E. High resolution SSFP imaging of the heart wall with projection reconstruction. Magn Reson Med. 2002;48:82–88. doi: 10.1002/mrm.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters D, Lederman R, Dick A, Raman V, Gattman M, Derbyshire J. Interactive undersampled projection reconstruction for active catheter imaging with adaptable temporal resolution and catheter-only views. Magn Reson Med. 2003;49:216–222. doi: 10.1002/mrm.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffler K, Hennig J. Reduced circular field-of-view imaging. Magn Reson Med. 1998;40:474–480. doi: 10.1002/mrm.1910400319. [DOI] [PubMed] [Google Scholar]
- 22.Glover GH, Pelc NJ. A rapid-gated cine MRI technique. In: Kressel HY, editor. Magnetic resonance annual. Raven Press; New York: 1988. pp. 288–333. [PubMed] [Google Scholar]
- 23.White R, Paschal C, Clampitt M, Spraggins T, Lenz G. Electrocardiograph-independent “wireless” cardiovascular cine MR imaging. J Magn Reson Imaging. 1991;1:347–355. doi: 10.1002/jmri.1880010313. [DOI] [PubMed] [Google Scholar]
- 24.Gai N, Axel L. Correction of motion artifacts in linogram and projection reconstruction MRI using geometry and consistency constraints. Med Phys. 1996;23:251–262. doi: 10.1118/1.597713. [DOI] [PubMed] [Google Scholar]
- 25.Wieben O, Barger A, Block W, Mistretta C. Correcting for translational motion in 3D projection reconstruction; Proceedings of the 9th Annual Meeting of ISMRM; Glascow. 2001. p. 737. [Google Scholar]
- 26.Kachelriess M, Sennst D, Maximoser W, Kalendar W. Kymogram detection and kymogram-correlated image reconstruction from subsecond spiral computed tomography scans of the heart. Med Phys. 2002;29:1489–1503. doi: 10.1118/1.1487861. [DOI] [PubMed] [Google Scholar]
- 27.Hardy C, Zhao L, Zong X, Saranathan M, Yucel E. Coronary MR angiography: respiratory motion correction with BACSPIN. J Magn Reson Imaging. 2003;17:170–176. doi: 10.1002/jmri.10250. [DOI] [PubMed] [Google Scholar]
- 28.Hardy C, Saranathan M, Zhu Y, Darrow R. Coronary angiography by real-time MRI with adaptive averaging. Magn Reson Med. 2000;44:940–946. doi: 10.1002/1522-2594(200012)44:6<940::aid-mrm16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Deimling M, Heid O. Magnetization prepared trueFISP imaging; Proceedings of the 2nd Annual Meeting of ISMRM; San Francisco. 1994. p. 495. [Google Scholar]
- 30.Jackson J, Meyer C, Nishimura D. Selection of a convolution function for Fourier inversion using gridding. IEEE Trans Med Imaging. 1991;10:473–478. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 31.Liang Z, Lauterbur P. Principles of magnetic resonance imaging: a signal processing perspective. IEEE Press; New York: 2000. [Google Scholar]
- 32.Dale B, Wendt M, Duerk J. A rapid look-up table method for reconstructing MR images from arbitrary k-space trajectories. IEEE Trans Med Imaging. 2001;20:207–217. doi: 10.1109/42.918471. [DOI] [PubMed] [Google Scholar]
- 33.Roemer P, Edelstein W, Hayes C, Souza S, Meuller O. The NMR phased array. Magn Reson Med. 1990;16:192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 34.Feinstein J, Epstein F, Arai A, Foo T, Hartley M, Balaban R, Wolff S. Using cardiac phase to order reconstruction [CAPTOR]: a method to improve diastolic images. J Magn Reson Imaging. 1997;7:794–798. doi: 10.1002/jmri.1880070505. [DOI] [PubMed] [Google Scholar]


