Abstract
Objectives
We investigated the effects of suppression of ETA receptors on bladder function and ET-1 levels in the bladder in rats with chronic spinal cord injury (SCI).
Methods
The spinal cord of female Spraque-Dawley rats was transected at the level of Th 8–9. Awake cystometrograms were performed 4 weeks after spinal cord transection. Cystometric parameters such as mean amplitudes of non-voiding contractions (NVCs), the number of NVCs, voided volume, voiding efficiency, and micturition pressure were evaluated before and after intravenous (i.v.) injection of ABT-627, an ETA antagonist, or A-19261, an ETB antagonist, in SCI animals. Four weeks after spinalization, the protein and mRNA levels of ET-1 in the bladder were also measured using enzyme-linked immunosorbent assay (ELISA) and quantitative real-time polymerase chain reaction (qRT-PCR).
Results
ABT-627 (1 mg/kg, i.v.), but not A-192621 (10 mg/kg, i.v.), significantly decreased the amplitude of NVCs and the number of NVCs in SCI rats. There were no significant changes in pressure threshold, maximum voiding pressure, voided volume or voiding efficiency. ELISA analysis for ET-1 showed significantly elevated protein concentrations in SCI rats compared with spinal cord intact rats. Significant upregulation of the ET-1 mRNA was also noted in SCI bladders.
Conclusions
These results suggest that upregulation of ET-1 is involved in the mechanism inducing bladder overactivity in chronic SCI rats and that an ETA receptor antagonist can suppress SCI-induced bladder overactivity as indicated by a reduction in NVCs. Thus, ETA receptor inhibition could be an effective treatment for neurogenic bladder overactivity in pathological conditions such as SCI.
Keywords: Endothelin, Bladder, Spinal cord injury, Capsaicin-sensitive C-fiber afferents
Introduction
Hyperexcitability of C-fiber bladder afferents, which are usually silent under the normal condition, is reported to be involved in the emergence of overactive bladder in various pathologic conditions including spinal cord injury.1, 2 Thus, suppression of the hyperexcitability is hypothesized to be an effective treatment of bladder overactivity in spinal cord injury (SCI). Intravesical application of capsaicin or resiniferatoxin, which desensitizes afferent neurons expressing TRPV1 receptors (vanilloid receptor 1), most of which are C-fibers, is considered to be one of the treatments for bladder overactivity.1, 2
Endothelin-1 (ET-1) is a 21-amino-acid endogenous vasoactive peptide1, 2 which binds to two receptor subtypes, the endothelin-A (ETA) and endothelin-B (ETB) receptors. ET-like immunoreactivity was identified in detrusor smooth muscles, epithelium, and vascular endothelium in the bladder.3 It is also reported that the ETA receptor is the predominant receptor subtype in the bladder.4 We previously reported that intravesical application of ET-1 induced bladder overactivity, which was suppressed by intravenous application of a selective ETA receptor antagonist as well as the pretreatment with capsaicin, which desensitizes C-fiber afferents, in normal rats.5 Therefore, it is assumed that ET-1 can directly activate nociceptive C-fiber afferent nerves in the bladder and that suppression of ETA receptors might be effective for the treatment of bladder overactivity by reducing hyperexcitability of C-fiber afferent pathways. Thus, in this study, we investigated the effects of inhibition of ETA or ETB receptors on bladder dysfunction and measured the bladder ET-1 levels using enzyme-linked immunosolvent assay (ELISA) and quantitative real-time polymerase chain reaction (qRT-PCR) in chronic SCI rats.
Material and Methods
Adult female Spraque-Dawley rats (250–350 gm) were used. All experiments were conducted in accordance with institutional guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Spinal cord transection
Spinal cord transection was performed at the level of Th8– Th9 spinal cord under halothane anesthesia. After T8 laminectomy, the dura and spinal cord were cut with scissors and a sterile Gelform sponge (The Upjohn Co., Kalamazoo, Michigan) was placed between the cut ends of the spinal cord. The overlying muscle and skin were sutured. Postoperatively, the animals were treated with ampicillin (50 mg/kg intramuscularly) for 7 days. The bladder of spinalized rats was manually emptied twice daily after spinalization until the experiments. In addition, bladders were harvested from spinal cord intact rats and SCI rats 4 weeks after spinalization and then frozen in dry ice and stored at −80°C until further processing for ELISA and qRT-PCR.
Cystometry
Four weeks after spinalization, cystometry in conscious rats was performed. Under halothane anesthesia, a PE-90 catheter was inserted via a midline abdominal incision into the bladder through the bladder dome. Intravenous (i.v.) injections were made through a cannula (PE-10) inserted into the right jugular vein. After surgery, rats were placed in a restraining cage and allowed to recover from anesthesia for 1 to 2 hours. The intravesical catheter was connected via a 3-way stopcock to a pressure transducer and a syringe pump for recording intravesical bladder pressure and infusing saline into the bladder, respectively. Saline at room temperature was infused at a rate of 0.08 ml per minute to elicit bladder contractions. Saline voided from the urethral meatus was collected and measured to determine voided volume (VV). After every bladder contraction, infusion was stopped and post-void residual volume (RV) was measured. Residual saline was withdrawn through the intravesical catheter by gravity. Bladder capacity (BC) was calculated using the formula, BC= VV + RV. Voiding efficiency (VE) was calculated using the formula, VE = ((VV/BC) × 100). Maximum voiding pressure and pressure threshold for voiding were also measured. The number and mean amplitudes of non-voiding contractions (NVCs) per voiding cycle were also measured during 2 min prior to micturition. NVCs were defined as rhythmic intravesical pressure increases greater than 7 cm water from baseline pressure without a release of fluid from the urethra. These cystometric parameters were obtained from 3 voiding cycles each before and after i.v. administration of ET receptor antagonists and averaged for comparison. ABT-627, an ETA antagonist or A-192621, an ETB receptor antagonist, were dissolved in 100% dimethyl sulfoxide (DMSO) immediately before the injection. ABT- 627 at doses of 0.1, 0.3, and 1.0 mg/kg was administered to each 6 SCI rats, and A-192621 at 10 mg/kg was administered to 5 rats. Vehicle (DMSO) was administered to 5 SCI rats as controls. ET receptor antagonists were provided by Abbott (Abbott Park, Illinois). The doses of ET antagonists were chosen based on the results of our previous study that used the same drugs.5
ELIZA for ET-1
ELISA measurements of ET-1 were performed on bladder homogenate from 6 spinal cord intact rats and 5 SCI rats 4 weeks after spinalization. Briefly, whole bladders that were frozen and stored at −80°C was homogenized with a Polytron homogenizer in 0.1 M phosphate buffer, pH 7.5, containing 0.9% NaCl, 0.1% bovine serum albumin, 0.1% Tween 20, and 0.01% thimerosal. The homogenate was centrifuged at 10,000 g for 30 minutes at 4°C and the supernatant was stored at −80°C until assayed. The bladder ET-1 level was measured using an ELISA kit specific for ET-1 (Amersham Biosciences, Piscataway, New Jersey) according to the manufacturer’s instructions. The levels of ET-1 were normalized to protein concentration, which was measured using a protein assay kit (Pierce, Rockford, Illinois), and the results were expressed as pg/mg total protein.
qRT-PCR of ET-1 mRNA
Measurement of ET-1 mRNA levels in the bladder was performed using 7 spinal cord intact rats and 5 SCI rats 4 weeks after spinalization. Total RNA of the bladder was extracted from frozen tissues using TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Reverse transcription was performed as previously described.6 The resulting cDNA was stored at −20°C until assayed by real-time PCR. Sequences of oligonuclotides used as primers for ET-1 were selected from the previous reports as follows.6 Genbank accession number is NM012548. ET-1 forward: CTCCTCCTTGATGGACAAGG. ET-1 reverse: CTTGATGCTGTTGCTGATGG. β-Actin purchased from Ambion (Austin, TX, USA) was used as the internal control. Real-time PCR was performed using the iCycler Thermal Cycler (Bio-Rad, Hercules, CA, USA) and carried out with QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA, USA) as previously reported.6 Specificity of ET-1 in the bladder was confirmed by melt curve analysis. Standard curves constructed from serial dilution of cDNA in each tissue were analyzed with the iCycler iQ Real-Time PCR Detection System (Bio-Rad). The quantitative values for ET-1 were normalized to that for β-actin obtained from the same samples.
Statistics
All data values are expressed as mean ± S.E. Statistical significance was determined with Mann-Whitney U test. P-values less than 0.05 are considered to be significant.
Results
Cystometry
During awake cystometry, all spinalized rats showed NVCs before large amplitude voiding bladder contractions occurred (fig. 1). The amplitude of NVCs increased as the bladder was filled with saline infusion. The mean amplitude and the mean number of NVCs per voiding cycle were 17.3 ± 3.6 cmH2O and 4.1 ± 0.6, respectively (table 1). ABT-627 (1mg/kg, i.v.) significantly decreased the amplitudes of NVCs to 13.3 ± 1.3 cmH2O (P < 0.01, n=6) and the number of NVCs to 2.6 ± 0.6 (P < 0.05, n=6) whereas DMSO (100%, n=5) or lower doses of ABT-627 (0.1 or 0.3 mg/kg, i.v., n=4 each) did not affect the amplitude and number of NVCs (fig. 2). There were no significant changes in pressure thresholds, maximum voiding pressure, VV, BC or VE before and after administration of vehicle nor ABT-627 at any doses (table 1). In addition, A-192621, an ETB receptor antagonist, did not affect any parameters including NVCs at doses up to 10 mg/kg (i.v.) (table 1).
Fig. 1.
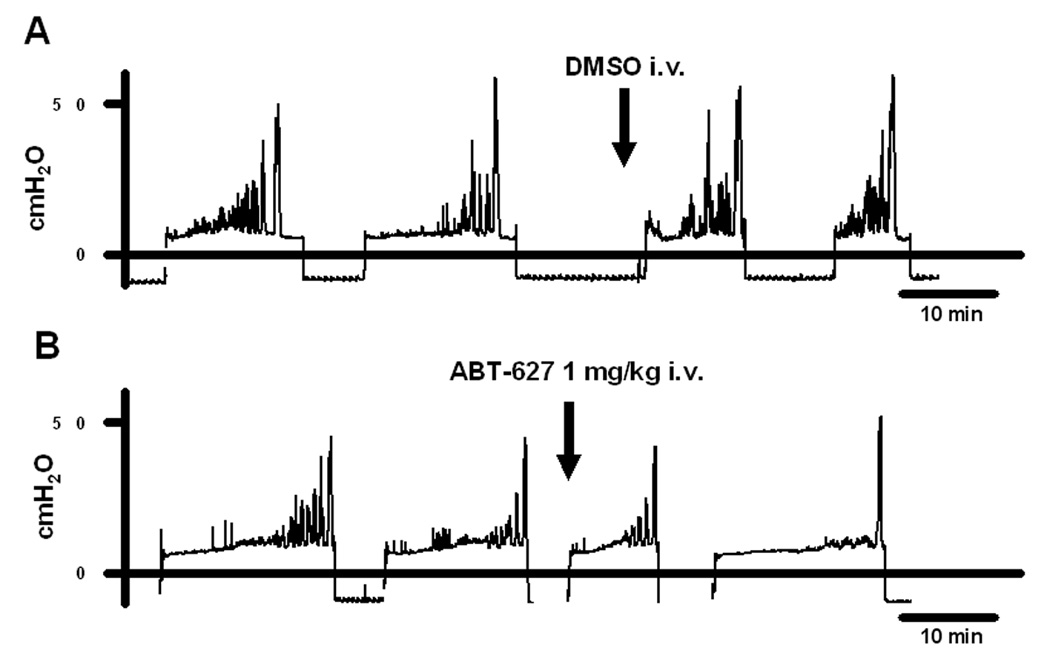
Effects of intravenous administration of (A) vehicle (DMSO) and (B) ABT-627, an ETA antagonist, on non voiding contractions in SCI rats. Arrows indicated the timing of drug administration
Table 1.
Changes in cystometric parameters before and after intravenous administration of vehicle (DMSO), ABT-627 or A-192621 in spinal cord injured rats
| Vehicle | ABT-627 (1mg/kg) | A-192621 (10 mg/kg) | ||||
|---|---|---|---|---|---|---|
| (n=5) | (n=6) | (n=5) | ||||
| Pre | Post | Pre | Post | Pre | Post | |
| Mean amplitude of NVCs (cmH2O) | 16.0 ± 2.3 | 17.1 ± 3.6 | 18.5 ± 2.0 | 13.3 ± 1.3** | 21.5 ± 10.0 | 21.9 ± 9.93 |
| Number of NVCs | 5.5 ± 1.1 | 4.1 ± 0.6 | 4.4 ± 0.7 | 2.6 ± 0.6* | 3.5 ± 0.4 | 3.1 ± 0.3 |
| Pressure threshold (cmH2O) | 9.4 ± 0.8 | 8.7 ± 0.6 | 8.4 ± 0.6 | 9.0 ± 0.9 | 13.5 ± 2.3 | 12.6 ± 1.5 |
| Maximum voiding pressure (cmH2O) | 31.8 ± 7.6 | 28.6 ± 4.8 | 27.0 ± 3.3 | 28.1 ± 3.7 | 50.6 ± 7.5 | 49.5 ± 9.2 |
| Voided volume (ml) | 0.63 ± 0.1 | 0.65 ± 0.1 | 0.74 ± 0.1 | 0.78 ± 0.1 | 0.66 ± 0.1 | 0.77 ± 0.3 |
| Bladder capacity (ml) | 0.82 ± 0.1 | 0.84 ± 0.1 | 0.93 ± 0.1 | 0.99 ± 0.1 | 1.22 ± 0.2 | 1.22 ± 0.3 |
| Voiding efficiency (%) | 75.3 ± 5.9 | 74.8 ± 6.7 | 80.8 ± 4.3 | 78.5 ± 4.9 | 54.1 ± 14.5 | 63.0 ± 11.8 |
p < 0.05
p < 0.01 compared with pre-drug values.
Fig. 2.
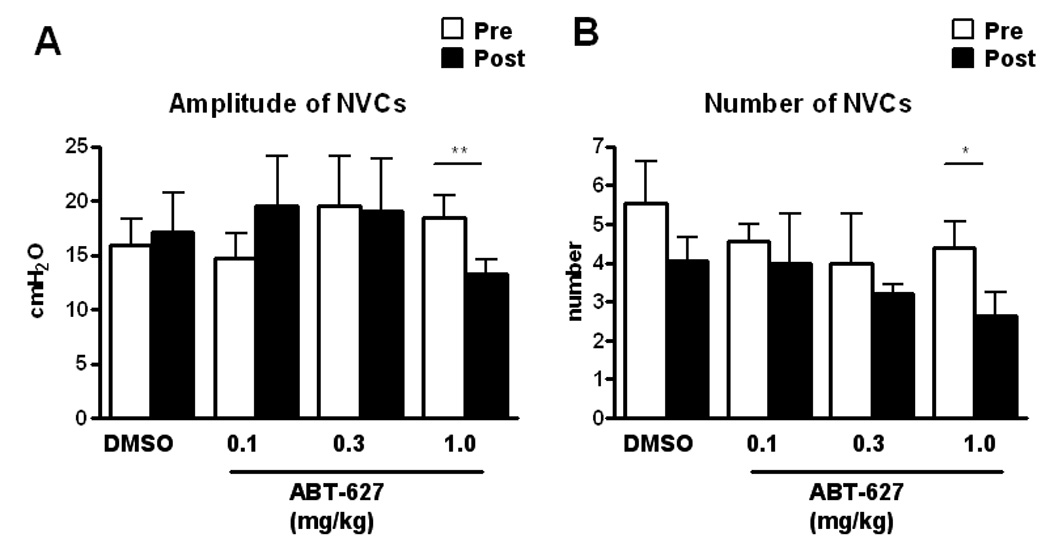
Amplitudes (A) and the number of non voiding contractions (B) in SCI rats before (Pre) and after (Post) intravenous administration of vehicle (DMSO) and ABT-627 (0.1–1.0 mg/kg). Each histogram represents mean ± S.E. **P < 0.01, *P < 0.05 compared with pre-drug values.
ELISA
The bladder ET-1 concentration was significantly increased in SCI rats compared with spinal cord intact rats (2.60 ± 0.07 vs. 2.08 ± 0.07 pg/mg protein: p < 0.01) (fig. 3A).
Fig. 3.
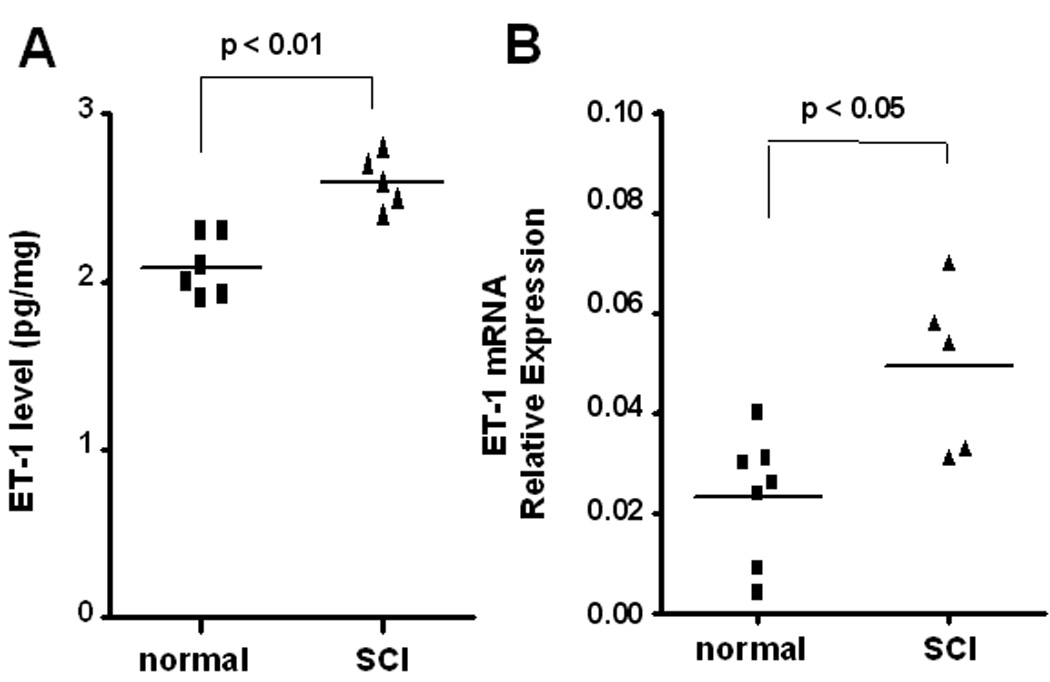
A: ELISA measurements of ET-1 in the bladder of normal rats (n=6) and SCI rats (n=5). ET-1 values were normalized to tissue protein and expressed in pg/mg total protein. B: Expression of ET-1 mRNA in the bladder of normal rats (n=7) and SCI rats (n=5). Relative expression levels of ET-1 mRNA were normalized against β-actin. Each bar represents mean ± S.E.
qRT-PCR
The relative expression level of ET-1 mRNA in the rat bladder is shown in fig. 3B. The ET-1 mRNA value was significantly higher in SCI rats than in spinal cord intact rats (p < 0.05).
Comment
The present study revealed that the protein and mRNA levels of ET-1 in the bladder are significantly increased in chronic SCI rats compared with spinal cord intact rats and that inhibition of ETA receptors, but not ETB receptors, suppressed bladder overactivity as evidenced by a reduction in the number and mean amplitude of NVCs in SCI rats. Thus, upregulation of the endothelin mechanism via increased expression of ET-1 and subsequent activation of ETA receptors in the bladder could contribute to the emergence of bladder overactivity in SCI rats.
Bladder overactivity after SCI is reportedly mediated by the emergence of a capsaicin-sensitive C-fiber-mediated spinal micturition reflex.7 In SCI rats, Aδ bladder afferents trigger the voiding reflex, and desensitizing TRPV1-expressing afferents, most of which are C-fibers by systemic capsaicin administration suppressed NVCs that occurred before voiding.8 We previously reported that, in normal rats, bladder overactivity induced by intravesically applied ET-1 was suppressed by intravenous application of a selective ETA receptor antagonist as well as by capsaicin pre-treatment, but not by a selective ETB receptor antagonist,5 indicating that activation of ETA receptor in capsaicin sensitive C-fiber afferents in the bladder induces bladder overactivity. However, this ETA receptor-C-fiber activation mechanism does not seem to be tonically active in the normal condition because, in our previous study, ABT-627 alone had no effect on the micturition reflex in normal rats.5 Thus, the present study is likely to indicate that locally increased ET-1 activates silent ETA receptors in capsaicin-sensitive C-fiber afferents to induce bladder overactivity after SCI and that targeting ETA receptors could be an effective treatment of C-fiber-mediated neurogenic bladder overactivity in pathological conditions.
ET-1 induces long lasting contractions of smooth muscle strips of the bladder dome has been shown to be mediated by the ETa receptor subtype.9, 10 It has been also suggested that ET-1 has a role in bladder hypertrophy. Bladder hypertrophy is often a situation in various pathological conditions such as benign prostatic hyperplasia, posterior urethral valves, and neurogenic bladder dysfunction including SCI. In vitro studies with smooth muscle cells obtained from obstructed rabbit bladders have shown that ETa receptor antagonists inhibit smooth muscle cell proliferation.11 Overall, ET-1 is likely to enhance smooth muscle contractility and proliferation in the bladder.
In the present study, mRNA levels of ET-1 in the bladder were measured using qRT-PCR, and β-actin was used as the internal control. It has recently been reported that there is a slight increase in β-actin in hypertrophied urinary bladder smooth muscle.12 Nevertheless, we found that bladder ET-1 levels normalized against potentially-increased β-actin were significantly higher in SCI bladders than in normal bladders, justifying our conclusion that SCI increased ET-1 mRNA levels in the bladder.
Schroder et al. have reported that oral administration of an endothelin converting enzyme (ECE) inhibitor (13 days) increased micturition intervals and bladder volume, and decreased NVCs in rats with bladder outlet obstruction (BOO), suggesting that the ECE inhibition can induce the protective effect on smooth muscle function.11 A recent study by Ukai et al. reported that YM598, another selective ETA receptor antagonist, reduced NVCs in BOO rats.13 Because NVCs in BOO rats are not affected by the treatment with the C-fiber neurotoxin resiniferatoxin,13 the emergence of bladder overactivity in this model is thought to be primarily due to myogenic changes, rather than neurogenic changes in C-fibers. Thus, it seems that the suppressive effect of ETA receptor antagonists or ECE inhibitors on BOO-induced NVCs is induced by inhibition of smooth muscle activity, which was enhanced by upregulated ET mechanisms in the obstructed bladder. The present study further indicates that suppression of ETA receptors is effective to suppress not only bladder overactivity of myogenic origin, but also that induced by neurogenic diseases such as SCI.
Conclusions
The present results indicate that inhibition of ETA receptors can inhibit non-voiding bladder contractions, which were associated with increased levels of ET-1 in the bladder, in SCI rats. Thus, ETA receptor antagonists could be useful for the treatment of bladder overactivity in neurogenic diseases such as SCI.
Acknowledgement
This work was supported by NIH grants DK57267, DK68557 and HD39768. ET receptor antagonists were kindly provided by Abbott.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA, editor. Nervous Control of the Urogenital System. vol. 3. London: Harwood Academic Publisher; 1993. pp. 227–290. [Google Scholar]
- 2.de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;vol. 41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 3.Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999;vol. 57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 4.Cheng CL, Ma CP, de Groat WC. Effects of capsaicin on micturition and associated reflexes in rats. Am J Physiol. 1993;vol. 265:R132–R138. doi: 10.1152/ajpregu.1993.265.1.R132. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;vol. 332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 6.Saenz de Tejada I, Mueller JD, de Las Morenas A, Machado M, Moreland RB, Krane RJ, et al. Endothelin in the urinary bladder. I. Synthesis of endothelin-1 by epithelia, muscle and fibroblasts suggests autocrine and paracrine cellular regulation. J Urol. 1992;vol. 148:1290–1298. doi: 10.1016/s0022-5347(17)36895-7. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa T, Kamo I, Pflug BR, Nelson JB, Seki S, Igawa Y, et al. Differential roles of peripheral and spinal endothelin receptors in the micturition reflex in rats. J Urol. 2004;vol. 172:1533–1537. doi: 10.1097/01.ju.0000139540.56916.0e. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura N, Chancellor MB. Current and future pharmacological treatment for overactive bladder. J Urol. 2002;vol. 168:1897–1913. doi: 10.1016/S0022-5347(05)64261-9. [DOI] [PubMed] [Google Scholar]
- 9.Raffa RB, Schupsky JJ, Jacoby HI. Endothelin-induced nociception in mice: mediation by ETA and ETB receptors. 1996:647–651. [PubMed] [Google Scholar]
- 10.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;vol. 9:2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 11.Schroder A, Tajimi M, Matsumoto H, Schroder C, Brands M, Andersson KE. Protective effect of an oral endothelin converting enzyme inhibitor on rat detrusor function after outlet obstruction. J Urol. 2004;vol. 172:1171–1174. doi: 10.1097/01.ju.0000133561.32285.23. [DOI] [PubMed] [Google Scholar]
- 12.Mannikarottu AS, Disanto ME, Zderic SA, Wein AJ, Chacko S. Altered expression of thin filament-associated proteins in hypertrophied urinary bladder smooth muscle. Neurourol Urodyn. 2006;25:78. doi: 10.1002/nau.20121. [DOI] [PubMed] [Google Scholar]
- 13.Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;vol. 21:999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


