Abstract
Visceral injury has been shown to alter somatic sensitivity, but little is known about the effect of somatic insult on the viscera. In the present study we examined: 1) the effect of colon inflammation on somatic sensitivity and 2) the affect of hindpaw incision on colon sensitivity. After intracolonic administration of trinitrobenzene sulfonic acid (TNBS) or zymosan, visceromotor responses (VMR) to colorectal distension (CRD) were increased to post-treatment day 8. Mechanical withdrawal thresholds in the hindpaw were decreased in TNBS- and in zymosan-treated rats until post intracolonic treatment day 2. There was no change in hindpaw heat withdrawal latency in either group. Plantar incision of the hindpaw resulted in a decrease in both hindpaw mechanical withdrawal threshold and heat withdrawal latency and significantly increased the VMR to CRD from post-incision day 1 to 8. The colon hypersensitivity was of longer duration than hyperalgesia at the site of hindpaw incision. These results support the hypothesis that somatic injury and visceral inflammation can alter central processing of visceral and somatic inputs, respectively.
Perspective
Surgical procedures are common and typically associated with hyperalgesia at and around the site of incision. This report establishes in a model of post-surgical pain and hyperalgesia that a long-lasting visceral hypersensitivity may also accompany post-surgical hyperalgesia.
Keywords: Colorectal distension, central sensitization, somatic pain, somatovisceral convergence, visceral pain, zymosan, incision
Introduction
Visceral pain is a common and typically difficult to manage form of pain, yet the majority of current pain research focuses on pain of non-visceral origin. Visceral pain is characterized as being poorly localized, diffuse in nature, accompanied by exaggerated motor and autonomic reflexes, and typically referred to cutaneous and sub-cutaneous sites.10,30 The poor localization, diffuse character and referral of visceral pain is explained in large part by the anatomical organization of the visceral innervation and the convergence of visceral and non- visceral inputs onto second order spinal neurons. Accordingly, clinical visceral pain conditions, including irritable bowel syndrome (IBS), have been associated with cutaneous hyperalgesia in areas of referral. 25,35,40,41,44
Changes in central processing are often implicated in cases of pain developing secondary to the primary site.3,22,24,46-48 Staud et al. 39 noted abnormalities in central nociceptive processing in subjects with fibromyalgia, including enhanced sensitivity to noxious cutaneous heat stimuli and enhanced temporal summation of repetitive C fiber stimulation. The coexistence of several pain conditions may also have a peripheral component. Several studies have indicated that axons may send their peripheral terminals to anatomically distinct segments, causing the sensation of pain distant to the primary site.11,23 Another anatomical explanation is that several primary sensory neurons converge onto second order neurons in the spinal cord and sensitization of one afferent pathway can alter input from other neurons at sites of convergence.21,28,31,32
If viscerosomatic convergence contributes to somatic hypersensitivity, then the converse should also be true. Somatic injury should enhance visceral sensitivity. Indeed, Miranda and co-workers26 reported that experimental muscle hypersensitivity increases colon sensitivity. In this study we utilized colon inflammation as a selective visceral insult and plantar incision as the somatic injury. As a model of post-surgical pain, the plantar incision model is particularly relevant because surgical procedures are relatively common and possible visceral hypersensitivity may also thus be a relatively common post-surgical event. This study addressed two questions: 1) Does colon inflammation in the rat sensitize responses to cutaneous/subcutaneous stimuli applied to the hindpaw? and 2) Does a plantar incision of the rat hindpaw sensitize responses to colon distension?
Methods
Animals
All experiments were performed with male Sprague-Dawley rats (300−350 g; Harlan, Indianapolis, Ind). Rats were housed one per cage, with food and water available ad libitum and maintained on a 12 h light-dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Iowa. After testing, all animals were euthanized by pentobarbital sodium overdose.
Surgical preparations
Rats were anesthetized with pentobarbital sodium (50 mg/kg, Nembutal, Abbott Labs, North Chicago, IL) administered intraperitoneally. Pairs of electrodes (Teflon-coated stainless steel wire; Cooner Wire Sales, Chatsworth, CA) were sutured into the external oblique musculature, just above the inguinal ligament, for electromyographic (EMG) recording as described previously.29 Electrodes were tunneled subcutaneously and externalized through the dorsal aspect of the neck for future access. The wounds were closed with 4−0 silk and covered with a local anesthetic ointment. After surgery, rats were given 5.0 ml of 5% dextrose in normal saline subcutaneously. Animals were housed separately and allowed to recuperate for at least three days prior to testing. Any animals with apparent motor defects or that lost significant weight were excluded from behavioral testing.
Plantar incision
A previously described plantar incision model was used.8 Rats were anesthetized with 2% halothane and the plantar aspect of the right hindpaw was prepared in a sterile manner. A 1-cm longitudinal incision was made with a number 11 blade through the skin and fascia of the plantar aspect of the hindpaw beginning 0.5 cm from the end of the heel. The flexor muscle was elevated and a longitudinal incision was made. Skin was closed with 5−0 nylon sutures and topical antibiotics were administered. Animals were allowed to recover in their cages and sutures were removed on the third post-operative day. Rats were acclimated to behavioral testing two days before testing began.
Hindpaw mechanical withdrawal threshold
Rats were placed individually on an elevated plastic mesh grid, covered with clear plastic cages and allowed to acclimate for a period of 1 h. Withdrawal threshold was assessed by applying calibrated Semmes-Weinstein von Frey filaments (Stoelting, Wood Dale, IL) to the plantar aspect of the hind paw in inactive rats. Testing began with 10 mN and increasing forces were applied until the hind paw was withdrawn. The maximum force applied was 250 mN. Rats were tested three times with a minimum of 10 min between tests. The lowest force required to induce withdrawal from the three tests was recorded as the mechanical withdrawal threshold. If no response was elicited by the 250 mN, the next highest filament, 586 mN, was recorded as the withdrawal threshold.
Heat withdrawal latency
Rats were placed individually on top of a 6.0 mm thick glass surface covered with a clear plastic cage and allowed to acclimate 45−60 min prior to testing. Withdrawal latencies to radiant heat were assessed by applying focused radiant heat from beneath the glass floor to the plantar aspect of the hindpaw of an inactive rat. The heat source consisted of a 50-W projector lamp with an aperture diameter of 6 mm. Intensity of the heat was adjusted to produce a withdrawal latency in naïve rats of 25−27 sec. Each rat was tested three times, with at least 10 min between tests. The average of the three trials was used for paw withdrawal latency. To prevent tissue injury, the heat stimulus was cut off at 32 s. A slow heat test rather than a fast heat test (e.g., 10 sec) was used to detect subtle facilitation of the heat withdrawal latency by both incision and CRD.
Visceromotor response to colorectal distension
Contraction of the abdominal wall in response to a noxious visceral stimulus can be quantified through EMG recordings. 29 As previously described,29 a flexible, lubricated latex balloon approximately 6 cm in length was attached to Tygon tubing (Akron, OH), inserted intra-anally into the descending colon and was kept in place by taping the catheter to the base of the tail. The colorectal balloon was distended for 20 s to 20, 40, 60, or 80 mm Hg, separated by 4-min intervals. Balloon pressure was monitored via a pressure transducer and held constant during distension. EMG activity of the external oblique muscle was amplified, rectified, and recorded for 10 s prior to colon distension (baseline EMG activity), 20 s during distension (response = increase above baseline) and 10 s after termination of balloon inflation. The EMG signal was integrated and normalized as a change over baseline activity using Spike-2/CED 1401 data acquisition (CED 1401; Cambridge Electronic Design, Cambridge, UK)
Experimental protocols
Colorectal distension (CRD) in unincised rats
Baseline CRD responses, heat withdrawal latencies and mechanical withdrawal thresholds were established. The next day colonic inflammation was induced by one of two different inflamogens that produce different magnitudes of monocyte infiltration into inflamed tissue. Rats were anesthetized with halothane and either trinitrobenzene sulfonic acid (TNBS 0.5 mL, 30 mg/kg in 50% ethanol, Sigma, St. Louis, MO), a hapten that produces transmucosal ulceration and inflammation, or zymosan (1 ml, 25 mg/ml), which is derived from a yeast cell wall and produces less ulceration, was instilled into the distal colon of different groups of rats (n=9) using a 16-gauge stainless steel feeding needle connected to 1 mL syringe. Rats in control groups (n=9) received an equal volume of sterile saline. Six hr after administration of TNBS, zymosan or saline, the visceromotor response (VMR) was recorded in response to CRD. After CRD testing, rats were allowed to acclimate for 60 min before heat withdrawal latencies and mechanical withdrawal thresholds were determined. The same sequence and methods of testing responses to colon distension, heat withdrawal latencies and mechanical withdrawal thresholds was repeated 1, 2, 4 and 8 days after colonic inflammation
Colorectal distension after plantar incision
EMG electrodes were implanted in rats as described above and baseline responses to colon distension, heat withdrawal latencies and mechanical withdrawal thresholds were established. The next day, a plantar incision was made and six hours later the VMR was recorded in response to CRD as described above. Rats were allowed to acclimate one-hour post CRD testing, then hindpaw heat withdrawal latencies and mechanical withdrawal thresholds were measured. The same sequence and methods of testing responses to colon distension, heat withdrawal latencies and mechanical withdrawal thresholds was repeated 1, 2, 4 and 8 days after hindpaw incision.
Data analysis
All changes in responses after incision and induced colon hypersensitivity are compared versus baseline. Statistical analysis was preformed using SigmaStat (Version 3.01 SPSS Inc., Chicago, IL) and Prizm (4.0, Graphpad Software, San Diego, CA). Results were analyzed using parametric two-way ANOVAs for repeated measures. Data were further analyzed by parametric and nonparametric ANOVA when indicated. The F values are reported. The treatment effects were compared vs the baseline immediately before intervention. When the 1-way ANOVA was significant, post hoc Dunnett'S or Dunn'S tests was performed vs baseline for parametric and nonparametric analyses, respectively.
A value of P < 0.05 was considered statistically significant. All data are given as mean +/− standard error or median and percentiles.
Results
Hindpaw and visceromotor responses after colon inflammation
When tested two days after intracolonic treatment, the stimulus-response functions to colorectal distension were significantly increased (F=166.8) in rats treated with TNBS (n = 9) or with zymosan (F=157.82, n=9) (both P < 0.01, repeated measures ANOVAs) relative to saline-treated counterparts (Figure 1). Thus, both intracolonic TNBS and zymosan produced significant colon hypersensitivity.
Figure 1.
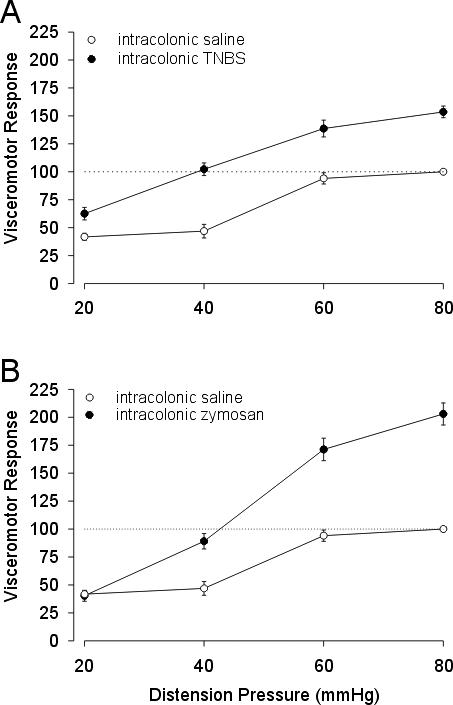
Mean VMR (as percent of baseline) to graded CRD (20−80 mm Hg) in rats receiving intracolonic administration of TNBS or saline vehicle (A). Data of mean VMR (as percent of baseline) to graded CRD (20−80 mm Hg) in rats receiving intracolonic administration of zymosan or saline vehicle (B). Intracolonic treatments were given 2 days before testing.
Before intracolonic administration of TNBS, the median hindpaw withdrawal threshold was 98 mN, which decreased significantly to 11 mN when first tested 6-hr after TNBS ( F=24.8 p<0.05 vs baseline, Figure 2B). Withdrawal thresholds increased until they were indistinguishable from baseline values at day 2. There was no change from baseline in hindpaw heat withdrawal latency in rats treated with intracolonic TNBS (F=3.6 p> 0.05 vs baseline, Figure 3B).
Figure 2.
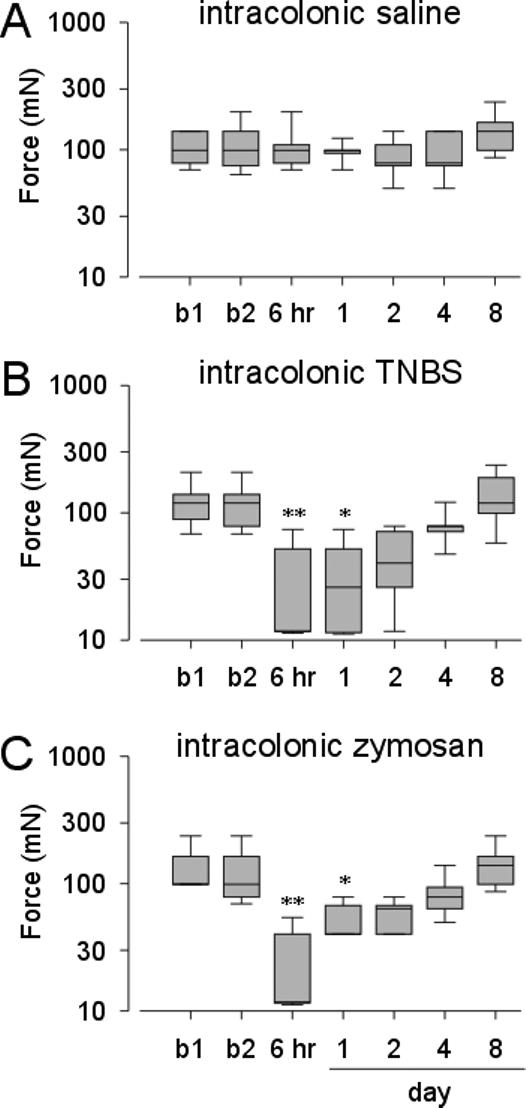
Hindpaw mechanical withdrawal threshold in rats treated with intracolonic administration of saline (A) or TNBS (B). Hindpaw mechanical withdrawal threshold in rats treated with intracolonic administration of zymosan (C). The results are expressed as median (horizontal line) with 1st and 3rd quartiles (boxes) and 10th and 90th percentiles denoted by error bars. b1=baseline 1. b2=baseline 2. (*P < 0.05 vs b2; **P < 0.01 vs. b2).
Figure 3.
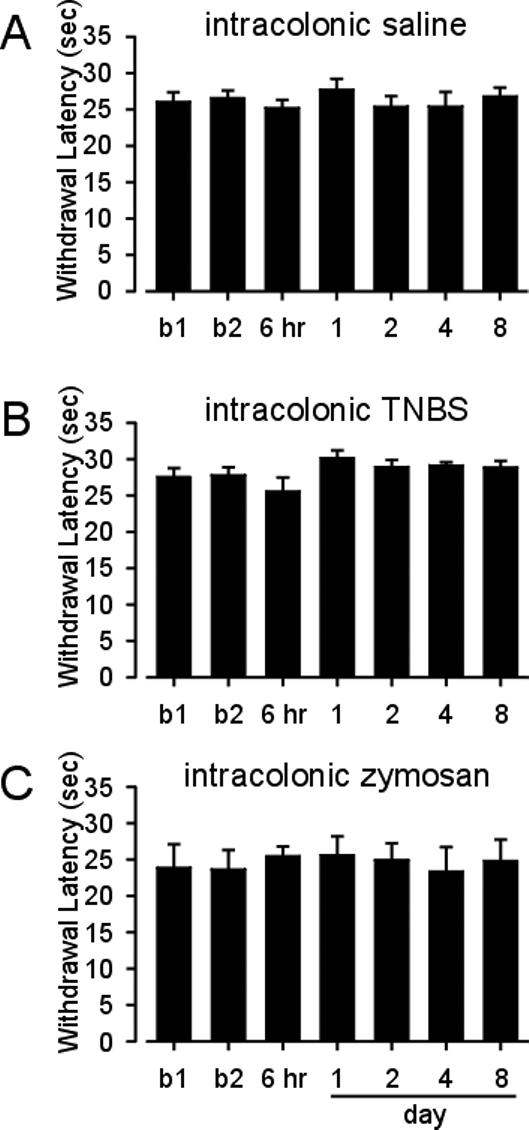
Mean hindpaw heat withdrawal latency of rats treated with intracolonic saline (A) or intracolonic TNBS (B). Mean hindpaw heat withdrawal latency of rats treated with intracolonic zymosan (C). Vertical lines are the standard error of the mean. b1=baseline 1. b2=baseline 2.
Similarly, in rats treated with intracolonic zymosan, the hindpaw withdrawal threshold significantly decreased from a median of 98 mN before zymosan administration to 11 mN 6 hr after zymosan administration (F=32.5 p< 0.05 vs baseline, Figure 2C). Withdrawal thresholds returned to baseline by day 2. As found following intracolonic TNBS treatment, there was no change in hindpaw heat withdrawal latency after intracolonic zymosan administration (F=1.2 p> 0.05 vs baseline, Figure 3C). There were no changes relative to baseline in either mechanical threshold (F=9.7 p> 0.05) or heat withdrawal latency (F=3.7, p> 0.05) after intracolonic saline treatment (Figures 2A and 3A).
Hindpaw and visceromotor responses after plantar incision
In the control group, the median withdrawal threshold (98mN) was unchanged after the sham incision (F=9.5 p>0.05 Figure 4A). After a plantar incision, however, the median withdrawal threshold was reduced from 98 mN to 11 mN when first tested 6 hr after the incision (Figure 4B). The withdrawal threshold remained decreased through postoperative day 2 ( F=38.2 p <0.05 vs baseline), and was indistinguishable from baseline thereafter. Hindpaw withdrawal latencies to heat were unchanged after the sham procedure ( 27.9 ± 4.1 to 25.4 ± 5.5; Figure 4C), whereas hindpaw withdrawal latency was significantly decreased to 12.3 ± 5.9 6 hr after incision (F=8.2 p<0.05 vs baseline) and remained significantly different from baseline through postoperative day 1 (Figure 4D). By four days after incision, the mean paw withdrawal latency returned to baseline values.
Figure 4.
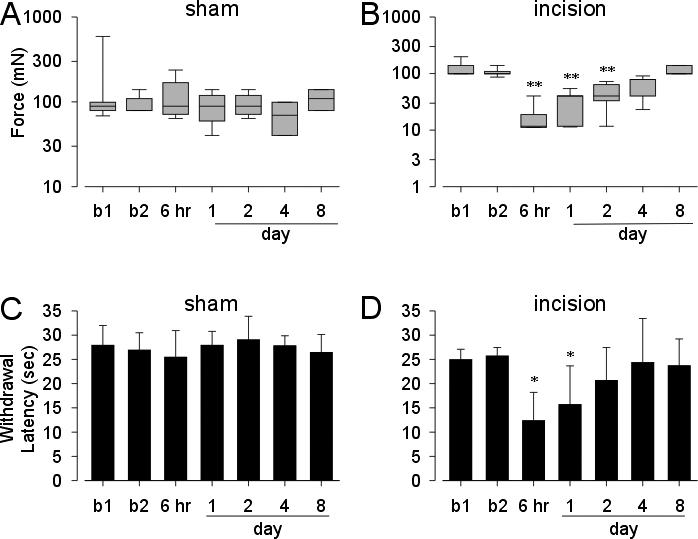
Withdrawal responses to punctuate mechanical stimuli in rats after sham incision or plantar incision (A and B). The results are expressed as median (horizontal line) with 1st and 3rd quartiles (boxes) and 10th and 90th percentiles. Mean hindpaw heat withdrawal latency in rats after plantar incision (C and D). Vertical lines are the standard error of the mean. Mean VMR (as percent of baseline) to graded CRD (20−80 mm Hg) in rats undergoing plantar incision in the hindpaw or sham surgery (E). Vertical lines are the standard error of the mean. b1=baseline 1. b2=baseline 2. (*P < 0.05 vs b2; **P < 0.01 vs. b2).
These same rats were tested for responses to graded CRD before and after either a sham incision or incision of the hindpaw. The VMR to distension was significantly increased at all times tested after hindpaw incision except when tested 6 hr after incision (F= 106.27. p<0.05 vs baseline; Figure 5A). Neither sham incision nor repetitive CRD testing was associated with colon hypersensitivity (Figure 5B). Accordingly, hindpaw incision produced colon hypersensitivity that persisted after mechanical and thermal hindpaw hyperalgesia had resolved (see Figure 4).
Figure 5.
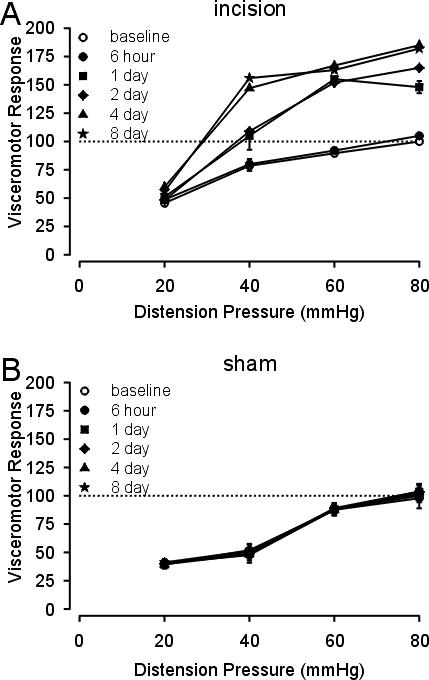
Mean VMR (as percent of baseline) to graded CRD (20−80 mm Hg) in rats after plantar incision (A) or sham incision (B). Vertical lines are the standard error of the mean. In A, P < 0.05 vs baseline control for all times of testing except 6 hr after hindpaw incision (see text).
Discussion
The principal findings of this study contribute to a growing literature supporting the importance of cross-sensitization between visceral and non-visceral structures following experimental injury. Thus, colon inflammation not only produces colon hypersensitivity to balloon distension, but also hindpaw hyperalgesia to mechanical stimuli. Conversely, hindpaw incision, a model of post-surgical nociception and hypersensitivity, not only produces hindpaw hyperalgesia to both thermal and mechanical stimuli, but increases responses to colon distension, an indication of colon hypersensitivity.
Referred hypersensitivity from the viscera is associated with increased tenderness to palpation and increased areas of referral and is attributed to sensitization in the central nervous system.12,16,33,37 Central sensitization results from increased input from sensitized and previously silent visceral afferents, which increases the excitability of central neurons upon which the afferents terminate.6 Spinal visceral afferent input terminates principally on neurons in superficial spinal laminae , laminae V and X around the central canal, but unlike non-visceral afferent input, visceral afferent input extends and arborizes significantly within the spinal cord to include multiple spinal segments both rostral and caudal to the segment of entry. In contrast, somatic inputs are generally limited to the segment of entry and immediately adjacent segments. Accordingly, the number of spinal neurons affected following visceral insult increases significantly 17 and contributes to increased excitability of spinal neurons that also receive convergent non-visceral inputs. Similarly, altered afferent input after injury of non-visceral tissues can cause heterosynaptic facilitation, sensitizing input from other areas of the body. Several studies have suggested that this central sensitization is N-methyl-D-aspartic acid (NMDA) dependent.26,49
Viscerosomatic convergence
The present study confirms previous reports of TNBS- and zymosan-induced colon hypersensitivity in the rat 9,14,15,27,38 and incision-induced thermal and mechanical hyperalgesia following a hindpaw incision.50 We also confirmed the development of non-visceral hyperalgesia following a visceral insult (see below) and, importantly, established a robust and long-lasting colon hypersensitivity following a distant non-visceral insult, hindpaw incision. The clinical implication of this finding relates to likely changes in visceral sensitivity and perhaps function following non-visceral surgery (or indeed other non-visceral insults).
As indicated above, the present data are consistent with previous studies demonstrating an increased sensitivity in non-visceral tissue after irritation or inflammation of the viscera in rodent models.5,7,18,20 For example, turpentine-induced inflammation of the bladder produced heat hyperalgesia in the hind limb for at least 24 h.19 In another study,18 bladder inflammation was associated with a referred mechanical and thermal hyperalgesia of the abdominal wall. Referral of visceral pain is not unique to animal models. In most interpretations of such experimental outcomes, convergence of inputs on the same or nearby spinal segments are considered to be the cause of cross-sensitization between visceral and non-visceral tissues. Afferent fibers from the colon project to thoracolumbar (T13-L2) and lumbosacral (L6-S1) segments of the rat spinal cord.10,28 Electrical stimulation of spinal nerves has shown that the plantar aspect of the hindpaw is predominately innervated by nerves entering spinal segments L4 and L5.42 Supraspinal sites are also likely involved (e.g., see 6, 43 ), but not commonly studied.
A specific effect of colon inflammation and hypersensitivity on hindpaw mechanical withdrawal threshold was noted. There was no effect of colon inflammation on responses to hindpaw heating, however. These results are consistent with other studies on central sensitization in which injury facilitates remote mechanical but not heat responses. For example, subcutaneous capsaicin injection produces remote secondary hyperalgesia to mechanical but not to heat stimuli in humans.4 Both hindpaw and gastrocnemius incision produce secondary mechanical but not heat hypersensitivity.34 This is consistent with enhanced responses of A-fibers but not C-fibers after injury.51 A mechanistic explanation for these outcomes is not obvious. Virtually all visceral afferent axons are either Aδ- or C-fibers and terminate centrally on neurons principally in the superficial laminae of the spinal dorsal horn. One mechanistic explanation that merits consideration and examination is that mechanosensitive hindpaw afferents converge on spinal neurons that receive visceral afferent input whereas thermosensitive hindpaw afferents synapse onto spinal neurons that do not receive a principal visceral convergent input. This remains to be directly tested, however.
Somatic referred hypersensitivity in humans also has been documented, for example in ureteric colic, renal infection, interstitial cystitis, and irritable bowel syndrome.3,48 Verne et al.44 found visceral hyperalgesia and cutaneous hyperalgesia in IBS patients that was greatest in lumbosacral dermatomes, corresponding to likely spinal hyperexcitiability in nearby spinal segments. Somatic hypersensitivity, however, has not been universally demonstrated in patients with irritable bowel syndrome when examining hypersensitivity to electrical stimulation of somatic structures or immersion of the hand in ice water.39 However, patients experiencing IBS and fibromyalgia had lower pain thresholds than IBS patients or healthy controls, suggesting the possible role of changes in central nervous system processing. 1,13,52
Somatovisceral convergence
Consistent with the convergence of visceral and non-visceral inputs onto spinal dorsal horn neurons, the visceromotor response to CRD was elevated one day after plantar incision (but not when tested at 6 hr) and remained elevated for eight days; we did not continue testing beyond this time. Elevated responses to noxious pressures were highest on postoperative days 4 and 8 when compared to baseline values, suggesting that colon sensitization peaks several days after hindpaw insult. Responses to distension were increased only within the noxious range of distending pressures (40, 60 and 80 mmHg). Although we did not test beyond day 8 after incision, unpublished results suggest that colon sensitization is present for up to two weeks. One interpretation of these findings is that neurons in supraspinal sites, principally those associated with descending modulation of spinal sensory input, have been sensitized and maintain visceral hypersensitivity after hindpaw insult. Irritable bowel syndrome and other ‘functional’ gastrointestinal disorders are characterized by discomfort and pain in the absence of visceral tissue inflammation or injury. It has become increasingly evident that supraspinal neurons and networks are important to maintenance of such functional disorders, but it remains to be determined what leads to these changes supraspinally. It has been shown that visceral insult in neonatal and young animals 2,36 leads to visceral hypersensitivity in the adult long after the neonatal insult has resolved. Similarly, neonatal paw injury alters visceromotor response in adult rats.45 Thus, functional visceral disorders characterized by hypersensitivity need not require a prior visceral insult, but could develop from an apparently unrelated non-visceral insult. The experimental design employed here examined insults that influenced spinal neurons in nearby or overlapping spinal segments, and given the known anatomical distribution of visceral and non-visceral afferent terminations in the spinal cord are understandable. What is unknown is whether and to what extent a distant non-visceral insult (e.g., to a forepaw, thoracic incision, etc.) might lead to colon hypersensitivity as described here.
Support
This work was performed in the Department of Pharmacology at the University of Iowa and supported in part by National Institutes of Health, Bethesda, Maryland grants GM-55831 and GM-067752 to T.J.B and NS 19912 to GFG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David M. Cameron, Department of Pharmacology, University of Iowa, Iowa City, Iowa
Timothy J. Brennan, Departments of Anesthesia and Pharmacology, University of Iowa, Iowa City, Iowa.
G. F. Gebhart, Departments of Anesthesiology, Medicine, Neurobiology and Pharmacology, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995;108:636–643. doi: 10.1016/0016-5085(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 2.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 3.Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: Unexplained associations with other chronic disease and pain syndromes. Urology. 1997;49:52–57. doi: 10.1016/s0090-4295(99)80332-x. [DOI] [PubMed] [Google Scholar]
- 4.Ali Z, Meyer RA, Campbell JN. Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin. Pain. 1996;68:401–411. doi: 10.1016/s0304-3959(96)03199-5. [DOI] [PubMed] [Google Scholar]
- 5.Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett. 2001;306:185–188. doi: 10.1016/s0304-3940(01)01906-1. [DOI] [PubMed] [Google Scholar]
- 6.Bielefeldt K, Gebhart GF. Visceral pain: Basic mechanisms. In: Koltzenburg M, McMahon S, editors. Textbook of Pain. 5th edition Churchill-Livingstone; London: 2005. pp. 721–736. [Google Scholar]
- 7.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: Species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- 8.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 9.Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: From basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. doi: 10.1016/s0016-5085(97)70056-8. [DOI] [PubMed] [Google Scholar]
- 10.Cervero F. Sensory innervation of the viscera: Peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- 11.Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA. Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain. 2007;128:235–243. doi: 10.1016/j.pain.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: Review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 13.Cook IJ, van Eeden A, Collins SM. Patients with irritable bowel syndrome have greater pain tolerance than normal subjects. Gastroenterology. 1987;93:727–733. doi: 10.1016/0016-5085(87)90434-3. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal nmda and non-nmda receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- 15.Duchmann R, Schmitt E, Knolle P. Meyer zum Buschenfelde, KH, Neurath, M: Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 16.Giamberardino MA. Recent and forgotten aspects of visceral pain. Eur J Pain. 1999;3:77–92. doi: 10.1053/eujp.1999.0117. [DOI] [PubMed] [Google Scholar]
- 17.Honore P, Kamp EH, Rogers SD, Gebhart GF, Mantyh PW. Activation of lamina I spinal cord neurons that express the substance p receptor in visceral nociception and hyperalgesia. J Pain. 2002;3:3–11. doi: 10.1054/jpai.2002.27001. [DOI] [PubMed] [Google Scholar]
- 18.Jaggar SI, Scott HC, James IF, Rice AS. The capsaicin analogue sdz249−665 attenuates the hyper-reflexia and referred hyperalgesia associated with inflammation of the rat urinary bladder. Pain. 2001;89:229–235. doi: 10.1016/s0304-3959(00)00366-3. [DOI] [PubMed] [Google Scholar]
- 19.Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 20.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 21.Lamb K, Zhong F, Gebhart GF, Bielefeldt K. Experimental colitis in mice and sensitization of converging visceral and somatic afferent pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G451–457. doi: 10.1152/ajpgi.00353.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lubrano E, Iovino P, Tremolaterra F, Parsons WJ, Ciacci C, Mazzacca G. Fibromyalgia in patients with irritable bowel syndrome. An association with the severity of the intestinal disorder. Int J Colorectal Dis. 2001;16:211–215. doi: 10.1007/s003840100299. [DOI] [PubMed] [Google Scholar]
- 23.Malykhina AP, Qin C, Foreman RD, Akbarali HI. Colonic inflammation increases na+ currents in bladder sensory neurons. Neuroreport. 2004;15:2601–2605. doi: 10.1097/00001756-200412030-00008. [DOI] [PubMed] [Google Scholar]
- 24.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 25.Mayer EA, Raybould HE. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 1990;99:1688–1704. doi: 10.1016/0016-5085(90)90475-g. [DOI] [PubMed] [Google Scholar]
- 26.Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Morteau O, Hachet T, Caussette M, Bueno L. Experimental colitis alters visceromotor response to colorectal distension in awake rats. Dig Dis Sci. 1994;39:1239–1248. doi: 10.1007/BF02093789. [DOI] [PubMed] [Google Scholar]
- 28.Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13-L2 spinal cord of the rat. J Neurophysiol. 1988;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- 29.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: Physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 30.Ness TJ, Gebhart GF. Visceral pain: A review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- 31.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991;66:20–28. doi: 10.1152/jn.1991.66.1.20. [DOI] [PubMed] [Google Scholar]
- 32.Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. Ii. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- 33.Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: Implications for the overlap of chronic pelvic pain disorders. Gastroenterology. 2005;128:1953–1964. doi: 10.1053/j.gastro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Pogatzki EM, Urban MO, Brennan TJ, Gebhart GF. Role of the rostral medial medulla in the development of primary and secondary hyperalgesia after incision in the rat. Anesthesiology. 2002;96:1153–1160. doi: 10.1097/00000542-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Price DD, Zhou Q, Moshiree B, Robinson ME, Nicholas Verne G. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar S, Hobson AR, Furlong PL, Woolf CJ, Thompson DG, Aziz Q. Central neural mechanisms mediating human visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1196–1202. doi: 10.1152/ajpgi.2001.281.5.G1196. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:175–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- 39.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 40.Stawowy M, Drewes AM, Arendt-Nielsen L, Funch-Jensen P. Somatosensory changes in the referred pain area before and after cholecystectomy in patients with uncomplicated gallstone disease. Scand J Gastroenterol. 2006;41:833–837. doi: 10.1080/00365520500463332. [DOI] [PubMed] [Google Scholar]
- 41.Stawowy M, Funch-Jensen P, Arendt-Nielsen L, Drewes AM. Somatosensory changes in the referred pain area in patients with cholecystolithiasis. Eur J Gastroenterol Hepatol. 2005;17:865–870. doi: 10.1097/00042737-200508000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory c-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- 43.Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 45.Wang G, Ji Y, Lidow MS, Traub RJ. Neonatal hind paw injury alters processing of visceral and somatic nociceptive stimuli in the adult rat. J Pain. 2004;5:440–449. doi: 10.1016/j.jpain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Weir PT, Harlan GA, Nkoy FL, Jones SS, Hegmann KT, Gren LH, Lyon JL. The incidence of fibromyalgia and its associated comorbidities: A population-based retrospective cohort study based on international classification of diseases, 9th revision codes. J Clin Rheumatol. 2006;12:124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
- 47.Wesselmann U, Burnett AL, Heinberg LJ. The urogenital and rectal pain syndromes. Pain. 1997;73:269–294. doi: 10.1016/S0304-3959(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 48.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: What are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 49.Willert RP, Delaney C, Kelly K, Sharma A, Aziz Q, Hobson AR. Exploring the neuropsychological basis of chest wall allodynia induced by experimental oesophageal acidification- evidence of central sensitization. Neurogastroenterol Motil. 2007;19:270–278. doi: 10.1111/j.1365-2982.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 50.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler EA, Magerl W, Meyer RA, Treede RD. Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to a-fibre nociceptor input. Brain. 1999;122(Pt 12):2245–2257. doi: 10.1093/brain/122.12.2245. [DOI] [PubMed] [Google Scholar]
- 52.Zighelboim J, Talley NJ, Phillips SF, Harmsen WS, Zinsmeister AR. Visceral perception in irritable bowel syndrome. Rectal and gastric responses to distension and serotonin type 3 antagonism. Dig Dis Sci. 1995;40:819–827. doi: 10.1007/BF02064986. [DOI] [PubMed] [Google Scholar]


