Abstract
Epidemiological data show that reproductive and hormonal factors are involved in the etiology of endometrial cancer, but there is little data on the association with endogenous sex hormone levels. We analyzed the association between prediagnostic serum concentrations of sex steroids and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition using a nested case–control design of 247 incident endometrial cancer cases and 481 controls, matched on center, menopausal status, age, variables relating to blood collection, and, for premenopausal women, phase of menstrual cycle. Using conditional regression analysis, endometrial cancer risk among postmenopausal women was positively associated with increasing levels of total testosterone, free testosterone, estrone, total estradiol, and free estradiol. The odds ratios (ORs) for the highest versus lowest tertile were 2.66 (95% confidence interval (CI) 1.50–4.72; P=0.002 for a continuous linear trend) for estrone, 2.07 (95% CI 1.20–3.60; P=0.001) for estradiol, and 1.66 (95% CI 0.98–2.82; P=0.001) for free estradiol. For total and free testosterone, ORs for the highest versus lowest tertile were 1.44 (95% CI 0.88–2.36; P=0.05) and 2.05 (95% CI 1.23–3.42; P=0.005) respectively. Androstenedione and dehydroepiandrosterone sulfate were not associated with risk. Sex hormone-binding globulin was significantly inversely associated with risk (OR for the highest versus lowest tertile was 0.57, 95% CI 0.34–0.95; P=0.004). In premenopausal women, serum sex hormone concentrations were not clearly associated with endometrial cancer risk, but numbers were too small to draw firm conclusions. In conclusion, relatively high blood concentrations of estrogens and free testosterone are associated with an increased endometrial cancer risk in postmenopausal women.
Introduction
Endometrial cancer comprises about 4% of all cancers in women worldwide (Parkin et al. 2002) and is the fourth most common cancer in women in westernized countries (Parkin et al. 2002, Bray et al. 2005). Obesity is an important risk factor for this disease in both pre- and postmenopausal women, accounting for ∼ 40% of the incidence (Bergstrom et al. 2001). The physiological mechanisms underlying this relationship are not completely understood, but may involve alterations in endogenous sex steroid metabolism (Kaaks et al. 2002).
The predominant hypothesis describing the relationship between sex steroid hormones and endometrial cancer risk is known as the ‘unopposed estrogen’ hypothesis (Henderson et al. 1982, Key & Pike 1988). The ‘unopposed estrogen’ hypothesis states that endometrial cancer risk is increased among women who have high circulating levels of bioavailable estrogens and low levels of progesterone, so that the mitogenic effect of estrogens is insufficiently counterbalanced by the opposing effect of progesterone (Henderson et al. 1982, Key & Pike 1988). The unopposed estrogen hypothesis is supported by the observations that the use of estrogen-only hormone-replacement therapy (HRT; Beral et al. 2005) and sequential oral contraceptives (OCs; Weiss & Sayvetz 1980) greatly increase endometrial cancer risk, while combined preparations of OCs and HRT (i.e., that contain progestagen as well as estrogen throughout the treatment period) have a protective effect (Cancer and Steroid Hormone Study 1987, Beral et al. 2005).
Most of the larger retrospective case–control studies (with 50 or more cases) have shown that blood levels of estrogens are higher in postmenopausal women with endometrial cancer than in healthy control subjects (Austin et al. 1991, Nyholm et al. 1993, Potischman et al. 1996). In premenopausal women, data on the association between circulating levels of sex steroid hormones and endometrial cancer risk are sparse, but suggest a role for elevated levels of estrone and androstenedione (Potischman et al. 1996). To date, only two prospective studies have examined the relationship of endometrial cancer risk with prediagnostic circulating levels of sex steroids, and these studies focused entirely on women who were postmenopausal at the time of blood donation (Zeleniuch-Jacquotte et al. 2001, Lukanova et al. 2004).
We report here the results from a case–control study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC), in which we examined the relationship between prediagnostic serum concentrations of sex steroids and endometrial cancer risk among pre- and postmenopausal women. In addition, we examined whether sex hormones might explain the association between obesity and risk. With a total of 247 incident cases of endometrial cancer and 481 matched controls, this is the largest prospective study to date on the relationships between endometrial cancer risk and serum levels of estrogens, androgens, and sex hormone-binding globulin (SHBG).
Subjects and methods
Study cohort
EPIC recruitment procedures and collection of questionnaire data, anthropometric measurements, and blood samples have been described in detail previously (Riboli et al. 2002). In brief, extensive standardized questionnaire data on dietary and non-dietary variables were collected between 1992 and 1998 from about 500 000 individuals across Europe, and a blood sample was collected from about 400 000 individuals. The present study includes endometrial cancer cases occurring after blood collection and matched control subjects from eight of the ten participating countries: Denmark, France, Germany, Greece, Italy, the Netherlands, Spain, and the United Kingdom. Norway was not included in the present study because blood samples have been collected only recently and very few cases of endometrial cancer have been diagnosed. Sweden was not included because of limited questionnaire information at the time of blood donation about menopausal status, past and current use of exogenous hormones, and phase of menstrual cycle (Malmö), and because endometrial cancer case patients had already been included in a previous study (Umeå; Lukanova et al. 2004).
A 30 ml blood sample was collected according to a standardized protocol. Filled syringes were kept at 5–10 °C, protected from light, and transferred to a local laboratory for further processing and aliquoting, with the exception of subjects recruited through the Oxford center. Here, blood samples were collected throughout the United Kingdom and transported to a laboratory in Norfolk by mail at ambient temperature. (The stability of serum sex steroid levels measured in blood transported at ambient temperatures or kept at ambient temperatures for 24 or 48 h has been documented previously (Hankinson et al. 1989, Murphy et al. 2000)). Blood fractions (serum, plasma, red cells, and buffy coat for DNA extraction) were aliquoted into 0.5 ml straws, which were then heat sealed and stored in liquid nitrogen tanks at −196 °C, except in Denmark where samples were stored in 1 ml tubes in nitrogen vapor at −150 °C.
Determination of menopausal status
Women were considered premenopausal at the time of blood donation when they reported having had regular menses over the past 12 months, or when they were <42 years of age, because 99.5% of EPIC women who had complete data were premenopausal below age 42. Women were considered postmenopausal when they reported not having had any menses over the past 12 months or when they reported a bilateral ovariectomy. Women who had incomplete data were considered postmenopausal when they were older than 55 years. Women who were between 42 and 55 years of age (inclusive) and had equivocal questionnaire data for menopausal status were classified as having an unknown menopausal status and were excluded from the subsequent analyses. Women who had had a hysterectomy were also excluded from the study. The methods used to determine the phase of menstrual cycle at blood donation from premenopausal women have been described in detail previously (Kaaks et al. 2005).
Follow-up for cancer incidence and vital status
In Denmark, Italy, the Netherlands, Spain, and the United Kingdom, incident cancer cases were identified through record linkage with regional cancer registries. In France, Germany, and Greece, follow-up was based on a combination of methods, including health insurance records, cancer, and pathology registries, and active follow-up through study subjects and their next of kin. Data on vital status in most EPIC study centers were collected from mortality registries at the regional or national level, in combination with data collected by active follow-up (Greece). For each EPIC study center, closure dates of the study period were defined as the latest dates of complete follow-up for both cancer incidence and vital status (dates varied between centers, from June 1999 to December 2003).
Selection of case and control subjects
Case subjects were women who developed endometrial cancer after the date of blood donation and before the end of the study period, defined for each study center by the latest date of follow-up. Case and control subjects were selected among women who had no previous diagnosis of cancer (except non-melanoma skin cancer) and were not currently using exogenous hormones at the time of blood donation. Among past HRT users, no information was available on the type or duration of HRT use.
In total, the eight sub-cohorts contributing to the present study included 135 953 women who were not taking exogenous hormones at the time of blood donation, of whom 301 had a diagnosis of endometrial cancer by the end of each center's follow-up period. After excluding cases with insufficient sera (n=8) or who had an unknown menopausal status (n=46), matched control subjects were identified for 247 cases. These included 54 cases in Denmark, 52 in Italy, 37 in Spain, 28 in the Netherlands, 32 in the UK, 20 in Germany, 16 in France, and 8 in Greece. Detailed tumor morphology was specified for 127 cases (51%), of which 118 cases (94%) were classified as type I (endometrioid adenocarcinomas) and 9 cases (6%) as type II (serous papillary, clear cell or squamous adenocarcinomas; Tavassoli & Devilee 2003).
For each case subject, up to two control subjects were chosen at random among appropriate risk sets consisting of all cohort members alive and free of cancer (except non-melanoma skin cancer) at the time of diagnosis of the index case. An incidence density sampling protocol for control selection was used, such that control subjects could include those who became a case later in time, while each control could also be sampled more than once. Matching criteria included: study center, menopausal status (premenopausal, postmenopausal), age at enrollment (±6 months), time of the day of blood collection (±1 h), and, for premenopausal women, phase of menstrual cycle (‘early follicular’ (days 0–7 of the cycle), ‘late follicular’ (days 8–11), ‘periovulatory’ (days 12–16), ‘midluteal’ (days 20–24), and ‘other luteal’ (days 17–19 or 25–40)). Case patients and control subjects were also matched on time between blood draw and last consumption of foods or drinks (<3, 3–6, >6 h), in view of further studies in which endometrial cancer risk will be related to endogenous insulin levels. All participants gave written consent for future analyses of their blood samples and the study was approved by the local ethics committees in the participating countries and the Internal Review Board of the IARC.
Hormone assays
Serum testosterone, dehydroepiandrosterone sulfate (DHEAS), androstenedione (A-dione), and SHBG levels were measured for all study subjects; serum estradiol and estrone levels were measured only in postmenopausal women because of its large intra-individual variation during the menstrual cycle among premenopausal women. Serum testosterone and DHEAS concentrations were measured by radio immunoassays (Immunotech, Marseilles, France). A-dione, estradiol, and estrone concentrations were measured by a radio immunoassay with a double-antibody system for the separation of free and bound antigen (Diagnostic Systems Laboratories Inc., Webster, TX, USA). Assays were performed in batches of 76 serum samples, analyzed together on the same day and with the same immunoassay kit. Serum samples of case and control subjects that were matched together were systematically analyzed within the same batch. SHBG was measured by a solid phase ‘sandwich’ IRMA (Cis-Bio International, Gif-sur-Yvette, France). Mean intra-batch coefficients of variation (CV) were estimated to be 5.5% (at 0.54 μmol/l) for DHEAS, 6.6% (at 1.4 nmol/l) for testosterone, 3.0% (at 3.5 nmol/l) for A-dione, 7.4% (at 55.4 pmol/l) for estrone, 8.4% (at 294 pmol/l) for estradiol, and 3.9% (at 30 nmol/l) for SHBG. Inter-batch CVs were 14% for DHEAS, 12% for testosterone, 8.4% for A-dione, 16% for estrone, 20% for estradiol, and 11% for SHBG. All hormone assays were performed by the laboratory of the Hormones and Cancer Team at IARC. The laboratory personnel performing the hormone assays were blinded as to the case–control status of the study subjects. Serum concentrations of free testosterone and free estradiol, unbound to SHBG or albumin, were calculated from the absolute concentrations of each of the steroids and SHBG using mass action equations, and assuming a constant serum albumin concentration of 43 g/l (Rinaldi et al. 2002).
Statistical analyses
In all analyses, levels of SHBG and sex steroids were log transformed to normalize their distributions. Differences in baseline characteristics between case patients and control subjects were compared using the paired t-test for continuous variables and the χ2-test for categorical variables. The statistical significance of case–control differences in geometric mean hormone concentrations were evaluated by paired comparisons (t-tests) of case values versus the average of the two matched control subjects in each case–control set (Rosner 1982). Pearson's partial correlation coefficients, adjusted for age, case–control status, and laboratory batch were calculated to assess the correlations between hormones and anthropometric indices. Analysis of covariance was used to investigate geometric mean differences in hormone levels by the following variables: country (Denmark, Italy, Spain, the Netherlands, UK, Germany, France, Greece); OC use (never, past); HRT use (never, past); smoking (never, past, current); body mass index (BMI; kg/m2; in quartiles); ever had a full-term pregnancy (no, yes), and in premenopausal women, phase of menstrual cycle that blood was taken (early follicular, late follicular, periovulatory, midluteal, other luteal), after adjusting for age (continuous variable), case–control status (case, control), and laboratory batch (categorical variable).
Relative risks (odds ratios (ORs)) for endometrial cancer in relation to serum hormone levels were calculated by conditional logistic regression models, using the PHREG procedure of the SAS software package (Version 9). Hormone levels were categorized into thirds for postmenopausal women to allow adequate numbers of subjects in each category. Cut points were based on the distribution of the hormones among the control subjects from all EPIC centers combined. Likelihood ratio tests were used to assess linear trends in ORs across the categories using the median hormone values for each category as the quantitative score of exposure and on a continuous scale. The 95% confidence intervals (CIs) were computed using the standard errors of the pertinent regression coefficients.
The effects of additional potential confounders (other than the matching criteria, controlled for by design) were examined by including additional regression terms into the logistic regression models. Potential confounders included: BMI (continuous variable), age at menarche (continuous variable), age at menopause (continuous variable), previous use of OCs (yes, no), previous use of HRT (yes, no), smoking (never, past, current), and physical activity (inactive, moderately inactive, moderately active, active). For all confounding variables considered in our analyses, the percentage of missing values was below 3% (except for age at menopause: 7%). To avoid exclusion of all of these women from our logistic regression analyses, we imputed missing values using a sequence of regression models as describe in Raghunathan et al. (2001). In this approach, normal linear regression models are used for continuous variables and polytomous regression models for categorical variables. All the models included age at blood donation, study center, and case–control status as regressors.
The effects of mutual adjustments between estrogens and testosterone were examined on a continuous scale. χ2-tests were used to examine heterogeneity of endometrial cancer risk associated with a doubling of hormone level by study country (eight countries), HRT use (never, former), and lag time between blood donation and diagnosis (<2 years, 2+ years). Absolute risks of developing endometrial cancer over a 10-year follow-up period were calculated using the method described by Gail et al. (1989). These calculations were performed for women in four different age categories (<55, 55–59, 60–64, and ≥65 years) and the age-specific hazard of dying from other causes than endometrial cancer were set at 1.7×10−3, 2.8×10−3, 4.3×10−3, and 4.5×10−3 respectively for each of these age four groups.
Results
This study includes 247 case patients diagnosed from recruitment until the end of follow-up: 55 women were premenopausal at recruitment and 192 were postmenopausal. The median age at recruitment was 49 years (5–95% range=40–54 years) in premenopausal women and 60 years (5–95% range=53–70 years) in postmenopausal women. Among cases, the median time between recruitment and cancer diagnosis was 3.5 years (5–95% range=0.3–7.3 years) for premenopausal women and 3.2 years (5–95% range=0.4–7.0 years) for postmenopausal women. The median age at cancer diagnosis was 52 years (5–95% range=42–58 years) among women who were premenopausal at recruitment and 63 years (5–95% range=55–74 years) among postmenopausal women.
Basic characteristics of the study participants are shown in Table 1. Overall, case patients were significantly heavier and had a higher BMI than control subjects, although this difference was found only in postmenopausal women. Case patients had a higher age at menopause and were more likely to be nulliparous, to have never used OCs and previously used HRT (Table 1). There were no significant differences in height, physical activity, age at menarche, age at first pregnancy, number of pregnancies, smoking, or self-reported prevalence of diabetes between case patients and control subjects.
Table 1.
Basic characteristics of endometrial cancer patients and control subjects: median (5th–95th percentile), unless otherwise stated
| Cases | Controls | P for differencea | |
|---|---|---|---|
| Number | 247 | 481 | |
| Menopausal status | |||
| Premenopausal | 55 | 107 | |
| Postmenopausal | 192 | 374 | |
| Age at blood donation | 58.4 (45.0–68.8) | 58.4 (44.9–68.6) | 0.48 |
| Age at diagnosis | 61.0 (47.0–72.0) | – | – |
| Height (m) | 160.0 (149.0–171.0) | 160.0 (149.5–171.0) | 0.48 |
| Weight (kg) | 68.6 (52.8–96.0) | 67.5 (51.6–88.5) | 0.0002 |
| BMI (kg/m2) All women | 27.4 (21.1–37.4) | 26.0 (20.2–34.8) | 0.0001 |
| Premenopausal | 25.2 (20.4–36.5) | 25.4 (19.5–33.6) | 0.53 |
| Postmenopausal | 27.7 (21.4–37.9) | 26.1 (20.6–35.5) | 0.0001 |
| Age at menarche | 13.0 (11.0–15.0) | 13.0 (11.0–16.0) | 0.17 |
| Nulliparous (%) | 20.7 | 9.6 | <0.0001 |
| Number of pregnanciesb | 2.0 (1.0–4.0) | 2.0 (1.0–5.0) | 0.16 |
| Age at first pregnancyb | 24.5 (18.0–31.0) | 25.0 (19.0–34.0) | 0.40 |
| Age at menopausec | 52.0 (45.0–56.0) | 50.0 (41.0–55.0) | 0.0004 |
| Previous OC use (%) | 32.4 | 39.5 | 0.03 |
| Previous HRT use (%) | 20.2 | 13.3 | 0.02 |
| Diabetes (%) | 4.1 | 4.2 | 0.90 |
| Physical activity (%) | |||
| Inactive | 11.7 | 9.2 | 0.30 |
| Moderately inactive | 27.9 | 23.9 | |
| Moderately active | 49.4 | 57.0 | |
| Active | 10.1 | 9.8 | |
| Unknown | 0.8 | 0.2 | |
| Smoking status (%) | |||
| Never smoker | 66.8 | 60.7 | 0.29 |
| Ex smoker | 19.0 | 21.6 | |
| Current smoker | 13.8 | 16.8 | |
| Unknown | 0.4 | 0.8 |
Two-sided paired t-test for continuous variables and χ2-test for categorical variables.
Among parous women only.
Among postmenopausal women only.
Differences in hormone levels according to baseline characteristics were evaluated after adjusting for age at blood collection, case–control status, and laboratory batch. Smoking, past HRT use, OC use, and parity were not significantly associated with serum hormone levels in all women combined, or in pre- or postmenopausal women separately (data not shown). There was no systematic variation over the menstrual cycle for testosterone, free testosterone, SHBG, DHEAS, or A-dione.
Pearson partial correlation coefficients between sex hormones, adjusted for age, case–control status, and laboratory batch, showed that all the androgens were positively correlated with each other (Table 2). In postmenopausal women, androgens were also positively correlated with estrone, estradiol, and free estradiol. As expected, SHBG was strongly inversely correlated with free estradiol and free testosterone. BMI was also inversely correlated with SHBG (r=−0.42 and −0.43 in pre- and postmenopausal women respectively) and, in turn, positively correlated with free testosterone (r=0.38 and 0.23) and free estradiol (r=0.41 in postmenopausal women). However, BMI was not correlated with A-dione or DHEAS in pre- or postmenopausal women.
Table 2.
Pearson's partial correlation coefficients for associations among endogenous hormone levels and BMI in premenopausal and postmenopausal women
| Testosterone | Free testosterone | Androstenedione | DHEAS | SHBG | Estrone | Estradiol | Free estraidiol | BMI | |
|---|---|---|---|---|---|---|---|---|---|
| Testosterone | 0.88‡ | 0.68‡ | 0.72‡ | −0.05 | 0.62‡ | 0.44‡ | 0.41‡ | 0.03 | |
| Free testosterone | 0.82‡ | 0.59‡ | 0.66‡ | −0.51‡ | 0.63‡ | 0.51‡ | 0.62‡ | 0.23‡ | |
| Androstenedione | 0.69‡ | 0.55‡ | 0.63‡ | 0.00 | 0.51‡ | 0.37‡ | 0.31‡ | 0.00 | |
| DHEAS | 0.74‡ | 0.70‡ | 0.58‡ | −0.09* | 0.65‡ | 0.46‡ | 0.42‡ | −0.03 | |
| SHBG | −0.17* | −0.70‡ | −0.04 | −0.23† | −0.20‡ | −0.24‡ | −0.58‡ | −0.43‡ | |
| Estrone | 0.64‡ | 0.62‡ | 0.21‡ | ||||||
| Estradiol | – | – | – | – | – | – | 0.93‡ | 0.28‡ | |
| Free estradiol | – | – | – | – | – | – | – | 0.41‡ | |
| BMI | 0.20* | 0.38‡ | −0.06 | 0.11 | −0.42‡ | 0.16* | – | – |
Analyses were based on 162 premenopausal women (not in bold) and 566 postmenopausal women (in bold). Analyses were done using log-transformed data and were adjusted for age, case–control status, and laboratory batch. Measurements were not available for estradiol or free estradiol among premenopausal women. BMI, body mass index; DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone-binding globulin. *P<0.05; †P<0.01; ‡P<0.0001 (two-sided tests).
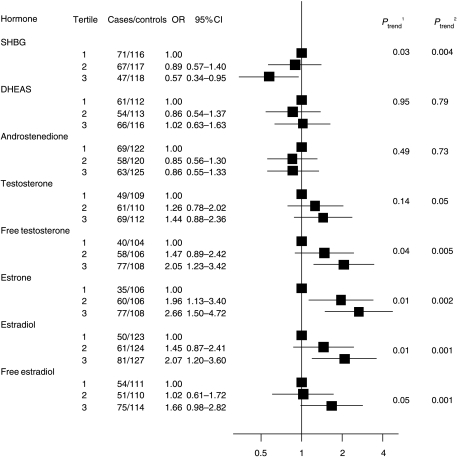
In women who were premenopausal at the time of blood donation, there were no significant differences in the geometric mean concentrations of SHBG or androgens between the 55 case patients and 107 control subjects (Table 3). In women who were postmenopausal at the time of blood donation, the geometric mean SHBG concentration was 11% lower among case patients than in control subjects (P=0.004; Table 3). Circulating mean concentrations of total testosterone, free testosterone, estrone, estradiol, and free estradiol were 10–18% higher in case patients than in control subjects (P≤0.05 for each hormone). Logistic regression analyses also showed strong associations between circulating serum concentrations of these sex hormones and endometrial cancer risk. A continuous linear trend test showed increases in endometrial cancer risk with decreasing levels of SHBG (Ptrend=0.004); when categorized into thirds, the OR for the highest versus lowest tertile was 0.57 (95% CI=0.34–0.95; Ptrend=0.03). By contrast, there were significant increases in endometrial cancer risk with increasing serum concentrations of total testosterone and free testosterone (Ptrend=0.05 for testosterone, and Ptrend=0.005 for free testosterone as continuous variables), although the relative risk estimate for the highest versus lowest tertile was significant only for free testosterone (OR=2.05; 95% CI=1.23–3.42; Ptrend=0.005), and not for total testosterone (OR=1.44; 95% CI=0.88–2.36; Ptrend=0.14). Endometrial cancer risk was also directly linearly related to circulating levels of estrone (Ptrend=0.002), total estradiol (Ptrend=0.001), and free estradiol (Ptrend=0.001). When categorized into thirds, ORs for the highest versus lowest tertile were 2.66 (95% CI=1.50–4.72; Ptrend=0.01) for estrone, 2.07 (95% CI=1.20–3.60; Ptrend=0.01) for estradiol, and 1.66 (95% CI=0.98–2.82; Ptrend=0.05) for free estradiol (Fig. 1). In this study population, the absolute risk of developing endometrial cancer for a postmenopausal women aged 60 years and followed up for 10 years was estimated at 0.9% for those in the highest tertile of serum estradiol and 0.5% for those in the lowest tertile. Similar absolute risks were found for estrone, free estradiol, and free testosterone (data not shown). A-dione and DHEAS had similar mean serum levels among postmenopausal case patients and control subjects and showed no association with endometrial cancer risk in logistic regression analyses.
Table 3.
Geometric means (95% confidence interval) of steroid hormone levels for endometrial cancer case patients and control subjects, by menopausal status at the time of blood donation
| Premenopausal women | Postmenopausal women | |||
|---|---|---|---|---|
| Hormone | Case patients | Control subjects | Case patients | Control subjects |
| SHBG (nmol/l) | 48.4 (42.2–55.5) | 51.0 (46.2–56.2) | 42.6 (39.7–45.7) | 47.9 (45.5–50.4)† |
| DHEAS (μmol/l) | 2.59 (2.19–3.06) | 2.41 (2.13–2.72) | 1.84 (1.66–2.05) | 1.89 (1.75–2.04) |
| Androstenedione (nmol/l) | 5.14 (4.65–5.69) | 4.97 (4.62–5.34) | 3.71 (3.49–3.95) | 3.75 (3.58–3.92) |
| Testosterone (nmol/l) | 1.39 (1.24–1.55) | 1.36 (1.25–1.47) | 1.32 (1.22–1.43) | 1.20 (1.14–1.28)* |
| Free testosterone (pmol/l) | 18.3 (15.7–21.2) | 17.4 (15.6–19.4) | 18.8 (17.2–20.7) | 15.9 (14.9–17.0)† |
| Estrone (pmol/l) | 138 (130–148) | 121 (116–127)† | ||
| Estradiol (pmol/l) | – | – | 98.8 (93.1–105) | 89.1 (85.3–92.9)‡ |
| Free estradiol (pmol/l) | – | – | 2.40 (2.24–2.58) | 2.09 (1.98–2.19)‡ |
*P<0.05, †P<0.01, ‡P<0.001. Analysis based on 162 premenopausal women (55 case patients and 107 control subjects) and 566 postmenopausal women (192 case patients and 374 control subjects). Measurements were not taken for estrone, estradiol, or free estradiol among premenopausal women. DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone-binding globulin.
Figure 1.
Odds ratio (and their corresponding 95% confidence intervals (CIs)) for endometrial cancer by serum levels of SHBG and sex steroids (in tertiles) among postmenopausal women. Solid squares represent odds ratios (ORs), estimated by conditional logistic regression, for tertiles of serum hormone concentrations (cut points based on the distribution of control subjects) and horizontal lines represent 95% CIs. Analysis based on 192 case patients and 374 control subjects. Case patients and control subjects were matched on EPIC (European Prospective Investigation into Cancer and Nutrition) recruitment center, age at blood donation, time of day at blood donation, and fasting status. DHEAS, dehydroepiandrosterone sulfate. 1Ptrend=P value for a test of linear trend across categories based on median hormone values for the three tertiles. 2Ptrend=P value for a test of linear trend based on log-transformed continuous variable.
BMI was significantly associated with endometrial cancer risk among postmenopausal women; compared with women of a normal BMI (<25 kg/m2), the ORs associated with being overweight (BMI 25–29 kg/m2) and obese (BMI 30+ kg/m2) were 1.26 (95% CI=0.81–1.96) and 2.67 (95% CI=1.63–4.37; Ptrend=0.0001) respectively. The association between BMI and endometrial cancer risk was moderately attenuated after adjusting for free estradiol; the risk associated with being obese compared with women of a normal BMI was reduced from 2.67 to 2.09 (95% CI=1.22–3.57; Ptrend=0.009) and the corresponding χ2-value declined by half from 15.23 to 7.24; adjustment for total estradiol and estrone had similar, albeit slightly weaker effects on the association between BMI and cancer risk. Adjustment for free testosterone or the other androgens did not appreciably alter the association between BMI and cancer risk in postmenopausal women (data not shown).
In turn, the associations between sex hormones and endometrial cancer risk were somewhat attenuated after adjustment for BMI, although the association with estrone remained statistically significant (OR for the highest versus lowest tertile=2.19 (95% CI=1.21–3.97; Ptrend=0.01; Fig. 2). The association for free testosterone with risk was attenuated further after adjustment for BMI, although it remained statistically significant (OR for the highest versus lowest tertile=1.74 (95% CI=1.02–2.96; Ptrend=0.04). Adjustment for BMI had a negligible effect on the association between total testosterone and endometrial cancer risk, although it weakened the association with SHBG, estradiol, and free estradiol, which were no longer statistically significant (Fig. 2). Further adjustments for age at menarche, parity, age at menopause, smoking, and physical activity made little difference to these findings (data not shown).
Figure 2.
Odds ratio (and their corresponding 95% confidence intervals (CIs)) for endometrial cancer by serum levels of SHBG and sex steroids (in tertiles) among postmenopausal women after adjustment for BMI*. Solid squares represent odds ratios (ORs), estimated by conditional logistic regression, for tertiles of serum hormone concentrations (cut points based on the distribution of control subjects) and horizontal lines represent 95% CIs. Analysis based on 192 case patients and 374 control subjects. Case patients and control subjects were matched on EPIC (European Prospective Investigation into Cancer and Nutrition) recruitment center, age at blood donation, time of day at blood donation, and fasting status. DHEAS, dehydroepiandrosterone sulfate. *BMI included as a continuous variable. 1Ptrend=P value for a test of linear trend across categories based on median hormone values for the three tertiles. 2Ptrend=P value for a test of linear trend based on log transformed continuous variable.
After adjustment for estrone and estradiol levels, the effect on endometrial cancer risk associated with a doubling of testosterone and free testosterone concentration was strongly attenuated, and no longer statistically significant (from 30 to 6% increased risk for testosterone and from 40 to 19% for free testosterone). The strength of the association with estrogens was also reduced after adjustment for testosterone levels but the models remained statistically significant. (The OR for a doubling of estrogen levels decreased from 2.02 (Ptrend=0.0002) to 1.79 (Ptrend=0.02) for estrone and from 2.29 (Ptrend=0.001) to 2.16 (Ptrend=0.02) for estradiol).
We next examined whether the associations between sex hormones and endometrial cancer risk varied according to the time between blood draw and cancer diagnosis in postmenopausal women. There was some evidence of heterogeneity in risk associated with a doubling of hormone concentration for case patients diagnosed <2 years since blood collection compared with cases diagnosed more than 2 years for most hormones, and which was statistically significant for estradiol (χ2=8.3; Pheterogeneity=0.004) and testosterone (χ2=5.2; Pheterogeneity=0.02). Overall, restricting the analyses to 134 postmenopausal women diagnosed at least 2 years after blood collection strengthened the associations between sex hormones and endometrial cancer risk; ORs for the highest versus lowest tertile of estrone, estradiol, and free estradiol were 4.17 (95% CI=1.98–8.80; Ptrend<0.0001), 3.19 (95% CI=1.61–6.32; Ptrend<0.0001) and 2.05 (95% CI=1.09–3.87; Ptrend<0.0001) respectively. The association between testosterone and free testosterone was also strengthened with ORs for the highest versus lowest tertile of 1.94 (95% CI=1.09–3.45; Ptrend=0.01) for testosterone and 2.34 (95% CI=1.30–4.22; Ptrend=0.002) for free testosterone. Restricting the analysis to case patients diagnosed 1 year after blood collection produced similar results, although the tests for heterogeneity were not statistically significant (data not shown).
When the analyses were restricted to postmenopausal women who never used HRT in the past, the association between estrone levels and endometrial cancer risk was strengthened (OR for the highest versus lowest tertile =3.68; 95% CI=1.79–7.58; Ptrend<0.0001). However, there was no evidence of heterogeneity of the association of hormones with endometrial cancer risk between past and never users of HRT or between the participating countries. Further, the results were similar when based on center-specific cut points instead of the cut points determined on the control subjects from all study centers combined (data not shown).
Discussion
This prospective study shows that circulating blood levels of estrogens and testosterone are positively associated with an increased risk of endometrial cancer in postmenopausal women. These results were statistically significant, similar between the eight European countries included in the analysis, and not altered by smoking, parity, and other known risk factors for endometrial cancer. These findings are consistent with data from two smaller prospective studies (that included 124 (Lukanova et al. 2004) and 57 postmenopausal cases (Zeleniuch-Jacquotte et al. 2001) respectively) and most of the larger case–control studies that have analyzed sex hormone levels in blood (Austin et al. 1991, Nyholm et al. 1993, Potischman et al. 1996) or urine (Baanders-van Halewijn & Poortman 1985) in relation to endometrial cancer risk.
In this multi-center study, there was a high degree of standardization across study centers of recruitment and blood collection protocols and questionnaire data. Furthermore, all the hormone measurements were made in one laboratory with samples from case patients and their matched control subjects assayed at the same time. The prospective design of our study minimizes the risk of ‘reverse causation’ bias that may occur when alterations in endogenous hormone levels are induced by metabolic effects of an existing tumor, anti-tumor treatments, psychological stress, or lifestyle changes after cancer diagnosis.
The finding that obesity is associated with increased endometrial risk in postmenopausal women is well established (Kaaks et al. 2002, Vainio & Bianchini 2002), and the prevailing hypothesis is that this association can be explained by increases in the amount of bioavailable estrogens in the circulation and the endometrial tissue (Kaaks et al. 2002). After the menopause, when ovarian production of both estrogen and progesterone ceases, the major source of estrogen is via peripheral conversion, mostly within adipose tissue, of androgens that continue being produced by the adrenal glands and ovaries. In addition, weight-related increases in insulin inhibit the synthesis of SHBG (Plymate et al. 1988), a protein that tightly binds estradiol and testosterone. In consequence, increasing adiposity is associated with increasing blood concentrations of estrogen, and with increasing fractions of estradiol (and also testosterone) unbound to SHBG, that can freely diffuse to target tissues. These well-known relationships of adiposity with circulating levels of total and bioavailable estradiol were clearly seen in our data. Although the association between BMI and endometrial cancer risk in postmenopausal women was only moderately weakened after an adjustment for free estradiol, the corresponding χ2-value declined by half and since hormone levels in single blood samples are only moderately representative of true long-term hormone levels (Potischman et al. 1994, Falk et al. 1997, Rinaldi et al. 2001), adjustment by the true hormone level would be expected to reduce the relative risk estimates for BMI, and corresponding χ2, even more (Fletcher et al. 1976). Residual confounding by hormone levels could therefore explain most of the association between obesity and endometrial cancer risk. However, one cannot exclude the possibility that obesity may, at least partly, influence endometrial cancer risk via mechanisms that are unrelated to bioavailable estrogen levels (e.g. that are related to chronic hyperinsulinemia and reductions in endometrial tissue concentrations of IGF-binding protein-1 (Kaaks et al. 2002) or to low adiponectin levels (Cust et al. 2007) or inflammatory factors (Modugno et al. 2005)).
Our study results also indicate that increasing circulating levels of free testosterone and, to a lesser extent, total testosterone are also associated with increasing postmenopausal endometrial cancer risk. These results are in line with those from a previous prospective study, which showed an association of risk with total testosterone (Lukanova et al. 2004). Since in this previous study, risk was also inversely related to SHBG levels, it is likely that risk would also be positively related to free testosterone levels, although no direct estimates for free testosterone were provided. Taken together, these results suggest that free testosterone may be an important determinant of endometrial cancer risk in postmenopausal women. The magnitude of risk associated with free testosterone was similar to that of the estrogens and because the levels of sex hormones are positively correlated with each other, it is difficult to identify which single hormone is most strongly associated with endometrial cancer risk. However, unlike estrogens, testosterone does not seem to have a direct stimulatory effect on endometrial cell proliferation (Tuckerman et al. 2000). Analyses with mutual adjustments between the androgens (testosterone and free testosterone) and estrogens (estrone, estradiol) suggest that, in this age group, the association of endometrial cancer risk with free testosterone levels could be a result of peripheral conversion of these androgens into estradiol. In contrast to previous prospective (Lukanova et al. 2004) and case–control studies (Austin et al. 1991, Potischman et al. 1996), we found no evidence that elevated levels of androgens of adrenal origin, such as A-dione and DHEAS, were associated with risk.
Free testosterone levels used in the present analyses were calculated on the basis of mass action law equations, which have been previously validated within the range of postmenopausal hormone concentrations, by theoretical simulations and direct comparison with free testosterone measurements obtained by an equilibrium dialysis method (Rinaldi et al. 2002). Additional simulations in our laboratory have shown that these same calculation methods will also provide valid results for premenopausal women, provided that their blood levels of testosterone, estradiol, SHBG, and dihydrotestosterone are all within the normal (non-pathological) range. A direct validation of calculated versus directly measured values of free testosterone in premenopausal women was published by Vermeulen et al. (1999).
This prospective study is the first to examine the association between endogenous androgens and endometrial cancer risk among women who were premenopausal at blood donation. It has been hypothesized that in premenopausal women, whose circulating estradiol levels generally are above a threshold of about 50 pg/ml (184 pmol/l), endometrial cancer risk is not related to between-subject differences in circulating estradiol levels, but that low progesterone levels may be a more important determinant of risk (Key & Pike 1988). However, this is difficult to examine directly in large-scale epidemiologic studies because of the marked cyclical variation in estradiol and progesterone levels in premenopausal women. Nevertheless, this hypothesis is consistent with the epidemiologic observations of a higher endometrial cancer risk among women with polycystic ovarian syndrome, a condition characterized by obesity, ovarian hyperandrogenism, anovulation, and progesterone deficiency (Hardiman et al. 2003). The present study, however, showed no association between obesity or circulating androgen levels and endometrial cancer risk in women who were premenopausal at blood donation, and which is consistent with some, but not all previous data (all from traditional case–control studies; Tornberg & Carstensen 1994, Potischman et al. 1996, Furberg & Thune 2003). It has been suggested that the increased risk associated with obesity in young women is restricted to women with high levels of BMI (Kaaks et al. 2002), and it is possible that the low numbers of cases who were obese in this study limited the power to detect a significant association.
A limitation of our study was the lack of data about menopausal status at the time of endometrial cancer diagnosis. However, the average follow-up time was still relatively short (∼3 years), and 71% of endometrial cancer case patients had their tumors detected <5 years after blood donation. Thus, for most women who had gone through the menopause between the time of blood donation and endometrial cancer diagnosis, the time since menopause must have been short and it is unlikely that risk would have changed markedly during that time. Another potential limitation is that the absolute concentrations of estradiol in postmenopausal women in this study are relatively high (geometric mean of 89 pmol/l in control subjects), considering their true values are expected to be <20 pmol/l. However, the steroid hormone assays used in the present study have been validated and showed very high correlations (>0.90) with values obtained by an indirect immunoassay after organic extraction and chromatographic pre-purification (Rinaldi et al. 2001), suggesting that the relative ranking of women according to their estrogen level is not affected using these assays. Finally, serum levels of androgens and estrogens may be poor indicators of total androgenic and estrogenic activities as they do not reflect local conversion of adrenal precursors (such as DHEA and DHEAS) into more active sex steroids in target tissue (Labrie et al. 2003). The true relative risks associated with the elevated serum and tissue concentrations of estrogens and testosterone therefore may have been underestimated in our study.
In conclusion, this case–control study nested with a prospective cohort provides strong evidence that elevated levels of estrogens (estrone, estradiol, and free estradiol) and free testosterone are associated with increased endometrial cancer risk in postmenopausal women. By contrast, androgens of predominantly adrenal origin were not related to risk in postmenopausal women. The positive association between BMI and endometrial cancer risk in postmenopausal women is most probably explained by weight-related increases in estrogen levels, although alternative (or complementary) physiological mechanisms cannot be ruled out. In premenopausal women, serum sex hormone concentrations were not associated with endometrial cancer risk, although there were too few numbers to draw firm conclusions.
Acknowledgements
Grant sponsors were Europe Against Cancer Programme of the European Commission (SANCO); Ligue contre le Cancer (France); Société 3M (France); Mutuelle Générale de l'Education Nationale; Institut National de la Santé et de la Recherche Médicale (INSERM); German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health; ISCIII Red de Centros RCESP C03/09, Spain; the participating regional governments and institutions of Spain; Cancer Research UK; Medical Research Council, UK; the Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; the Wellcome Trust, UK; Greek Ministry of Health; Greek Ministry of Education; Italian Association for Research on Cancer; Italian National Research Council; Dutch Ministry of Public Health, Welfare and Sports; Dutch Ministry of Health; Dutch Prevention Funds; LK Research Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skane, Sweden; Norwegian Cancer Society; and University of Sydney, Cancer Institute NSW, Australia. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Austin H, Austin JM, Jr, Partridge EE, Hatch KD, Shingleton HM. Endometrial cancer, obesity, and body fat distribution. Cancer Research. 1991;51:568–572. [PubMed] [Google Scholar]
- Baanders-van Halewijn EA, Poortman J. A case–control study of endometrial cancer within a cohort. Maturitas. 1985;7:69–76. doi: 10.1016/0378-5122(85)90036-2. [DOI] [PubMed] [Google Scholar]
- Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–1551. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- Bergstrom A, Pisani P, Tenet V, Wolk A, Adami HO. Overweight as an avoidable cause of cancer in Europe. International Journal of Cancer. 2001;91:421–430. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1053>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Bray F, Loos AH, Oostindier M, Weiderpass E. Geographic and temporal variations in cancer of the corpus uteri: incidence and mortality in pre- and postmenopausal women in Europe. International Journal of Cancer. 2005;117:123–131. doi: 10.1002/ijc.21099. [DOI] [PubMed] [Google Scholar]
- Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- Falk RT, Dorgan JF, Kahle L, Potischman N, Longcope C. Assay reproducibility of hormone measurements in postmenopausal women. Cancer Epidemiology, Biomarkers and Prevention. 1997;6:429–432. [PubMed] [Google Scholar]
- Fletcher CM, Peto R, Tinker CM, Speizer FE. The Natural History Bronchitis and Emphysema. Oxford University Press; Oxford: 1976. [Google Scholar]
- Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. International Journal of Cancer. 2003;104:669–676. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of National Cancer Institute. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, Sacks FM, Stampfer MJ. Effect of transport conditions on the stability of biochemical markers in blood. Clinical Chemistry. 1989;35:2313–2316. [PubMed] [Google Scholar]
- Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–1812. doi: 10.1016/s0140-6736(03)13409-5. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Research. 1982;42:3232–3239. [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiology, Biomarkers and Prevention. 2002;11:1531–1543. [PubMed] [Google Scholar]
- Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L, Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) Journal of National Cancer Institute. 2005;97:755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- Key TJ, Pike MC. The dose–effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. British Journal of Cancer. 1988;57:205–212. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocrine Reviews. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Micheli A, Arslan A, Ferrari P, Rinaldi S, Krogh V, Lenner P, Shore RE, Biessy C, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. International Journal of Cancer. 2004;108:425–432. doi: 10.1002/ijc.11529. [DOI] [PubMed] [Google Scholar]
- Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiology, Biomarkers and Prevention. 2005;14:2840–2847. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Browne RW, Hill L, Bolelli GF, Abagnato C, Berrino F, Freudenheim J, Trevisan M, Muti P. Effects of transportation and delay in processing on the stability of nutritional and metabolic biomarkers. Nutrition and Cancer. 2000;37:155–160. doi: 10.1207/S15327914NC372_6. [DOI] [PubMed] [Google Scholar]
- Nyholm HC, Nielsen AL, Lyndrup J, Dreisler A, Hagen C, Haug E. Plasma oestrogens in postmenopausal women with endometrial cancer. British Journal of Obstetrics and Gynaecology. 1993;100:1115–1119. doi: 10.1111/j.1471-0528.1993.tb15176.x. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. IARC Press; Lyon, France: 2002. [Google Scholar]
- Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. Journal of Clinical Endocrinology and Metabolism. 1988;67:460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- Potischman N, Falk RT, Laiming VA, Siiteri PK, Hoover RN. Reproducibility of laboratory assays for steroid hormones and sex hormone-binding globulin. Cancer Research. 1994;54:5363–5367. [PubMed] [Google Scholar]
- Potischman N, Hoover RN, Brinton LA, Siiteri P, Dorgan JF, Swanson CA, Berman ML, Mortel R, Twiggs LB, Barrett RJ, et al. Case–control study of endogenous steroid hormones and endometrial cancer. Journal of National Cancer Institute. 1996;88:1127–1135. doi: 10.1093/jnci/88.16.1127. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;27:85–95. [Google Scholar]
- Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, et al. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutrition. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Dechaud H, Biessy C, Morin-Raverot V, Toniolo P, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Secreto G, Ciampi A, et al. Reliability and validity of commercially available, direct radioimmunoassays for measurement of blood androgens and estrogens in postmenopausal women. Cancer Epidemiology, Biomarkers and Prevention. 2001;10:757–765. [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiology, Biomarkers and Prevention. 2002;11:1065–1071. [PubMed] [Google Scholar]
- Rosner B. Ageneralization of the paired t-test. Applied Statistics. 1982;31:9–13. [Google Scholar]
- Tavassoli FA & Devilee P 2003 eds. Pathology and genetics – tumours of the breast and female genital organs. World Health Organization Classification of Tumours, pp 217–258. Lyon: IARC.
- The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. Combination oral contraceptive use and the risk of endometrial cancer. JAMA. 1987;257:796–800. [PubMed] [Google Scholar]
- Tornberg SA, Carstensen JM. Relationship between Quetelet's index and cancer of breast and female genital tract in 47 000 women followed for 25 years. British Journal of Cancer. 1994;69:358–361. doi: 10.1038/bjc.1994.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckerman EM, Okon MA, Li T, Laird SM. Do androgens have a direct effect on endometrial function? Fertility and Sterility. 2000;74:771–779. doi: 10.1016/s0015-0282(00)00711-1. [DOI] [PubMed] [Google Scholar]
- Vainio H, Bianchini F. Weight Control and Physical Activity. IARC Press; Lyon, France: 2002. [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. Journal of Clinical Endocrinology and Metabolism. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. New England Journal of Medicine. 1980;302:551–554. doi: 10.1056/NEJM198003063021004. [DOI] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Akhmedkhanov A, Kato I, Koenig KL, Shore RE, Kim MY, Levitz M, Mittal KR, Raju U, Banerjee S, et al. Postmenopausal endogenous oestrogens and risk of endometrial cancer: results of a prospective study. British Journal of Cancer. 2001;84:975–981. doi: 10.1054/bjoc.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]