Abstract
Asthma has been associated with atherosclerotic disease in several studies with some evidence that this association may be limited to women. However, most previous studies have failed to account for the heterogeneity of asthma subtypes. We previously reported increased carotid intima medial thickness among women with adult-onset asthma. In this study, we examine the association of adult and child-onset asthma with incident coronary heart disease (CHD) and stroke. Subjects were classified according to self-report of physician diagnosed asthma and age of asthma onset. We used Cox proportional hazards models to test the association of adult and child-onset asthma with incident CHD and stroke, testing for gender interaction. Subanalysis was also performed using only neversmokers. Women with adult-onset asthma experienced a 2-fold increase in incident CHD and stroke which was independent of other risk factors including smoking, body mass index, and physical activity and persisted when the analysis was restricted to never-smokers. No significant association was found among women with child-onset asthma or among men. In conclusion, adult-onset asthma may be a significant risk factor for CHD and stroke among women but not men.
Keywords: ASTHMA, CORONARY DISEASE, STROKE, SEX FACTORS
Asthma has been associated with vascular disease, carotid atherosclerosis, coronary heart disease (CHD), or stroke in at least nine studies.1–9 Among studies that present results stratified by gender, there is a suggestion that the association may be stronger among or entirely limited to women.1–4 However, asthma is not a single disease but rather a collection of distinct underlying subtypes, with somewhat differing etiologies.10,11 Child and adult-onset asthma differ in regards to asthma triggers11, gender distribution11, and systemic inflammation.12 We previously reported an association between carotid intima medial thickness and adult-onset asthma among women in the Atherosclerosis Risk in Communities (ARIC) study.4 This association was not observed among women with child-onset asthma or among men with either adult or child-onset asthma. In this study, we examined the association of asthma age of onset phenotypes with incidence of CHD and stroke according to gender within the ARIC cohort.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective study of the etiology of atherosclerotic, cardiovascular, and cerebrovascular disease in 4 U.S. communities in North Carolina, Mississippi, Minnesota, and Maryland.13 The study population of 15,792 men and women ages 45 to 64 years includes both black and white participants. Subjects completed a baseline clinic visit during 1987 to 1989 and were followed for incidence of CHD and stroke events. We used publicly available data with follow-up available through 2001 for 15,732 participants. We excluded subjects missing data for asthma status (n=28) or who reported ever having asthma but did not report physician diagnosis of asthma (n=131). We also excluded subjects with self reported history of stroke (n= 320) or prevalent CHD (n=692), defined as history of MI, silent MI, or revascularization surgery at baseline. This left 14,567 subjects for analysis.
Baseline assessment of asthma status and other covariates
Based on self report of physician diagnosed asthma and age of asthma onset, subjects were classified as having “adult-onset asthma” if age of onset was 21 years or above, or “child-onset asthma” if onset was before age 21. Smoking was measured by self-reported smoking status (former, current, or never). Classification of diabetes was based on at least one of the following: fasting plasma glucose greater than 126 mg/dL, non-fasting plasma glucose greater than 200 mg/dL, self reported diabetes, or taking diabetes medications. Low density lipoprotein (LDL) and high density lipoprotein (HDL) cholesterol were included in models as continuous variables. All laboratory tests were run at centralized chemical, hemostasis and lipid laboratories, and hematological tests were run at local laboratories. Hypertension was defined as a diastolic blood pressure greater than or equal to 90 mm Hg, a systolic blood pressure greater than 140 mm Hg, or self report of use of anti-hypertensive drugs.14 Physical activity was assessed according to the Baecke scale based upon frequency, duration, and intensity of physical activity.15 Education level was classified according to the number of years of school completed (<12 years, 12–16 years, or>16 years). Asthma medication use (beta adrenergic and oral glucocorticoids) was classified according to use during the 2 week period prior to the baseline clinic visit and was ascertained by having subjects bring all prescription and non-prescription medications used during this period to the clinic during their baseline visit. Forced expiratory volume in one second (FEV1) and forced expiratory vital capacity (FVC) were assessed using spirometry according to the ARIC study protocol. 16 This protocol includes strict quality control procedures to ensure recorded spirometry measurements are technically acceptable and reproducible.16 FEV1 was categorized for use in multivariable analysis according to gender-specific quartiles. Chronic bronchitis and emphysema were classified based upon self report of physician diagnosis.
Ascertainment of incident CHD and stroke events
For our primary analysis, incident CHD was defined as definite or probable myocardial infarction (MI) or fatal CHD. We also performed subanalyses in which incident CHD events included revascularization procedures and silent MI detected through electrocardiogram (ECG). Incident strokes included both ischemic and hemorrhagic strokes. Potential CHD and stroke events were identified in cohort members through annual follow-up, survey of area hospital discharge lists, and state vital statistics. Where discharge summaries indicated diagnosis codes for cardiovascular disease, diabetes, stroke, or included stroke related keywords, hospital records were abstracted by trained study personnel. Out-of-hospital deaths were investigated by means of death certificates, interview with one or more next of kin, and a physician questionnaire, coroner reports or autopsy reports. MI events were classified based upon chest pain, cardiac enzyme levels, and ECG results. Fatal CHD classification was based on chest pain symptoms, cause of death from the death certificate, and available hospital information and medical history, including ARIC clinic visits. For stroke events, records were reviewed in detail by a member of the ARIC study Stroke-Mortality and Morbidity Classification Committee and the patient was classified according to the type of stroke that occurred (ischemic or hemorrhagic). The outcome ascertainment process has been described in further detail by ARIC investigators.17
Analysis
Analysis was completed using SAS version 9. Baseline covariates were compared by asthma history among men and women separately using chi-square tests, Fisher exact tests, and pooled or unpooled t-tests. Missing covariate values, which occurred at <3% for any covariate, were imputed using multiple imputation methods.18 Crude incidence density rates of CHD and stroke were calculated among men and women for non-asthmatics, child-onset asthmatics, and adult-onset asthmatics. Crude and multivariate hazard ratios comparing each asthma subtype to non-asthmatics were computed using Cox proportional hazards models. Multivariate models were adjusted for age, body mass index (BMI), black race, smoking status, diabetes, hypertension, education level, low and high density lipoprotein levels, and leisure physical activity. We tested for interaction between asthma and gender in crude and multivariate models using Wald chi-square tests. In subanalyses, we used an expanded definition of CHD to include revascularization and silent MI.
We also performed additional analyses to investigate the impact of asthma medications, lung function, and the respiratory comorbidities chronic bronchitis and emphysema on the association of asthma with cardiovascular outcomes. To further examine the possible confounding effect of smoking and the possible misclassification of chronic obstructive pulmonary disease (COPD) as asthma on our results, we repeated the analysis in the subgroup of individuals who never smoked in the past. Finally, we also performed age-adjusted analysis among women free of diabetes, hypertension, emphysema, and chronic bronchitis. In all of these additional analyses we used a combined outcome of incident CHD or stroke to maximize the number of events in the model.
Results
The distribution of asthma age of onset among men and women is shown in figure 1. The prevalence of child-onset asthma was higher among men (3.3%) than women (2.5%), while adult-onset asthma was more common among women (3.4%) compared to men (2.0%). Compared to their non-asthmatic counterparts, men and women with adult-onset asthma were older, had a higher prevalence of diabetes and hypertension, more pack-years of smoking, higher fibrinogen levels, and a higher prevalence of beta adrenergic and glucocorticoid steroid asthma medication use at baseline (table 1). Women with adult-onset asthma also had significantly higher body mass index (BMI), lower physical activity, and were more often post-menopausal than women without a history of asthma. Compared to nonasthmatics, FEV1, % expected FEV1, and FEV1/FVC were lower among men and women with either asthma subtype but were lowest among those with adult-onset asthma. Likewise, chronic bronchitis, emphysema, and use of asthma medications were more prevalent among all asthmatics but were most prevalent among adult-onset asthmatics (table 1).
Figure 1.
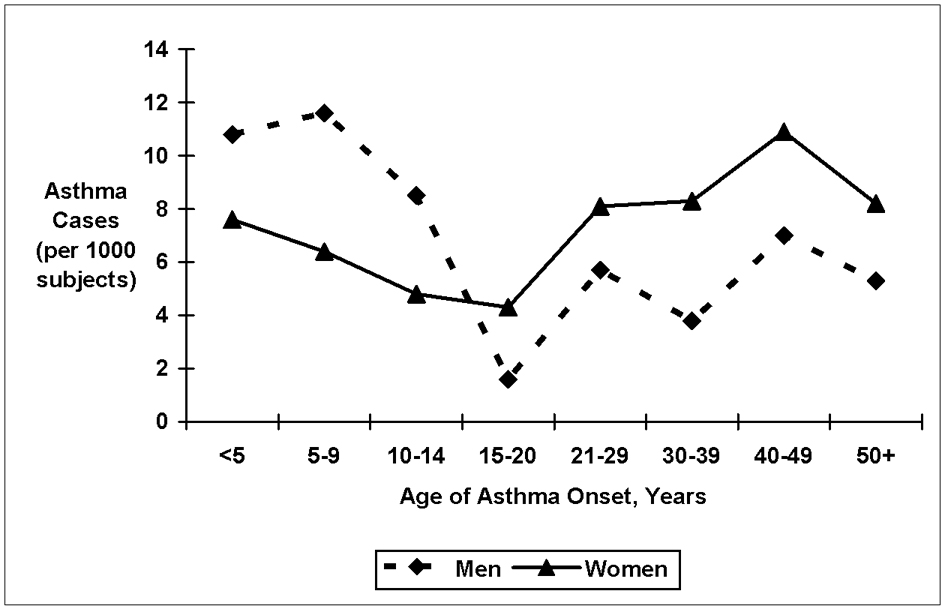
Self-reported asthma age of onset among men and women.
Table 1.
Baseline comparison of men and women according to self-reported asthma history
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variable | No Asthma | Child Onset* Asthma | Adult Onset* Asthma | No Asthma | Child Onset* Asthma | Adult Onset* Asthma |
| (n=5931) | (n=210) | (n=131) | (n=7809) | (n=203) | (n=283) | |
| Age (years) | 54.3 | 53.3† | 55.4† | 53.7 | 52.9† | 54.3 |
| Black Race | 23.3% | 19.1% | 15.3%† | 29.6% | 30.1% | 33.2% |
| High School Graduate | 77.5% | 82.3% | 65.7%† | 77.2% | 76.4% | 69.3%† |
| Body Mass Index (kg/m2) | 27.4 | 27.2 | 27.3 | 27.8 | 28.3 | 28.9† |
| Diabetes Mellitus | 10.5% | 10.6% | 14.5% | 10.8% | 17.2%† | 17.8%† |
| Hypertension | 32.4% | 29.2% | 37.4% | 34.1% | 33.0% | 43.6%† |
| Chronic Bronchitis | 4.0% | 15.1%† | 27.9%† | 9.2% | 39.6%† | 43.1%† |
| Emphysema | 1.9% | 4.3%† | 8.4%† | 0.9% | 3.9%† | 5.3%† |
| Current Smoker | 27.6% | 22.4% | 21.4% | 24.6% | 22.8% | 29.0% |
| Pack Years | 21.7 | 20.1 | 24.3 | 10.0 | 11.2 | 13.8† |
| Leisure Physical Activity Index | 2.34 | 2.35 | 2.35 | 2.38 | 2.33 | 2.29† |
| One Second Forced Expiratory Volume (liters) | 3.37 | 3.11† | 2.74† | 2.44 | 2.20† | 2.05† |
| One Second Forced Expiratory Volume (% predicted) | 91.4% | 83.2%† | 74.6%† | 97.1% | 86. 5%† | 83.3%† |
| One Second Forced Expiratory Volume / Forced Expiratory Vital Capacity | 73.5 | 68.3† | 63.5† | 75.9 | 71.1† | 70.3† |
| Low Density Lipoprotein Cholesterol (mg/dL) | 139 | 135 | 139 | 136 | 133 | 134 |
| High Density Lipoprotein Cholesterol (mg/dL) | 45 | 45 | 47† | 58 | 58 | 58 |
| Albumin (mg/dL) | 3.92 | 3.96 | 3.91 | 3.83 | 3.78† | 3.81 |
| Fibrinogen (mg/dL) | 295 | 294 | 306 | 307 | 315 | 316† |
| Current Hormone Replacement Therapy | - | - | - | 19.2% | 22.6% | 15.7% |
| Post-Menopausal or Hysterectomy | - | - | - | 67.1% | 58.9%† | 75.9%† |
| Beta Adrenergic Asthma Medication Use | 0.6% | 6.2%† | 29.0%† | 0.4% | 13.3%† | 22.6%† |
| Oral Glucocorticoid Asthma Medication Use | 0.5% | 2.9%† | 12.2%† | 1.0% | 3.5%† | 9.2%† |
Child onset = age < 21 years; adult onset = age ≥ 21 years;
p<0.05 comparing subjects within asthma subtype to subjects reporting no history of asthma within each gender
Women, but not men, with adult-onset asthma experienced a 2-fold increase in rate of CHD compared to their non-asthmatic counterparts (table 2). This association was attenuated but remained significant after adjustment for age, BMI, black race, smoking status, diabetes, hypertension, education level, low and high density lipoprotein cholesterol levels, and physical activity (table 2). Child-onset asthma was not significantly associated with incident CHD among women or men. Tests of interaction between gender and adult-onset asthma were significant (p<0.05) in all CHD models, while interaction tests of child-onset asthma with gender were not. Results were similar in subanalyses where incident CHD also included revascularization procedures and silent MI, with an adjusted hazard ratio (HR) of 1.86 (95%Confidence Interval [CI]: 1.31 to 2.63) for women with adult-onset asthma and nonsignificant associations observed among all other asthma-gender subgroups.
Table 2.
Incident coronary heart disease rates and hazard ratios among men and women according to asthma history
| Males | Females | |||||
|---|---|---|---|---|---|---|
| No History of Asthma | Childhood Onset Asthma | Adult-Onset Asthma | No History of Asthma | Childhood Onset Asthma | Adult-Onset Asthma | |
| Crude Rate* | 7.85 | 8.52 | 6.22 | 3.52 | 3.49 | 7.34 |
| (Cases / Person-Years) | (565 / 72006) | (22 / 2582) | (10 / 1607) | (348 / 98963) | (9 / 2580) | (25 / 3407) |
| Crude Hazard | 1.0 (Ref) | 1.08 | 0.80 | 1.0 (Ref) | 0.99 | 2.10 |
| Ratio (95% Confidence Interval) | (0.71, 1.66) | (0.43, 1.49) | (0.51, 1.93) | (1.40, 3.16) | ||
| Multivariate | 1.0 (Ref) | 1.25 | 0.71 | 1.0 (Ref) | 0.95 | 1.78 |
| Adjusted† | (0.82, 1.92) | (0.38, 1.32) | (0.49, 1.83) | (1.18, 2.67) | ||
| Hazard Ratio (95% Confidence Interval) | ||||||
Per 1000 person years
Adjusted for age, body mass index, black race, diabetes mellitus, hypertension, education level, low and high density lipoprotein cholesterol levels, and physical activity
Similar to the results for CHD, adult-onset asthma was associated with incident stroke among women but not men with a significant gender interaction (p<0.05) (table 3). The association of adult-onset asthma with stroke in women remained significant after adjustment for demographic variables and established CHD risk factors (table 3). The small numbers of stroke events precluded multivariate analysis among men. Child-onset asthma was not significantly associated with incident stroke among men or women and the interaction of child-onset asthma and gender was nonsignificant (table 3).
Table 3.
Incident stroke rates and hazard ratios among men and women according to asthma history
| Males | Females | |||||
|---|---|---|---|---|---|---|
| No History of Asthma | Childhood Onset Asthma | Adult-Onset Asthma | No History of Asthma | Childhood Onset Asthma | Adult-Onset Asthma | |
| Crude Rate* | 3.50 | 2.27 | 1.22 | 2.39 | 3.51 | 5.57 |
| (Cases / Person-Years) | (257 / 73489) | (6 / 2639) | (2 / 1634) | (238 / 99443) | (9 / 2565) | (19 / 3411) |
| Crude Hazard | 1.0 (Ref) | 0.65 | 0.35 | 1.0 (Ref) | 1.47 | 2.36 |
| Ratio (95% Confidence Interval) | (0.29, 1.45) | (0.09, 1.41) | (0.76, 2.87) | (1.48, 3.76) | ||
| Multivariate | 1.0 (Ref) | - | - | 1.0 (Ref) | 1.25 | 2.08 |
| Adjusted† | (0.64, 2.44) | (1.30, 3.32) | ||||
| Hazard Ratio (95% Confidence Interval) | ||||||
Per 1000 person years
Adjusted for age, body mass index, black race, diabetes mellitus, hypertension, education level, low and high density lipoprotein cholesterol levels, and physical activity
Because of the similarity of results for the CHD and the stroke outcomes, we performed additional analyses using a combined endpoint of incident cardiovascular disease, including CHD or stroke (figure 2). In the fully adjusted model including covariates for asthma medications, FEV1, chronic bronchitis, and emphysema, adult-onset asthma was significantly associated with this combined outcome among women (HR = 1.68, 95% CI: 1.21 to 2.35). Results remained robust in analyses restricted to never-smokers, which again confirmed a significant association of adult-onset asthma among women but not among men or among women or men with child-onset asthma (table 4). Finally, in age-adjusted analysis among women free of diabetes, hypertension, emphysema, and chronic bronchitis, adult-onset asthma was strongly associated with incidence of CHD or stroke (HR = 3.93, 95% CI: 2.01 to 7.02) while child-onset asthma was not significantly associated (HR = 1.80, 95% CI: 0.67 to 4.87).
Figure 2.
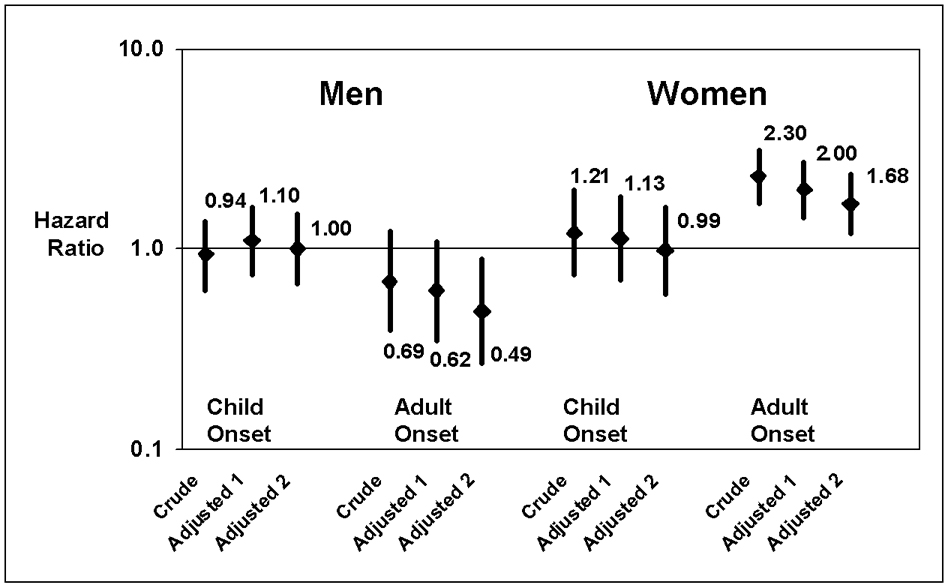
Hazard ratios for incident coronary heart disease or stroke according to asthma age of onset and gender. Adjusted 1 model includes age, body mass index, black race, smoking status, diabetes mellitus, hypertension, education level, LDL and HDL cholesterol, and physical activity; Adjusted 2 model includes model 1 covariates plus forced expiratory volume in 1 second, chronic bronchitis, emphysema, and use of glucocorticoid or beta adrenergic medicines.
Table 4.
Never-smokers only - Incident combined coronary heart disease or stroke event rates and hazard ratios among men and women according to asthma history
| Males | Females | |||||
|---|---|---|---|---|---|---|
| No History of Asthma | Childhood Onset Asthma | Adult-Onset Asthma | No History of Asthma | Childhood Onset Asthma | Adult-Onset Asthma | |
| Crude Rate* | 8.31 | 5.49 | 10.69 | 4.75 | 5.40 | 10.59 |
| (Cases / Person-Years) | (181 / 21757) | (5 / 910) | (5 / 468) | (251 / 52868) | (7 / 1296) | (18 / 1699) |
| Crude Hazard | 1.0 (Ref) | 0.65 | 1.31 | 1.0 (Ref) | 1.14 | 2.24 |
| Ratio (95% Confidence Interval) | (0.27, 1.58) | (0.54, 3.18) | (0.51, 2.43) | (1.39, 3.62) | ||
| Multivariate | 1.0 (Ref) | 0.74 | 1.04 | 1.0 (Ref) | 1.10 | 2.05 |
| Adjusted† | (0.30, 1.80) | (0.43, 2.53) | (0.52, 2.33) | (1.28, 3.31) | ||
| Hazard Ratio (95% Confidence Interval) | ||||||
Per 1000 person years
Adjusted for age, body mass index, black race, diabetes mellitus, hypertension, education level, low and high density lipoprotein cholesterol levels, and physical activity
Discussion
In this large, community-based follow-up study, women with adult-onset asthma experienced a nearly 2-fold increase in the rate of CHD and stroke, which was independent of other risk factors including smoking, BMI, and physical activity and persisted when the analysis was restricted to never-smokers. This result is consistent with our previous finding that women, but not men, with adult-onset asthma have increased carotid intima medial thickness compared to their nonasthmatic counterparts 4 and with other reports suggesting a role of asthma in atherosclerotic disease among women but not men.1–3
This is the first study to test the association of asthma age of onset subtypes with cardiovascular outcomes. It is recognized that “asthma” is not a uniform disease, but rather a constellation of distinct conditions.10,11,19 Adult-onset asthma differs from childonset asthma in several aspects, including its distribution among men and women11, and its immunological and inflammatory pathophysiology.10,19 Nevertheless, previous studies of asthma and atherosclerotic outcomes have generally ignored asthma subtypes. Three previous studies have presented gender specific results for the association between asthma and CHD. Toren et al. reported an age-adjusted standardized mortality ratio for ischemic heart disease of 1.4 (95%CI: 0.8 to 2.0) among asthmatic men and 2.5 (95%CI: 1.7 to 3.3) among asthmatic women.1 In a retrospective cohort study of a large insurance cohort, Iribarren et al. reported multivariate adjusted hazard ratios of 1.22 (95% CI: 1.14 to 1.31) among asthmatic women and 0.99 (95% CI: 0.93 to 1.05) among asthmatic men.2 Similarly, an earlier report from the ARIC study found an elevated risk of stroke in asthmatic women, but not men, compared to non-asthmatic subjects, although no association was found with CHD outcomes in either women or men.3 None of these previous reports distinguish among asthma subtypes. Thus, the lack of association of asthma with CHD reported in the previous ARIC study probably reflects a mixing of the heterogeneous effects of adult and child-onset asthma subtypes. 3 By separating asthma subtypes, we have uncovered an important risk associated with adult-onset asthma among women.
The precise mechanisms underlying the association between adult-onset asthma and atherosclerotic vascular disease in women are unclear. Asthma may predispose to atherosclerosis through specific pathophysiologic pathways perhaps linked to the chronic inflammatory response of this disorder. Alternatively, the association between asthma and atherosclerosis may be due to an inherent joint susceptibility to both diseases through shared inflammatory pathways. For example, cysteinyl leukotrienes, potent inflammatory mediators, are implicated in the pathogenesis of both asthma20 and atherosclerosis21. Why is the association between asthma and cardiovascular disease only observed in women with adult-onset asthma? Estrogen levels, which increase at puberty, modulate the release of proinflammatory cytokines from activated monocytes, macrophages22, and vascular cells23,24 and also regulates the production of leukotrienes from mast cells.25 The incidence rate of asthma among women is temporally associated with shifts in estrogen levels, with incidence increasing after puberty26 and peaking during the onset of menopause 27 (figure 1). Thus, we may speculate that the women who develop asthma during these hormonal life events may be particularly susceptible to estrogen-modulated alterations in inflammatory cytokine and leukotriene regulation.
Our study has several strengths. Foremost, the ARIC cohort is large, multiracial and prospective, and includes rich and high quality subject data. We were able to control for potentially important confounding variables such as smoking, physical activity, and asthma medication use and we further addressed confounding by smoking and other confounders by performing sub-analyses restricted to never smoking subjects and subjects free of diabetes, hypertension, emphysema, and chronic bronchitis. The major weakness of our study is the fact that asthma status was based upon self-report of physician diagnosis. Although there is some evidence to suggest that self-reported asthma yields high specificity28, there is also literature to suggest that misdiagnosis of COPD as asthma occurs frequently, and more so among women29. Because approximately 85% of COPD cases have a history of smoking, the persistence of the association of adult-onset asthma with CHD among never smoking women suggests that the observed association is not likely to be due to misdiagnosed COPD cases. Nonetheless, there remains the need for further research in which asthma classification is objectively determined through established clinical guidelines. Furthermore, we did not have information on IgE levels, asthma triggers, or presence of allergies to differentiate asthma according to allergic status. Other limitations include small numbers of events, particularly stroke, among men with adult-onset asthma and lack of data for inflammatory markers such as C - reactive protein. Finally, because our study is observational we cannot exclude the influence of unmeasured confounding factors or residual confounding because asthma is associated with many known cardiovascular risk factors.
Acknowledgments
The authors wish to thank the ARIC researchers for their outstanding work and contributions to the study of cardiovascular disease. Special thanks to Mr. Sean Coady for his help in data acquisition.
The “Atherosclerosis Risk in Communities (ARIC)” study is conducted and supported by the NHLBI in collaboration with the ARIC Study Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the ARIC Study group or the NHLBI.
Primary work for this study was performed while Stephen Onufrak was a doctoral student at Emory University.
Funding: This study was supported by grants from the National Institutes of Health K24 HL077506. Dr. Onufrak received funding through a predoctoral fellowship from the American Heart Association (Award Number: 0615219B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References
- 1.Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol. 1996;25:617–620. doi: 10.1093/ije/25.3.617. [DOI] [PubMed] [Google Scholar]
- 2.Iribarren C, Tolstykh IV, Eisner MD. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33:743–748. doi: 10.1093/ije/dyh081. [DOI] [PubMed] [Google Scholar]
- 3.Schanen JG, Iribarren C, Shahar E, Punjabi NM, Rich SS, Sorlie PD, Folsom AR. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60:633–638. doi: 10.1136/thx.2004.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onufrak S, Abramson J, Vaccarino V. Adult-onset asthma is associated with increased carotid atherosclerosis among women in the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis. 2007;195:129–137. doi: 10.1016/j.atherosclerosis.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zureik M, Kony S, Neukirch C, Courbon D, Leynaert B, Vervloet D, Ducimetiere P, Neukirch F. Bronchial hyperresponsiveness to methacholine is associated with increased common carotid intima-media thickness in men. Arterioscler Thromb Vasc Biol. 2004;24:1098–1103. doi: 10.1161/01.ATV.0000128128.65312.05. [DOI] [PubMed] [Google Scholar]
- 6.Liss GM, Tarlo SM, Banks D, Yeung KS, Schweigert M. Preliminary report of mortality among workers compensated for work-related asthma. Am J Ind Med. 1999;35:465–471. doi: 10.1002/(sici)1097-0274(199905)35:5<465::aid-ajim3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Liss GM, Tarlo SM, Macfarlane Y, Yeung KS. Hospitalization among workers compensated for occupational asthma. Am J Respir Crit Care Med. 2000;162:112–118. doi: 10.1164/ajrccm.162.1.9906108. [DOI] [PubMed] [Google Scholar]
- 8.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128:2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 9.Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY studies. Arch Intern Med. 2005;165:2521–2526. doi: 10.1001/archinte.165.21.2521. [DOI] [PubMed] [Google Scholar]
- 10.Bel EH. Clinical phenotypes of asthma. Curr Opin Pulm Med. 2004;10:44–50. doi: 10.1097/00063198-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 12.Olafsdottir IS, Gislason T, Thjodleifsson B, Olafsson I, Gislason D, Jogi R, Janson C. Creactive protein levels are increased in non-allergic but not allergic asthma: a multicentre epidemiological study. Thorax. 2005;60:451–454. doi: 10.1136/thx.2004.035774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 15.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 16.The ARIC Investigators. Chapel Hill, NC: National Heart Lung and Blood Institute, National Institutes of Health; ARIC Protocol 4. Pulmonary Function Assessment. Version 7. 1987
- 17.The ARIC Investigators. Atherosclerosis Risk in Communities (ARIC) Study surveillance component procedures protocol 3, version 4. Chapel Hill, NC: Collabrative Studies Coordinating Center, University of North Carolina; 1987. [Google Scholar]
- 18.Barnard J, Meng XL. Applications of multiple imputation in medical studies: from AIDS to NHANES. Stat Methods Med Res. 1999;8:17–36. doi: 10.1177/096228029900800103. [DOI] [PubMed] [Google Scholar]
- 19.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy. 2001;56 Suppl 66:7–11. doi: 10.1034/j.1398-9995.56.s66.2.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med. 2004;14:191–195. doi: 10.1016/j.tcm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis Rheum. 2004;50:1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- 23.Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF-{alpha}-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am J Physiol Heart Circ Physiol. 2007;292:H2607–H2612. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 24.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 25.Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T. Estradiol activates mast cells via a nongenomic estrogen receptor-alpha and calcium influx. Mol Immunol. 2007;44:1977–1985. doi: 10.1016/j.molimm.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Marco R, Locatelli F, Sunyer J, Burney P. Differences in incidence of reported asthma related to age in men and women. A retrospective analysis of the data of the European Respiratory Health Survey. Am J Respir Crit Care Med. 2000;162:68–74. doi: 10.1164/ajrccm.162.1.9907008. [DOI] [PubMed] [Google Scholar]
- 27.Balzano G, Fuschillo S, Melillo G, Bonini S. Asthma and sex hormones. Allergy. 2001;56:13–20. doi: 10.1034/j.1398-9995.2001.00128.x. [DOI] [PubMed] [Google Scholar]
- 28.Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. 1993;104:600–608. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 29.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest. 2001;119:1691–1695. doi: 10.1378/chest.119.6.1691. [DOI] [PubMed] [Google Scholar]


