Abstract
Previous treatments of biological evolution have concentrated upon either the general appearance or habits of organisms or the sequences of molecules, such as their proteins and DNA (RNA), within species. There is no consideration of the changing relationship of the chemistry of organisms to the elements and energy available from the environment. In essence, organisms at all times had to accumulate certain elements while rejecting others. Central to accumulation were C, N, H, P, S, K, Mg and Fe while, as ions, Na, Cl, Ca and other heavy metals were largely rejected. In order to form the vital biopolymers, C and H, from CO2 and H2O, had to be combined generating oxygen. The oxygen then slowly oxidized the environment over long periods of time. These environmental changes were relatively rapid, unconstrained and continuous, and they imposed a necessary sequential adaptation by organisms while increasing the use of energy. Then, evolution has a chemical direction in a combined organism/environment ecosystem. Joint organization of the initial reductive chemistry of cells and the later need to handle oxidative chemistry has also forced the complexity of chemistry of organism in compartments. The complexity increased to take full advantage of the environment from bacteria to humans in a logical, physical, compartmental and chemical sequence of the whole system. In one sense, rejected material can be looked upon as waste and, in the context of this article, leads to the consideration of the importance of waste from the activities of humankind.
Keywords: systems: chemical, evolution: chemical, chemistry of evolution, organisms: evolution, biological chemistry, compartments of cells
1. Introduction
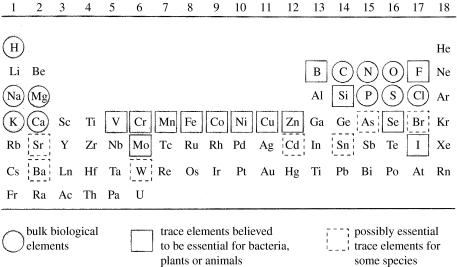
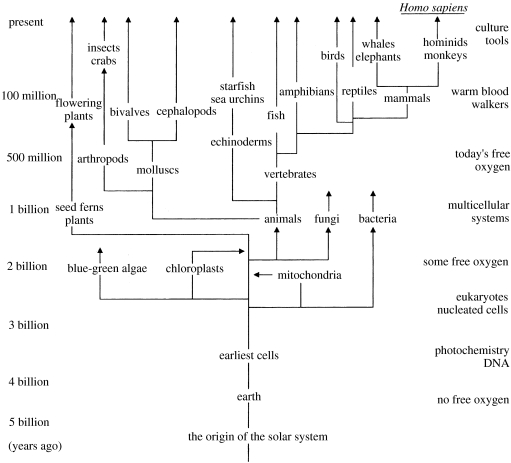
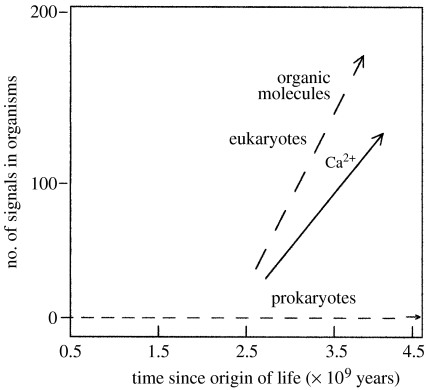
Evolution of our whole system from the gross level of the Universe to very local happenings has to be seen as a consequence of either initial local inevitable and generalizable events traceable in a time sequence from the Big Bang or local ‘accidents’, chance one-off events, which occur at a given time and in a given place and which are not open to a logical treatment. There are three major events in the evolution of the Universe, which have this ‘one-off’ nature. The first is the Big Bang itself for which we can offer only a very speculative ‘explanation’. The second is the formation of Earth with the Sun's other planets. Here, we can give a possible physical explanation of an accident 4.5 Gyr ago, but we do not know the chance of a very closely similar event. Exploration may be possible here, but any definite evidence about another Earth/Sun creation in the Universe has not yet been found and it is not likely to be easily obtained. The third case is the origin of life, critically dependent on the Earth/Sun relationship. We have no evidence except a probable time, 3.5–4.0 Gyr ago, for this happening and there is no parallel system. The nature of these three singular events is certainly outside the scope of this article for the further very good reason that I have no ability to say anything useful about them. However, a logical analysis has been given for the evolution over all time of the first two, the material and energy of the Universe and those of Earth. Physical/chemical studies have given rise to collections of observable materials and observations of changes in them and then to rational explanation. The way forward is usually termed reductive analysis, a seeking for the basic ingredients of today's material and its energies from given origins in the Universe and on Earth. This has led to concepts of variables, such as pressure and temperature, and a description of them in terms of the properties of basic materials in phases. The materials have been broken down into particles, atoms in the Periodic Table in chemical studies (e.g. figure 1), together with the description of the energy of their interactions in compounds and their movements at all levels. Meanwhile, energy has been analysed in a somewhat different ‘particulate’ analysis of quanta with a duality of mass and wave characteristics. By contrast, the evolution of living objects has frequently been treated as if there is some principle operative, which is outside the usual run of physics and chemistry. There is a clash with religions and beliefs outside experimental tests, and also even the scientific approaches of biologists' analyses are not on the same basis as those used in the study of the Universe and Earth. Organisms are said to have evolved as species by chance improvement of survival, and the possibility of a logical description of their evolution, even in large classes based on chemistry and physics, has not been examined. Species are described as having arisen in a set of material bodies either with shapes and behaviours (classical biology) or with these and other characteristics related to molecule sequences (molecular biology). The observed similarities in these properties have allowed the development of evolutionary trees of seemingly independent organisms (figure 2). The trees can be made extremely detailed beyond the level of classes to that of individual species of organisms. The changes of the environment of organisms in the branches of trees are not considered when they are described. The whole is then a driven branching growth for the success of particular organisms in a struggle for survival. These views put forward by Darwin have been reaffirmed recently in a book by Maynard-Smith & Szathmàry (1995) and in the proceedings of a recent Royal Society Discussion (Cavalier-Smith et al. 2006). Maynard-Smith & Szathmàry conclude, in agreement with many biologists, that although organisms increase in complexity, this is neither ‘universal nor inevitable’. Although features of neo-Darwinism have some element of environmental determination, there is no physical/chemical analysis following the style of those of the nature of today's Universe and Earth. Here, as stated, the examination and experimentation reach back to, in fact beyond, the level of atoms in materials and their energy. As all biological materials are made from atoms in and energy from the environment, the surface of Earth and the Sun, it seems appropriate here to go back to the same features and their known changes in time to see if we can work forward in the manner of a physical/chemical examination in a search for a rational physical/chemical explanation of the evolution of living systems (Morowitz 1992, 2002; de Duve 2002). We start from an analysis of the energy inputs to the environment and organisms treating them as one system.
Figure 1.
The Periodic Table showing the elements required by all life. Note how almost the whole possible variety of chemistry is covered, in that there are representative elements from 15 of the 17 chemically active groups in cells.
Figure 2.
A standard evolutionary tree based on the characteristics of organisms or their DNA which appears to indicate an independence of different branches of life.
2. Energy from the environment
The energy driving evolution on Earth, both of geological and biological objects, is in two parts. (i) Energy moving from the high-temperature interior to the surface at a lower temperature of 300 K. This flow itself is only sufficient to maintain a surface temperature of approximately 250 K. It has been able to do so for all the period of Earth's existence, since the mainly oxidic rocks gave Earth a hard heat-insulating mantle with water covering it around 4.0 Gyr ago. The deep interior of Earth, temperature greater than 5000 K due in part to radioactive decay of thorium, uranium and potassium, maintains this heat flow, but there are also energy transfers from the decomposition of unstable minerals on or near the surface, especially in ocean vents, for example black smokers. This surface heating has been augmented by (ii) energy from the Sun which is absorbed at the solid/liquid surface of Earth and in the atmosphere. Together, the two raise the surface temperature from 250 K, mentioned above, to the average ambient temperature of approximately 300 K. The loss of heat from Earth has fallen somewhat over time due to loss of some of the greenhouse gases, while the Sun has gained in radiative strength with the result that the surface temperature of Earth has remained roughly constant for all the period from ca 4.0 Gyr ago to date with fluctuations of some ±25 K in certain periods of millions of years. We shall assume therefore that material evolution has taken place effectively in a thermostat at 300 K (Cavalier-Smith et al. 2006). The thermostat kept water as a liquid on the surface, which permits much of all the material flow.
The chemicals mentioned so far are minerals, including water, so-called inorganic compounds, but the effect of the energy flow has also allowed the formation and loss of unstable organic chemicals in synthesis/degradative cycles in cells.
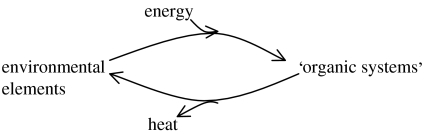
The organic systems are not just based on H, C, N, O, S and P, but, as we shall see, they contain a considerable number of mineral elements. The energy trapping in such a system from high-energy chemicals or the Sun can create unstable ‘organic’ chemicals either of high-energy content, for example in the reaction
or in the form of physical gradients of concentration of charge or particles, inorganic or organic, across boundaries. It is important to remember that only certain elements can be energized chemically for considerable periods of time as their chemical bonds have sufficient kinetic stability and so can give rise to particular organic compounds of long life. We shall categorize these compounds and also analyse the way in which the energized materials can be trapped physically in isolated volumes of various sizes, which could also have a considerable lifetime. The total system has increased in its ability to absorb energy, as the environment/organism system on Earth's surface has evolved.
In summary, evolution is driven by energy flow and degradation as numbers of high-energy quanta (light for example) are degraded to larger numbers of low-energy quanta, heat (entropy gain), while environmental material goes in the above cycle through life and largely returns to the environment (but see waste below). The material cycle increases the rate of energy degradation. We expect the system as a whole to increase its energy absorption, so that energy and material flux increases as the system increases in complexity. In effect, if there had been no material loss, once the cycle was in steady-state flow, the only change would have been entropy production and there would have been no evolution. We illustrate the general principles with simple non-organic systems before we consider the important case of systems which cycle in part but create waste.
3. Basic material flow, physical and chemical, on Earth
Energy flow is inevitably from a hot source to a cold sink, but it can go through intermediate stages in which material that has absorbed energy is physically, in two ways, or chemically transformed. The pattern of physical transformations arises from increase in random motion (temperature) and/or increase in directed flow (momentum). The first appears as a change in equilibrated or steady-state structures, solid→liquid→gas, associated with increased random kinetic energy, while the second appears as directed kinetic energy in steady-state organized patterns of flow. The second is related to the creation of gradients. The two are readily distinguished. On Earth, there are phases of ice and liquid water in containers of fixed structures and water vapour with apparently unimpeded random thermal motion in the atmosphere. Both the first two phases have ordered physical boundaries, and solids also have ordered internal structure, giving rise to static shapes. A very different picture of water in the atmosphere is observed in clouds, which are examples of material in organized flow. The clouds come in many closely, not exactly, reproducible dynamic shapes, so that just as crystals are given symmetry titles, so clouds are given names based now on their ill-defined shapes. Cumulus clouds are formed from low-down turbulent flow of water droplets falling under gravity but dispersing again at higher temperature as vapour that rises to reform droplets again at lower temperatures. Droplets and vapour in high stratus clouds circulate in streaming motion which is not turbulent. Therefore, clouds are classified descriptively in a collection of types, but it is hardly possible to classify the huge numbers of kinds within types, i.e. species or individuals, except by drawing them. We shall see how, when we replace the energization flow process of physical condensation and vaporization by chemical flows in which hundreds of chemicals take part in cyclic energized transformations from the environment to cells and back, it is equally possible to group large numbers of species and individuals into types. Here, species and individuals within types are characterized by small differences in shapes, behaviour or certain chemical molecular sequences, where there may be no clear reason for their existence beyond survival strength. We shall then show that there are ways of rationalizing the physical/chemical characteristics of these types, large groups, of organisms, such as those of prokaryotes (bacteria) and eukaryotes (protocysts, fungi), plants and animals, as they evolved. The environment here will be both that experienced in the formation of clouds, i.e. physical fields and radiant energy, and the chemicals in it. If, at any time, such a physical/chemical system reached a complete steady state, no further evolution could arise.
The circulation of water can be described further as clouds fall as rain, forming streams and rivers in pathways on land. These shapes are much more persistent than the shapes of clouds but are not permanent. River flow is contained by short-range forces, due to the solid minerals of riverbanks, while flow in a cloud is only contained by long-range fields. Now, as a river flows, it gradually erodes the minerals off its banks and causes them to flow towards the sea whence the water came originally before being energized into clouds. The eroded material settles as the river flow slackens forming new shapes of riverbanks and deltas by the sea. This is a non-cycling waste product of flow. This finely divided deposited material is referred to as sediment or soil, and its evolution with the continuous water flows has allowed much organic life on land to evolve. The material flow, apart from the water, is not cyclic and it evolves. Note how geo- and biosciences are intimately connected in an ecosystem and there are very different time-scales of change in both.
We could go on to describe the evolution of Earth's surface on a still slower scale, where land masses, in different sized units surrounded by the sea, change in novel ways due to ‘organized’ flow of molten minerals beneath the surface. We shall not do so, but only observe that the nature of ‘organization’, guided flow and its development can have quite different time constants and ill-defined sizes and shapes. Clouds, rivers and land show that organisms are far from the only systems with the characteristics of developing organized flow.
The simplest chemical system, illustrating organization and order, is the formation of the ozone layer due to the action of sunlight on oxygen, which generates different ozone depths in different latitudes, so that its layered depth has shape, organization, while O3 is more ordered than O2. The ozone layer has evolved in the last 1 Gyr and it is vital for life on land. It would have remained in a steady state, but humans have introduced chemicals interfering with the flow. There are yet other ‘inorganic’ chemical systems giving such organization on a small scale.
An important conclusion is that these systems are in general, but not in particular, cases predictable and they follow physical/chemical principles (Corning 1995, 2001; de Duve 2002; Morowitz 2002). The general behaviour is not random but gives rise to a steady state of flow, which can die but then recur, as the environment fluctuates. This steady state is one of the maximum flow and maximum energy degradation. We shall take this as the final outcome of any energized flow noticing how different it is from an equilibrium state and that it may take a very long time to reach the final steady-state condition (Corning 1995, 2001; Morowitz 2002). These examples show that characteristics such as shape can readily arise in steady states due to field boundary conditions affecting the flow. We now approach the environment/organism ecosystem looking for similar physical/chemical characteristics linked to organic chemical flow. The essence of life is organic—a mixture of energized flows of inorganic and organic atoms and molecules. It is an energized organized chemical system, with molecular physical boundaries, cells, and is very complicated. (Note that a virus is excluded from living systems as it is an ordered assembly without deployment of energy and without flow.)
As an aside, some authors describe the appearance of organization with internally ordered units such as molecules as emergent systems. Order itself can arise through an energetically favourable process but organized flow cannot. In the process of energy absorption before its inevitable degradation, an unstable system with chemical and physical properties can emerge but it can degrade with time if new features are introduced.
4. Organic systems
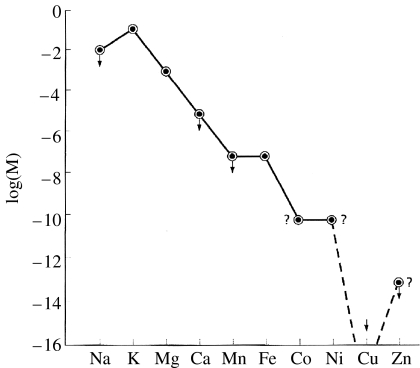
We are not yet ready to refer to living organisms which are reproductive rather than recurrent, for we have to see the origin of life in principle before it became reproductive (de Duve 2002; Russell & Hall 2006). There has to be some primitive organization before reproductive life, since it requires a code that can only ‘code’ some pre-existing system. So what material was available to make an energized beginning? This question takes us back to Earth's formation from a star and then farther back to the earliest stars and then to the Big Bang. Running forward quickly, at an intermediate stage some time after the Big Bang, the giant stars formed and then the chemical elements arose, 92 of them, which fall in the Periodic Table of atoms (figure 1). They were formed in a predictable pattern of abundances (Fraústo da Silva & Williams 2001). It was their dispersal at high temperature that later, due to cooling with condensation, gave rise to the planetary system including Earth around the Sun. As we have explained, the energy flow, of interest, is to the surface of this planet and we shall consider Earth's surface as including an irradiated sea with an atmosphere containing certain elements in particular concentrations and very basic inorganic combinations (figure 3). Therefore, we need to consider only the elements on the surface which through abundance and chemistry were made available and so were irradiated. These were mainly: (i) in the atmosphere, and somewhat dissolved in the sea, C (CO, CO2, CH4), H (H2, H2O, H2S), N (NH3, N2), S (H2S) and (ii) in minerals, which are soluble to some very different degrees (figure 4), giving the following ions also in the sea: Na+; K+; Mg2+; Ca2+; Mn2+; Fe2+; Co2+; Ni2+; Cu+; Zn2+; Mo or W ( or ); Se (H2Se); P ; V(VO2+); and Cl−. These elements, with perhaps one or two others, are the only ones of interest in the possible initial organic systems. Of the almost 20 elements listed, there are representatives of some 14 of the possible 17 groups of elements with strong chemical differences in the Periodic Table (figure 1). Hence, they can generate very much of the possibilities of chemistry. It is these 20 elements which we shall show are found to be essential to life and evolution and much of the ecosystem. We call the system in an organization, organic. A feature of the availabilities in the environment is that they have not been constant over time in either concentrations or chemical forms (Williams & Fraústo da Silva 2006). This evolution has occurred in both the environment and the organisms and both have developed cooperatively through the very nature of energization of the system of molecules (see Note (i)). The evolution was immediately directional once the flow became to and from compartment cells and in all probability prior to coding simply by the energization of organic material in kinetically stable reduced states of carbon.
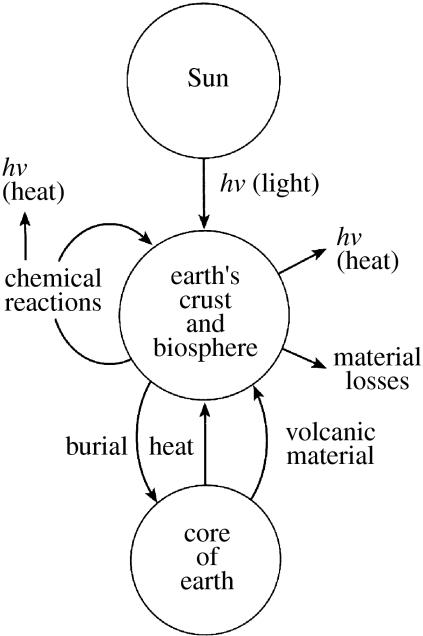
Figure 3.
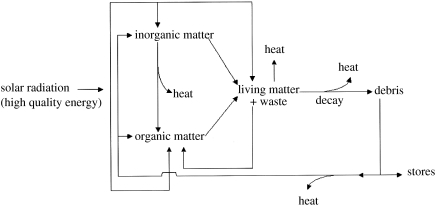
Basic unity of the ecosystems' energy and material. Chemical reactions here include all organism activity (figure 5).
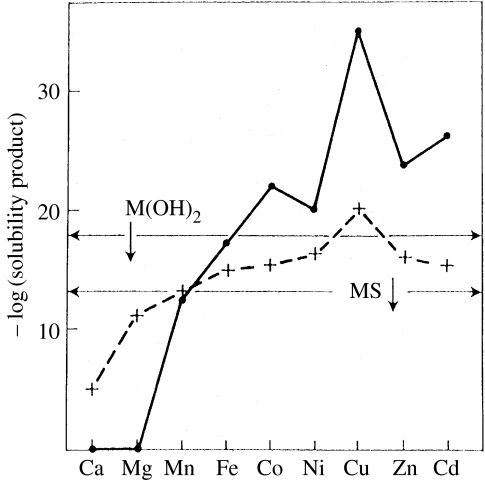
Figure 4.
Insolubility of divalent metal ion sulphides and hydroxides at pH 7 shown as solubility products. Continuous line with dots, sulphides; broken line with crosses, hydroxides; two horizontal lines, solubility products which allow a free metal ion of micromolar concentration. Cu, Zn and Cd are virtually not available in the sulphide era while all the ions are available in water at pH 7, Cu weakly so (see also figure 5). Note Fe2+ is not available in oxidizing conditions and life had to scavenge for Fe3+.
4.1 Basic features of kinetic stability and organic energization
A system of irradiated or otherwise energized chemicals has to have a considerable lifetime if it is to enter into a flow system of any complexity and it must not become dispersed. Molecules made from H, C, N and O, and to a lesser extent including S and P, have the ability to be persistent. It is doubtful whether any other elements can build similar water-soluble polymers. All such chemicals made from these basic building blocks are, in fact, thermodynamically unstable but kinetically stable at 300 K and are reduced relative to very simple environmental compounds, such as CO2 and H2O. Therefore, it is extremely probable that the original constructs of a living cell were uniquely associated with larger organic molecules of this kind, which we see today, i.e. lipids, proteins, saccharides and nucleic acids, and it may well be that there is no other way to create life. Then, it is still the case that there is an essential set of pathways from the elementary starting materials of the environment to these biopolymers in all forms of life. A characteristic of this perhaps unique set of organic molecules is that: (i) their elements in the compounds are reduced relative to CO (CO2) and N2 by the incorporation of hydrogen and (ii) they are mostly negatively charged to keep many of them soluble in water. However, some, the lipids, are insoluble in water and readily form vesicle membranes which prevent dispersion of internal contents, thus they could generate the precursors of cells. These vesicular protocells are postulated to give rise to the origin of life for which we offer no other positive explanation (Russell & Hall 2006). We are only concerned here with the evolution of the ecosystem. The reductive chemistry, which later produced kinetically stable cells, simultaneously generated oxidized waste and rejected some other materials into the environment.
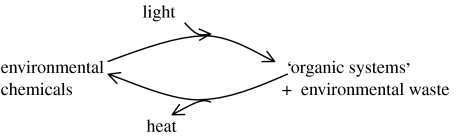
(Light is not the only possible source of energy (see §2) but it has become dominant.) The cycles are shown in more complicated form in figure 5. Here, one waste had to be oxidized material such as sulphur from H2S or oxygen from H2O, where the amount and availability of water made itself the preferred source of hydrogen quite early in evolution. Another waste is in energy applied to reject some available elements that would be poisonous to the system. The necessity for selective rejection is also part of the origin of protocells and life. It is the adaptation to waste which drives evolution allowing greater use of energy.
Figure 5.
Energized cycle of material in cells driven by solar energy, which degrades to heat. Note waste which causes evolution (see §2 for alternative energy sources).
The kinetics of compounds made from inorganic ions in water, here the sea, are usually quite different from those of organic compounds in that generally they equilibrate quickly between free ionic forms, different oxidation states and between bound conditions in organic molecules (Fraústo da Silva & Williams 2001). Even their compounds of high thermodynamic stability are usually of quite low kinetic stability. We shall treat all the ions Na+, K+, Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Cu+ (Cu2+), Zn2+ and Cl− as in effective but different equilibria with their compounds in different oxidation states in both protocells (and later in cells) and the environment even though distribution to binding sites may require carriers. (There are some exceptions to this statement.) All these ions exist in the sea and cells in controlled amounts to date, though not in the same amounts in all cells and certainly differently in the sea and cells at different times during evolution (Fraústo da Silva & Williams 2001). In the protocell, they had to be, as they are today, energized in their concentrations by pumps as they cannot cross membranes. A general rule is that much as one group of energized, synthesized organic molecules of the same kind is to be found in the central parts of all cells, the cytoplasm, so certain free concentrations of ions are present there in fixed concentrations to the best of our knowledge (figure 6; Fraústo da Silva & Williams 2001; Outten & O'Halloran 2001). (Metal compounds are not present equally in all cell cytoplasms, of course.) The implication is that in the steady state of all protocells and cells, the inward/outward pumping to/from the cytoplasm maintains very similar kinetic gradients of free ions across all outer cell membranes and this includes H+. Particular interest lies in the fact that protocells, like all cells, had to reject Na+, Ca2+ and Cl− (and perhaps heavy metal ions especially Mn2+) to the sea so as to reduce osmotic pressure (Na+, Cl−) and prevent conglomeration and precipitation of organic molecules (Ca2+). K+ and Mg2+ are then allowed into the cells to balance the mainly negatively charged soluble organic molecules. There are also restrictions, largely outward pumping, upon several other M2+ ion concentrations although these ions in bound states are required for catalytic or structural functions but a switch in evolutionary conditions later has made it necessary to pump these ions selectively in new ways either in or out of cells more effectively. Note especially a grave problem with the maintenance of iron levels in cells, since in oxidizing environmental conditions, free iron ions are in very low concentrations, 10−17 M Fe(III). Finally, we can list the minimal element requirement of life and element availability as far as we know them (table 1).
Figure 6.
Estimated free monovalent and divalent metal ion concentrations in all cell cytoplasm. The values from Na to Ca are well known (Fraústo da Silva & Williams 2001). Values from Fe to Zn are possibly too low. Free Fe2+ may well be nearer to micromolar, and free Zn2+ nearer to −log[M]=10. The literature contains variable data but the trend is clear (Outten & O'Halloran 2001). Note how it compares with the insolubility of sulphides in figure 4 and that for obvious reasons it matches the inverse of stability constants of model complexes with thiolate donor atoms (see Note (ii)).
Table 1.
Absolute minimal element content of primitive life. (See Williams & Fraústo da Silva 2006.)
| elementa | availability | use |
|---|---|---|
| H | H2S (air), HS− (sea) | organic molecules, energy capture |
| C | CH4, CO or CO2 (air) | organic molecules |
| N | NH3, HCN (sea) | organic molecules |
| O | H2O, CO, CO2 (air) | organic molecules |
| Na+, K+, Cl− | sea salts | electrolyte balance, osmotic control |
| Ca2+, Mg2+ | sea salts | structure stabilization, weak acid catalyst (Mg2+) |
| P() | sea salts | organic molecules, energy transfer |
| S | H2S (air), HS− (sea) | element transfer, energy metabolism |
| Fe | Fe2+/Fe3+/S2− (sea) | catalysis |
These 12 elements were incorporated of necessity into any vesicle formed in the sea in the period around 3–4 Gyr ago to give the biopolymers of life. Others that were present in reasonable amounts but perhaps not incorporated of necessity initially were Al, Si, V, Mn, Co, Ni, Mo, W, and perhaps Se and Br. The primitive organisms we know, such as archaebacteria, have approximately 20 elements.
Among non-metal chemistry, there is another dominant chemical feature apart from reduction and pumping, which requires energy, namely condensation and removal of water, which leads not only to most metabolites from C, H, N and O basic materials but also to all biopolymers, nucleotides, proteins, saccharides and lipids. The removal of water is driven by pyrophosphate hydrolysis in the form of nucleotide triphosphates. Apart from the fact that pyrophosphate hydrolysis drives these reactions, phosphate is also incorporated into all nucleotides and many lipids, and is a component of many metabolites. It requires energy to form all these phosphorylations again, demanding the prior formation of pyrophosphate. Clearly, this chemical was required before any complex biomolecule could be formed and there is no known alternative possibility. Pyrophosphate is synthesized from two phosphates using the energy of a proton gradient in the first condensation reaction. The proton gradient is made directly or indirectly from light (Williams 1961), demanding an electron transfer circuit, which also gives rise to reduction. Underlying condensation and the formation of many cellular chemicals are then the need for phosphate. In the sea, phosphate cannot exceed approximately 10−6 M due to the presence of calcium ions. The cellular requirement for phosphate can only be met therefore by pumping it into cells, where free calcium is reduced in concentration. Here, energy is again required. A general point can now be made regarding biological chemistry. The selection of each of the 20 chemical elements, phosphorus is one in cells, is one of the necessities where no other available element has equal advantageous properties for a particular chemistry. In other words, life as we know is fashioned by chemical limitations in an unavoidable manner in a system. Is it unique? Consider a second example.
We have already stressed the requirements for many elements in cells, including especially their functions in reduction and condensation, and in general physical stability. In the first two of these, we have seen how many organic elements are incorporated in compounds by kinetically stable bond formation and/or by binding at equilibrium after pumping of ions in or out of cells. There is a particular example of incorporation, that of oxygen, now using oxidation, not yet mentioned, which was and is needed in all life. For this purpose, organisms require either molybdenum or tungsten as a catalyst. The chemical problem they solve is the incorporation of oxygen in the formation of, say, carboxylates from aldehydes. These elements have the special ability of being able to exchange oxygen atoms with water readily at low oxidation/reduction potential which existed before there was oxygen (Williams & Fraústo da Silva 2002). It is very surprising at first that life depends from its beginnings upon not just C, H, N, O, S, P and common metal ions but on such elements as molybdenum and tungsten (figure 1). We ask again ‘Is the system unique?’
At this stage, we stress that we have not needed to introduce anything but physical/chemical principles applied to both inorganic and organic materials. We consider, as shown experimentally, that lipids when agitated give rise to vesicles and postulate that these vesicles can concentrate material. We have shown that shapes, compartments, arise, but we have had no need to refer to codes. A code will become essential only when we come to reproductive life which we shall put on one side as an accident. We shall show by element analysis how the system, coded (we say subsequently) for reproduction, which guaranteed continuity provided that the system already had sufficient persistence to give time for it, could evolve keeping physical/chemical principles for explanations. We can then envisage the whole environment/organism ecosystem development of chemical types in a rational fashion, which includes the impingement of changing organism waste in the environment upon the organisms themselves (figure 5).
5. The markers of system evolution
This account of the evolution of the environment/organism ecosystem is very different from any previous analysis, in that the useful markers of change cannot be the traditional organic chemicals, e.g. DNA, RNA, proteins or complicated metabolites. The break with tradition is forced, since we treat the environment/organism ecosystem as a unity and there are no such organic chemical markers in the environment. In fact, there are only the very small carbon molecules, CO2 and CH4, which could be called organic but are usually called inorganic in studies of global atmosphere changes and are not controllable in cells. The evidence for the changes in cells of these two chemicals over the period from 4.0 Gyr ago to date is complicated and of indifferent quality. N2, NH3, O2 and the other compounds related to the dominant non-metal elements S and P, i.e. and with their minerals in the environment, are usually also classed as inorganic compounds. Now, the changes of some of the compounds of these elements and their isotopic composition, e.g. O2, H2S and , are useful long-period markers of evolutionary change in the environment related to cellular evolution. Most importantly, the long-period changes of the metal elements (ions) and the isotopes of non-metals (Holland 2006) can be followed in sedimentary mineral deposits such as red bands (figure 7) and baryte, BaSO4. The changes in the use of these and other inorganic elements in cells, mostly bound with successively introduced organic proteins, make certain proteins also strong markers of long-period evolution in organisms which can be directly related to newly expressed parts of the coded DNA. We shall be particularly concerned with following the changes in availability in the environment and the use of Ca2+, Zn2+, Mn2+, Fe2+, Co2+, Ni2+ and Cu+ (Cu2+) and their proteins in cells, all within the ecosystem as oxidation of the environment increases (figure 5) and then cellular content changes. We can follow them against the known background of O2 increases and the appearance of, for example, (figure 8) and and some other non-metal compounds. For all these reasons, the changes of the 20 available elements and a broad view of them and their compounds in cells are the best markers of the organisms/environment system evolution.
Figure 7.
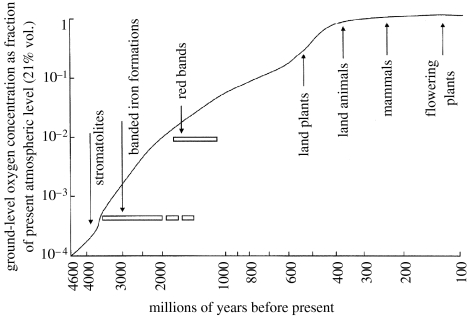
Deposition of geological iron oxide bands in time as the oxygen level rises. Note the ‘coincidence’ of the fall in iron, rise of oxygen, and the introduction of eukaryotes in evolution (figure 10).
Figure 8.
Rise in and Zn2+ in organisms with time. Note the time of the rise from single-cell to multicellular eukaryotes in figure 10.
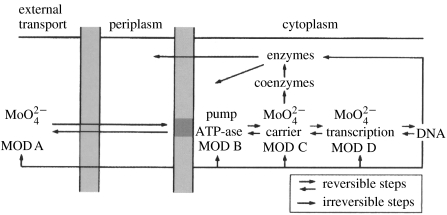
A great advantage in following inorganic ions is that their connections between the environment and cells are made using a particular required series of recognizable proteins, including inward pumps, internal carriers (sometimes required), transcription factors for their protein partners, the protein partners themselves, outward pumps, ion channels and homeostatic cellular buffers (sometimes required). The chemistry of selective equilibrium binding of all the ions is now well understood (Fraústo da Silva & Williams 2001) and we can therefore find and rationalize the most suitable ‘signature’ amino acid sequences in the proteins for different metal ions. They were devised in the earliest cells and have been maintained with only some novel additions. The set of individual time patterns of ion-binding proteins and enzymes can therefore be found in the genome. Hence, it is possible to give a development pattern of both for different ions, which is related to the introduction of chemotypes, cells evolving with different elements, and their protein partners, differently organized (Dupont et al. 2006). An example of the connection to the genome is shown in figure 9 for molybdenum and of the ability to follow an element in evolution by the calcium-binding sites (tables 4 and 6).
Figure 9.
Genetic structure of the molybdenum uptake and incorporation of proteins. The labels are for the genes which occur in a sequence after a promoter. Mod A protein carries the molybdenum in the extracellular fluids.
Table 4.
Involvement of elements in homeostasis during evolution.
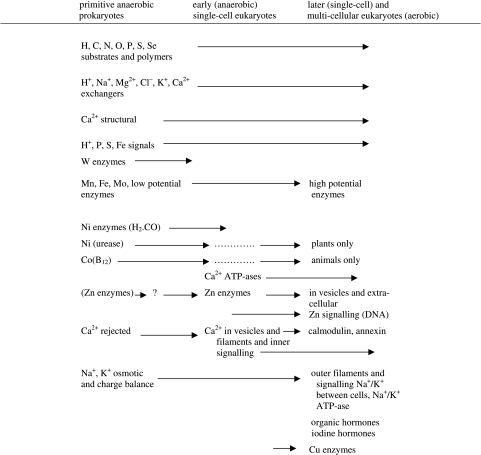 |
Table 6.
Some classes of calcium proteins.
| protein | location and function |
|---|---|
| calmodulina | cytoplasm, trigger of kinases, etc. |
| calcineurina | cytoplasm, trigger of phosphatases |
| annexins | internal, associated with lipids, trigger |
| C-2 domains | part of several membrane-linked enzymes |
| S-100a | internal and external: buffer, messenger, trigger |
| EGF-domains | external growth factor but general protein assembly control, e.g. fibrillin |
| GLA-domains | external, associated with bone |
| cadherins | cell–cell adhesion |
| calsequestrin | calcium store in reticula |
| ATPases | calcium pumps |
EF-hand proteins.
Returning to the non-metals H, C, N and P, which are very much involved in the general chemistry in cells, their compounds did not change greatly in kind in the cytoplasm although there are many changes of detail. This is a consequence of the conservative nature of all life in its essential production of nucleotides, proteins, saccharides and lipids, all products of considerable reduction of the atmospheric compounds CO, CO2 and N2. The nature of P is almost invariably in phosphate compounds. The major studies of the C chemistry in evolution are of isotopic fractionation, and those of both C and N chemistry are of molecular sequences (Holland 2006). There was however a relatively small change in carbon chemistry after oxygen increased in the atmosphere, which we shall note in messengers and some extracellular proteins, in higher organisms, and a larger change of nitrogen in the environment; table 2 shows the essential primitive metal elements and their activities in life, as far as we can estimate them from existing anaerobes. They are the main metal ions which we shall follow as early markers of evolution together with changes of O, N and S, and then later new markers appear, e.g. Cu and Zn.
Table 2.
Essential primitive roles of metal ions. (Note that K+/Na+/Cl− control osmotic and charge balance while Ca2+, Zn2+ and Cu have very little internal role, but they and their use are all excellent markers of evolution.)
| metal ion | some roles |
|---|---|
| Mg2+ | glycolytic pathway (enolase) |
| all kinases and NTP reactionsa | |
| signalling (transcription factors) | |
| DNA/RNA structures | |
| light capture | |
| Fe2+ | reverse citric acid cycle |
| CO2 incorporation | |
| signalling transcription factors | |
| control of protein synthesis (deformylation) | |
| light capture | |
| W(Mo) | O-atom transfer at low potential |
| Mn | O2-release |
| Ni/Co | H2, CH3-metabolism |
| Na+, K+ | osmotic/electrolyte balance |
| Ca2+ | stabilizing membrane and wall, some signalling? |
Almost all synthesis pathways.
As our stress is equally on the environment, which is much more rapidly affected by oxidation than organisms, we turn to its evolution first.
6. The effect of oxygen on the environment
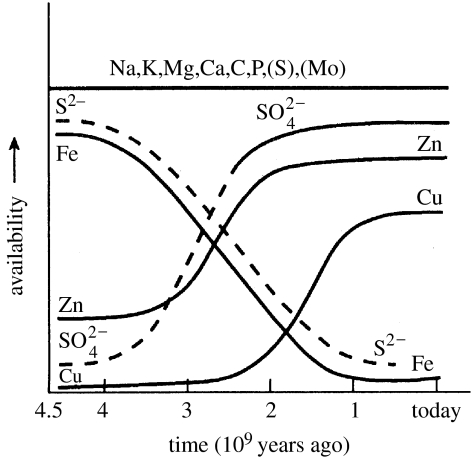
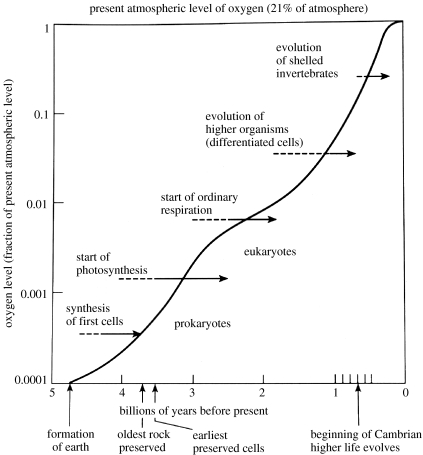
Waste oxygen from biological activity did not accumulate quickly in the atmosphere, since the large quantities of reduced inorganic materials in the environment rapidly equilibrated with it to give oxidized states of elements. Thus, the O2 pressure was buffered for a long time. The major materials concerned, which are powerful fast reductants, were the abundant free H2S and Fe2+ from sulphides. They were oxidized to and Fe3+, respectively. As mentioned, we can follow their conversion in time by the precipitation of Fe3+ iron bands in surface geological minerals and changes in isotope ratios of sulphur in sulphates (Holland 2006). It took some 2 Gyr for the free oxygen concentration to rise to a significant degree, say to 1% of present levels (figure 10; Kasting & Siefert 2002). Subsequently, the oxidation potential of O2 rose steadily with its partial pressure, perhaps for some 1 Gyr, reaching present levels say 500 Myr ago. In these later periods, oxidation of metal compounds, especially the sulphides of Co, Ni, Cu and Zn gathered pace, but vast quantities have remained buried and are still being made slowly available (Saito et al. 2003). The elements vanadium and molybdenum are special cases as they were more readily oxidized from sulphides. The sea became quite rich in these two elements early in evolution. Now, non-metals other than sulphur were oxidized too (figures 8 and 11) and especially the original CO and any NO or NH3 (Miyakawa et al. 2002) were replaced by CO2 and , as in our atmosphere and seas today, and we shall have occasion to refer to the newly formed selenate and iodine. A peculiarity is that CH4, formed by organic degradation, reacts slowly with O2 so that some of it became buried, which led to the formation of oils and coal. However, the majority of organic debris materials from cell activity were oxidized by cells. We emphasize that the loss of availability of many elements and the gain of many others placed great stress on pre-existing chemical flows in organisms. The early organisms gradually faced a more and more hostile environment, always present in the form of the Na+, Cl− and Ca2+ in the sea, but increasingly from loss of access to C, N, S, Se and Fe2+ and lastly from the directly poisonous increasing presence of amounts of O2 and its non-metal products and several, slowly more available, transition metal ions. It is this systematic, close to equilibrated change, in the environment (figure 11) that forced a directional evolution of types of organism, as we shall show. We cannot use single species to describe this change but only large groups of species which are affected simultaneously until humans appeared. Since we are describing chemical change we shall refer to chemotypes as the classes of species, while genotype relates to the individual species and specific genes define individuals. It is only the change of chemotypes that can be given a physical/chemical system explanation.
Figure 10.
Rise in oxygen levels and evolution of the groups of chemotype species (compare figures 7 and 8).
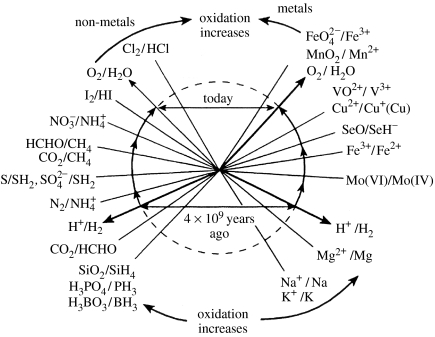
Figure 11.
Redox potentials of the elements at pH 7, where that for H+/H2 is −0.42 and that for O2/H2O is +0.8. The sequential change of the sea will follow these redox potentials if the environment is at equilibrium with oxygen. The redox potential of the environment reached around +0.1 some 1.5 Gyr ago and is now approaching the O2/H2O line. The dissolution of sulphides depends on their solubility products. A sulphide solubility product change of logSP=10, increased solubility, will move the sulphide equilibrium by +0.30 V.
Here, it is convenient to draw attention to the ideas put forward by Lovelock (2000) that Earth has developed in its chemistry as a changing steady state of organic chemicals of carbon as oxygen increased until today when a fixed biological steady state has evolved in which organic matter production by organisms is an effective final buffer of oxygen pressure. The Earth's ecosystem is then given the title ‘Gaia’ with this implication. We cannot agree with this interpretation. Our view is that the organic chemistry of organisms through its coded character is extremely conservative, so that it is only dragged unwillingly forward by the oxidation of its environment which is much closer to being in true equilibrium, not a steady state, than are organisms. The environment also has important inorganic parts not covered in Gaia. The process of evolution, as we see, is a simple directed external chemical change due to biological waste, forcing slow adaptive change upon organisms, the whole increasing energy absorption and degradation. Initially, the buffering of the O2 was purely environmental and inorganic by fast Fe2+ and H2S reactions when organisms hardly changed for 2 Gyr, while today the short-term buffering is indeed largely organic (as stated by Lovelock 2000), but it is now the residual inorganic changes of minerals which are slower to adjust but will demand adaptation to them as the environment changes. In addition, there is the ongoing slow oxidation of methane, oil and coal. Moreover, humankind's industry is today on such a scale as to complicate carbon/oxygen buffering further as recognized in Gaia and by many authors. The outcome is quite unclear and most species are not at risk (except for their populations), but it has always been so and will continue until at some time Gaia could be reached in theory much though this may not be possible in practice. Adaptation is a hazardous activity and flexibility of the system is surely limited but unknown while environmental changes continue. Bacteria can adapt quickly to long-lasting poisons, organic (certain halogenated compounds) and inorganic (Hg, Pb, Cd, etc.), but it is very doubtful if higher animals can adapt to such changes. Global warming is not a threat to any species that can migrate, but it will force readjustment of all populations in different places. Population and more general pollution may be worse problems than global warming, great though this threat is, and may be equally immediate. These considerations apply to all life and have deep implications for humankind's activities (mentioned later).
7. The chemotype classification of organisms
We shall not attempt to follow the changes with time of the concentrations of H, C, N and O in their compounds as the problem in cells is so complicated (Kasting & Ono 2006) and the environment is linked to them only through simple inorganic compounds such as CO2, N2, H2 and H2O which cannot be homeostatically controlled. (It is true that the study of small metabolites in different cells, metabolomics, is advancing but it is likely to be species related.) We shall be concerned, however, with the changes in general availability of these four elements and some oxidized modifications of them and of S, I and Se compounds, as the chemotypes have diversified. As stated earlier, it is the metal ion concentrations and components, metallomes, which have changed considerably with time and which form an easily seen close link between cells and the environment due to the relatively fast reactions in both compartments. There is then a basic analytical content of both cell and environment compartments linked to their free metal ion concentrations through equilibria, very different in the two, which can be used to describe evolutionary changes. The best linkage to H, C, N and O chemistry is then via metalloproteins and then of these proteins to DNA sequences. As stated, these proteins have a homeostatic link to the metal ions they bind. We can use the gene to tell us which binding proteins can be expressed and the metalloprotein concentration in a compartment to tell us which are formed quantitatively. Often, only the DNA of a cell type has been characterized and we know there are differences between DNA and metal/protein characteristics of the major types of organisms (Morgan et al. 2004; Dupont et al. 2006; Williams & Fraústo da Silva 2006). For example, in the most primitive single cell anaerobes, unavailable dioxygen and copper cannot be used and their protein partners are absent; in single cell aerobic prokaryotes, there is little, if any, use of calcium and few of its proteins while calcium signalling becomes valuable for single-cell eukaryotes, in which novel calcium-binding proteins appear (table 5); multicellular organisms make use of more extensive Ca, Zn, Cu and Fe proteins and many elements outside cells in controlled fluids, where further regulated novel proteins occur. Animals with nerves found a quite new use of Na+ and Cl− in these fluids requiring new pump proteins (figure 12). All these features can be traced back to new DNA sequences. Simultaneous with this use of chemicals in organisms, including humans, came increasing use of necessary compartments, cytoplasmic, periplasmic, vesicular, extracellular and then those outside the cell in the environment. These very basic physicochemical features of an organism/environment system allow us to divide evolution into a few kinds of chemical organization in a systematic way. We shall describe the classes, in turn, noting how they arise through adaptations to allow survival value of the sum of the random species within them in a gradually changing environment. However, while the environment links to chemotype changes systematically, the new element chemistry will be seen to demand also compartment separation in organisms giving increased compartmental complexity (table 3)—all part of a directed total system. The difficulties of complexity are then relieved by symbiosis in this total ecosystem. Finally, humankind employs a vast range of new chemical elements outside the organism itself with no DNA connection and creates quite a novel stress for evolution. Table 4 is given here to enable the reader to have an overview of the connection between the use of elements as they became available in time and evolution from the earliest prokaryotes to the later eukaryotes as described in the next sections.
Table 5.
Distribution of different Ca2+-binding protein motifs in organisms. (The table is based on the total number of all proteins in the DNA sequences available in 2004. The activities of calcium proteins are indicated in table 6. Adapted from Morgan et al. 2004.)
| binding proteins | ||||||
|---|---|---|---|---|---|---|
| Excalibur | EF-hand | C-2 | annexins | calreticulum | S-100 | |
| archaea | — | 6a | — | — | — | — |
| bacteria | 17 | 68a | — | — | — | — |
| yeasts | — | 38 | 27 | 1 | 4 | — |
| fungi | — | 116 | 51 | 4 | 6 | — |
| plants | — | 499 | 242 | 45 | 40 | — |
| animals | — | 2540 | 762 | 160 | 69 | 107 |
These proteins have single EF-hands and are not signalling proteins; all the remainder are for signalling.
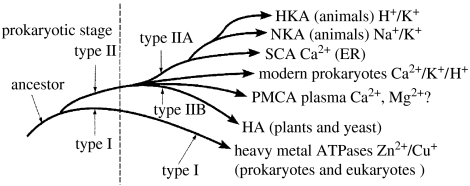
Figure 12.
Evolution of the metal ion pumps. The variety of Ca2+ pumps rose with the coming of eukaryotes and those for Na+/K+ rose with the evolution of animals with nerves. Heavy metal ion pumps have been necessary from very primitive cell times.
Table 3.
Major stages of evolution (Alberts et al. 2002).
| (a) | Prebiotic. They include a variety of energized flow systems of chemicals leading to precursors of the primary chemicals of later stages. |
| (b) | Prokaryotic cells. They came first and are of more than one variety today. There is only one major compartment, the cytoplasm, contained by one major membrane. The cell activities are already coded and concerted. The varieties of these cells and their metabolism have increased with time, anaerobes and aerobes. Their sensing of the environment is not advanced. |
| (c) | Single eukaryotic cells. They have many internal compartments, vesicles and organelles, and many types of such cells exist. The basic metabolism in the cytoplasm is much like that of prokaryotes. They are all aerobic large cells with a much increased organization internally and an increased ability to recognize environmental factors. |
| (d) | Multicellular eukaryotes. They are often classified as fungi, plants and animals. They are all aerobes. There is a great increase in organizational complexity signalling between differentiated cells and organs. They have an increased ability to sense the environment. |
| (e) | Animals with brains. The development of the nervous system with a brain allowed the animal to be informed about the environment and remember experiences. The fast responses were increasingly independent with reference to DNA. Organization in groups is seen in patterns of behaviour. |
| (f) | Mankind. The further development of the brain led to the understanding of the chemical and physical environment and many features of organisms. Hence, the environment became usable in constructs independent of inheritance in the DNA. External equipment could perform many desired functions. Information was passed down the generations through external and internal recording. Organization expanded enormously externally. |
7.1 The first chemotypes: anaerobic single cell organisms, prokaryotes
We wish to relate the organization in organisms to thermodynamic system quantities not simple descriptive qualities such as genes. Here, we shall not refer to intensive DNA sequences, or a description of organisms, except where its organization or expression is controlled by chemical elements. All other cellular components, including proteins, occur as extensive (concentration dependent) quantities belonging to the thermodynamics of system biology in the above cycle. (Do remember, however, that as DNA is destroyed after cell death, it too cycles.)
Types of anaerobic organisms are generally accepted to be the most primitive organisms in two groups, bacteria and archaea (Woese 2002). All these cells, the chemistry of which we deduce to have existed from knowledge of modern anaerobes, have a free concentration of K+(10−1 M), Mg2+ (10−3 M) and Fe2+ (10−7 M), and they use these elements, Fe2+ mostly bound, together with a little Co and Ni but very little Mn and Zn and probably no Cu or Ca ions internally. The archaea appear to differ quite strongly in their use of Ni, note especially the Ni-porphyrin, F430. All the cells reject Na+ (10−3 M), Cl− (10−3 M) and Ca2+ (10−6 M) relative to concentrations in the sea. The cytoplasmic concentrations given are approximate and we can give only even more uncertain values of free Ni and Co ions, while we give maximum levels of Zn and Cu ions from knowledge of equilibria with organic sulphides. An outline of the probable free element cytoplasmic concentrations was given earlier in figure 5. Note that the order of free ion concentration is certainly in the reverse order to that of the Irving–Williams stability series of complex ions and observe the connection to the inverse of sulphide solubility products (figure 4). It can be explained from knowledge of dissociation constants of metalloproteins and other model ligands (Fraústo da Silva & Williams 2001). It would appear that these concentrations in the cytoplasm, the only compartment of the earliest cells, are closely maintained there throughout evolution. Note that the control of relative concentrations of free ions is vital for otherwise fast exchange would destroy the system by poisoning. Hence, any increases in free metal ions in the environment can be allowed to give rise to new metal-binding proteins but not to change in free ion concentrations in cells. This emphasizes the unique character of the cytoplasmic metal ion chemistry of the systems of all organisms which is also consistent with the unique pattern of the major organic chemicals in this compartment of all cells. Cells in steady state must have concentrations of proteins, including pumps, with binding side chains limiting free transition metal concentration in the above fixed free order. While this does not limit the number of different complexes of a given metal ion and their concentrations, they must all have similar binding constants if they are in equilibrium. The amounts of binding proteins and molecules are controlled by feedback from these ion concentrations to the DNA (Williams & Fraústo da Silva 2006). For example, it appears that adenosine triphosphate (ATP) is always close to 10−3 M, very similar to free Mg2+ concentration, and it is MgATP which is the active agent in most reactions. This homeostasis also applies to the mobile coenzymes such as NADH and certainly to the cytoplasmic pH of cells. The free cytoplasmic Ca2+ ion concentration is always less than 10−6 M and little Ca2+ is bound (Carafoli & Klee 1999). There are no intracellular Ca2+-binding proteins in early cells. Certain cellular gradients were therefore established from the beginning of life and they included the use of proton gradients to drive many energized activities (Mitchell 1961; Williams 1961).
Even before the environment changed, there must have been development of a few other coenzymes. In this article, special interest lies in the four coenzymes linked to the porphyrin skeleton and metal ions: haem (Fe); coenzyme B12 (Co); chlorophyll (Mg); and factor F430 (Ni). In effect, these four synthesized components with non-exchanging metal ions act as if they were four new elements. Porphyrin may have arisen even before there were cells, but in any case these four give new markers of evolution. Note that F430 (Ni) appears only in archaea that do not make chlorophyll (Mg), thus making a subchemotype distinction. Another example of coenzyme development is that of the dithiolate complexes of tungsten (W) and molybdenum (Mo), where the (W) complex is found in archaea. In very early times, Mo was precipitated as its sulphide MoS2. There is little further development of Ni, Co and W chemistry throughout evolution and they are lost from many higher organisms.
Before going further, we stress again that this analysis of chemotype is quite different from that of genetic information. Here, we are concerned with expressed concentrations controlled in a network to give homeostasis in a cell's steady state. These are characteristics of a system and not properties of single molecules, such as DNA. Each chemotype system does have one set of common genes with many variations, species, which separate it from all other chemotypes. However, in a general sense, gene development must follow chemical change in the environment and give protective homeostatic concentrations of free metal ions based on unavoidable equilibrium constants while generating novel useful proteins. In so far as the environment change is systematic so the organism evolution must be, and we now show that it has been so.
7.2 Aerobic prokaryotes
The bacteria required hydrogen and, as mentioned already, they discovered water as a source of it, giving waste oxygen. It is this rejection of oxygen together with that of Na+, Ca2+ and Cl− (and maybe Mn2+), which created the environmental pressures which drove evolution of the ecosystem in a particular direction chemically. The earliest effect of oxygen is as a poison especially through its partially reduced products, superoxide and peroxide. Before the use of oxygen or these derivatives, protection from excess of them arose. There are enzymes for removing O2 (based on reduction by NADH), (superoxide dismutases based on Fe and Mn) and H2O2 (catalases based on Fe or Mn), which occur very early in ‘anaerobes’. Later in evolution, all three products find a use and we shall often see the progression
Biological pollution forced adaptative evolution to use self-generated poisons.
Certain prokaryotes have developed new metabolic pathways to use the poisons derived from oxygen including new states of non-metals, such as and , and increasingly, newly available metals such as Zn and Cu. Zinc was used in novel extracellular degradative enzymes and copper in oxidation outside the cytoplasm (Williams & Fraústo da Silva 2006). Nitrogen fixation from N2 needed quite novel enzymes too, but we do not know the time of appearance of the known vanadium (first?) and molybdenum enzymes. The order of oxidation from sulphide is V before Mo. Some new subclasses of aerobic chemotypes appeared including sulphate-, nitrogen- and nitrate-dependent bacteria. There was also separation of cell chemotypes which used a primary source of energy, light, giving out oxygen from those using only secondary energy sources such as oxidation of reduced debris by oxygen or oxidized compounds. This separation led much later to the evolution of plants and animals as very distinct subclasses of chemotypes among multicellular eukaryotes. The second group (animals) are totally dependent on the first for sources of reduced carbon and nitrogen. The divisions of labour in unconnected cells among prokaryotes reduce complexity (genetic load) in each chemotype in a total ecosystem, but it is clearly not a very effective organization. Competition for survival is largely internal to species of very similar chemistry, though clearly the most independent organisms (bacteria) can challenge even the most sophisticated but later organisms (humans).
As a consequence of oxidation, Fe(II) in the sea was replaced by Fe(III), very largely precipitated (figures 7 and 8). The devised scavenging agents, siderophiles, for Fe(III) are oxidized organic molecules and the complexity of this system evolves in higher and higher organisms (Fraústo da Silva & Williams 2001), but all the time they must seek a balance in an ecosystem.
There are some examples of extravesicular compartments in bacteria. A striking case is that of the thylakoid, where localized proton production occurs as part of a vastly complex weaving vesicle membrane system for light capture (Williams 1961). This enables its internal pH locally to be below 5.0, while maintaining the pH of the cytoplasm slightly above 7.0. The later device for oxidative phosphorylation uses an outer membrane potential rather than a pH gradient to avoid the pH problem (Mitchell 1961). A second example is in the bacteria anammox which oxidizes toxic ammonia with nitric oxide within a separate vesicle. In the next sections, we shall see how development of compartments had to be a major feature of new chemotypes all the way to humans.
Furthermore, there are observed small pieces of circular DNA, plasmids, separate from the main DNA, to which we shall have occasion to refer again. The plasmids have the novel genes for combating new poisons, e.g. drugs and heavy metal ions. Why are they separate? They are a new code compartment reducing complexity.
7.3 The coming of unicellular eukaryotes
Around 2 Gyr ago, the first truly complex multicompartment single cells arose apparently simultaneously in all the lines of development from early aerobic bacteria and archaea leading later to plants (light using), fungi and animals (Cavalier-Smith et al. 2006). Their arrival has no thorough genetic or shape connection to previous organisms. Apart from the fact that they now have a central separate nuclear compartment, they are all large cells with a novel flexible membrane, many new internal filaments, several new vesicular compartments (some of a long weaving character, including organelles; see below) and new signalling methods. The flexibility of their outer membrane, due to the inclusion of cholesterol (Summons et al. 2006), allowed them to digest prokaryotes, hence the need to synthesize the chemicals essential to both was unnecessary. This led increasingly to symbiosis in which the higher organism was a source of certain basic foods required by the lower organism, and the lower organism often supplied the higher organism with more complicated molecules, coenzymes, e.g. flavin or materials such as ammonia which were difficult to make from N2. The uptake of bacteria into eukaryotes also led to the creation of organelles, in the form of new compartments (stripped-down bacteria) capable of generating cell energy in a chemically transformed compound, ATP (Margulis 1998; Andersson et al. 2003). The organelles take up certain material from the higher organism's cytoplasm to aid photosynthesis or oxidative phosphorylation. Now, there is DNA in three different compartments in some organisms. Other compartments were for (i) digestion of bacteria and large molecules, lysosomes, (ii) synthesis of large glycosylated and sulphated molecules often for export through the flexible membrane in the Golgi, (iii) synthesis of minerals for export in microcrystalline form to give protective outer shells, and (iv) oxidation by enzymes for special metabolism of certain organic molecules in peroxisomes or in signalling vesicles. As the organic chemical complexity of these cells increased, we can readily distinguish their chemotypical changes. The organelles contained high levels of Mg2+ and Mn2+ (in chloroplasts), Fe and Cu (in both organelles) and mitochondria synthesized not only ATP, but also haem, chlorophyll and Fe/S cofactors; the lysosomes are of unusual acidity (H+); the Golgi has high concentrations of Mn2+ and ; the mineral synthesis vesicles have high amounts variously of Ca2+, Sr2+, Fe3+, and sometimes Ba2+ with ; the peroxisomes have high quantities of haem (Fe). The high concentration of Ca2+ in endoplasmic vesicles is used in a messenger system, while zinc has increased in novel transcription proteins and Cu enzymes are largely periplasmic. The inorganic chemistry is quite different from that of prokaryotes.
Symbiotic internal cooperativity gave increasing efficiency, while still relying on some external symbiotic activity. Now, all the compartmental separations, including internal organelles, had to be coordinated by internal messages and it was also to the advantage of these large long-lived eukaryotic cells to have knowledge of the dangers and advantages of the environment through message systems. In prokaryotes (bacteria), there were already internal controls through feedback links to DNA to give homeostasis of cytoplasmic activity. The messengers here were small molecules and ions in this single compartment connected to protein receptor transcription factors bound to DNA. Apart from the many organic molecules of the metabolomes, especially phosphates, there was strong control signalling from Fe2+ and Mg2+ (Williams & Fraústo da Silva 2006). All of these links had to be maintained in eukaryotes. The rejected elements Na+, Cl− and Ca2+ played little or no part in signalling in prokaryotes, but we shall see how their use increased in eukaryotes. As stated, the prokaryotes were forced to create a steep gradient in Ca2+ from approximately 10−3 M external to less than 10−6 M internal. In eukaryotes, the Ca2+ ion was allowed to be 10−3 M in the endoplasmic reticulum vesicles, but the same cytoplasm/environment gradient had to be kept (Carafoli & Klee 1999). These huge gradients are ideal for conversion to use in new signalling devices (table 6), especially when coupled to the internal cytoplasmic signalling, for example, by phosphates. We observe that eukaryotic cells obtained knowledge of the changes of the external environment, advantageous or disadvantageous, by the opening of Ca2+ input channels to the cytoplasm (figure 13). Following cytoplasmic diffusion, this input released additional Ca2+ from the reticulae, which acted as an amplifier. Together, the Ca2+ ions activated responses of metabolism and filament tension, which changed shape, and they also activated the organelles to increase usable forms of energy, ATP. Thus, both the feeding needs and protection of the eukaryotes were then met by Ca2+ signalling and consequential responses. A fascinating novel chemical feature had therefore appeared in organisms. While the rigid cells of prokaryotes had some knowledge of the changes of their outside environment, their major response, apart from chemotaxis, had remained by relatively slow mutation and development of their genes. The flexible cells of eukaryotes evolved with a very fast response to external events. Their changes are independent of changes of genes so that they could change shape and metabolism rapidly and reversibly. We shall see that this is part of a gradually developing greater environment/organism integration, which increases in evolution. It is a way to increase energy capture while not requiring short-time involvement of DNA.
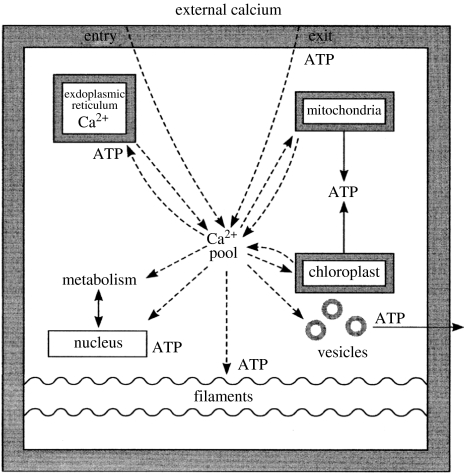
Figure 13.
Calcium ion signalling system of eukaryotes. An external event triggers calcium entry which activates simultaneously a wide variety of physical, shape and metabolic changes. The time of activation can be as short as 1 ms and no record of the changes need to be retained by the cell and there is no interaction with DNA.
There were also other element changes, in that new Zn2+-dependent transcription factor proteins evolved (Klug 2005) and a new protective superoxide dismutase enzyme based on Cu and Zn appeared. (The free zinc in cells is still somewhat below 10−10 M and free copper remained at some 10−15 M of necessity.) Note that prokaryote superoxide dismutases were and are still based largely on Fe or Mn even in organelles. We shall turn to more extensive uses of both copper and zinc enzymes in the section on multicellular eukaryotes. Before doing so, we stress the dramatic effect that the exposure to and use of the poisonous element production in environment had on the evolution of organisms. There is the use, especially of oxygen and non-metal oxides, the heavy metal ions that oxygen generated, and calcium, all poisonous elements or compounds rejected of necessity from the cytoplasm at first, some even from the beginning of life. The next changes are then further adaptation to unavoidable enforced changes of the chemical element content of the environment. It was this that drove the evolution of eukaryotes and prokaryotes as organisms improved the overall total rate of energy use and degradation, with strong survival due to compartmental coordinated development and symbiosis. The changes in genes or of the linked shapes and sizes of cells in species offer by themselves no rational explanation for development as they are said to be random, only coupled to a general strengthening of survival. Note that, in part, species gene size and load increased, but it is observed that it decreased in some ways. No more striking examples of the reduction of genetic load can be found than the relationship of eukaryotes to bacteria. The eukaryotes came to depend on bacteria for the element as essential as carbon for life, namely nitrogen. Eukaryotes cannot obtain nitrogen from N2 and many cannot carry out denitrification from nitrate. Again as noted earlier, the synthesis of porphyrin and Fe/S proteins occurs in mitochondria. As mitochondria are really internal bacteria, it is not only the transduction of energy (ATP) from oxygen or in chloroplasts from light, which are in separate cell compartments, but also that of several essential cofactors for the eukaryote cytoplasm. In essence, these cofactors are similar to the later so-called vitamins, since, for example, many of the vitamins, essential chemicals for humans, are coenzymes made externally or internally to this organism by prokaryotes. There is no sense of competition here between prokaryotes and eukaryotes, but only cooperation, although some bacteria can attack eukaryotes. Later in this article, we shall stress the great dependence of the highest organisms upon lower organisms for other essential (to all life) chemicals and note that the lower organisms also gained in symbiosis with higher organisms, in that, for example, the higher plants supply reduced carbon to lower organisms, fungi, that do not photosynthesize but scavenge so effectively in their soil environment. As we stressed before, the whole is a greater system for material and energy uptake and degradation than the parts, a feature of evolution not visible in figure 2. Note that throughout the above, we are describing physical/chemical pressures driving evolutionary chemotype change which can only be achieved by DNA changes in species to discover the code for advance in a direction.
Clearly, single cell eukaryotes are quite new major chemotypes with vast numbers of probably random species in a few subclasses. They are quite distinct from prokaryotic anaerobes and aerobes in chemical element use. While they could not have appeared de nouveau, there is not good evidence for intermediate cases which must have existed. The intermediates must have had sufficient stability to give continuity (see Cavalier-Smith et al. 2006 for an extensive analysis).
7.4 Multicellular eukaryotic evolution
The next development, of multicellular organisms, is not so difficult to appreciate as much of the in-cell chemistry is only changed in the quantity and division in different cells of the already present chemical components in the unicellular eukaryotes. The big chemical changes are in extracellular chemistry of external filaments assisting cell/cell space organization, of communication between cells via the extracellular space, and to some degree, in the chemistry of differentiation. This extracellular space is occupied by fluids which became more and more well controlled as the organisms grew in size within an outermost ‘skin’. Now, there are some general chemical element changes which we can list. In the cytoplasm, there are a vastly increased number of zinc transcription factors moving towards 5% of the total number: there is a decreasing use of nickel, so that higher animals do not have nickel proteins coded in their genome, and cobalt, where there is no function for coenzyme B12 in higher plants (replaced by Zn enzymes), and this cobalt compound has as a precursor, a vitamin, in higher animals: there is some doubt about whether there is coding of t-RNA for selenium amino acids in these eukaryotes. Outside the cells, the cross-linking of animal filaments, such as collagen, is done by oxidation using novel copper enzymes and the breakdown of filaments, necessary for growth, is largely due to zinc proteases, much like early digestive enzymes. Digestion also uses more zinc enzymes in extracellular fluids. Many of the extracellular filaments have numbers of oxidized protein side chains and are glycosylated, and they frequently bind calcium. Sulphated polysaccharides are also found outside cells. Turning again to the extracellular fluids, they gradually developed as fluids very well controlled in free Na+, K+, Mg2+, Ca2+ and Cl− and in transport proteins for heavy metal ions such as Zn2+, Cu2+ and Fe3+. In vesicles, one development is now especially of oxidized organic molecules, e.g. adrenaline and hydroxytryptamine and amidated peptides, as fast cell to cell transmitters, many of which are synthesized with the aid of copper enzymes (figure 14). Zinc enzymes often assist later in the external hydrolysis of the peptides. Zinc ions from vesicles in some organisms in external fluids also act as messengers. All the above organic molecules are fast transmitters, but there are also the slower-acting extracellular hormones many of which are produced by a vastly increased number of haem (Fe) oxidases, hydroxylases and peroxidases (cytochromes P450 and the thyroxine producing peroxidase). The reactions take place in the cytoplasm in such a way that the enzymes do not release the intermediates and H2O2. These hormones such as sterols, thyroxine and retinoic acid have the zinc transcription factors as their receptors. Zinc thus became a general factor linked to growth and metamorphism and is probably an internal homeostatic connector between these hormone receptors so that they act together. (Zinc deficiency causes general growth and metamorphic diseases, for example on an extremely zinc-deficient diet, humans become diminutive and do not go through puberty.) Much of the novel organic molecule communication system is then connected to the increasing availability of the two metal ions, zinc and copper (figure 8). The selectivity of the fast transmitters at receiving cells is largely based on receptors linked to Ca2+ input as before and then responses occur much as in single-cell eukaryotes. There is a remarkable increase in Ca2+ receptor proteins (figure 15; tables 5 and 6; Morgan et al. 2004). Note that these metal ion-based synthesis and novel messenger systems are common to plants, fungi and animals so that very distinct families of unrelated chemotypes, including vast numbers of species, have similar systems based on copper, calcium and zinc to a large degree. Given that the changes of all these functional uses of metal ions occur almost simultaneously in time in all the three branches of multicellular organisms, plants, animals and fungi, which are connected only distantly through different branches of unicellular organisms to bacterial origins, it could hardly be that random mutation led to simultaneous appearance of these similar novelties in all of them (tables 7 and 8). The common factor is the environment change, noting here especially the rise in copper and zinc. How does gene loss (Ni and Co) and addition (Zn, Ca, Cu and Fe) occur with environmental change while all elements increase in availability? (The data for the above statements are all to be found with references in Williams & Fraústo da Silva 1996.)
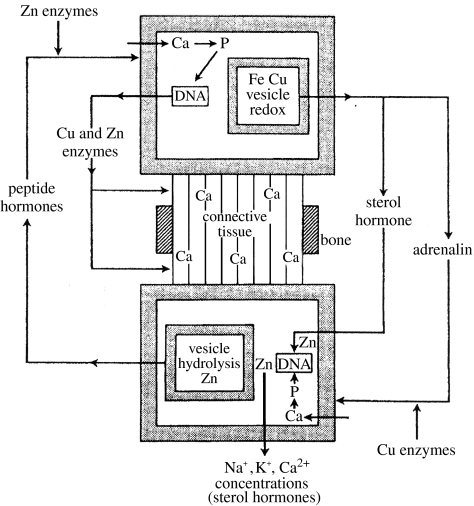
Figure 14.
Simplified diagram showing the involvement of the increasingly available zinc and copper ions as well as that of calcium in multicellular organisms. Note the difference between fast transmitters, very new oxidized molecules, made by the action of copper ions in vesicles and the hormonal slow activators, oxidized molecules by the earlier haem iron enzymes in the cytoplasm.
Figure 15.
Increase in signalling Ca2+-proteins and that of the kinds of organic signalling molecules with time. All of these proteins and molecules arose as oxygen increased above the level which, with certain novel element uses, gave rise to increasingly sophisticated eukaryotes.
Table 7.
Metalloprotein sets in two organisms.
| protein set | yeast | worm (C. elegans) | metal |
|---|---|---|---|
| nuclear hormone receptor (Zn)a | 0 | 270 | Zn |
| binuclear GAL cluster (Zn)a | 54 | 0 | Zn |
| metalloproteases | 0 | 94 | Zn |
| Na+ channelsa | 0 | 28 | Na |
| Mg2+ adhesiona | 4 | 43 | Mg |
| calmodulin-like proteinsa | 4 | 36 | Ca |
| K+ channels (voltage gated)a | 0 | 135 | Ca |
| EGF, Ca2+-binding cysteine-rich repeatsa | 0 | 135 | Ca |
| kinases (tyrosine) | 15 | 63 | Mg |
| cytochrome P450 | 3 | 73 | Fe |
Absent in bacteria (Williams & Fraústo da Silva 2006).
Table 8.
New element biochemistry after the advent of dioxygen.
| element | biochemistry |
|---|---|
| copper | most oxidases outside higher cells; connective tissue finalization; production of some hormones, dioxygen carrier, N/O metabolism |
| molybdenum | two-electron reactions outside cells; , , aldehyde metabolism |
| manganese | higher oxidation state reactions in vesicles, organelles, and outside cells; lignin oxidation (note especially plants); O2 production |
| nickel | virtually disappears from higher organisms |
| vanadium | new haloperoxidases outside cells |
| calcium | calmodulin systems; γ-carboxyglutamate links; general value outside cells and in cell–cell links |
| zinc | zinc fingers connect to hormones produced by oxidative metabolism |
| selenium | detoxification from peroxides, deiodination? |
| halogens | new carbon–halogen chemistry, poisons, hormones |
| irona | vast range of especially membrane-bound and/or vesicular oxidases; peroxidases for the production of hydroxylated and halogenated secondary metabolites; dioxygen carrier and store |
Note that iron can act as an agent responding to the redox potential changes in the cytoplasm.
If we ask why organization should develop in this way (tables 4 and 7), then system analysis of the environment/organism ecosystem suggests that the placing of different functions in more controlled connected relationships, different organs, in space allows greater effectiveness. The resultant increase is in the capture of energy and material for synthesis and the overall degradation of energy, light to heat, with cycling of material (figure 3). It is as if plants strain to gain total area for light capture by increase of height and spread while animals and fungi evolve to be more effective consumers of plants and plant debris assisting material turnover with heat (entropy) production. They do so by using a wider diversity of elements in new ways, including sensing, within increasing numbers of compartments. It is especially the increased availability of a few elements, note especially Zn and Cu, which allowed the development. But all this complexity is at a price of a heavier need for the lessening of genetic and control function loads. As mentioned previously, this is relieved to a considerable degree by symbiosis. Plants rely on bacteria for nitrogen supply and fungi for minerals, giving to the symbionts carbon compounds in return. The life of higher animals depends totally on plants for food and bacteria for digestion and coenzymes (vitamins) allowing animals to lose several metabolic paths. Animal debris in turn feeds plants. Chemical cooperation is the essence of advance in organization of an effective total ecosystem and it increases in evolution (but see humans below). Why otherwise did organisms lose genes so easily?
8. Animals with nerves and brains
An additional step in communication systems appeared in the larger scavenging animals. In these animals, awareness using refined senses is coupled to the filamentous and muscular structures for mobility, now in several separate organs. The coupling is managed, as it should be, by the fastest possible biological communication network, which comprises nerve cells. (Note that cellular material does not allow long-range electronic connection.) They have thin long cylindrical extensions able to conduct depolarizing ionic currents, effectively in wires. In this way, biological organisms are enabled to make long-range fast physical, electrolytic, connections based on the most mobile ions Na+, K+ and Cl−. The currents arise mostly from inward movement of Na+ in exchange for K+. These ions bind to very few organic molecules and hence cannot activate changes at nerve cell termini. At nerve/muscle or nerve/nerve synapse junctions, it is therefore necessary to revert to earlier very short range communication using the activity effective ions, Ca2+, and fast organic transmitters, which do bind to receptors much as described previously. The feature to be noted here is that the nerve cell electrolytic message system is based upon the most primitive cell gradients of Na+, K+ and Cl−, which were necessarily created by the earliest prokaryotes for osmotic protection due to the ‘poisonous’ nature of the sea. It is this depolarization current which opens the calcium channels at synapses. A new Na+/K+ ATPase pump is involved in gradient recovery (figure 12), in which we show how pumps evolved sequentially. We also note how the use of elements such as Ca, Zn, Na and Mg has increased from yeast to worms (table 7). (It is not absolutely the case that K+ ions do not bind to organic molecules and K+ has found some special use in DNA and a few enzymes.)
The scavenging ability of animals was further increased by the organization of the nervous system in the brain firstly in primitive worms and then increasingly up to the arrival of humankind. The earliest brain gave automatic responses to the searching power for plant material, food, and enable avoidance of predators, mostly rather similar animal species. The next stage in the progress of the brain was to organize the nerves to form a unit not only to coordinate many inputs and outputs (automatic response), but also to remember events. It then learnt to control appropriately the best action opposite a previously met experience. This is not a genetic response, though genes create the basic framework of the brain but with poor connectivity. Connectivity now comes from experience. Detailed shaping of a retained memory has no code.
We can follow the very fast development of the brain in evolution, but as yet the reasons for the rate of change and for the inorganic element and organic transmitter distributions and its many compartments are unknown (Williams 2003). It is both the new uses of Na+, K+ and Cl− together with the stored element and selected compound distribution in the brain, and their consequences that make these animals a new chemotype. It is not possible to give an explanation of the brain's rapid evolution by reference to genes.
The use of the brain now made it possible to construct externally using natural environmental material. We observe animals building nests, making barriers in streams and digging holes. Moreover, the animals are able to protect and feed their offspring. Finally, they are able to map the world outside themselves. A moot point is the degree to which they have self-recognition and self-consciousness, but they do have suggestive intriguing behaviour patterns. Many of these additional details help to define species, but they do not define a new chemotype as the chemistry has not changed much in this physical activity. It was increase in awareness of the nature of the chemical environment that did allow one species to be very decidedly a quite new chemotype, Homo sapiens.
Now, we may ask what kind of information is stored in the brain. It is clearly very different from that in the one-dimensional chemical sequences of DNA related to genes. It is created from nerve messages originating in the senses as well as a basic underlying structure of nerve tissue laid down under genetic control. The stored image can be recalled and used in activity. It gives rise to controlled external activity much as DNA information gives rise to controlled internal activity. The image is a consequence of initial electrical pulses from the senses, but these pulses are converted into synaptic chemical and charge stores, e.g. of Na+, K+. If the same senses are stimulated repeatedly, they cause synapse growth and long-term memory. This is the equivalent of a new gene in a bacterium, but it is a system's energized property. Now, an image has a boundary zone in the brain (Kull 2000), and inside the boundary there is activity detected as electric waves. The only immediate parallel the author can see is with the boundaries of clouds and their constant physical circulation. Here, the images (or the reality in the cloud) are not coded in a manner related to DNA or even to digital storage in a computer. They are a steady state of flow phenomenon.
9. Humankind
While the use of the Na+/K+ gradients and the whole apparatus of the brain allowed animals with brains to be classified as a separate chemotype, it is very clear that the evolution of humankind today is to a different chemical organizational level. While the most sophisticated animals use the environment in learnt physical ways, humans use it by rapidly introducing understanding and to active innovation in chemistry. The novel chemistry and physics using empirical methods, starting from around 1600 and extending to date, is the employment of the whole range of 92 chemical elements in the Periodic Table and a few synthesized elements, not the 20 or so of which the genome is aware. The consequences of this knowledge and that of physical properties of materials, and the accompanying understanding of them, have led to huge industries. It is the exploitation of chemicals not only in construction, but also in the necessary novel modes of transport and communication which is a vast extension of external ‘biological’ activity heading towards a complete control over energy and all species and minerals on Earth's surface and the remote possibility of a final steady state (Gaia?). There is no doubt that this single species has the attributes of a vastly different chemotype, which has evolved amazingly rapidly with little change in genetic complement. This chemotype is a striking witness to the ever increasingly deep involvement of the environment, material and energy, and organisms in one system, which had to be to increase energy degradation. Starting from the origin of life, organisms based on environmental material and energy have increasingly changed the environment and then, of necessity, they have become more and more interactive with that through senses and hence an ability to adapt to and use information gained from that (Kull 2000). At the same time, consistent with the systematic increase in organization internally is the systematic reflective use of the extension of symbiosis. The latest product, humans, is the least effective chemical factory dependent on supplies of many vitamins, amino acids and sugars from earlier organisms. Through logical thought, humans are making more striking environmental changes rapidly. All the time, the environmental change reacts back and will force organisms to change (but note that the fastest genetic readjustment lies with prokaryotes alone). Every new chemotype in the environment/organism ecosystem forces change on it all (tables 4 and 8), but we must see this as a logical development of the pressure to increase energy and material adsorption and energy degradation: life itself creates the changes.
Before closing this account, we have to recognize that humans' fast developing chemical activities have grave risks. Biological systems are DNA coded and cannot change as quickly as the DNA-independent functions, which are now linked to the gene-independent activity of the brain. Humans must become the caretaker but not the spoiler of the ecosystem (figure 16) and must be aware not only of physical effects, such as global warming, but also of the whole range of chemical problems for organisms which is being created. A great advance but a great danger is protection of any organism creating comfort but overpopulation now using chemistry not related to genes. The selfish gene cannot lead to a balanced environment/organism ecosystem, which has to be a global tolerance of many species and individuals in harmony. This can only be achieved by humans using their ability to rationalize collectively.
Figure 16.
Conical figure of the evolution of chemotypes. The axis of the cone indicates the time from the origin to date and simultaneously the increase of oxidized chemicals in both the organisms and the environment. Circular sections of the cone and its divisions shows the development of chemotypes all increasing in species numbers and chemical variety from the outside of the cone which is associated with anaerobic conditions to the inside which is increasingly in oxidized conditions. The cone then represents the chemical space in which organisms can exist. Outside the cone is a vast range of chemistry which is now being explored by humans. Compare the pictures of evolutionary trees (figure 2).
10. Addendum: genetics and the environment
There is now a puzzle. How do we make a synthesis of the logical history of chemotypes (figure 16) and the notion of the random appearance of species? The answer must lie in the interaction between the environment and genes or gene products since for organisms to evolve genes had to change with time as the organisms caused environmental change. There is no doubt that much change of DNA is random and we cannot see a rationale for the appearance of each of so many different species of similar organisms, for example among four-legged animals. Then, this is not a question of environmental pressure acting on genes to create similar species. However, the major changes in evolution which we have classified under chemotypes clearly arise as a response to earlier environmental changes. These changes are in a particular chemical direction, oxidation, produced by the very nature of the reductive metabolism of organisms. It is time to look again for a directed long-term effect of the environment on genetic change. One possibility is that the change of genes is in localized regions connected to the initial adverse effect of novel environments. Is this why bacteria have separate regions of DNA plasmids which carry so much of adaptation? It may be that completely random search by mutation is not general but constrained to some extent as appears to be the case in the immune response (Neuberger et al. 2003). We must leave this possibility to experimental test (Jablonka & Lamb 1995; Turner 2001; Caporale 2004).
It could well be that alternatively the gene changes are not affected so directly by the environment but that random variation of DNA, speciation, is the only way that DNA can find the direction of the drive towards optimal energy use and degradation in tune with the ecosystem. Such variation can be, in part, misdirected and individuals (and species) can behave selfishly. However, the overall direction remained inevitable until humans appeared. Through chemistry, and increasingly through control of breeding and genetics, humans have become freed from the development by random gene variation matching the environmental changes. The use of brain and knowledge obtained about the environment has made a quite new chemistry possible. However, this activity has risks for all organisms totally dependent on DNA for control over a more limited chemistry. Can humans handle the risks? Humans must see the nature of the whole of the environment/organism ecosystem and conform to its direction in the search for a cyclic steady state.
Acknowledgments
The data for the tables and the figures are obtained from Williams & Fraústo da Silva (2006). Very extensive references are given there to all topics in this article. The author is extremely grateful to two referees for their comments and aid in clarification of the text.
Notes
Environmental element concentrations and time. In order to obtain concentrations in the sea, we assume that all available inorganic ions equilibrate with potential partners and have done so at all times. Included are the oxidation states of the elements. A general proof of these statements follows a consideration of complex ion chemistry and the solubility products of the oxides (hydroxides), sulphides (figure 4), carbonates and sulphates. Evidence from iron mineral deposits and isotopes of sulphur dates the rise of oxygenation and loss of hydrogen sulphide (becoming sulphate) from the oceans. The loss of Fe2+ and HS− then allowed the further oxidation of less readily soluble transition metal sulphides. We have used the oxidation/reduction poise as it changed with time to estimate the increases of zinc and copper concentrations with time (Fraústo da Silva & Williams 2001).
The inorganic ions in cells, free and bound, have been measured in many cell types representing organisms from the earliest times to date. Data on free K+, Na+, Mg2+, Ca2+, Fe2+ and Zn2+ are now robust. Recently, a number of binding proteins for several important elements have become available from genetic analysis in these cells (Dupont et al. 2006). From studies of model systems and direct examination of protein metal ion exchange, we know that over time periods of a few microseconds to a few minutes the large majority of sites of ion binding come to equilibrium. All evidence gives rise to the picture of free ion concentrations in figure 5 and tables 1 and 2 (Williams & Fraústo da Silva 2006). The chemical composition is partly linked to element in and out pumping and partly to gene expression controlled by feedback linkage to the free element concentration.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. 4th edn. Garland Publishing; New York, NY: 2002. Molecular biology of the cell. [Google Scholar]
- Andersson S.G, Karlberg O, Kanback B, Kurland C.G. On the origin of mitochondria: a genomics perspective. Phil. Trans. R. Soc. B. 2003;358:165–177. doi: 10.1098/rstb.2002.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale L.H. McGraw-Hill; New York, NY: 2004. Darwin in the genome. [Google Scholar]
- Carafoli E, Klee C, editors. Calcium as a cellular regulator. Oxford University Press; New York, NY: 1999. [Google Scholar]
- Cavalier-Smith T, Brasier M, Embley T.M. Molecular steps in cell evolution: palaeontological, molecular and cellular evidence of their timing and global effects. Phil. Trans. R. Soc. B. 2006;361:843–1083. [PubMed] [Google Scholar]
- Corning P.A. Synergy and self-organisation in the evolution of complex systems. Syst. Res. 1995;12:89–121. [Google Scholar]
- Corning P.A. Control information. Kybernetes. 2001;30:1272–1288. [Google Scholar]
- de Duve C. Oxford University Press; New York, NY: 2002. Life evolving. [Google Scholar]
- Dupont C.L, Young S, Palenik B, Bourne P.E. Modern proteomes contain imprints of ancient shifts in ocean chemistry. Proc. Natl Acad. Sci. USA. 2006;103:17 822–17 827. doi: 10.1073/pnas.0605798103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraústo da Silva J.J.R, Williams R.J.P. 2nd edn. Oxford University Press; Oxford, UK: 2001. The biological chemistry of the elements—the inorganic chemistry of life. [Google Scholar]
- Holland H.D. The oxygenation of the atmosphere and oceans. Phil. Trans. R. Soc. B. 2006;361:903–916. doi: 10.1098/rstb.2006.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E, Lamb M. Oxford University Press; New York, NY: 1995. Epigenetic inheritance and evolution. [Google Scholar]
- Kasting J.F, Ono S. Palaeoclimates: the first two billion years. Phil. Trans. R. Soc. B. 2006;361:917–929. doi: 10.1098/rstb.2006.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasting J.F, Siefert J.L. Life and the evolution of Earth's atmosphere. Science. 2002;296:1066–1068. doi: 10.1126/science.1071184. [DOI] [PubMed] [Google Scholar]
- Klug A. The discovery of zinc fingers and their development for practical applications in gene regulation. Proc. Jpn Acad. Ser. B. 2005;81:87–102. doi: 10.2183/pjab.81.87. [DOI] [PubMed] [Google Scholar]
- Kull K. Organisms can be proud to have been their own designers. Cybern. Hum. Knowing. 2000;7:45–55. [Google Scholar]
- Lovelock J. Oxford University Press; Oxford, UK: 2000. Homage to Gaia. [Google Scholar]
- Margulis L. Basic Books; New York, NY: 1998. Symbiotic planet. [Google Scholar]
- Maynard-Smith J, Szathmàry E. W.H. Freeman; New York, NY: 1995. The major transitions of evolution. [Google Scholar]
- Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Miyakawa S, Yamanashi H, Kobayashi K, Cleaves H.J, Miller S.L. Prebiotic synthesis from CO atmospheres: implications for the origin of life. Proc. Natl Acad. Sci. USA. 2002;99:14 628–14 631. doi: 10.1073/pnas.192568299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.P, Martin-Almedina S, Gonzalez-Florezm M.I, Fernandez M.P. Calcium proteins in organisms of different classes. Biochim. Biophys. Acta. 2004;1742:133–140. doi: 10.1016/j.bbamcr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Morowitz H.J. Yale University Press; New Haven, CT: 1992. Beginnings of cellular life. [Google Scholar]
- Morowitz H.J. Oxford University Press; New York, NY: 2002. The emergence of everything. [Google Scholar]
- Neuberger M.S, Harris R.S, Di Noia J.M, Petersen-Mahrt S.K. Immunity through DNA. Trends Biochem. Sci. 2003;28:305–312. doi: 10.1016/S0968-0004(03)00111-7. [DOI] [PubMed] [Google Scholar]
- Outten C.E, O'Halloran T.V. Femtomolar sensitivity of metallo regulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. N.B. The quantitative values may be too low. [DOI] [PubMed] [Google Scholar]
- Russell M.J, Hall A.J. The onset and early evolution of life. Geol. Soc. Am. Memoir. 2006;198:1–32. [Google Scholar]
- Saito M.A, Sigman D.M, Morel F.M.M. The bio-inorganic chemistry of the ancient oceans. Inorg. Chim. Acta. 2003;356:308–320. doi: 10.1016/S0020-1693(03)00442-0. [DOI] [Google Scholar]
- Summons R.E, Bradley A.S, Jahnke L.L, Waldbauer R. Steroids, triterpenoids and molecular oxygen. Phil. Trans. R. Soc. B. 2006;361:951–968. doi: 10.1098/rstb.2006.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B.M. Blackwell Science; London, UK: 2001. Chromatin and gene regulation—molecular mechanisms in epigenetics. [Google Scholar]
- Williams R.J.P. Possible functions of chains of catalysts. J. Theor. Biol. 1961;1:1–17. doi: 10.1016/0022-5193(61)90023-6. [DOI] [PubMed] [Google Scholar]
- Williams R.J.P. The biochemical chemistry of the brain and its possible evolution. Inorg. Chim. Acta. 2003;356:27–40. doi: 10.1016/S0020-1693(03)00471-7. [DOI] [Google Scholar]
- Williams R.J.P, Fraústo da Silva J.J.R. Clarendon Press; Oxford, UK: 1996. The natural selection of the chemical elements—the environment and life's chemistry. [Google Scholar]
- Williams R.J.P, Fraústo da Silva J.J.R. The involvement of molybdenum in life. Biochem. Biophys. Res. Commun. 2002;292:293–299. doi: 10.1006/bbrc.2002.6518. [DOI] [PubMed] [Google Scholar]
- Williams R.J.P, Fraústo da Silva J.J.R. Elsevier; Amsterdam, The Netherlands: 2006. The chemistry of evolution: the development of our ecosystem. [Google Scholar]
- Woese C.R. On the evolution of cells. Proc. Natl Acad. Sci. USA. 2002;99:8742–8747. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]