Abstract
Inbred strains are genetically stable across time and laboratories, allowing scientists to accumulate a record of phenotypes, including physiological characteristics and behaviors. To date, the C57/C58 family of inbred mouse strains has been identified as having the highest innate ethanol consumption, but some lineages have rarely or never been surveyed. Thus, the purpose of the present experiment was to measure ethanol preference and intake in 22 inbred mouse strains, some of which have never been tested for ethanol consumption. Male and female mice (A/J, BALB/cByJ, BTBR+Ttf/tf, BUB/BnJ, C57BL/6J, C57BLKS/J, C58/J, CZECH/Ei, DBA/2J, FVB/NJ, I/LnJ, LP/J, MA/MyJ, NOD/LtJ, NON/LtJ, NZB/B1NJ, NZW/LacJ, PERA/Ei, RIIIS/J, SEA/GnJ, SM/J, and 129S1/SvlmJ) were individually housed and given unlimited access in a two-bottle choice procedure to one bottle containing tap water and a second containing increasing concentrations of ethanol (3%, 6%, 10%), 0.2% saccharin, and then increasing concentrations of ethanol (3%, 6%, 10%) plus 0.2% saccharin. Mice were given access to each novel solution for a total of 4 days, with a bottle side change every other day. Consistent with previous studies, C57BL/6J (B6) mice consumed an ethanol dose of > 10g/kg/day while DBA/2J (D2) mice consumed < 2g/kg/day. No strain voluntarily consumed greater doses of ethanol than B6 mice. While the C58 and C57BLKS strains showed high ethanol consumption levels that were comparable to B6 mice, the BUB and BTBR strains exhibited low ethanol intakes similar to D2 mice. The addition of 0.2% saccharin to the ethanol solutions significantly increased ethanol intake by most strains and altered the strain distribution pattern. Strong positive correlations (rs ≥ 0.83) were determined between consumption of the unsweetened versus sweetened ethanol solutions. Consumption of saccharin alone was significantly positively correlated with the sweetened ethanol solutions (rs = 0.62 – 0.81), but the correlation with unsweetened ethanol solutions was considerably lower (rs = 0.37 – 0.45). These results add new strains to the strain mean database that will facilitate the identification of genetic relationships between voluntary ethanol consumption, saccharin preference, and other phenotypes.
Keywords: alcohol, saccharin, preference, male, female
INTRODUCTION
No single animal model can duplicate a phenotype as complex as alcoholism in its entirety. Nonetheless, animal models of oral ethanol self-administration are widely used for examining specific aspects of behavior and physiology relevant for understanding alcoholism (Cunningham et al., 2000). The most common way to examine oral ethanol consumption is the two-bottle choice procedure, in which animals have continuous free access to two bottles containing water or a dilute ethanol solution. By measuring the volume of ethanol and water consumed, one can elucidate behavioral patterns amongst rodents of different genetic backgrounds.
It has long been established that some inbred strains prefer to drink an ethanol solution (McClearn and Rodgers, 1959), while others do not. However, there are more than one hundred inbred mouse strains derived from seven lineages (Petkov et al., 2004), and only a handful have been examined for this phenotype (e.g., Rodgers, 1966; Belknap et al., 1993). These two studies measured voluntary two-bottle choice preference drinking in 14–15 inbred strains, in which an animal was given a choice between a bottle containing a dilute ethanol solution (often 10% v/v in tap water) and a bottle containing tap water. Consistent with previous reports, C57BL/6 (B6) mice voluntarily consumed an ethanol dose > 10 g/kg/day, whereas DBA/2 (D2) consumed < 2 g/kg/day, patterns that have often been replicated (e.g., Fuller, 1964). However, some lineages have rarely or never been surveyed (e.g., Japan NZ lineage or wild-derived strains).
There are several advantages of testing multiple inbred strains. Once established, each strain remains genetically stable over time and across laboratories because same-sex individuals within an inbred strain are genetically identical and possess two identical alleles at each locus (discussed in Crabbe, 1999). This allows geneticists to build a cumulative database. When multiple inbred strains are compared for a behavioral response under carefully controlled environmental conditions, the difference among strain means is largely genetic in origin. Thus, another advantage to inbred strain studies is that testing multiple strains that exhibit a range of behavioral responses will allow estimation of genetic correlations. For example, recent work documented that preference for a 10% ethanol solution is highly stable over a period of 40–50 years, with most strain correlations across studies in the range of 0.89 – 0.98 (Wahlsten et al., 2006). (n.b., this comparison included some of the 10% ethanol preference data reported in the current manuscript). It should be noted that inbred strains have the limitation that all loci are fixed homozygous so that any effects of heterozygosity cannot be captured. This also limits their capacity to model individual differences in human populations, which feature not only heterozygosity but also rare alleles that have not been eliminated from the population by drift or selection pressures. Thus, decisions on the most appropriate animal model to examine depend on research goals. Since we wished to add to the cumulative strain mean database, we reasoned that testing a panel of inbred strains was the best strategy to achieve this goal.
When considering oral consumption of ethanol in humans and animals, sensory modalities such as taste, olfaction and chemosensory irritation are three independent systems that each play an important role in the acceptance or rejection of the alcohol solution (Bachmanov et al., 2003). Previous work looking at possible contributions to voluntary ethanol consumption in B6 and D2 mice have examined preabsorptive qualities of ethanol (e.g., taste and odor; Kampov-Polevoy et al., 1990) as well as strain differences in acceptance of an ethanol solution when water is not an alternative (Belknap et al., 1978). An examination of these mechanisms can aid in our understanding of strain differences in ethanol consumption. Previous data have shown that some strains avoid an ethanol solution unless it was masked by the sweet taste of saccharin (e.g., Belknap et al. 1993). Certainly, operant ethanol self-administration studies pioneered by Samson (1986) demonstrated that introducing ethanol into a sweet solution and then fading out the sweet solution produced reliable responding for and consumption of ethanol in approximately 2 months (e.g., Samson, 1986; Weiss et al., 1990). Interestingly, recent work found that mutant mice with a deletion of a gene critical in taste transduction (e.g., α-gustducin (Gnat3), Tas1r3 or Trpm5) significantly decreased preference for alcohol and saccharin solutions without altering consumption of sodium chloride solutions (Blednov et al. 2007). These findings are consistent with studies demonstrating a strong association between the consumption of ethanol and sweetened fluids (e.g., Belknap et al., 1993; Kampov-Polevoy et al., 1999; Blizard and McClearn, 2000).
The purpose of the present study was to examine a panel of 22 inbred strains, some of which have never been tested, for their consumption of ethanol solutions at different concentrations in the presence or absence of saccharin, with water freely available. In general, the method described in Belknap et al. (1993) was utilized with a small modification, as this method elicited three patterns of strain responses regarding their voluntary consumption of ethanol, ethanol plus 0.2% saccharin, or 0.2% saccharin solutions. By following essentially the same protocol used by Belknap et al. (1993), we reasoned that the resulting data could be combined with the Belknap data when added to the Mouse Phenome Database (MPD), greatly strengthening subsequent correlational analyses with other traits (Grubb et al., 2004). An additional advantage to allowing mice 4 days to consume each solution is that recent work indicated that two-bottle choice tests (conducted across a range of 1 – 6 days) were most sensitive to detect strain differences when they lasted 4 days (Tordoff and Bachmanov, 2002). The 0.2% saccharin solution was chosen based on early work indicating that it is the maximally preferred concentration for both the B6 and D2 inbred strains (Fuller, 1974). Additional goals were to: 1) Estimate the coefficient of genetic determination (or heritability) for these consumption traits (i.e., the proportion of observed variability that is genetically determined); 2) Examine genetic correlations, or the degree to which two phenotypes show common genetic influence, across other ethanol-related behaviors and archived data; and 3) Identify strains that exhibit very high or very low ethanol consumption.
METHODS
Subjects
Studies were conducted on drug-naïve, male and female mice from 22 inbred strains. Mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and shipped to Portland, Oregon at 6–8 weeks of age. The number of animals per strain was as follows, with nearly equal numbers of each sex tested per strain: A/J (N = 16), BALB/cByJ (N = 16), BTBR+Ttf/tf (N = 16), BUB/BnJ (N = 10), C57BL/6J (N = 16), C57BLKS/J (N = 15), C58/J (N = 15), CZECH/Ei (N = 10), DBA/2J (N = 16), FVB/NJ (N = 16), I/LnJ (N = 16), LP/J (N = 16), MA/MyJ (N = 16), NOD/LtJ (N = 16), NON/LtJ (N = 16), NZB/B1NJ (N = 16), NZW/LacJ (N = 16), PERA/Ei (N = 14), RIIIS/J (N = 16), SEA/GnJ (N = 16), SM/J (N = 16), and 129S1/SvlmJ (N = 16). The strains were tested in six passes (A–F), with each pass comprised of 42 – 64 mice. The order of testing the strains was dictated by the availability from Jackson Laboratory, and strain shipments were staggered so that 4 strains were tested in passes A–D and 3 strains were tested in passes E–F. Testing occurred across the months of July 2002 – February 2003. Mice were group housed (4 to a cage, separated by sex and strain) in the Department of Veterans Affairs Veterinary Medical Unit in clear polycarbonate/polysulfone cages (28 × 18 × 13 cm) on Bedicob bedding. Animals were allowed to acclimate an average of 1 month to the temperature (22 ± 1°C) and humidity controlled environment with a 12:12 hour light/dark cycle (lights on at 0600) prior to experimentation, which was due in part to the manner in which strain shipments were received. Thus, the age range when ethanol access began varied for each pass: Pass A, B & F (60 – 80 days), Pass C (90 – 100 days), Pass D (120 – 130 days) and Pass E (90 – 140 days). Rodent chow (Purina 5001) and water were given ad libitum throughout the study. To acclimate to the drinking bottles used in the study, the mice were individually housed 1 day before the start of the study with rodent chow and continuous access to 2 bottles (25 ml graduated cylinders) containing tap water. All procedures followed the United States Public Health Service and National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care, and were approved by the local Institutional Animal Care and Use Committee.
General Procedures
All mice had 24 hr access to two inverted bottles with metal sippers placed on stainless steel cage tops. Food was distributed near both bottles to avoid food associated tube preferences. One tube always contained tap water and the other contained increasing concentration of ethanol (3%, 6%, 10% v/v in tap water; 3E, 6E, 10E, respectively), 0.2% w/v saccharin (0.2S) in tap water, and then increasing concentrations of ethanol (3E, 6E, 10E) plus 0.2% w/v saccharin in water (3E+0.2S, 6E+0.2S, 10E+0.2S, respectively).
Mice had access to each novel solution for 4 days; with bottle positions alternated every other day. Fresh fluids were provided each time the concentration was changed. Body weight was recorded simultaneously with each solution change, and cages were changed every 8 days. Each morning, daily fluid intake (to the nearest 0.2 ml) was recorded from both bottles by measuring the level of the meniscus on the graduated drinking tube. Ethanol ± saccharin and water tubes placed on two empty cages allowed for the measurement of leakage and evaporation from the tubes. The average volume depleted from these “control” tubes was subtracted from the individual drinking volumes each day.
Data Analysis
The primary dependent measure was dose consumed, expressed as g/kg per day (ethanol) or mg/kg per day (saccharin). Other dependent measures were volume of ethanol, saccharin or water consumed (mls), body weight, and preference ratio (calculated as the volume of ethanol, ethanol+saccharin, or saccharin consumed divided by the total volume consumed). Drinking data were analyzed with our standard laboratory procedure. In brief, the average of the 2nd and 4th day of each set of solution pairing conditions was used, as this allowed the mouse to stabilize its consumption after a concentration and/or tube position change. Analysis of variance (ANOVA) assessed strain, sex and concentration (repeated measure) effects on the dependent variables. Pass was not used as a factor in the analyses because it was unequally represented (i.e., separate strains were tested in each pass). Significant interactions were pursued with one-way ANOVAs at each concentration and post-hoc tests. Results from the one-way ANOVA also provided an estimate of genetic effect size (sum of squares between strains from the ANOVA divided by sum of squares total). Correlational analyses were used to examine the relationship between strain mean values for consumption of the various ethanol concentrations in the presence and absence of 0.2% saccharin with other ethanol-related behaviors [database maintained by the Portland Alcohol Research Center (PARC); http://sunweb1.ohsu.edu/parc/index.htm] and non-ethanol-related behaviors [MPD maintained by Jackson Laboratory; http://www.jax.org/phenome].
Data are presented as the mean ± SEM. The significance criterion was established at P ≤ 0.05. Statistical analyses were conducted with the computer program SYSTAT (version 11, SYSTAT Software, Inc., Richmond, CA).
RESULTS
Ethanol Consumption
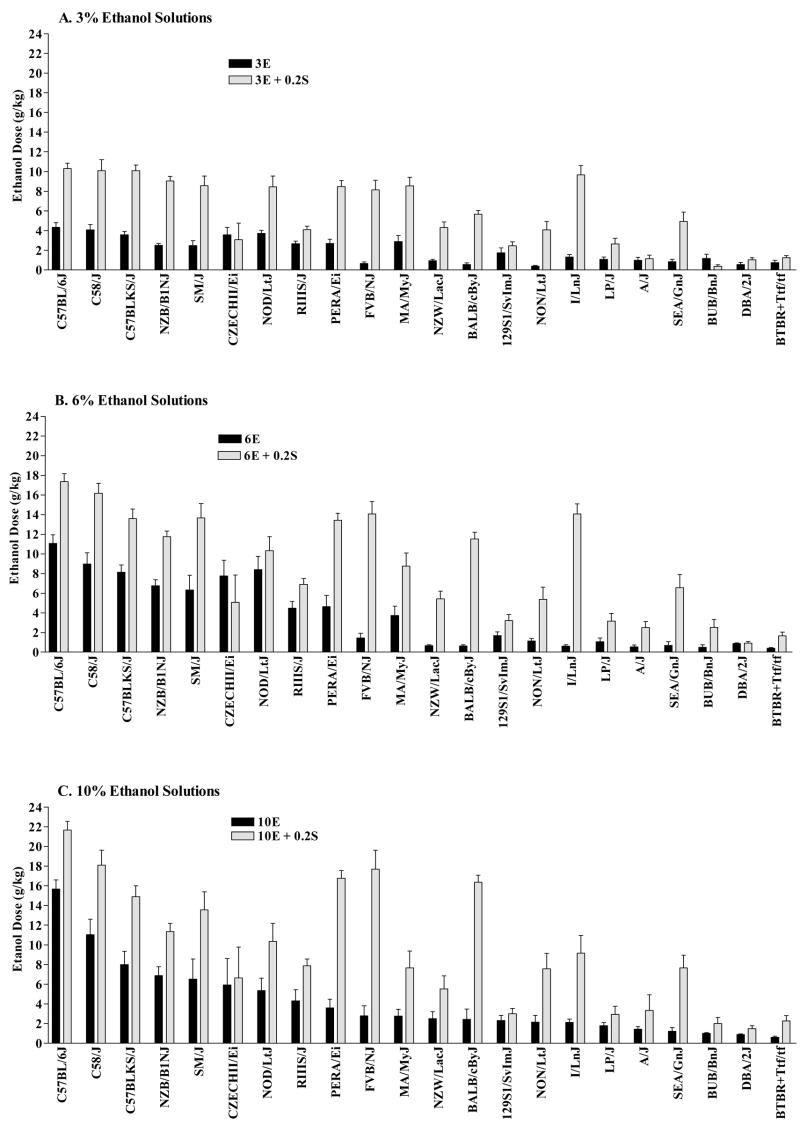
The ethanol dose consumed from the different ethanol solutions in the presence and absence of saccharin for the 22 inbred mouse strains is depicted in Figure 1. Strains are ranked from the highest to lowest ethanol dose consumed, based on consumption of 10E (Figure 1C). Analysis of the ethanol solutions (3E, 6E, 10E) confirmed that there were significant strain differences in the ethanol dose consumed [F(21,290) = 31.238, P < 0.001]. Estimates of genetic effect size suggested that 47%, 60% and 49% of the variance in ethanol dose consumed from the 3E, 6E and 10E solutions, respectively, could be explained by genotype. The main effect of sex on ethanol dose [F(1,290) = 57.505, P < 0.001] suggested that consumption was significantly higher in female than in male mice. Repeated measures ANOVA indicated that consumption of the different ethanol solutions significantly influenced the ethanol dose consumed [F(2,580) = 86.323, P < 0.001]. The interactions between sex and strain [F(21,290) = 4.042, P < 0.001], sex and ethanol solution [F(2,580) = 8.322, P < 0.001], as well as among sex, strain and solution [F(42,580) = 1.584, P < 0.02] suggested that the higher ethanol intake in the female versus male mice was not consistent across all strains and ethanol solutions.
FIGURE 1. Voluntary intake of a (A) 3% ethanol, (B) 6% ethanol, or (C) 10% ethanol solution in the presence or absence of 0.2% saccharin in mice from 22 inbred strains.
Values represent the mean ± SEM ethanol intake (g/kg/day) for 15–16 mice/strain, except for CZECH and BUB (n = 10) or PERA (n = 14). Strains are rank ordered for consumption of 10% ethanol (Figure 1C).
This conclusion was supported by the separate analyses of each ethanol solution. For each solution, there were main effects of sex [Fs(1,291) ≥ 21.225, Ps < 0.001] and strain [Fs(21,291) ≥ 16.001, Ps < 0.001], with significant interactions between main effects [Fs(21,291) ≥ 2.302, Ps ≤ 0.001]. Subsequent analyses confirmed that the number of strains in which the ethanol dose consumed was significantly higher in female than in male mice varied for the different solutions: consumption of the 3E solution was significantly higher in females in 5 strains (CZECH, MA, NOD, PERA and SM); consumption of the 6E solution was significantly higher in female mice from 6 strains (B6, CZECH, NOD, PERA, RIIIS and SM), with a trend for an increase in female mice from 2 strains (MA and NZB); whereas consumption of the 10E solution was significantly higher in female mice from 3 strains (NZB, PERA and SM), with a trend for an increase in female B6 mice.
The addition of saccharin to the ethanol solutions changed the strain distribution pattern for ethanol dose consumed, as clearly illustrated in Figure 1. Saccharin produced a dramatic increase in consumption of some (e.g., BALB, PERA, I/Ln) but not all (e.g., D2, BUB, LP) inbred strains that initially exhibited low ethanol consumption in the absence of saccharin. But 0.2S also increased intake in strains with high ethanol intake in the absence of sweetener. The ethanol dose consumed was significantly influenced by strain [F(21,282) = 35.912, P < 0.001] and the concentration of the sweetened solution [F(2,564) = 205.569, P < 0.001]. Estimates of genetic effect size suggested that 58%, 64% and 58% of the variance in ethanol dose consumed from the 3E+0.2S, 6E+0.2S and 10E+0.2S solutions, respectively, could be explained by genotype. Consumption of the sweetened ethanol solutions was significantly higher in female than in male mice [F(1,282) = 90.663, P < 0.001], and there was a significant interaction between sex and strain [F(21,282) = 2.920, P < 0.001] as well as between sex and solution [F(2,564) = 10.048, P < 0.001]. However, the 3-way interaction was not significant.
Ethanol Preference
Preference ratios for all solutions tested in the 22 inbred strains are depicted in Table 1. Strains are ranked from the highest to lowest preference ratio, based on preference for 10E. Estimates of genetic effect size suggested that 44%, 60% and 45% of the variance in preference for the 3E, 6E and 10E solutions, respectively, could be explained by genotype, similar to estimates for the ethanol dose consumed (see above). As with ethanol dose, the ethanol preference ratio was significantly influenced by strain [F(21,291) = 26.973, P < 0.001], sex [F(1,291) = 25.134, P < 0.001], and concentration of the ethanol solution [F(2,582) = 32.10, P < 0.001]. While the interactions between sex and strain [F(21,291) = 2.573, P < 0.001], sex and solution [F(2,582) = 3.416, P < 0.05] and strain and solution [F(42,582) = 4.882, P < 0.001] were significant, the 3-way interaction was not significant.
TABLE 1.
Preference for ethanol and saccharin solutions in 22 inbred strains
| Inbred Strain | 3E | 6E | 10E | 0.2S | 3E+0.2S | 6E+0.2S | 10E+0.2S |
|---|---|---|---|---|---|---|---|
| C57BL/6J (n = 16) | 54.8 ± 6.5 | 77.0 ± 5.5 | 79.4 ± 4.6 | 94.5 ± 2.9 | 96.5 ± 1.3 | 97.7 ± 0.5 | 97.3 ± 0.4 |
| C58/J (n = 12–15)a | 58.4 ± 6.1 | 65.3 ± 7.9 | 53.3 ± 8.1 | 87.7 ±6.3 | 88.4 ± 5.2 | 93.5 ± 3.4 | 84.8 ± 5.0 |
| NZB/B1NJ (n = 16) | 47.0 ± 3.7 | 59.9 ± 4.3 | 44.2 ± 6.1 | 93.8 ± 1.6 | 95.9 ± 0.6 | 89.9 ± 3.2 | 64.9 ± 4.2 |
| C57BLKS/J (n = 15) | 50.2 ± 4.5 | 68.7 ± 6.6 | 37.5 ± 7.3 | 85.3 ± 5.6 | 97.9 ± 0.7 | 93.1 ± 6.0 | 78.7 ± 6.2 |
| SM/J (n = 16) | 29.7 ± 6.1 | 41.5 ± 9.3 | 30.5 ± 9.2 | 90.1 ± 4.4 | 82.3 ± 7.7 | 79.6 ± 7.2 | 54.6 ± 7.0 |
| RIIIS/J (n = 16) | 55.3 ± 4.7 | 47.8 ± 6.9 | 26.2 ± 7.6 | 84.6 ± 5.1 | 76.7 ± 6.4 | 69.3 ± 5.9 | 47.6 ± 5.1 |
| NOD/LtJ (n = 16) | 48.5 ± 2.3 | 54.6 ± 7.8 | 22.5 ± 5.2 | 91.6 ± 5.0 | 84.0 ± 6.1 | 73.1 ± 9.2 | 51.6 ± 9.0 |
| NZW/LacJ (n = 16) | 20.7 ± 3.3 | 6.6 ± 1.2 | 18.2 ± 6.4 | 93.0 ± 4.4 | 70.8 ± 6.5 | 49.4 ± 6.4 | 29.4 ± 6.3 |
| CZECH/Ei (n = 8–10)b | 43.9 ± 8.8 | 49.8 ± 11.0 | 17.0 ± 7.0 | 38.9 ± 10.3 | 24.8 ± 12.5 | 26.0 ± 13.8 | 27.0 ± 13.5 |
| PERA/EiJ (n = 14) | 41.9 ± 6.0 | 36.9 ± 8.7 | 16.7 ± 4.2 | 89.0 ± 4.2 | 97.6 ± 0.3 | 94.6 ± 1.5 | 89.5 ± 3.0 |
| FVB/NJ (n = 15–16)c | 13.9 ± 4.3 | 11.8 ± 3.6 | 15.3 ± 5.8 | 78.3 ± 7.5 | 85.8 ± 6.7 | 91.7 ± 4.0 | 82.7 ± 5.9 |
| NON/LtJ (n = 16) | 7.9 ± 1.4 | 8.6 ± 1.7 | 15.2 ± 6.3 | 98.2 ± 1.0 | 53.1 ± 8.4 | 45.2 ± 8.5 | 43.6 ± 8.8 |
| BALB/cByJ (n = 15–16)d | 10.2 ± 3.1 | 5.9 ± 1.0 | 14.0 ± 6.5 | 84.3 ± 7.4 | 91.8 ± 3.5 | 91.7 ± 3.5 | 90.1 ± 4.3 |
| 129S1/SvlmJ (n = 15–16)e | 24.2 ± 5.0 | 21.0 ± 5.1 | 13.2 ± 3.5 | 65.2 ± 6.3 | 48.9 ± 7.8 | 35.2 ± 7.4 | 17.4 ± 3.8 |
| MA/MyJ (n = 16) | 36.8 ± 7.9 | 25.3 ± 6.8 | 10.8 ± 3.1 | 82.4 ± 4.9 | 86.2 ± 4.7 | 62.6 ± 8.9 | 34.4 ± 7.5 |
| I/LnJ (n = 13–16)f | 22.8 ± 3.3 | 5.0 ± 1.2 | 10.4 ± 2.1 | 70.8 ± 6.4 | 94.4 ± 3.0 | 93.0 ± 1.6 | 49.1 ± 9.1 |
| LP/J (n = 16) | 19.1 ± 4.7 | 9.7 ± 3.5 | 8.5 ± 1.9 | 73.1 ± 6.0 | 39.3 ± 7.4 | 28.8 ± 7.6 | 16.5 ± 5.5 |
| A/J (n = 15–16)g | 22.4 ± 7.3 | 5.4 ± 1.5 | 8.4 ± 1.6 | 55.8 ± 9.5 | 25.5 ± 7.3 | 28.4 ± 7.6 | 13.0 ± 4.8 |
| SEA/GnJ (n = 16) | 14.0 ± 4.0 | 4.8 ± 2.6 | 7.8 ± 3.6 | 95.5 ± 2.3 | 54.4 ± 9.2 | 46.6 ± 9.7 | 31.8 ± 6.2 |
| BUB/BnJ (n = 10) | 22.4 ± 8.9 | 3.6 ± 1.6 | 5.3 ± 0.5 | 67.3 ± 8.2 | 8.6 ± 4.8 | 26.3 ± 8.8 | 16.3 ± 6.2 |
| DBA/2J (n = 15–16)h | 5.3 ± 1.4 | 5.0 ± 0.4 | 4.2 ± 1.0 | 78.5 ± 6.8 | 13.6 ± 3.6 | 5.8 ± 1.1 | 7.9 ± 3.2 |
| BTBR+Ttf/tf (n = 15–16)i | 15.5 ± 4.4 | 6.2 ± 3.1 | 2.5 ± 0.5 | 55.4 ± 3.3 | 20.5 ± 4.2 | 10.9 ± 1.7 | 10.4 ± 3.1 |
Values represent the mean ± SEM for each strain and solution, collapsed across sex, for the number of animals in parenthesis. In the cases where there was a range in the number of animals per strain, footnotes denote the number of animals per solution. The attrition in numbers for all strains (except CZECH) was due to bottles leaking (from animals chewing on the rubber stopper), resulting in an inaccurate measurement for that day. The attrition in the CZECHs was due to 2 animals that escaped from their cage when body weights were measured (this wild strain was notoriously hard to handle). Based on preference for 10E (bold font), strains are listed from highest to lowest preference ratio.
C58: n = 15, except for 10E+0.2S (n = 12)
CZECH: n = 10, except for 6E+0.2S (n = 9) and 10E+0.2S (n = 8)
FVB: n = 16, except for 6E+0.2S and 10E+0.2S (n = 15)
BALB: n = 16, except for 0.2S and 3E+0.2S (n = 15)
129: n = 16, except for 3E+0.2S (n = 15)
I/Ln: n = 16, except for 3E+0.2S (n = 15) and 0.2S (n = 13)
A/J: n = 16, except for 10E (n = 15)
DBA: n = 16, except for 3E+0.2S, 6E+0.2S and 10E+0.2S (n = 15)
BTBR: n = 16, except for 10E+0.2S (n = 15)
Preference ratio for the ethanol + saccharin solutions also was significantly influenced by strain [F(21,281) = 33.891, P < 0.001], sex [F(1,281) = 15.578, P < 0.001], and concentration of the sweetened ethanol solution [F(2,562) = 111.841, P < 0.001]. There was a significant interaction between strain and sex [F(21,281) = 2.899, P < 0.001] as well as between strain and solution [F(42,562) = 4.50, P < 0.001]. However, the 3-way interaction was not significant. Estimates of genetic effect size suggested that 63%, 61% and 61% of the variance in ethanol preference for the 3E+0.2S, 6E+0.2S and 10E+0.2S solutions, respectively, could be explained by genotype, again similar to estimates for the consumption of the sweetened ethanol solutions.
Saccharin Consumption and Preference
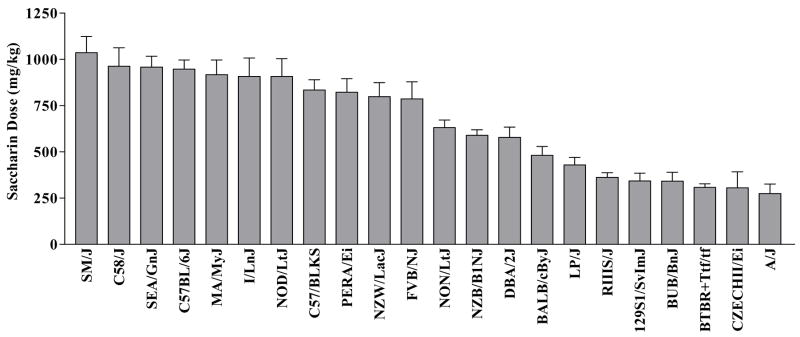
Strains differed significantly in consumption of saccharin [F(21,292) = 18.752, P < 0.001], as depicted in Figure 2. The dose of saccharin consumed also was significantly higher in female than in male mice [F(1,292) = 59.625, P < 0.001]. However, there was only a trend for an interaction between sex and strain on saccharin consumption [F(21,292) = 1.446, P < 0.10]. Estimates of genetic effect size suggested that 51% of the variance in saccharin dose consumed could be explained by genotype.
FIGURE 2. Voluntary intake of 0.2% saccharin in mice from 22 inbred strains.
Strains are rank ordered from highest to lowest consumption of saccharin. Values represent the mean ± SEM saccharin intake (mg/kg/day) for the mice depicted in Figure 1.
Saccharin preference ratio was significantly influenced by strain [F(21,288) = 6.404, P < 0.001] and is depicted in Table 1. About 31% of the variance in saccharin preference could be explained by genotype. There was no sex difference in saccharin preference, nor was the interaction between sex and strain significant.
Genetic Correlations
Correlational analyses (see Table 2) of the ethanol and saccharin consumption data found highly significant positive correlations between consumption of the three unsweetened ethanol solutions (rs = 0.83 – 0.95, Ps < 0.001, n = 22) and consumption of the three sweetened ethanol solutions (rs = 0.84 – 0.95, Ps < 0.001, n = 22). Strong positive correlations also were found between consumption of saccharin and sweetened ethanol solutions (rs = 0.62 – 0.81, Ps < 0.01, n = 22), but the correlations between consumption of saccharin and unsweetened ethanol solutions were lower (rs = 0.37 – 0.45, Ps < 0.10, n = 22).
TABLE 2.
Correlations for ethanol and saccharin consumption
| 3E | 6E | 10E | 0.2S | 3E+0.2S | 6E+0.2S | 10E+0.2S | |
|---|---|---|---|---|---|---|---|
| 3E | 1.00 | ||||||
| 6E | 0.95*** | 1.00 | |||||
| 10E | 0.83*** | 0.91*** | 1.00 | ||||
| 0.2S | 0.37+ | 0.41+ | 0.45* | 1.00 | |||
| 3E+0.2S | 0.61** | 0.65*** | 0.67*** | 0.81*** | 1.00 | ||
| 6E+0.2S | 0.57** | 0.63** | 0.71*** | 0.73*** | 0.95*** | 1.00 | |
| 10E+0.2S | 0.52** | 0.62** | 0.72*** | 0.62** | 0.84*** | 0.94*** | 1.00 |
Correlations were conducted between strain means (n = 22), collapsed across sex, for the doses of ethanol (g/kg), ethanol + saccharin (g/kg) or saccharin (mg/kg) consumed. The Pearson correlation matrix is depicted.
P < 0.10,
P < 0.05,
P≤ 0.01,
P≤ 0.001
Correlations also were calculated between the current data and consumption/preference measures reported from previous work in which common strains were evaluated. Consumption of 10E was significantly correlated between the present study and previous work by Belknap et al. (1993; r = 0.91, P < 0.05, n = 5), as was consumption of 10E+0.2S (r = 0.91, P < 0.05, n = 5), though there were few strains in common between the studies. Consumption of 10E in the present study also was significantly correlated with 10E intake in a recent study by Bachmanov (r = 0.88, P < 0.001, n = 14; see supplemental data in Wahlsten et al., 2006). Additional correlations between the present findings and those of Belknap et al. (1993) found that consumption of 10E was significantly positively correlated with consumption of 0.2S and 3E+0.2S (rs = 0.91–0.93, Ps < 0.05, n = 5). Similarly, 10E preference in the present study was highly correlated with 10E preference in early work (Rodgers, 1966; r = 0.98, P < 0.05, n = 5) as well as with the study by Bachmanov (r = 0.79, P < 0.001, n = 14).
With regard to other ethanol-related behaviors in the PARC database, we examined the intake of 10E (the most commonly used concentration across studies in the literature) and 10E+0.2S to see if intake was correlated. In all, we examined 14 other variables related to ethanol intoxication, ataxia, acute functional tolerance, anxiety, and withdrawal severity in this database, and only 4 correlations approached significance (data available upon request). Intake of 10E was positively correlated with intake of 20E in a single bottle “drinking in the dark” procedure (Rhodes et al., 2007) in both male (r = 0.80, P < 0.05, n = 8) and female (r = 0.81, P < 0.05, n = 8) mice. Consistent with multiple, previously reported genetic data sets (Metten et al., 1998), the dose of unsweetened and sweetened 10E was negatively correlated with chronic ethanol withdrawal severity, measured by the area under the withdrawal curve for handling-induced convulsions following 72 hr of continuous ethanol vapor inhalation (Metten and Crabbe, 2005). However, this correlation was not significant, possibly due to the low number of strains in common (r = −0.65 and −0.70, P ≤ 0.16 and 0.12, respectively, n = 6). Intake of sweetened 10E tended to be negatively correlated with one index of ethanol-induced ataxia, impairment of performance on the fixed speed rotarod (3 rpm) at 30 min after injection of a 1.75-g/kg ethanol dose (r = −0.74, P < 0.06, n = 7). It should be noted that there were usually only 7 strains in common, limiting the power of such correlational analyses to a large extent. Also, we made no correction for multiple comparisons, as our goal in these analyses was exploratory.
Correlations between the present data and non-ethanol-related variables in MPD yielded some interesting findings (Table 3). Here we explored all MPD variables (nearly 600) versus our 7 consumption measures for which there were at least 8 strains in common (potential for about 4000 correlations). The significance values reported are not corrected for multiple comparisons, as these analyses were frankly exploratory. Consumption of unsweetened ethanol solutions was positively correlated with preference for potassium chloride (rs = 0.65 – 0.98, Ps < 0.05, n = 13) and sodium chloride (r = 0.67, P < 0.05, n = 13) solutions, whereas consumption of sweetened ethanol solutions were negatively correlated with preference for calcium chloride solutions (rs = −0.66 – −0.73, P < 0.01, n = 13). With regard to the various activity measures depicted in Table 3, consumption of the sweetened and unsweetened ethanol solutions was positively correlated with the activity phenotypes (e.g., ambulatory activity, total activity, rearing; see Table 3 for details). Likewise, distance traveled in an open field over a 5 min test was positively correlated with consumption of unsweetened (rs = 0.67 – 0.68, Ps < 0.05, n = 12) and sweetened (rs = 0.75 – 0.79, P < 0.01, n = 12) ethanol solutions, with similar correlations when the distance traveled was separated into 1 min bins (data not shown). In contrast, the number of fecal boli during the 5 min open field test, which is often taken as an index of anxiety, was negatively correlated with consumption of sweetened and unsweetened ethanol solutions (rs = −0.63 – −0.66, P < 0.05, n = 12). Finally, consumption of sweetened and unsweetened ethanol solutions in our study was negatively correlated with bone mineral density (rs = −0.63 – −0.68, P < 0.01, n = 20) in female mice and positively correlated with aortic lesion size (rs = 0.68 – 0.83, P < 0.01, n = 19) in male mice fed an atherogenic diet for 8 weeks.
TABLE 3.
Mouse Phenome Database correlations with ethanol ± saccharin consumption
| Phenotype | 3E | 6E | 10E | 0.2S | 3E+0.2S | 6E+0.2S | 10E+0.2S |
|---|---|---|---|---|---|---|---|
| Preference | |||||||
| 450 mM NaCl | 0.67** (n = 13, ♂) | ||||||
| 300 mM KCl
200 mM KCl 100 mM KCl |
0.83**(n = 13, ♂)
0.80**(n = 13, ♂) |
0.87** (n = 13, ♂)
0.88** (n = 13, ♂) |
0.98**(n = 13, ♂)
0.71** (n = 13, ♂) 0.65* (n = 13, ♂) |
||||
| 25 mM CaCl2
30 mM CaCl2 75 mM CaCl2 |
−0.63* (n = 13, ♂) −0.68** (n = 22, ♀) |
−0.73** (n = 13, ♂) |
−0.66** (n = 22, ♂)
−0.69** (n = 13, ♂) |
||||
| Activity/Anxiety | |||||||
| Ambulatory, (breaks/min X 12 hr light)
Ambulatory, (24 hr breaks/min) Total, (12 hr light period) Rearing, (12 hr light period) |
0.67*(n = 8, ♀) |
0.69* (n = 8, ♀) 0.72* (n = 8, ♀) |
0.64+ (n = 8, ♀) |
0.89**(n = 8, ♀)
.74* (n = 8, ♀) 0.84** (n = 8, ♀) 0.79* (n = 8, ♀) |
0.72* (n = 8, ♀)
0.65+ (n = 8, ♀) 0.63+ (n = 8, ♀) |
0.79* (n = 8, ♀)
0.64+(n = 8, ♀) 0.70* (n = 8, ♀) 0.63+(n = 8, ♀) |
0.79*(n = 8, ♀)
0.67*(n = 8, ♀) 0.71* (n = 8, ♀) |
| Distance, (5 min open field) | 0.68** (n = 12, ♀) | 0.67* (n = 12, ♀) | 0.81** (n = 12, ♀) | 0.78**(n = 12, ♀) | 0.79** (n = 12, ♀) | 0.75** (n = 12, ♀) | |
| # Fecal boli, (5 min trial) | −0.63* (n = 12, ♀) | −0.77** (n = 12, ♀) | −0.66* (n = 12, ♀) | −0.63* (n = 12, ♀) | |||
| Thigmotaxis, (5 min trial) | −0.67* (n = 12, ♀) | ||||||
| Atherogenic diet (8 wk) | |||||||
| Aortic lesion size | 0.71** (n = 19, ♂) | 0.75** (n = 19, ♂) | 0.83**(n = 19, ♂)
0.65** (n = 20, ♀) |
0.68** (n = 19, ♂) | |||
| Bone mineral density | −0.64** (n = 20, ♀) | −0.69** (n = 20, ♀) | −0.68** (n = 20, ♀) | −0.63** (n = 20, ♀) | |||
Correlations were conducted between strain mean data points for the doses of ethanol (g/kg), ethanol + saccharin (g/kg) or saccharin (mg/kg) consumed versus phenotypes in the mouse phenome database (MPD) with a minimum of 8 strains in common. The Pearson correlation matrix is depicted for male (♂) and female (♀) mice.
P < 0.10,
P < 0.05,
P ≤ 0.01
DISCUSSION
The present findings add to the database of genetic information on strain differences in ethanol preference and intake (e.g., Rodgers et al., 1966; Belknap et al. 1993). It should be noted that the design we employed offered relatively short (4 day) periods of access to each solution, since recent findings indicated that two-bottle preference tests (range of 1 – 6 days) were most sensitive to detect strain differences when they lasted 4 days (Tordoff and Bachmanov, 2002). Additionally, we adopted this approach in order to compare the results with previous work more directly. However, we cannot exclude a potential role for temporal changes in preference in our results. That is, some strains may simply show patterns of increasing or decreasing preference for ethanol over many days, and the results might have been different for any particular concentration offered if we had tested for more days or without the prior history of solutions preceding it as in our design. With this in mind, ethanol consumption was significantly and positively correlated with previous work (e.g., Belknap et al., 1993), as was ethanol preference ratio (e.g., Rodgers, 1966). These robust correlations among studies conducted over a span of 40 years provide evidence for the stability of the ethanol preference drinking phenotype (discussed in more detail in Wahlsten et al., 2006).
Estimates of genetic effect size for ethanol consumption and preference were comparable, suggesting that the continuous access, two-bottle choice procedure provides similar information regarding the contribution of genetic factors to measures of consumption and preference. It is notable that the present findings suggest that 44–60% of the variance in consumption/preference of unsweetened ethanol solutions and 58–64% of the variance in consumption/preference of sweetened ethanol solutions could be explained by genotype. These estimates are comparable to those in Belknap et al. (1993) and provide strong support for the conclusion that all of the consumption/preference measures were highly and significantly genetically determined.
The finding that alcohol consumption/preference measures share substantial common genetic influence suggests that identifying specific genes influencing any one of these traits (through QTL mapping or gene expression profiling, for example) is likely to be informative for the other traits. The utility of this strategy is provided by recent work that employed a meta-analysis of microarray data from different genetic models of high and low alcohol consumption in their analysis of alcohol preference (Mulligan et al., 2006). This analysis identified genes covering a range of cellular pathways, such as cellular homeostasis and neuronal function, as well as genes with uncharacterized functions. It is not surprising that genes related to neuronal adaptation and homeostasis would be important for alcohol-related responses following varying levels of consumption.
Strains ranked highest in terms of 10E dose consumed were the three C57/58 family strains (B6, C58 and C57BLKS). At the opposite end of the spectrum, the D2 (Little’s DBA + lineage), BUB (Swiss lineage) and BTBR strains consumed the lowest doses of ethanol. The BTBR strain derives from Castle’s strains, most of whose members are the various substrains of 129 strain mice that serve as the embryonic stem cell donor strains for most null mutant and transgenic over-expression lines. One consideration is that the current design, like previous experiments, does not allow us to identify the nature of the motivation of each strain to consume particular ethanol concentrations. One high-preferring strain may be seeking the taste of ethanol, while another seeks its pharmacological effect. Clearly, relative preference for saccharin also can influence the strain-specific responses to saccharin adulteration of ethanol solutions.
The addition of saccharin to the ethanol solutions increased ethanol intake in most genotypes, but it also changed the strain distribution pattern of ethanol intake. In general, strains that exhibited < 80% preference for 0.2S did not significantly increase their intake of the sweetened versus unsweetened ethanol solutions. The exceptions were the FVB and I/Ln strains, which significantly increased their intake of sweetened versus unsweetened ethanol solutions while preference for 0.2S was 78% and 71%, respectively. Interestingly, the addition of saccharin to the ethanol solutions increased the ethanol dose consumed in the SM, PERA, FVB and BALB strains to levels comparable to that in the C57/58 family strains. Based on the range in overall saccharin consumption (see Figure 2 for strain distribution) as well as preference for 0.2S in these strains (from 90% in SM to 78% in FVB; Table 1), it is difficult to determine whether the increased intake of sweetened ethanol solutions was due to the strong relative avidity for saccharin (so that the strains would tolerate ethanol’s aversive effects more than other strains) or the reduced taste of ethanol when given in saccharin.
Another pattern of consumption was observed in strains with low consumption of unsweetened and sweetened ethanol (LP, A, BUB, BTBR and D2), which could be due to a combination of factors (discussed in Belknap et al., 1993). Early work has documented that D2 mice were more sensitive to the aversive taste or odor of ethanol, when compared to B6 mice (Belknap et al., 1978), but the specific role of odor or taste in determining strain-specific alcohol intake has not been thoroughly tested across multiple strains. Saccharin consumption in these five strains fell in the mid to low end of the strain distribution pattern (Figure 2), while preference for 0.2S ranged from 78% (D2) to 55% (BTBR). Even though saccharin should have changed the taste of alcohol, the addition of saccharin did not produce a marked increase in ethanol consumption, suggesting that these five strains may have been reacting primarily to the odor of ethanol in maintaining their relative avoidance of the ethanol solutions. It also is possible that a higher concentration of saccharin might have masked the taste of the ethanol solutions more effectively in these strains. However, recent findings indicate that mice with a null mutation in one of three genes that are important for taste transduction (Gnat3, Tas1r3, or Trpm5) exhibited reduced consumption of and preference for alcohol (6 – 12%) and saccharin (0.033 – 0.066%) solutions (Blednov et al., 2007). Since polymorphisms in the Tas1r3 gene (believed to be identical to the mouse Sac locus, important for saccharin preference) were strongly associated with saccharin preference in a panel of 30 inbred strains (Reed et al., 2004), it is possible that polymorphisms in genes important for taste transduction contribute to the low alcohol consumption in select inbred strains. It should be noted that some strains may respond to adulteration of ethanol solutions with other compounds by increasing intake, as D2 mice have been shown to increase their intake of and two-bottle preference for ethanol when it was added to non-alcoholic beer (Grisel et al., 2007). Finally, a recent study has shown that in rats, sucrose-responsive neurons in the nucleus of the solitary tract showed a much larger, concentration-dependent response to an oral ethanol stimulus than sucrose-unresponsive cells (Lemon et al., 2004). This suggests a possible physiological basis for differential modulation of ethanol’s tase-related cue properties in saccharin preferring versus non-preferring genotypes.
Although B6 exhibited the highest consumption and preference for all ethanol solutions examined, recent work determined that preference for 10 – 30% ethanol solutions was significantly increased over that of B6 in an F1 hybrid cross of B6 and FVB mice (Blednov et al., 2005). This finding appeared to be selective for 2-bottle preference procedures, as the B6 and F1 hybrids did not differ in ethanol consumption with the “drinking in the dark” procedure, where mice had 2 hr of access to a single ethanol bottle containing a 20% ethanol solution (Crabbe and Rhodes, unpublished). Nonetheless, additional 2-bottle preference studies determined that consumption of ethanol solutions (12 – 36%) also was significantly increased in F1 hybrid crosses of the FVB or SJL strains with B6 (Blednov, unpublished). These findings suggest that other F1 crosses with B6 may show overdominance with regard to consumption of ethanol in a 2-bottle choice procedure and suggest that a single gene does not control preference drinking.
With the exception of saccharin alone, consumption of the sweetened and unsweetened ethanol solutions was significantly influenced by sex. In general, ethanol intake was higher in female than in male mice, but not in all genotypes. An examination of sex differences across all three unsweetened ethanol solutions determined that there was a consistent and significant increase in ethanol intake in female mice from the PERA and SM strains. There was a significant sex difference in the consumption of the 3E and 6E solutions in the CZECH strain. Female B6 mice had significantly greater intake of 6E, with a trend for an increase in 10E, when compared to their male counterparts. Nonetheless, these findings are consistent with clinical and preclinical studies documenting the existence of sex differences in sensitivity to a number of alcohol-related behaviors associated with neuroadaptation and reinforcement (e.g., Devaud et al., 2003; Finn et al., 2004a, 2004b; Green et al., 1999; Hashimoto and Wiren, 2007; Lancaster, 1995; Middaugh and Kelly, 1999; Middaugh et al., 1999; Rhodes et al., 2007; Vivian et al., 2001; Wiren et al. 2006).
Genetic correlations, or the degree to which two phenotypes show common genetic influence, were conducted. In addition to the high positive correlations between preference and consumption in the present and earlier 2-bottle choice studies, continuous access 10E preference drinking was significantly, positively correlated with consumption of 20E over a 4 hr period in a single bottle test (Rhodes et al., 2007). This finding suggests that genetic differences in propensity to consume alcohol may be comparable across various ethanol concentrations, access periods and choice conditions. Consistent with a recent meta-analysis that reported a genetic relationship between high ethanol withdrawal and low ethanol consumption (Metten et al., 1998), ethanol intake in the current study tended to be negatively correlated with chronic ethanol withdrawal severity (Metten and Crabbe, 2005). Ethanol intake also was negatively correlated with impaired performance on the rotarod following injection of an ataxic dose of ethanol. This finding may be specific to the rotarod, as strain correlational studies have shown that different assays of ethanol intoxication tend to be influenced by different genes (Crabbe et al., 2005). It is of interest, however, as a substantial human literature has found a genetic relationship between low sensitivity to alcohol and eventual diagnosis of alcohol dependence in offspring with a positive family history (Schuckit, 1994; Schuckit et al., 2004).
Correlations between ethanol consumption and MPD variables also suggest the pleiotropic effect of genes related to preference for other solutions (sodium chloride, potassium chloride, calcium chloride), activity level and anxiety, as well as bone mineral density. Since the MPD variables reflect data in alcohol naive strains, the genetic relationship between alcohol consumption and measures of activity and anxiety may reflect the strain differences in locomotor activity and anxiety that exist (e.g., Finn et al., 2003; Trullas and Skolnick, 1993; Wahlsten et al., 2003). With regard to bone mineral density, limited evidence suggests that there is no consistent relationship between high bone mass or strength with selection for high alcohol consumption and/or preference in rat lines (Alam et al., 2005), whereas data from animal models of chronic or excessive alcohol intake suggest that chronic exposure to high alcohol doses can inhibit bone formation (e.g., Wahl et al., 2006). All these correlational analyses must be viewed with the caveat that the significance values reported are uncorrected for multiple comparisons. Nonetheless, these correlations provide some suggestions for future examinations of genetic relationships between alcohol consumption/preference and other phenotypes.
In conclusion, the present findings add new strains to the existing strain mean database on genetic differences in consumption of, and preference for, sweetened and unsweetened ethanol solutions as well as saccharin. Although there was no strain identified that consumed higher doses of ethanol than the B6, estimates of genetic effect size indicated that up to 60% or 64% of the variance in consumption/preference of unsweetened or sweetened ethanol solutions, respectively, could be explained by genotype. The fact that the measures of ethanol preference and consumption were highly and significantly correlated genetically indicates that the growing strain mean databases will be an important resource for future studies aimed at the identification of genes important for high (or low) alcohol consumption.
Acknowledgments
Supported by NIAAA INIA Consortium Grants AA13478 and AA13519, NIAAA Grant AA10760 and the Dept. of Veterans Affairs. We thank Andy Cameron for assistance with the correlations with variables in the PARC database and Michelle Bobo and Steven Grubb (The Jackson Laboratory) for arranging the MPD portal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam I, Robling AG, Weissing S, Carr LG, Lumeng L, Turner CH. Bone mass and strength: phenotypic and genetic relationship to alcohol preference in P/NP and HAD/LAD rats. Alcohol Clin Exp Res. 2005;29:1769–1776. doi: 10.1097/01.alc.0000183005.28502.4f. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Keifer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences and consumption. Alcohol Clin Exp Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Coleman RR, Foster K. Alcohol consumption and sensory threshold differences between C57BL/6J and DBA/2J mice. Physiol Psychol. 1978;6:71–74. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2007 Mar 21; doi: 10.1111/j.1601-183X.2007.00309.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Association between ethanol and sucrose intake in the laboratory mouse: Exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res. 2000;24:253–258. [PubMed] [Google Scholar]
- Crabbe JC. Animal models in neurobehavioral genetics: Methods for estimating genetic correlation. In: Jones BC, Mormede P, editors. Neurobehavioral Genetics: Methods and Applications. New York: CRC Press; 1999. pp. 121–138. [Google Scholar]
- Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Alele P, Chadda R. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15:41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: sex and strain differences. Pharmacol Biochem Behav. 2004a;78:435–443. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Finn DA, Rutledge-Gorman M, Crabbe JC. Genetic animal models of anxiety. Neurogenetics. 2003;4:109–135. doi: 10.1007/s10048-003-0143-2. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck M, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004b;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Measurement of alcohol preference in genetic experiments. J Comp Physiol Psychol. 1964;57:85–88. doi: 10.1037/h0043100. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Single locus control of saccharin preference in mice. J Heredity. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- Green KL, Szeliga KT, Bowen CA, Kautz MA, Azarov AV, Grant KA. Comparison of ethanol metabolism in male and female cynomolgus macaques (Macaca fasicularis) Alcohol Clin Exp Res. 1999;23:611–616. [PubMed] [Google Scholar]
- Grisel JE, Metten P, Williams S, Allen SA, Chesler EJ. Genetic analysis of beer consumption in BXD RI mice. Alcohol Clin Exp Res. 2007;31:138A. [Google Scholar]
- Grubb SC, Chruchill GA, Bogue MA. A collaborative database of inbred mouse strain characteristics. Bioinformatics. 2004;20:2057–2059. doi: 10.1093/bioinformatics/bth299. [DOI] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: Expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology. 2007 Jun 27; doi: 10.1038/sj.npp.1301494. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 1990;7:83–86. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Lancaster FE. Gender differences in animal studies: implications for the study of human alcoholism. Recent Dev Alcohol. 1995;12:209–215. [PubMed] [Google Scholar]
- Lemon CH, Brasser SM, Smith DV. Alcohol activates a sucrose-responsive gustatory neural pathway. J Neurophysiol. 2004;92:536–544. doi: 10.1152/jn.00097.2004. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Rodgers DA. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–695. [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119:911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM. Operant ethanol reward in C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:185–194. doi: 10.1016/s0741-8329(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004;14:1806–1911. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Backmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rodgers DA. Factors underlying differences in alcohol preference among inbred strains of mice. Psychosom Med. 1966;28:498–513. [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res. 2004;28:1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. Influence of test duration on the sensitivity of the two-bottle test. Chem Senses. 2002;27:759–768. doi: 10.1093/chemse/27.9.759. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology. 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomologous monkeys (Macaca fascicularis) Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Wahl EC, Liu L, Perrien DS, Aronson J, Hogue WR, Skinner RA, Hidestrand M, Ronis MJ, Badger TM, Lumpkin CK., Jr A novel mouse model for the study of the inhibitory effects of chronic ethanol exposure on direct bone formation. Alcohol. 2006;39:159–167. doi: 10.1016/j.alcohol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Hendricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Weiss F, Mitchiner M, Bloom FE, Koob GE. Free-choice responding for ethanol versus water in Alcohol-Preferring (P) and unselected Wistar rats is differentially altered by naloxone, bromocriptine and methysergide. Psychopharmacology. 1990;101:178–186. doi: 10.1007/BF02244123. [DOI] [PubMed] [Google Scholar]
- Wiren KM, Hashimoto JG, Alele PE, Devaud LL, Price KL, Middaugh LD, Grant KA, Finn DA. Impact of sex: Determination of alcohol neuroadaptation and reinforcement. Alcohol Clin Exp Res. 2006;30:233–242. doi: 10.1111/j.1530-0277.2006.00032.x. [DOI] [PubMed] [Google Scholar]