Summary
Bax, a pro-apoptotic Bcl-2 family protein, translocates to mitochondria during apoptosis, where it brings about mitochondrial outer membrane permeabilisation (MOMP). MOMP releases pro-apoptotic factors, such as cytochrome c and SMAC/Diablo, into the cytosol where they activate caspases. It is often inferred that Bax activation occurs in a single step, a conformational change in the protein bringing about its translocation and oligomerisation into high molecular weight membrane pores. However, a number of studies have shown that Bax translocation to mitochondria does not necessarily induce MOMP. Indeed, Bax translocation can occur several hours prior to release of cytochrome c, indicating that its regulation may be a complex series of events, some of which occur following its association with mitochondria. We have examined endogenous Bax in epithelial cells undergoing anoikis, a physiologically relevant form of apoptosis that occurs when normal cells lose contact with the extracellular matrix (ECM). Using blue native polyacrylamide gel electrophoresis (BN-PAGE) we show that Bax forms a 200 kDa complex before caspase activation. Furthermore, Bax in this 200 kDa complex is not in the active conformation, as determined by exposure of N-terminal epitopes. These results indicate that Bax oligomerisation is an event that must be interpreted differently from the currently held view that it represents the apoptotic pore.
Keywords: apoptosis, anoikis, Bax, blue native PAGE, mitochondria
Introduction
Apoptosis is a precisely regulated process for removing damaged or unwanted cells. In most cells, apoptosis occurs when mitochondrial outer membrane permeabilisation (MOMP) releases into the cytosol factors, including cytochrome c and SMAC/Diablo, which result in the activation of caspases. The Bcl-2 family mediate MOMP, in particular the pro-apoptotic proteins Bax and Bak [1]. Mice deficient for Bax and Bak have multiple developmental defects [2], and cells isolated from these mice are resistant to most apoptotic stimuli [3]. Current models indicate that Bax and Bak induce MOMP by forming pores in the outer mitochondrial membrane (OMM) [4]. In healthy cells, Bax is found predominantly as a monomer in the cytosol [5-7]. Numerous apoptotic stimuli, including growth factor withdrawal, anoikis, and DNA damage, as well as cytotoxic compounds like staurosporine, result in Bax translocating to the OMM, where it assembles into high molecular weight complexes [8-10]. Bax undergoes a conformational change during this process, which can be followed by exposure of cryptic antibody epitopes. In contrast to Bax, Bak is always present on the OMM, but it also undergoes a conformational change and oligomerises during apoptosis, suggesting similarities in the mechanism of activation [11, 12].
It is often inferred that Bax oligomerisation represents the formation of the pores that drive MOMP [13]. Chemical cross-linking and the use of synthetic liposomes have suggested that Bax assembles into dimers and tetramers which release cytochrome c [5, 14, 15]. Studies largely based upon reconstituting Bax into purified liposomes indicate that pore formation does not require other mitochondrial proteins [15, 16], and Bax monomers appear to oligomerise in vitro following membrane insertion [17]. Recombinant Bax activated by the BH3-only protein Bid forms a 100 kDa complex in vitro which can release mega-Dalton sized dextrans from liposomes [16]. However, in vivo experiments suggest a different model. Bax forms a 200 kDa complex on mitochondria during apoptosis [5, 7]. Studies with isolated mitochondria indicated that other mitochondrial proteins were required for its association with the OMM [18]. These discrepancies between in vivo and in vitro studies are yet to be resolved, and the nature of native Bax complexes during apoptosis remains unclear.
Anoikis is a physiological form of apoptosis that occurs when cells are not attached to the correct extracellular matrix (ECM) [19]. Anoikis functions to maintain tissue and organ integrity, and if defective can contribute to processes such as tumour metastasis [20]. We have shown in epithelial cells that loss of ECM attachment is followed within minutes by the accumulation of Bax on mitochondria [7, 8, 21]. Despite this, MOMP and cell death do not occur for several hours. Bax translocation to the OMM during anoikis does not irreversibly commit cells to apoptosis, and if they reattach to ECM Bax redistributes back to the cytosol. Following MOMP, Bax accumulates in large aggregates adjacent to mitochondria [7, 22]. The transition between these pre- and post-cytochrome c release states is rapid, and occurs concomitant with a change in Bax conformation. These data suggest that Bax is held in an inactive state following translocation to mitochondria but prior to cytochrome c release.
Most studies examining mitochondrial Bax have utilised isolation procedures based upon the finding that non-ionic detergents could activate cytosolic Bax, whereas detergents such as CHAPS did not [10, 23]. Subsequently, studies on Bax have used CHAPS as a modus operandi without critically examining whether isolation conditions based upon the soluble, cytosolic protein were suitable to determine the quaternary structure of its membrane inserted form. We have re-examined Bax oligomerisation using blue-native polyacrylamide gel electrophoresis (BN-PAGE). Our data indicate that the integrity of the native Bax complexes is significantly affected by CHAPS. We show that following translocation to mitochondria, endogenous Bax assembles into a high molecular weight complex prior to OMM permeabilisation and cell death. Native Bax in this complex is inactive, as judged by N-terminal epitope exposure. We suggest that in vivo, Bax forms complexes prior to cell death that are distinct from the MOMP inducing pore, and that these may represent an important point of regulation.
Experimental
Cell culture and transfection
Fsk-7 cells, a mouse mammary epithelial cell line, were cultured as previously described [24]. Human colon tumour cells (HCT116) deficient for Bax, kindly provided by B. Vogelstein [25], were grown in McCoy’s 5A media (Invitrogen) with 10% foetal calf serum. Expression vectors for YFP and YFP-Bax were transfected into FSK-7 and HCT116 cells using Lipofectamine Plus as previously described [7, 21]. Apoptosis was induced by 10 μM staurosporine (STS; Calbiochem) for 4 hrs; control cells were treated with vehicle (DMSO) alone.
Reagents and antibodies
Acrylamide and sodium dodecyl sulphate (SDS) were from Bio-Rad. β-mercaptoethanol, dithiothreitol (DTT), Tricine, Bis/Tris, aminocaproic acid, CHAPS, n-octylglucoside, Coomassie brilliant blue G-250 and poly-2-hydroxyethyl methacrylate (poly-HEMA) were from Sigma. Triton X-100 was from BDH. Protease Inhibitor Cocktail (539131), staurosporine and zVADfmk were from Calbiochem.
Antibodies used
Bax monoclonals 6A7 (B8429) and 5B7 (B9054) were from Sigma. Polyclonal BaxΔ21 (sc-6236) was from Santa Cruz. Bad (AF819) and Bid (AF860) were from R&D Systems. Bak (B5929) was from Sigma. Hsp60 (SPA-806) was from Stressgen. TOM20 was a gift from David Breckenridge (McGill). Bcl-XL (2762) and cleaved caspase 3 (9661) were from Cell Signalling Technology. Anti-14-3-3 (06-511) was from Upstate Biotechnology.
Cell fractionation and size exclusion chromatography
Adherent and detached FSK-7 cells were subfractionated into cytosolic and membrane fractions as previously described [7]. In some experiments, the membrane fractions were extracted in RIPA buffer (50 mM Tris/Cl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% NP-40, 1% deoxycholate, 0.1% SDS). Size-exclusion chromatography was carried out on a SephacryS100-HR column (1.5 × 25 cm, Amersham Biosciences), with 10 mM HEPES.Cl, pH 7.6, 150 mM NaCl, 4 mM CHAPS [7]. Alternate fractions were separated by SDS-PAGE prior to immunoblotting.
Chemical cross-linking
For cross-linking of endogenous Bax with the homobifunctional reagent BS3 (Pierce), cytosolic extracts were incubated with 5 mM BS3 at room temperature for 30 min. Reactions were quenched with SDS sample buffer prior to analysis by SDS-PAGE.
BN-PAGE and immunoblotting
Blue-native electrophoresis was performed according to Brookes et al. [26]. Adherent and detached Fsk-7 cells or adherent HCT116 cells were subfractionated into a cytosolic and membrane fractions as described [7]. Membrane pellets were extracted in 1% CHAPS, 10% glycerol, 0.5 M aminocaproic acid in 50 mM Bis/Tris, pH 7.0 containing protease inhibitors and left on ice for 10 min prior to centrifuging at 100000xg for 30 min. Protein content of the cytosol and the CHAPS-soluble membrane fraction were measured using the BCA assay (Pierce), with BSA as standard. To 100 μl of supernatant was added 6.3 μl of 5% w/v Coomassie brilliant blue G-250 in aminocaproic acid (0.5 M). Samples were then kept on ice for no longer than 30 min prior to electrophoresis. The proteins (40 μg /lane) were resolved on a 3-15% gradient gel that was electrophoresed at 2-4 mA overnight at 4°C. The anode buffer comprised 50 mM Bis/Tris ph 7.0 and the cathode buffer comprised 50 mM Tricine, 15mM Bis/Tris pH 7.0, and 0.002% Coomassie Blue G-250. The gel was equilibrated in transfer buffer for 20 min and the proteins electroblotted to PVDF membrane (Millipore). BN-PAGE was calibrated with the following proteins: Keyhole Limpet Haemocyanin (KLH; 400 kDa), β-amylase (200 kDa, albumin (66 kDa), carbonic anhydrase (29 Kd) and cytochrome c (14 Kd), which were visualised by silver staining.
For the second dimension (2D) SDS-PAGE, a lane from BN-PAGE was cut into 16 slices, from high to low molecular weight, and each boiled in modified SDS sample buffer (2% SDS, 50 mM Tris-Cl, pH 6.8, 0.2M DTT, 2M Urea). These fractions were resolved by 12.5% SDS-PAGE. 2-D gels were electroblotted to nitrocellulose (Bio-Rad) membrane. For both the 1D and 2D BN-PAGE, immunodetection was performed by SuperSignal chemiluminesence (Pierce). For re-probing, immunoblots were stripped in 62.5 mM Tris-Cl, pH 6.8, 2% SDS, 0.7 % β-mercaptoethanol at 50 °C for 30 min with constant agitation.
Quantitative immunoblotting was employed to measure the amount of Bax in the cytosolic and CHAPS-extracted membrane fraction of both adherent and detached (60 min) Fsk 7 cells. Recombinant mouse Bax (ABC 1359, Santa Cruz) was used to generate a standard curve. Samples and standards were analysed by SDS-PAGE and immunoblotted with 5B7. Bound antibody was detected using Alexa Fluor 680 goat anti-mouse IgG (Invitrogen) and subsequently quantified by infrared imaging (Odyssey, Li Cor Biosciences).
Immunofluorescence and imaging
For imaging the distribution and reactivity of endogenous and YFP conjugated Bax, cells on coverslips were fixed with 4% formaldehyde in PBS and permeabilised with 0.5% Triton X-100/PBS. Cells were immunostained with anti-Bax 5B7 followed by Rhodamine-RX conjugated donkey anti-mouse IgG (Jackson Immunoresearch). Nuclei were stained with 1 μg/ml Hoechst 33258. Images were collected on an Olympus IX70 microscope, equipped with a Deltavision imaging system, using a 100 x PLAN-APO 1.4NA objective. Images were processed by constrained iterative deconvolution on softWoRx™ software (Applied Precision).
Results
Conventional size-exclusion chromatography indicates differential association of Bcl-2 proteins with high-molecular weight complexes
Gel-filtration chromatography initially identify high-molecular weight Bax complexes in apoptotic HeLa cells [5]. These studies indicated that mitochondrial Bax was distributed between a monomeric form and a 200 kDa complex. Extraction of mitochondria in Triton-X100, in contrast, resulted in Bax exclusively present in a high-molecular weight complex in both apoptotic and healthy cells.
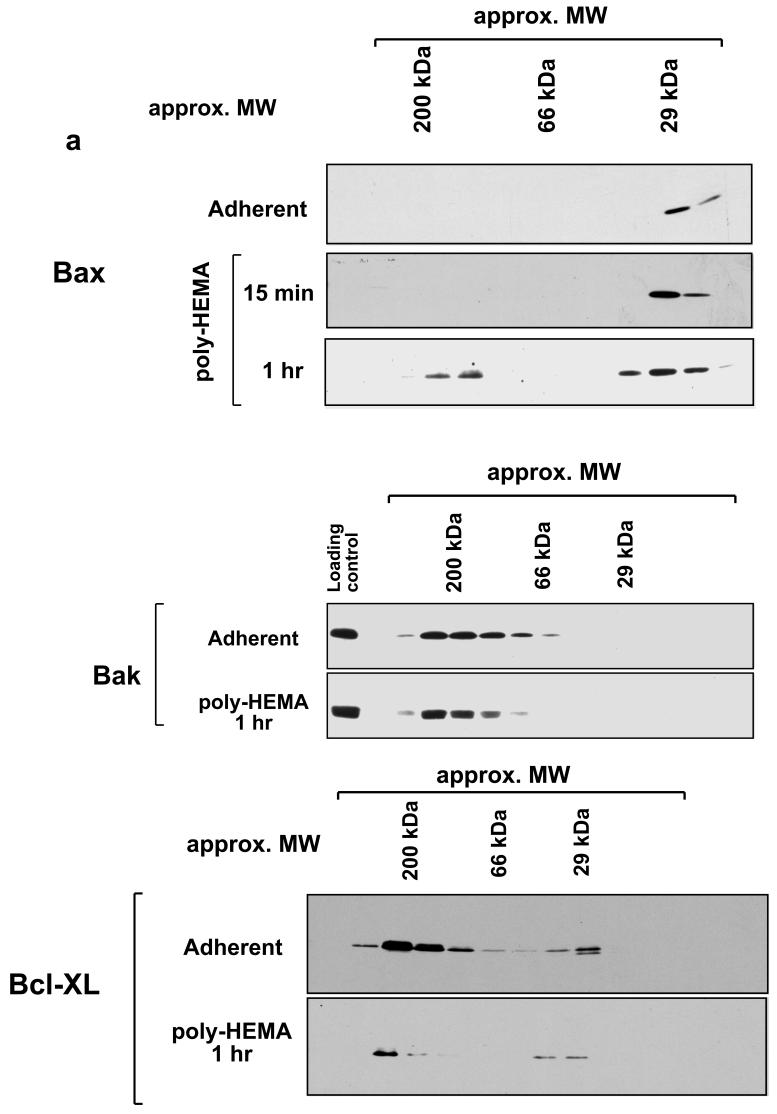
We re-examined Bax oligomerisation during anoikis. Membrane fractions from adherent and detached mammary epithelial cells were extracted in CHAPS and separated by Sephacryl S100 gel filtration (Fig. 1a). As we had previously shown, some Bax was present on the OMM of adherent FSK-7 cells, and this eluted exclusively as a monomer [7]. Following detachment of cells from ECM, Bax accumulated on the OMM after 15 minutes, and this also eluted primarily as a monomer. Prolonged loss of ECM (1 hr) resulted in higher molecular weight complexes of Bax becoming apparent, although these always represented the minority of Bax on the OMM. Distinct distributions were seen with other Bcl-2 proteins. Mitochondrial Bcl-XL and Bak were present in high molecular weight complexes in adherent and detached cells (Fig. 1a). Bak has been shown to exist in a complex with VDAC-2 [12]. More Bcl-XL was present on mitochondria of adherent cells than in detached cells. Gel filtration of whole cell lysates extracted in CHAPS indicated that Bid, which also associates with mitochondria during anoikis [27], eluted exclusively as a monomer (Fig. 1b). The relative amount of monomeric and oligomeric Bax varied by some considerable degree between experiments (data not shown). In contrast, Bak was exclusively in a 200 kDa complex. This variability in the amount of oligomeric Bax was previously noted in HeLa cells following either 16 hr treatment with staurosporine or UV irradiation [5].
Fig. 1.
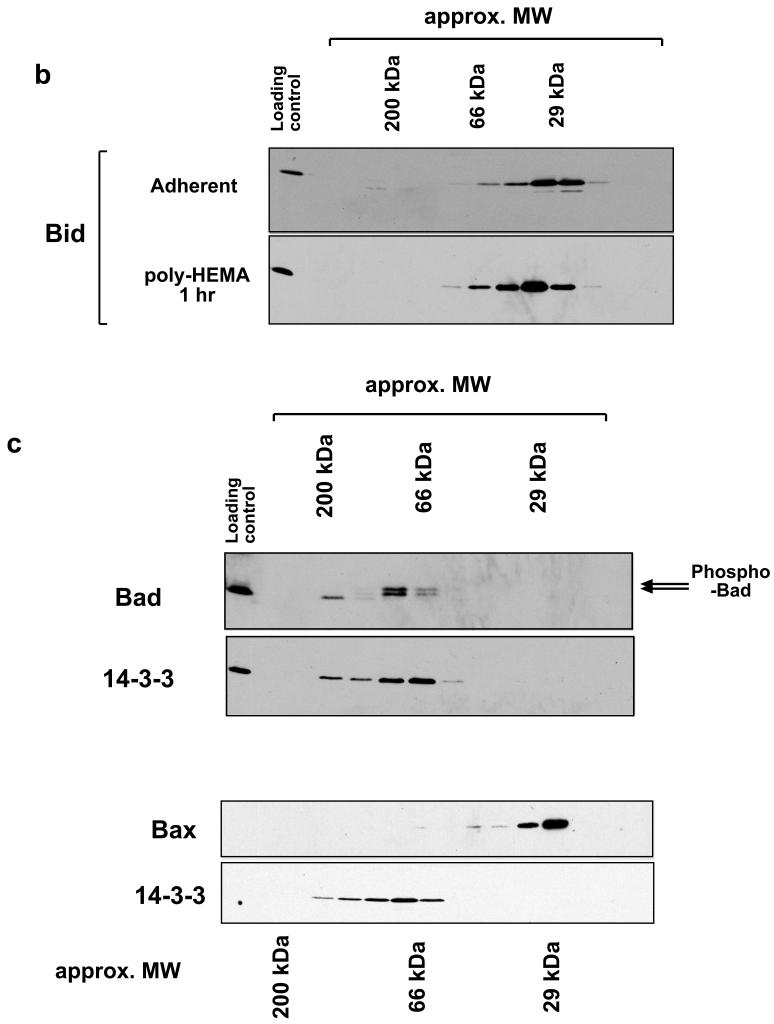
Size exclusion chromatography of Bcl-2 proteins. a) Membrane fractions were prepared from adherent or detached FSK-7 mammary epithelial cells. Detached cells had been maintained on the non-adhesive substrate poly-HEMA for 15 minutes or 1 hour. Membrane fractions were extracted in 4 mM CHAPS and separated on a Sephachryl S100-HR column. Fractions were analysed by SDS-PAGE and immunoblotting for Bax, Bak and Bcl-XL as indicated. b) Whole cell lysates of adherent or detached FSK-7 cells were prepared in 4 mM CHAPS and separated by gel-filtration as in a). Fractions were analysed by SDS-PAGE and immunoblotting for Bid. c) Cytosolic fractions from adherent FSK-7 cells separated on a Sephachryl S100-HR column in detergent free conditions. Column fractions were analysed by immunoblotting for Bad and Bax. The immunoblots were then stripped and reprobed for 14-3-3 proteins. Phosphorylated Bad can be detected by a shift in its mobility by SDS-PAGE, indicated by the arrows. Note that phosphorylated Bad co-elutes with 14-3-3 proteins. Bax elutes exclusively as a monomer.
We also examined cytosolic extracts from adherent cells (Fig. 1c). In the absence of any detergent, cytosolic Bax eluted exclusively as a monomer. In contrast, cytosolic Bad eluted in higher molecular weight fractions. In agreement with published data, the phosphorylated form of Bad (indicated by the upper arrow) co-eluted with 14-3-3 proteins [28]. Bax has been suggested in the literature to be sequestered in the cytosol through an interaction with 14-3-3 [29]. We observed no overlap between Bax and 14-3-3 containing fractions by gel-filtration.
Together, these data indicate that Bcl-2 family proteins exist in distinct complexes on the OMM. Furthermore, they suggest that during anoikis Bax is largely monomeric on the OMM but over time assembles into a high molecular weight complex.
Blue-native PAGE indicates that the majority of Bax on the OMM is associated with a high molecular weight complex
The inconsistent nature of the Bax oligomers seen in cells undergoing anoikis was in contrast to that seen with Bak. We asked if the manner of isolation and analysis was masking the true nature of Bax complexes on mitochondria. BN-PAGE has been used to isolate native protein complexes from mitochondria, including the protein translocase [30] and respiratory chain [31] complexes. We adapted BN-PAGE to examine endogenous Bax isolated from mitochondria of epithelial cells during anoikis.
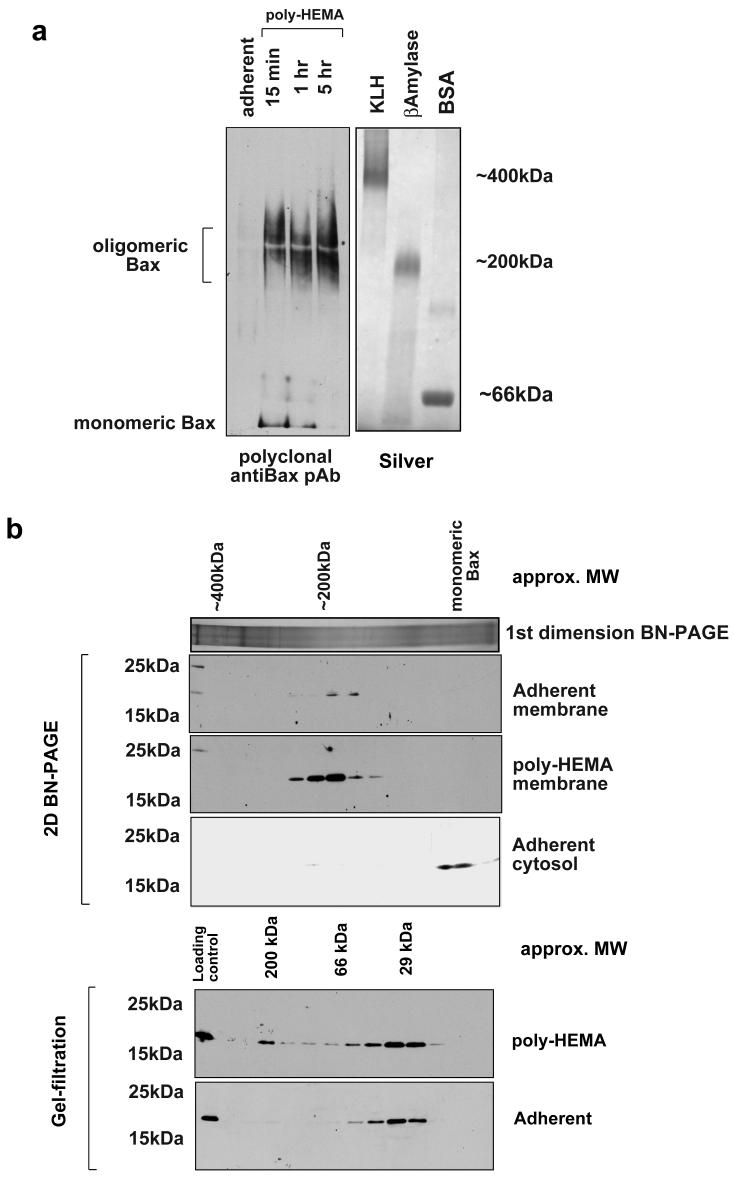
Adherent or detached FSK-7 cells were separated into cytosolic and membrane fractions. Isolated membrane fractions were extracted in 1% CHAPS/0.5M aminocaproic acid. Zwitterionic salts such as aminocaproic acid can improve solubility of large membrane complexes [26, 31]. Solubilised membrane fractions were added to BN-PAGE sample buffer and separated on a 3-15% gradient gel. Immunoblotting the separated fractions with a polyclonal anti-Bax antibody indicated that the majority of Bax on mitochondria following detachment was present in a complex of approximately 200 kDa (Fig. 2a). Detectable amounts of monomeric Bax were seen on the membrane 15 minutes following detachment, but this was substantially reduced after 1 hour.
Fig. 2.
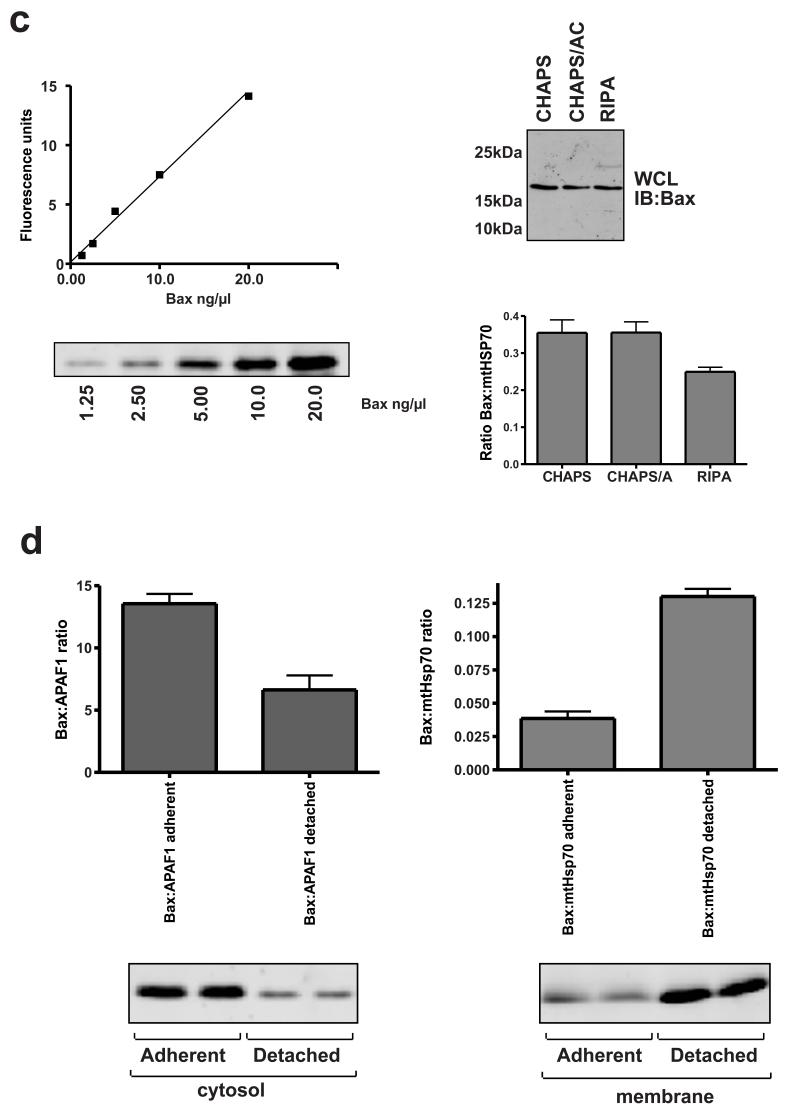
BN-PAGE analysis of Bax complexes. a) Membrane fractions were prepared from adherent or detached FSK-7 cells. Detached cells had been maintained on poly-HEMA for 15 minutes, 1 hour or 5 hours. Samples were separated on a 3-15% gradient gel as described in the methods section. Following transfer to PVDF, Bax was analysed by immunoblotting with a polyclonal anti-Bax antibody. The silver stained gel shows size markers to show approximate molecular weights - keyhole limpet haemocyanin (KLH) ∼400 kDa; β-amylase 200 kDa; bovine serum albumin (BSA) 66 kDa. b) Bax separated by BN-PAGE separated in a 2nd dimension by SDS-PAGE (upper panel), compared with Bax separated by gel-filtration (lower panel). Fractions from adherent or detached (1 hour on poly-HEMA) FSK-7 cells were separated by each method and immunoblotted for Bax. A representative 1st dimension BN-PAGE lane of the mitochondrial fraction following silver staining is shown in the upper panel. c) Increasing amounts of recombinant Bax were separated by SDS-PAGE and quantitative immunoblotting performed, shown in arbitrary fluorescence units. Equal amounts of membrane fraction from cells detached for 1 hour were extracted in the indicated detergents and immunoblotted as for the recombinant Bax standards. Membrane extracts were also probed for mtHsp70, and the amount of Bax normalised to this is shown. d) Adherent and detached FSK-7 cells were separated into cytosolic and membrane fractions. Quantitative immunoblotting was performed as in c). Cytosolic fractions were normalised to APAF1, and membrane fractions were normalised to mtHsp70. The data is the mean of three separate experiments. Error bars represent standard error of the mean. The fluorescent Bax immunoblots show duplicate samples run in one of these experiments.
To improve resolution and sensitivity, BN-PAGE of mitochondria from cells detached for 1 hour were separated in a 2nd dimension by SDS-PAGE and immunoblotted for Bax. The 2D analysis indicated that the vast majority of mitochondrial Bax was present in a complex of approximately 200 kDa (Fig. 2b). 2D BN-PAGE of cytosolic extracts from adherent cells indicated only monomeric Bax was present (Fig. 2b). We noted that gel-filtration of membrane fractions from adherent cells indicated that some monomeric Bax was present on mitochondria in adherent cells. Although 1D BN-PAGE did not detect Bax on the membranes of adherent cells (probably due to the sensitivity of antibody detection with the diffuse bands obtained with this gel system), it was detected when samples were run in a second dimension by SDS-PAGE. Interestingly, 2D BN-PAGE indicated that the Bax on membranes in adherent cells was exclusively present in the 200kDa complex.
The discrepancy in the amount of monomeric Bax detected by gel-filtration and BN-PAGE may be due to either the efficiency of extraction under the different conditions, or because the complexes disassociate during gel-filtration. To investigate this, membrane fractions from cells detached for 1 hour were extracted in either 1% CHAPS alone, 1% CHAPS/0.5M aminocaproic or RIPA. Quantitative immunoblotting, using recombinant Bax as a standard, showed no difference in the amount of Bax extracted in CHAPS alone or CHAPS/0.5M aminocaproic (Fig. 2c). The reduction in the ratio with RIPA is due to more efficient extraction of mtHsp70 in this buffer (data not shown). We determined the relative amount of Bax in the cytosol and membrane of cells before and after detachment (Fig. 2d). Detachment from ECM produced an approximate four-fold increase in the amount of Bax present in the membrane fraction. The ratio of cytosolic to membranous Bax was approximately 4:1 in adherent cells, which was reversed to a 1:4 ratio in favour of membrane associated Bax within 1 hour of detachment.
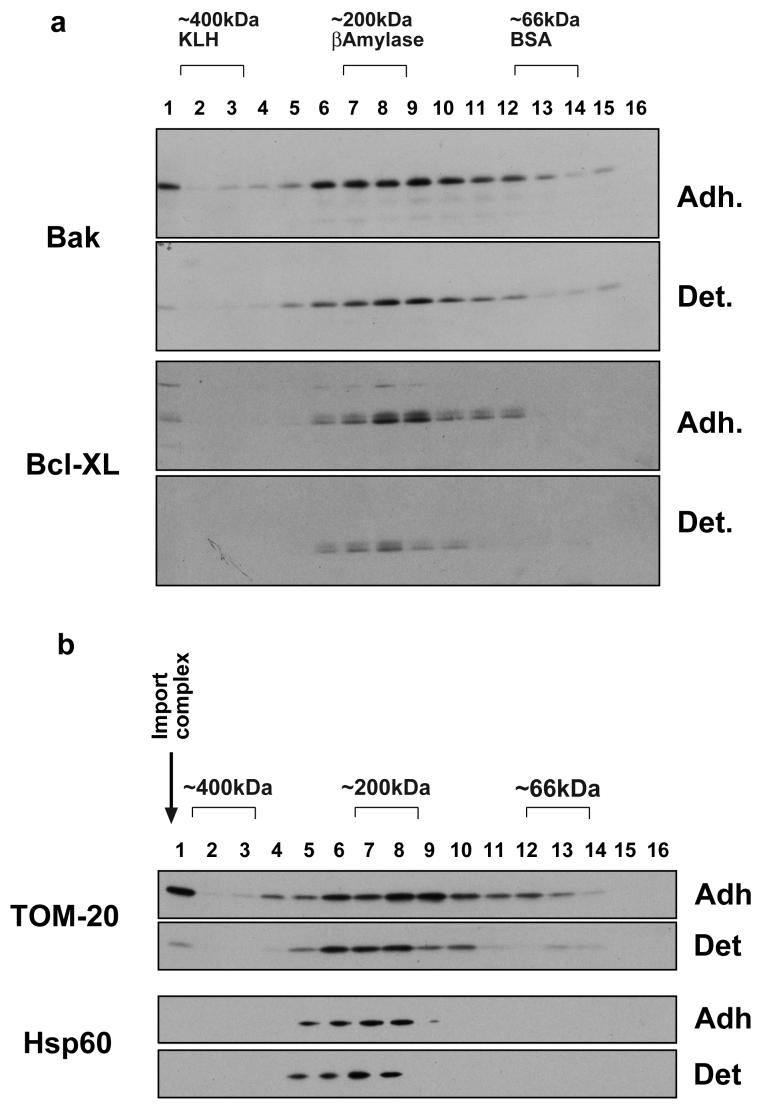
We examined Bax and Bcl-XL by BN-PAGE to determine if their association with high-molecular weight complexes differed from that observed by gel-filtration. Isolated mitochondria from both adherent and detached FSK-7 cells were separated by 2D BN-PAGE, before immunoblotting (Fig. 3a). For both Bax and Bcl-XL the estimated molecular weights of complexes was similar to that observed by gel-filtration. We also observed the same loss of mitochondrial Bcl-XL in detached cells that was observed by gel-filtration. To confirm the reliability of BN-PAGE, we examined two other mitochondrial proteins (Fig. 3b). TOM-20 acts as a receptor for signal sequence containing proteins and directs them to the general import pore (GIP). TOM-20 has been shown to exist in two populations on the OMM, at approximately 400 kDa (the GIP fraction) and at lower molecular weights (TOM20 dissociated from GIP) [30]. Both populations were readily identifiable in our BN-PAGE analysis and at the molecular weight previously shown. Less TOM 20 was present in the GIP in detached cells, perhaps indicative of a reduction in protein import. In contrast, Hsp60 was detected in complexes that did not alter with loss of cell/ECM adhesion.
Fig. 3.
a) 2D BN-PAGE analysis of various Bcl-2 family proteins in adherent (Adh) and detached (Det) FSK-7 cells. Detached cells were maintained on poly-HEMA for 1 hour. The BN-PAGE gel was cut into 16 slices for separation by SDS-PAGE, as described in the methods. These were analysed by immunoblotting for Bak and Bcl-XL. As with the gel-filtration, Bcl-XL is significantly reduced in the detached membrane. b) Membrane fractions prepared as those in a) were immunoblotted for TOM-20 and Hsp60. The arrow indicates the expected size of the general import pore complex.
Together, BN-PAGE suggests that the majority of Bax associated with mitochondria following an apoptotic stimulus is present in a high molecular weight complex.
Bax complexes form prior to cell death and caspase activation
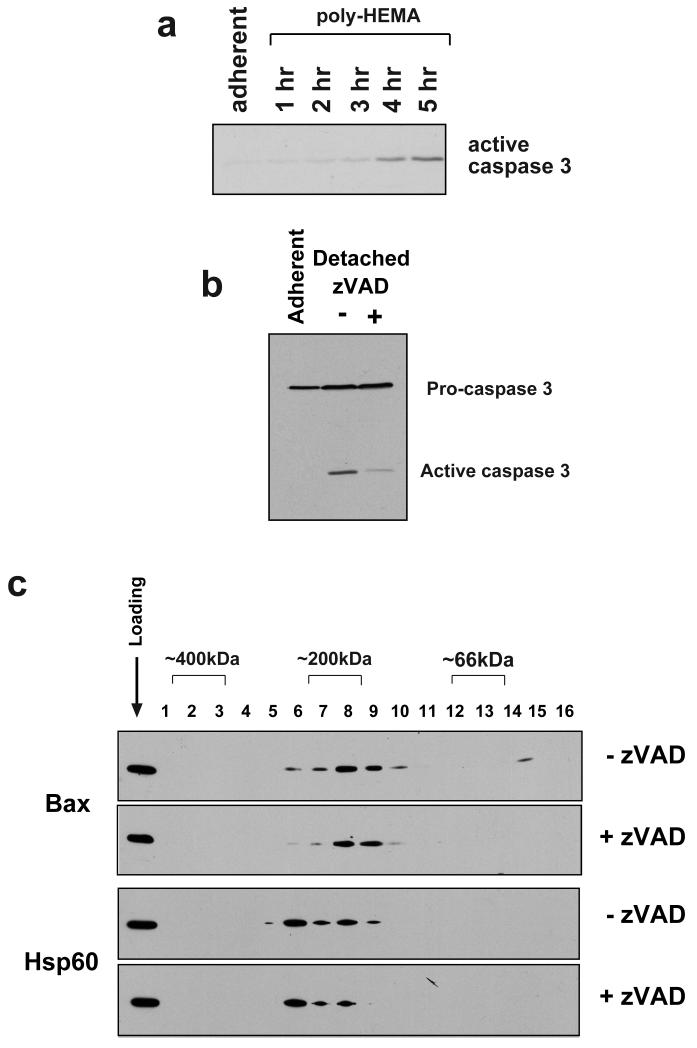
Our previous studies have shown that the association of Bax with the OMM occurs some time before the release of cytochrome c and cell death [7, 8, 21]. The majority of epithelial cells do not undergo MOMP until several hours following loss of cell/ECM adhesion. However, Bax complexes are clearly seen after just 15 minutes detachment, concomitant with its translocation, which would imply that the complexes are forming prior to Bax forming an active, cytochrome c releasing pore.
Bax complexes form prior to cell death, as confirmed by immunoblotting whole cell lysates of detached FSK-7 cells with an antibody against activated caspase 3 (Fig. 4a). Although complexes are seen after only 15 minutes detachment (Fig. 2a), active caspase 3 is not detectable until after at least 2 hours, in agreement with our previous data on the kinetics of anoikis [7]. Furthermore, to show that Bax complexes are independent of caspase activation, we detached FSK-7 cells from ECM in the presence or absence of the broad-spectrum caspase inhibitor, zVAD. zVAD significantly inhibited the processing of caspase 3 (Fig. 4b) seen after 5 hours detachment. However, Bax membrane complexes were still seen in detached cells treated with zVAD (Fig. 4c).
Fig. 4.
The formation of Bax complexes is independent of caspase activity. a) Lysates of adherent FSK-7 cells or those detached for the indicated times were separated by SDS-PAGE and immunoblotted for active caspase 3. There is no detectable active caspase 3 after 1 hour of detachment, which is when Bax complexes are readily detected. b) Processing of caspase 3 is abrogated during anoikis by zVAD. FSK-7 cells were detached from ECM for 5 hours in the presence or absence of zVAD. Lysates were immunoblotted to indicate caspase 3 processing. c) 2D BN-PAGE analysis of equal amounts of membrane fractions from FSK-7 cells detached from ECM for 1 hour in the presence or absence of zVAD. Treatment with zVAD did not inhibit formation of Bax complexes. Hsp60 and whole cells lysates were immunoblotted as controls for gel loading and running of the BN-PAGE.
BN-PAGE maintains the native conformation of Bax and allows determination of N-terminal epitope conformation
The N-terminus of Bax has been shown to undergo a conformational change during apoptosis [9, 10, 21]. This conformational change has been suggested to be involved in regulating both the translocation of Bax to the OMM and its oligomerisation into pores [32, 33]. We recently demonstrated that the conformational change of Bax is not associated with its translocation to the OMM [21]. Given the early time point at which Bax oligomers form, we were intrigued by the implications this had with regard to the conformational change of Bax. BN-PAGE can separate enzymatically active protein complexes isolated from mitochondria [31]. We therefore asked if we could use BN-PAGE to determine the conformation of Bax biochemically, both as a monomer and, more significantly, in mitochondrial complexes.
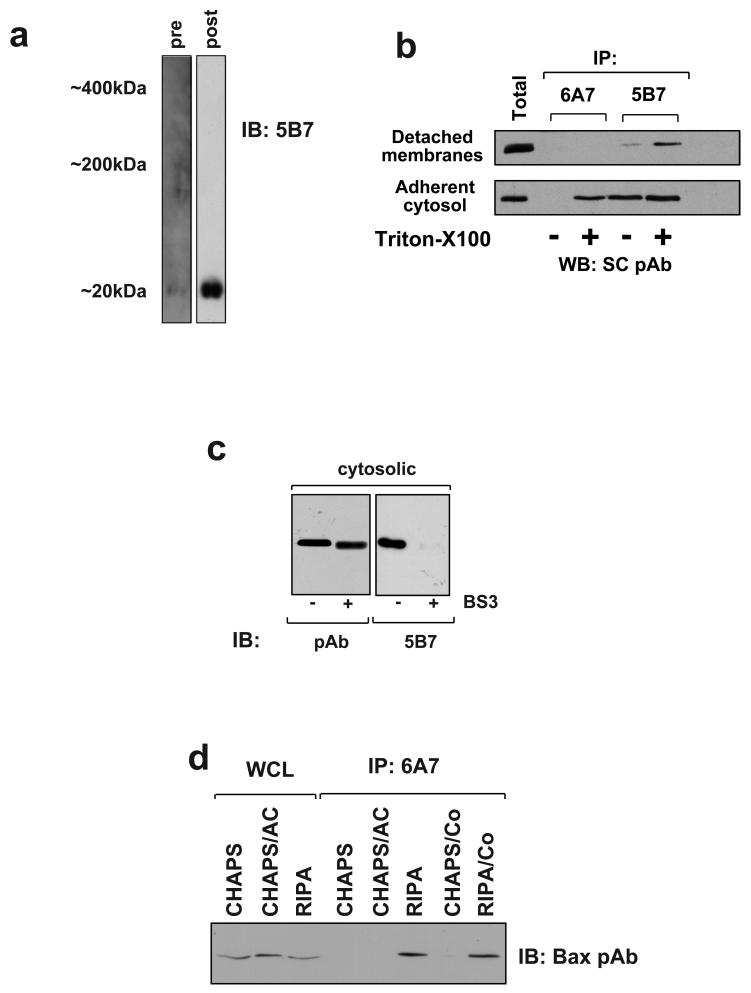
We first asked if BN-PAGE could maintain the conformation of cryptic N-terminal epitope of cytosolic Bax seen in adherent (i.e. non-apoptotic) cells. Cytosolic extracts of adherent FSK-7 cells were separated by BN-PAGE in the absence of detergent, and immunoblotted with anti-Bax monoclonal antibody (mAb) 5B7, which recognises an epitope within the N-terminus of the protein. Cytosolic Bax separated by BN-PAGE and immunoblotted to PVDF membrane was not reactive with mAb 5B7 (Fig. 5a). We then denatured proteins on the membrane and re-probed it again with mAb 5B7 (Fig. 5a). mAb 5B7 now detected monomeric Bax on the same immunoblot. This result indicated that cytosolic Bax separated by BN-PAGE was in a conformation in which the extreme N-terminus was not recognised by mAb 5B7.
Fig. 5.
BN-PAGE maintains the native N-terminal conformation of Bax. a) The cytosolic fraction of adherent FSK-7 cells was prepared without detergents. This was then separated by BN-PAGE, transferred to PVDF and immunoblotted using monoclonal anti-Bax 5B7. The immunoblot was then denatured using SDS/β-mercaptoethanol before reprobing with 5B7. Bax was not detected with 5B7 on the native membrane (pre), but was detected after denaturing (post). “Pre” exposure to film was 30 minutes. “Post” exposure was 30 seconds. Blot is representative of three separate experiments. b) Adherent FSK-7 cells were separated into cytosolic and membrane fractions. The membrane fractions were extracted as for BN-PAGE followed by dialysis to reduce the concentration aminocaproic acid. The cytosolic fraction was detergent free. Equal amounts of each sample were immunoprecipitated with either mAb 6A7 or 5B7, with or without addition of 1% Triton X100. Immunoprecipitates were separated by SDS-PAGE and immunoblotted with a polyclonal anti-Bax antibody (SC pAb). A sample of the membrane and cytosolic fractions used for each immunoprecipitation was included in the immunoblot (Total). c) Equal amounts of detergent free cytosolic fraction from adherent FSK-7 cells were either left untreated or treated with the homobifunctional crosslinker BS3. Samples were then separated by SDS-PAGE and immunoblotted with either a polyclonal anti-Bax (pAb), or with monoclonal anti-Bax 5B7. Both untreated and crosslinked Bax were detected using the polyclonal antibody, whereas only untreated Bax was detected with 5B7. d) Membrane fractions from cells detached for 1 hour were extracted in CHAPS, CHAPS/aminocaproic acid (AC) or RIPA. Equal amounts were immunoprecipitated with mAb 6A7, either in extraction buffer alone or following the addition of coomassie (CO). Immunoprecipitates were separated by SDS-PAGE and immunoblotted with polyclonal anti-Bax. e) Membrane fractions from adherent (A) or detached (D) cells were extracted in 1% CHAPS. The samples were then maintained in either CHPAS or 1% Triton X100 and separated by BN-PAGE before immunoblotting for Bax. f) Equal amounts of FSK-7 cytosolic fractions were treated with 1% CHAPS, 1% octylglucoside (OG) or 1% Triton X100 before separating by BN-PAGE and immunoblotting using mAb 5B7. N-terminal epitope exposure was induced by either OG or Triton X 100. A partial increase in reactivity was seen with CHAPS. The detergent free sample was run on a separate gel to avoid any possible effect due to the added detergents. Blots are representative of three separate experiments. g) FSK-7 cells and Bax deficient HCT116 cells were treated with staurosporine (STS) for the indicated times. Cells were transiently expressing either YFP-Bax or YFP alone, as indicated. Cells were then fixed and immunostained with anti-Bax mAb 5B7. Positive staining was only seen in FSK-7 cells following STS treatment, and then only in the individual cells that had apoptotic nuclei (indicated by the arrows). Apoptotic HCT116 cells expressing YFP alone did not immunostain with mAb 5B7, but did when expressing YFP-Bax. In the HCT116 cells, arrows indicate examples of punctate YFP-Bax and co localising 5B7 immunostaining.
mAb 5B7 has previously been shown to immunoprecipitate cytosolic Bax from non-apoptotic cells, so it was therefore surprising that it did not detect Bax following BN-PAGE [10]. Another monoclonal antibody, 6A7, only immunoprecipitated Bax during apoptosis or following treatment with Triton-X100, both of which bring about a conformational change exposing the epitope [10, 23]. 6A7 did not detect Bax by immunoblotting (data not shown). In agreement with previously published results, 5B7 immunoprecipitated Bax from cytosolic fractions of FSK-7 cells in the presence or absence of Triton-X100 (Fig. 5b). In contrast, 6A7 only immunoprecipitated cytosolic Bax in the presence of Triton-X100. However, when we attempted to immunoprecipitate Bax from membrane fractions of cells detached for 1 hour extracted using our BN-PAGE protocol, only 5B7 immunoprecipitated Bax, and then only following treatment with Triton X100. These data suggest that, rather than one epitope being exposed and the other cryptic, both show different degrees of exposure depending upon the assay conditions used.
To further investigate the native conformation of Bax, cytosolic extracts from adherent cells were split into equal proportions which were either cross-linked with BS3 as previously described or left untreated [7]. These were separated by SDS-PAGE and immunoblotted with either a polyclonal anti-Bax antibody or mAb 5B7 (Fig. 5c). The polyclonal antibody detected Bax both before and after cross-linking, with cross-linked Bax migrating slightly faster. In contrast, 5B7 only detected Bax in the untreated extracts. These data suggest that although the 5B7 epitope is more accessible than other epitopes under some conditions, this region of Bax may not be exposed in the native molecule.
We needed to exclude the possibility that BN-PAGE might activate Bax that had associated with mitochondria. Bax can be activated in vitro by a variety of factors, including detergents, pH and temperature [23]. We asked if the conformation of Bax extracted from mitochondria was affected by either aminocaproic acid or coomassie. Membrane fractions of cells detached for 1 hour were extracted in either CHAPS, CHAPS/aminocaproic acid, or RIPA. Equal amounts of each were then immunoprecipitated with anti-Bax mAb 6A7, and immunoblotted with a polyclonal anti-Bax antibody (Fig. 5d). 6A7 did not immunoprecipitate membrane extracted Bax in either CHAPS alone or the CHAPS/aminocaproic acid, but did in RIPA. We asked if addition of coomassie had any affect on Bax activation. In the presence of coomassie mAb 6A7 immunoprecipiated RIPA extracted Bax, indicating that the dye did not mask the epitope, but did not immunoprecipitate CHAPS extracted Bax (Fig. 5d). To determine if BN-PAGE resulted in Bax oligomerisation, we extracted mitochondrial fractions in CHAPS alone, which should result in the complexes dissociating into monomers based upon gel-filtration. We then added BN-PAGE sample buffer, separated the extract and immunoblotted for Bax (Fig. 5e). Bax extracted in CHAPS alone was monomeric on BN-PAGE, indicating that if the Bax complexes had dissociated, they did not oligomerises due to the aminocaproic acid and coomassie. In contrast, membranes extracted in Triton-X100 contained large Bax complexes. Thus, CHAPS/aminocaproic acid and coomassie did themselves induce Bax oligomerisation or N-terminal epitope exposure.
To ask if Bax maintained its N-terminal conformation during BN-PAGE, we exposed cytosolic fractions to CHAPS, Triton X100 or n-octylglucoside. These were then separated by BN-PAGE and immunoblotted with 5B7 (Fig. 5f). No immunoreactive Bax was seen in untreated lysates, consistent with the earlier results. 1% CHAPS increased the immunoreactivity of Bax to 5B7 slightly. Triton X100 and n-octylglucoside each induced a significant increase in 5B7 reactivity along with a reduction in mobility. These detergents can induce dimerisation of cytosolic Bax, and this might account for the altered size seen by BN-PAGE [7, 23]. This indicated that if the N-terminus of Bax was exposed, it maintained this exposure during BN-PAGE and it could be subsequently detected following transfer to PVDF.
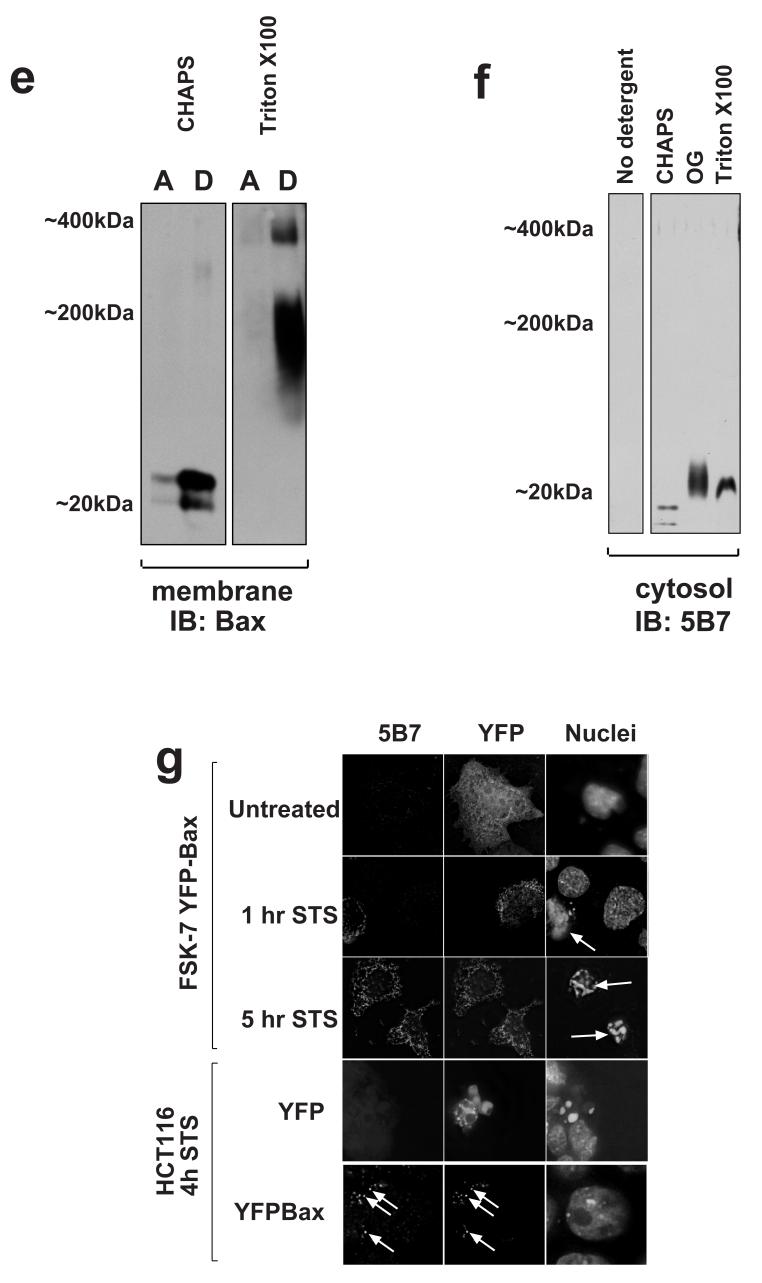
To confirm the native conformation of the 5B7 epitope by an independent method, we examined single cells transiently expressing YFP-Bax. Cells were treated with staurosporine for 1 hour or 4 hours before fixing and immunostaining with 5B7 (Fig. 5g). In untreated cells, cytosolic YFP-Bax did not show 5B7 immunoreactivity. In cells treated with staurosporine, 5B7 immunoreactivity was only seen in cells that had apoptotic nuclear morphology. Note that in the panel showing cells treated for 1 hr, the non-transfected cell clearly has an apoptotic nucleus and endogenous Bax stains positively for 5B7. In contrast, the YFP-Bax expressing cell has a punctate distribution of YFP-Bax that does not react with 5B7. In the panel showing cells treated for 5 hours, both cells have apoptotic nuclei and show colocalisation between 5B7 immunoreactivity and YFP-Bax. Finally, to confirm that YFP-Bax did react with 5B7, we repeated the experiment in Bax deficient HCT116 cells [25]. No 5B7 immunoreactivity was seen following staurosporine treatment when HCT116 cells were transiently transfected with YFP alone. However, YFP-Bax expressing HCT116 cells that had apoptotic nuclei showed 5B7 staining colocalised with the YFP (Fig. 5e).
Together, these experiments demonstrate that mAb 5B7 recognises native Bax on mitochondria, but only in cells that have undergone apoptosis. Furthermore, BN-PAGE maintains the native conformation of Bax as seen in vivo.
Endogenous Bax complexes assemble on the OMM during apoptosis prior to N-terminal epitope exposure
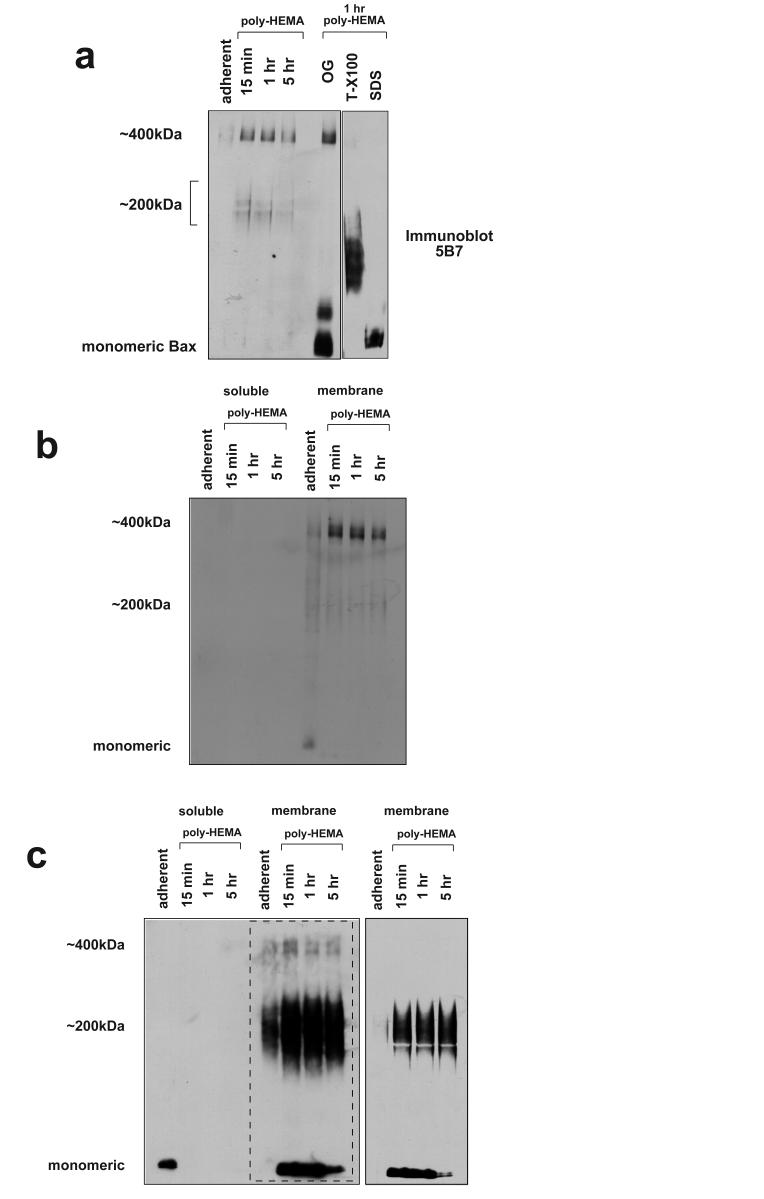
We next asked if we could determine the N-terminal conformation of endogenous Bax in the high-molecular weight complexes separated by BN-PAGE. FSK-7 cells were detached from ECM for various times and membrane fractions separated by 1D BN-PAGE. These were then immunoblotted using mAb 5B7 (Fig. 6a). 5B7 detected a pattern of immunoreactive bands distinct from that seen using the polyclonal Bax antibody (compare with Fig. 2a). No Bax was detected in the membrane fraction of adherent cells. 5B7 appeared to detect a 400kDa species, however this was not observed following a second dimension separation by SDS-PAGE (Figs. 2b, 4c), suggesting it represents non-specific immunoreactivity. Interestingly, the 200kDa Bax complex previously seen was detected only very weakly. Prolonged detachment from ECM did not show an increase in the amount of Bax that was detected by 5B7. This may be explained by our previous observation that following MOMP, Bax becomes insoluble in CHAPS, which corresponds to the large clusters previously described [7, 21, 22]. This CHAPS resistant Bax could be extracted in urea. Activated Bax may not, therefore, be present in the fractions run by BN-PAGE here.
Fig. 6.
N-terminal epitope exposure of endogenous Bax in membrane complexes during anoikis. a) Membrane fractions isolated from adherent cells and cells detached for various times were extracted separated by BN-PAGE before immunoblotting with mAb 5B7. 5B7 predominantly detected a 400kDa species in the membrane fraction of detached cells, but 2D-analysis suggests that this is non-specific. Equal amounts of the membrane fraction from cells detached for 1 hour were treated with octylglucoside, Triton X100 or SDS before BN-PAGE and immunoblotting with 5B7. All three detergents resulted in an increase in immunoreactive Bax. b) Cytosolic and membrane fractions from adherent and detached FSK-7 cells were separated by BN-PAGE and transferred to PVDF. The native membrane was immunoblotted with mAb 5B7. c) The same immunoblot as shown in panel B was denatured and reprobed with mAb 5B7 a second time. Bax was now detected as a monomer and in the 200kDa complex. The area of the immunoblot within the dotted line is shown at a lower exposure in the adjacent panel to allow comparison. Note that the distribution of Bax seen with 5B7 in the denatured blot is similar to that shown earlier with a polyclonal anti-Bax antibody in a native blot (Fig. 2a). All blots are representative examples of at least three experiments.
To detect the total amount of Bax present, equal amounts of membrane fractions isolated from cells detached for one hour were treated with n-octylglucoside, Triton-X100 or SDS. These were the separated by 1D BN-PAGE and immunoblotted with 5B7 (Fig. 6a). Bax was detected by mAb 5B7 in all the detergent treated samples. The size of the Bax complexes observed varied with each detergent. N-octylglucoside increased immunoreactivity at a molecular weight that approximates to monomeric and dimeric Bax. SDS resulted in exclusively monomeric Bax. Triton-X100 generated a higher molecular weight complex.
As Bax in the 200 kDa complex was only weekly immunoreactive with 5B7, we speculated that the N-terminal epitope was not exposed. To test this, cytosolic and membrane fractions from adherent and detached cells were separated by 1D BN-PAGE as before and immunoblotted with 5B7 (Fig. 6b). The same immunoblot was then denatured and probed a second time with 5B7 (Fig. 6c). Probing the native proteins showed no specific immunoreactivity in either the cytosolic or membrane fractions of adherent or detached cells. Following denaturation, however, 5B7 detected monomeric Bax in the cytosol of adherent cells. Bax was not detected in cytosolic fractions of cells detached from ECM. In marked contrast to the native blot, following denaturation 5B7 detected both monomeric and 200 kDa Bax species in the membrane fractions. Furthermore, the relatively small amount of Bax present in the membrane fraction of adherent cells was present in a 200 kDa complex, in agreement with Fig. 2b. As previously seen with the polyclonal anti-Bax antibody (Fig. 2a), the amount of membrane associated monomeric Bax decreased in cells detached from ECM for longer time periods (see shorter exposure of the same immunoblot shown in Fig. 6c).
Together, these data indicate that contrary to current models of how Bax functions, both translocation to mitochondria and association into high molecular weight complexes occurs without exposure of N-terminal epitopes.
Discussion
Bax translocation, N-terminal epitope exposure and oligomerisation are often discussed as a single event, resulting in Bax switching from a benign, monomeric protein in the cytosol to one that has formed channels in the OMM [1]. Indeed, many papers equate Bax oligomerisation with cytochrome c release and cell death [5, 14, 15, 34, 35]. However, several studies suggest that Bax activation involves a number of distinct regulatory steps [8, 9, 33, 36-38]. In this paper we report conditions that allow extraction and analysis of endogenous Bax complexes from mitochondria. By using epithelial anoikis as a model, we were able to examine cells in which Bax translocation to mitochondria had occurred but most cells had yet to undergo MOMP [7, 21]. We demonstrate that endogenous Bax translocates to mitochondria and assembles into protein complexes prior to the conformational change associated with MOMP. Thus, characterising native Bax complexes provides novel insights into the mechanism of the apoptotic process.
It was previously proposed that dimerisation between pro- and anti-apoptotic Bcl-2 proteins represented a key aspect of their regulation [39-41]. In a series of seminal papers, Hsu and Youle demonstrated that interactions between Bcl-2 proteins were influenced by detergents used to generate cell lysates [10, 23]. In particular, NP-40 induced monomeric, cytosolic Bax to dimerise with other Bcl-2 proteins, whereas CHAPS did not. Subsequently, studies have almost exclusively used CHAPS. However, during apoptosis Bax undergoes a profound change in its biochemical properties. Whereas cytosolic Bax is soluble and can be extracted without detergent, during apoptosis it becomes an integral membrane protein. Most studies have examined membrane-inserted Bax using CHAPS, i.e. conditions chosen for their ability not to alter the conformation of cytosolic Bax. No studies to date have asked if this approach gives a true reflection of the nature of native, membrane associated Bax.
We asked what conditions were required to isolate native Bax from the OMM of cells undergoing apoptosis. Our data indicate that conditions chosen on the basis that they do not alter the conformation of cytosolic Bax are not suited for determining the quaternary structure of membrane associated Bax. CHAPS solubilised mitochondria separated by gel filtration suggested that the majority of Bax following detachment from ECM was monomeric. In contrast, BN-PAGE indicated that even at early time points following Bax association with mitochondria, the majority was in high-molecular weight complexes. Zwitterionic detergents like CHAPS can disrupt protein/protein interactions during purification of membrane complexes [42]. Throughout gel-filtration, membrane proteins are solubilised in CHAPS, increasing the chance of complexes dissociating. During BN-PAGE, membrane proteins are solubilised by the anionic Coomassie dye, thus being detergent free and minimising dissociation.
Chemical cross-linkers have been used to characterise Bax oligomerisation, identifying dimers, trimers and tetramers [5, 14, 17, 43], and supporting in vitro studies indicating a Bax tetramer could release cytochrome c [15]. Crosslinkers require care in interpretation. They have defined spacer arm lengths that require reactive groups on adjoining proteins to be within the necessary distance [44]. Furthermore, reaction conditions, particularly in complex protein mixtures, do not necessarily occur stoichiometrically. When attempting to cross-link a multi-protein complex, therefore, the likely outcome is a mixture of the different components each covalently linked to different extents. Rather than one large complex, one is likely to see a mixture of smaller complexes. BN-PAGE and gel-filtration, both in this study and others, show no indication that Bak or Bax exist as dimers or trimers on mitochondria [5, 7, 21, 45].
The studies by Hsu and Youle established that Bax underwent a conformational switch during activation that could be followed using antibodies against cryptic epitopes. Two monoclonal antibodies, 5B7 and 6A7, have subsequently been used follow Bax activation in numerous studies. 5B7 will immunoprecipiate cytosolic Bax in the absence of detergent, whereas 6A7 does not, indicating that the epitope for the former was accessible prior to activation. We have recently demonstrated that Bax translocation to mitochondria is not accompanied by the conformational change in the N-terminus [21]. Instead, the conformational change is associated with mitochondrial permeabilisation, during which activated Bax assembles into detergent resistant clusters [7, 22]. Other studies have also separated Bax activation and translocation. In myc deficient cells, etoposide induced Bax translocation but not N-terminal epitope exposure or cytochrome c release [37]. In another study, a single amino acid substitution within the C-terminal tail of Bax induced constitutive localisation to mitochondria, but not N-terminal epitope exposure until apoptosis was induced with staurosporine [36].
We found that although mAb 5B7 can immunoprecipitate Bax under detergent free conditions, it was unable to recognise Bax in fixed cells or extracts separated by BN-PAGE. The epitopes for both monoclonal antibodies 5B7 and 6A7 overlap within amino acids 7-19 of Bax [10]. Substituting proline 13 with alanine abolished immunoreactivity with both 6A7 and 5B7 (Upton and Gilmore, unpublished data). Although the solution structure of monomeric, recombinant Bax shows this region to be extremely flexible, it may actually be more restricted in vivo [32]. Cross-linking of cytosolic Bax suggested that the N-terminal conformation is less flexible, with a shift to a faster migrating form which did not react with mAb 5B7 following treatment with BS3. We can discount the possibility of direct modification of the epitope by the crosslinker, it being devoid of lysine. The difference between these results and immunoprecipitation may be explained if this region of the molecule can switch between open and closed conformations. Cross-linking would fix Bax in the closed state, whereas binding to an antibody would “fix” it in the open conformation.
In conjunction with our previous work [7, 21], our findings suggest that Bax complexes form prior to MOMP. Furthermore, Bax is not exclusively cytosolic in healthy cells, some being present on mitochondria in adherent cells. BN-PAGE indicates that in healthy cells, this mitochondrial Bax is already in a 200 kDa complex. Thus, Bax “oligomers” may not represent the active pore. However, oligomerisation of membrane associated Bax monomers has been shown to drive MOMP [37]. In that study, using myc deficient cells treated with etoposide, mitochondrial Bax eluted as a monomer following gel-filtration. However, in the presence of myc, etoposide induced Bax assembly into large complexes and cells died. Our own gel filtration experiments indicated mitochondrial Bax eluted as a monomer immediately following loss of ECM attachment, but at later times it eluted in 200 kDa complexes. One possibility is that the complexes we see by BN-PAGE at early times during anoikis are distinct from those seen by gel-filtration following cell death. Bax may be held in an inactive state prior to MOMP through interactions with other mitochondrial proteins, in a similar way to Bak. Bak interacts with VDAC2, which inhibits its pro-apoptotic function [12]. During apoptosis, Bak dissociates from VDAC2. Cells lacking VDAC2 are more susceptible to Bak dependent MOMP, whereas VDAC2 over expression inhibits it. Bax can also bind to components of the permeability transition pore (PTP) [46, 47]. It may be that interactions with these proteins are disrupted by CHAPS, whereas later interactions associated with pore formation are not.
Numerous studies and reviews on Bax assume a number of facts as “given”. These include that Bax undergoes a conformational change that regulates its translocation to mitochondria where it oligomerises into a pore. However, there is little biochemical data on native, endogenous Bax on the OMM during apoptosis. Our data show that Bax translocation and assembly into complexes occurs independently of the N-terminal conformational change associated with MOMP and cell death. These findings also highlight the importance of characterising the native protein in a physiological model of apoptosis.
Acknowledgments
We thank Mauro Esposti, Tom Owens, Fiona Foster and Jennefer Lyndsay for comments on the manuscript. This work was supported by The Wellcome Trust.
Abbreviations
- BN-PAGE
blue native polyacrylamide electrophoresis
- BS3
bis(2-[succinimidooxy-carbonyloxy]ethyl)sulfone
- CHAPS
[3-(3 Cholamidopropyl)-diamethylammonio]-1-propanesulphonate]
- ECM
extracellular matrix
- GIP
general import complex
- MOMP
mitochondrial outer membrane permeabilisation
- OMM
outer mitochondrial membrane
- SMAC
second mitochondrial activator of caspases
- poly-HEMA
poly-2-hydroxyethyl methacrylate
- TOM
translocase outer membane
- YFP
yellow fluorescent protein
- zVADfmk
benzyloxycarbonyl-Val-Ala-Asp-fluoromethane
References
- 1.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 2.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol. Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 5.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 6.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J. Cell Biol. 2003;162:599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore AP, Metcalfe AM, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 2000;149:431–445. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desagher S, Osen Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu YT, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths GJ, Corfe BM, Savory P, Leech S, Esposti MD, Hickman JA, Dive C. Cellular damage signals promote sequential changes at the N-terminus and BH-1 domain of the pro-apoptotic protein Bak. Oncogene. 2001;20:7668–7676. doi: 10.1038/sj.onc.1204995. [DOI] [PubMed] [Google Scholar]
- 12.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 13.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 14.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. Embo. J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat. Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- 16.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 17.Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem. J. 2002;368:915–921. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 20.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 21.Upton JP, Valentijn AJ, Zhang L, Gilmore AP. The N-terminal conformation of Bax regulates cell commitment to apoptosis. Cell Death Differ. 2007;14:932–942. doi: 10.1038/sj.cdd.4402092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J. Cell Biol. 2001;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- 24.Kittrell FS, Oborn CJ, Medina D. Development Of Mammary Preneoplasias Invivo From Mouse Mammary Epithelial-Cell Lines Invitro. Cancer Res. 1992;52:1924–1932. [PubMed] [Google Scholar]
- 25.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 26.Brookes PS, Pinner A, Ramachandran A, Coward L, Barnes S, Kim H, Darley-Usmar VM. High throughput two-dimensional blue-native electrophoresis: a tool for functional proteomics of mitochondria and signaling complexes. Proteomics. 2002;2:969–977. doi: 10.1002/1615-9861(200208)2:8<969::AID-PROT969>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Valentijn AJ, Gilmore AP. Translocation of full-length bid to mitochondria during Anoikis. J. Biol. Chem. 2004;279:32848–32857. doi: 10.1074/jbc.M313375200. [DOI] [PubMed] [Google Scholar]
- 28.Zha JP, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine Phosphorylation Of Death Agonist Bad In Response to Survival Factor Results In Binding to 14-3-3 Not Bcl-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 29.Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y. 14-3-3 Interacts directly with and negatively regulates pro-apoptotic Bax. J. Biol. Chem. 2003;278:2058–2065. doi: 10.1074/jbc.M207880200. [DOI] [PubMed] [Google Scholar]
- 30.Dekker PJ, Ryan MT, Brix J, Muller H, Honlinger A, Pfanner N. Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol. Cell. Biol. 1998;18:6515–6524. doi: 10.1128/mcb.18.11.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 33.Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C. Conformational control of Bax localization and apoptotic activity by Pro168. J. Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345(Pt 2):271–278. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell. Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soucie EL, Annis MG, Sedivy J, Filmus J, Leber B, Andrews DW, Penn LZ. Myc potentiates apoptosis by stimulating Bax activity at the mitochondria. Mol. Cell. Biol. 2001;21:4725–4736. doi: 10.1128/MCB.21.14.4725-4736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, Hanada M, Bodrug S, Irie S, Iwama N, Boise LH, Thompson CB, Golemis E, Fong L, Wang HG, Reed JC. Interactions Among Members Of the Bcl-2 Protein Family Analyzed With a Yeast 2-Hybrid System (Vol 91, Pg 9238, 1994) Proc. Natl. Acad. Sci. USA. 1995;92:1794–1794. doi: 10.1073/pnas.91.20.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. U S A. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton SL, Hawkes MJ, Brush K, Cook R, Chang RJ, Smilowitz HM. Subunit composition of the purified dihydropyridine binding protein from skeletal muscle. Biochemistry. 1989;28:7820–7828. doi: 10.1021/bi00445a044. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 44.Mattson G, Conklin E, Desai S, Nielander G, Savage MD, Morgensen S. A practical approach to crosslinking. Mol. Biol. Rep. 1993;17:167–183. doi: 10.1007/BF00986726. [DOI] [PubMed] [Google Scholar]
- 45.Grinberg M, Schwarz M, Zaltsman Y, Eini T, Niv H, Pietrokovski S, Gross A. Mitochondrial carrier homolog 2 is a target of tBID in cells signaled to die by tumor necrosis factor alpha. Mol. Cell. Biol. 2005;25:4579–4590. doi: 10.1128/MCB.25.11.4579-4590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 47.Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl. Acad. Sci. USA. 1998;95:14681–14686. doi: 10.1073/pnas.95.25.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]