Abstract
Running increases neurogenesis in the dentate gyrus of the hippocampus, a brain structure that is important for memory function. Consequently, spatial learning and long-term potentiation (LTP) were tested in groups of mice housed either with a running wheel (runners) or under standard conditions (controls). Mice were injected with bromodeoxyuridine to label dividing cells and trained in the Morris water maze. LTP was studied in the dentate gyrus and area CA1 in hippocampal slices from these mice. Running improved water maze performance, increased bromodeoxyuridine-positive cell numbers, and selectively enhanced dentate gyrus LTP. Our results indicate that physical activity can regulate hippocampal neurogenesis, synaptic plasticity, and learning.
New neurons are added continuously to certain areas of the adult brain, such as the hippocampus and olfactory bulb (1, 2). The functional significance of new hippocampal cells is not clear. In birds, food storage and retrieval experience correlate with changes in hippocampal size and neurogenesis (3). In mice, neurogenesis in the dentate gyrus increases with exposure to an enriched environment, and it is associated with improved learning (4). Similarly, voluntary physical activity in a running wheel enhances the number of new hippocampal cells (5). Although it is not known whether running also affects learning, it has been shown that physical activity facilitates recovery from injury (6) and improves cognitive function (7). Furthermore, trophic factors, associated with progenitor cell survival and differentiation (8), alterations in synaptic strength (9), long-term potentiation (LTP) (10), and memory function (11), are elevated after exercise (12, 13). At the cellular level, wheel running enhances the firing rate of hippocampal cells in a manner that correlates with running velocity (14). Thus, exercise may increase synaptic plasticity and learning, as well as neurogenesis. We designed the following experiments to test this hypothesis.
Materials and Methods
Subjects.
Thirty-four female C57BL/6 mice, 3 months old (The Jackson Laboratory) were divided into two groups of 17, the controls and the runners. The runners had free access to a running wheel equipped with an electronic counter. During the first 10 days animals received one 10-mg/ml intraperitoneal injection of 5-bromodeoxyuridine (BrdU; Sigma), dissolved in 0.9% NaCl, filtered sterile at 0.2 μm, at 50 μg/g of body weight per day to label dividing cells.
Spatial Learning.
The mice were trained on a Morris water maze (15) with either two or four trials per day for 6 days. The platform was hidden 1 cm below the surface of water; it was made opaque with white nontoxic paint. The starting points were changed every day. Each trial lasted either until the mouse had found the platform or for a maximum of 40 s. At the end of each trial, the mice were allowed to rest on the platform for 10 s. The time to reach the platform (latency), the length of swim path, and the swim speed were recorded semi-automatically by a video tracking system (San Diego Instruments).
Electrophysiology.
The animals were coded so that the experimenter was blind to the identity of individual mice. LTP experiments were done in the dentate gyrus and CA1 subfields of the hippocampus, both in the presence and in the absence of d-2-amino-5-phosphonovaleric acid (APV). The sequence of the experiments was randomized to prevent any possible order effects. Statistical analyses were carried out between pooled, rather than all, slices from individual mice. Data from all slices tested in the same condition from the same mouse were averaged to give a single value, whereas data from slices tested in different conditions from the same mouse were considered independent values.
Mice were anesthetized with fluorothane and decapitated, and the brains were quickly removed into chilled artificial cerebrospinal fluid (125.0 mM NaCl/2.5 mM KCl/1.25 mM NaH2PO4/25.0 mM NaHCO3/2 mM CaCl2/1.3 mM MgCl2/10.0 mM dextrose/0.001 mM bicuculline methobromide, at pH 7.4), and continuously bubbled with 95% O2/5% CO2. One hemisphere was immediately placed in 4% paraformaldehyde in 0.1 M PBS and kept for histological analysis. The second hemisphere was affixed to a vibratome and cut into 400-μm slices. The slices were heated at 32°C for 30 min in a circulating perfusion chamber and then maintained at room temperature. Individual slices were transferred to the recording chamber as needed, and experiments were performed in artificial cerebrospinal fluid maintained at 32–34°C.
A sharpened tungsten, bipolar stimulating electrode and a 1-MΩ recording electrode filled with 3 mM KCl or 1 mM NaCl were used for testing LTP. For the dentate gyrus, electrodes were positioned in the middle third of the molecular layer, whereas for CA1 responses, a recording electrode and a stimulating electrode (toward CA3) were positioned in the stratum radiatum of field CA1, aided by a microscope (Olympus BX50wi) with a ×40 objective lens. Responses were evoked with single biphasic current pulses (10–400 μA), adjusted to yield a response ≈30% of maximum. All evoked responses were initially tested with paired-pulse stimuli (at 50, 100, 200, and 500 ms). For dentate recordings, only the responses that did not show paired-pulse facilitation were used to ensure that medial perforant path synapses were being examined (16, 17). Individual synaptic responses were elicited at 15-s intervals. After at least 10 min of stable baseline responses, LTP was induced by a burst of 50 pulses at 100 Hz; bursts were repeated four times at 30-s intervals. Recordings continued for 45 min after LTP induction.
Immunohistochemistry.
Immunohistochemistry for BrdU and immunofluorescent triple labeling for BrdU, the neuronal marker NeuN, and the glial marker calcium-binding protein S100β were performed on free-floating 40-μm coronal sections that were pretreated by denaturing DNA, as described previously (5). The antibodies were mouse anti-BrdU (Boehringer Mannheim), 1:400; rat anti-BrdU ascites fluid (Accurate, Harlan Sera-Lab, Loughborough, England) for triple labeling, 1:100; rabbit anti-S100β (Swant, Bellinoza, Switzerland) 1:2500; and mouse anti-NeuN (kindly provided by R. J. Mullen, University of Utah), 1:20. To determine the number of BrdU-labeled cells, we stained for BrdU by using the peroxidase method (ABC system, with biotinylated donkey anti-mouse IgG antibodies and diaminobenzidine as chromogen; Vector Laboratories). The fluorescent secondary antibodies used were FITC-labeled anti-mouse IgG, Texas red-labeled anti-rat IgG, and Cy5-labeled anti-rabbit IgG (Jackson ImmunoResearch), 6 μl/ml.
Stereology.
BrdU-positive cells were counted in a one-in-six series of sections (240 μm apart) through a ×40 objective (Leitz) throughout the rostro caudal extent of the granule cell layer. A one-in-six series of adjacent sections stained with 0.5 mg/ml Hoechst 33342 in Tris-buffered saline (Molecular Probes) for 15 min was used to measure granule cell layer volume. We used a semiautomatic stereology system (StereoInvestigator, MicroBrightfield) and a ×10 objective to trace the granule cell area. The granule cell reference volume was determined by summing the traced granule cell areas for each section multiplied by the distance between sections sampled. The number of BrdU-labeled cells was then related to granule cell layer sectional volume and multiplied by the reference volume to estimate total number of BrdU-positive cells.
Results
Mice were assigned to either control (n = 17) or runner (n = 17) conditions. Mice in the runner group ran an average distance of 4.78 ± 0.41 kilometers per day. During the first 10 days, animals received one intraperitoneal BrdU injection per day to label dividing cells. Thereafter, animals continued in their respective experimental conditions for 2 to 4 months. Mice were tested on the water maze task between day 30 and day 49. Between day 54 and day 118, mice were anesthetized with fluorothane and decapitated. One half of the brain was used for electrophysiological experiments. The other half was kept for immunocytochemistry (Fig. 1).
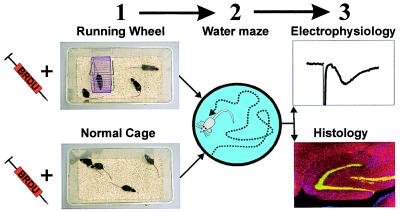
Figure 1.
Flowchart of the experiment. Controls were housed in standard 30- by 18-cm cages, whereas runners had 48- by 26-cm housing with free access to a running wheel (1). Mice in both conditions received BrdU (50 μg/g per day) injections for the first 10 days after their housing assignment. After 1 month in their respective environments, mice were tested in the water maze (2) between days 30 and 36 or between days 43 and 49. Mice were anesthetized and decapitated between days 54 and 118; one hemisphere was used for electrophysiology; the other was used for immunocytochemistry (3).
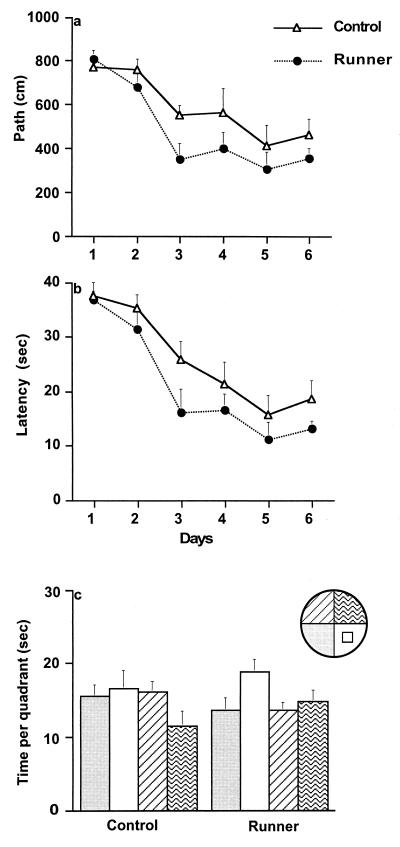
To assess spatial learning, mice were tested in the Morris water maze over 6 days. Mice were trained with four trials per day (controls, n = 8, and runners, n = 8) between days 30 and 36, or two trials daily (controls, n = 9 and runners, n = 9) between days 43 and 49. When mice were trained with four trials per day, ANOVA with repeated measures (days) showed no difference between the groups in path length (F(1,14) = 0.56, P > 0.47), latency (F(1,14) = 0.08, P > 0.79), or swim speed (F(1,14) = 1.5, P > 0.24). However, when mice were trained by using the more challenging two-trials-per-day paradigm, acquisition of the task was significantly better in the runners than in the controls, showing decreased path length (F(1,16) = 4.99, P < 0.04; Fig. 2a) and latency (F(1,16) = 4.61, P < 0.047; Fig. 2b) to the platform. These results were not confounded by swim speed, because there was no significant difference between the groups in this regard (F(1,16) = 0.59, P > 0.29). To test retention of the task, the platform was removed for a 60-s probe test 4 hr after the last trial on day 6. Mice that had been trained with four trials per day spent more time in the platform quadrant than in all others (controls, F(3,28) = 57.17, P < 0.0001; runners, F(3,28) = 16.70, P < 0.0001). Mice trained with two trials per day did not show a significant preference for the platform quadrant (controls, F(3,32) = 1.53, P > 0.23; runners, F(3,32) = 2.56, P < 0.07), although for runners, the trend appeared stronger (Fig. 2c). Taken together, our results indicate that running enhances acquisition on the water maze task.
Figure 2.
Water maze learning in controls and runners trained with two trials per day (four-trial data are not shown here, but see description in Results). Mice were trained over 6 days to find the hidden platform in the Morris water maze. A significant difference developed between the groups (P < 0.04) in path length (a) and (P < 0.047) in latency (b). Results of probe test 4 hr after the last trial on day 6 (c).
Exercise can affect steroid hormone and stress levels, which in turn could influence learning, LTP, and neurogenesis (18–20). Therefore, after completion of behavioral testing, blood samples were collected retro-orbitally (controls, n = 8; runners, n = 9) on day 53 at 14:00 hr under isoflurane (4%) anesthesia. Radioimmunoassay for plasma corticosterone was performed by using the manufacturer’s protocol for a commercial kit (ICN). No differences between the groups were observed (controls, 59 ± 13.1 ng/ml; runners, 49.4 ± 7.7 ng/ml; t(15) = 0.65, P > 0.52). However, the possibility remains that running induces noncognitive, affective variables that could influence behavior.
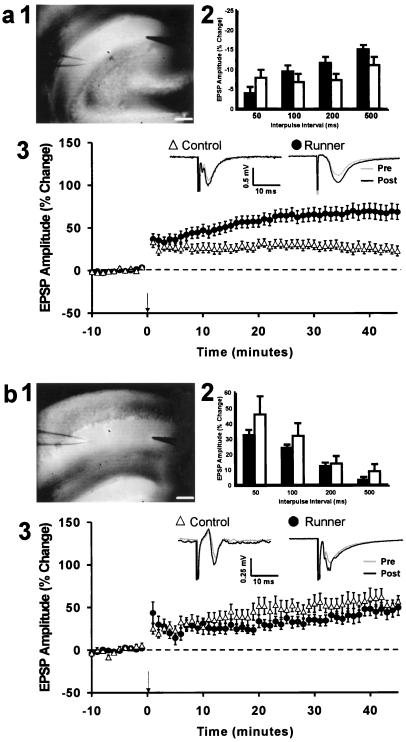
To determine whether running affects synaptic plasticity, LTP was studied in hippocampal slices from the same 5- to 7-month-old controls and runners that had received BrdU and had been tested in the water maze. Recordings were made in the dentate gyrus, because this is where running-induced changes in cell proliferation and survival occur (5), and in area CA1. For dentate recordings only medial perforant path synapses were examined (refs. 16 and 17; Fig. 3a2). No differences were found between the groups in the initial excitatory postsynaptic potential (EPSP) amplitudes, suggesting no effect of running on basal synaptic efficacy (dentate gyrus: controls, 0.39 ± 0.04 mV, runners, 0.42 ± 0.05 mV (t(22) = 0.45, P > 0.66); CA1: controls, 0.35 ± 0.07 mV, runners, 0.32 ± 0.08 mV (t(12) = 0.29, P > 0.78)). The recordings in the dentate gyrus showed that EPSP amplitude was significantly increased 45 min after the administration of high-frequency stimuli (controls, 17 slices from 10 mice (t(9) = 2.34, P < 0.047); runners, 20 slices from 14 mice (t(13) = 3.82, P < 0.002); paired t test)). The groups were significantly different from each other (F(1,22) = 4.66, P < 0.042), demonstrating that running increased dentate gyrus LTP (Fig. 3a3). Robust LTP was also elicited in the CA1 Schaffer collateral pathway (controls, 12 slices from 7 mice (t(6) = 3.59, P < 0.011); runners, 7 slices from 7 mice (t(6) = 2.55, P < 0.044); paired t test). However, there was no difference in the magnitude of CA1 LTP between controls and runners (F(1,12) = 0.22, P > 0.65), (Fig. 3b3).
Figure 3.
LTP in dentate gyrus (a) and area CA1 (b). (a1, b1) Digital images of hippocampal slices showing the position of the stimulation and recording electrodes. (a2, b2) Paired-pulse facilitation at 50-, 100-, 200-, and 500-ms interpulse intervals. EPSP, excitatory postsynaptic potential. There was no difference between slices from controls and runners (P > 0.91). (a3, b3) Time course of LTP in slices from controls (▵) and runners (●) . In addition, representative examples are shown of evoked responses immediately before (Pre) and 30 min after (Post) induction of LTP. Example waveforms are the average of 20 responses recorded over a 5-min period. Population spikes were apparent in some animals in each group after LTP induction. (Scale bars under a1 and b1 indicate 250 μm.)
LTP in the medial perforant path-to-dentate granule cells and in Schaffer collateral CA1 synapses has been shown to depend on the activation of N-methyl-d-aspartate (NMDA) receptors (21). To determine whether LTP in runners was mediated by NMDA receptors, 50 μM NMDA receptor antagonist APV was added to the bath at the onset of recordings. APV completely blocked the induction of LTP, as demonstrated by the lack of an increase of percent baseline amplitude from values before induction [dentate gyrus: controls, four slices from four mice (t(3)= 0.51, P > 0.65), runners, 11 slices from six mice (t(5)= 0.27, P > 0.79); CA1: controls, five slices from five mice (t(4)= 0.44, P > 0.68), runners, five slices from five mice (t(4) = 0.26, P > 0.81); paired t test].
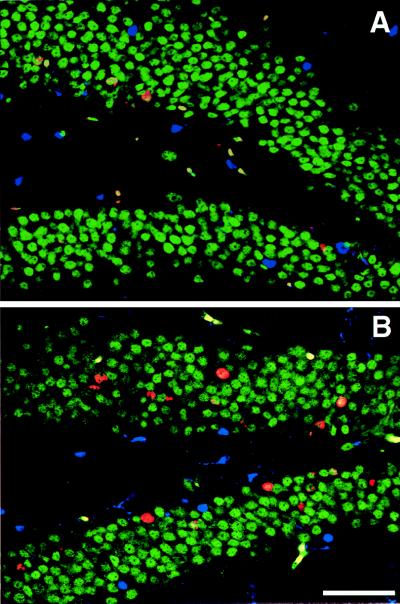
BrdU-positive cells were analyzed in the remaining hemisphere of animals used for electrophysiology. The total number of BrdU-labeled cells was significantly greater in runners than in controls. In addition, 50 cells per animal were analyzed by confocal microscopy (Zeiss, Bio-Rad) for coexpression of BrdU and NeuN for neuronal phenotype and S100β for glial phenotype. Runners had a significantly higher percentage of BrdU-positive cells that colabeled for NeuN. The groups did not differ with regard to astrocytic fate of the newborn cells (Fig. 4; Table 1).
Figure 4.
Confocal images of BrdU-positive cells in control (A) and runner coronal sections (B). Sections were immunofluorescent triple-labeled for BrdU (red), NeuN, indicating neuronal phenotype (green), and S100β, selective for glial phenotype (blue). (Scale bar indicates 50 μm.)
Table 1.
BrdU-positive cell number and phenotype
| Group | No. with BrdU label | % NeuN+ | % S100β+ | % Neither | Volume, mm3 |
|---|---|---|---|---|---|
| Control | 1,409 ± 132 | 81.5 ± 2.4 | 5.5 ± 1.9 | 13.5 ± 3.2 | 0.438 |
| Runner | 3,746 ± 800* | 92.8 ± 1.7* | 1.8 ± 0.8 | 5.5 ± 1.6* | 0.481 |
Controls and runners received BrdU (50 μg/g per day) from day 1 to day 10. Survival of BrdU-labeled cells was determined 2 to 4 months after the last BrdU injection. Runners (n = 8), as compared to controls (n = 8), had significantly more BrdU-positive cells (t(14) = 2.88, P < 0.012). The percentages of cells double-labeled for NeuN were greater in runners than in controls (t(14) = 3.82, P < 0.002), and a lower percentage of BrdU-positive cells that were labeled for neither S100β nor NeuN was observed in runners than in controls (t(14) = 2.26, P < 0.04). Means are ± SEM. *, Significantly different from controls.
Discussion
Running enhances neurogenesis, water maze performance, and LTP in medial perforant path-to-dentate gyrus synapses. Spatial navigation in running mice was improved as compared with controls when mice were trained with two trials rather than four trials daily. Research by others on the effects of forced treadmill exercise on water maze learning in C57BL/6 mice also showed no difference with four trials daily, whereas tasks that are more complex demonstrated that exercising mice perform better (7). It is possible that increased neurogenesis in the runners contributes to learning. Indeed, several factors that elevate production of new neurons are also associated with enhanced learning. Both running and living in an enriched environment double the number of surviving newborn cells (5) and improve water maze performance (4). In addition, survival of cells born prior to spatial training may be enhanced by hippocampal-dependent learning (22). Moreover, treatment with hormones, such as estrogen, increases cell proliferation (23) and improves memory function (24). In contrast, factors that reduce neurogenesis, such as corticosterone treatment, stress, and aging, are associated with diminished performance on spatial learning tasks (20, 25). In our present study, corticosterone levels were similar in controls and runners; however, it is possible that other steroid hormone levels changed and influenced neurogenesis and learning.
The characteristics of LTP, including rapid formation, stability, synapse specificity, and reversibility, make it an attractive model for certain forms of learning and memory (26). Basic mechanisms underlying LTP in the dentate gyrus and CA1 subfields appear unchanged in runners and controls. Induction of LTP was completely blocked by the NMDA receptor antagonist APV (21). In addition, paired-pulse facilitation characteristics were similar to those in previous reports (16, 17), suggesting no running-induced alterations in presynaptic transmitter release. However, running did induce a specific increase in dentate gyrus LTP, concurrent with enhanced neurogenesis and improved water maze performance, raising the possibility that newborn granule cells play a role in increased dentate gyrus LTP. Although newborn cells are a small percentage of the total cells in the granule cell layer, it is possible that new neurons affect hippocampal physiology more than do mature cells (27). Indeed, in immature rats, dentate gyrus LTP lasts longer than in adults (28). Newborn cells in the adult brain may be similar to those generated during development.
The hypothesis that these new cells may mediate increased synaptic plasticity and improved learning is an attractive one; however, several other variables may be important as well. Increased exercise may result in elevated trophic factor production (12, 13), angiogenesis (29), and serotonin levels (30). Some of these variables may exert multiple effects, influencing learning, LTP, and neurogenesis. For example, increased serotonin levels enhance cell proliferation [B. L. Jacobs, P. Tanapat, A. J. Reeves, and E. Gould (1998) Soc. Neurosci. Abstr. 24, 796.4]. Reduction of serotonin by 5,7-dihydroxytryptamine lesions diminishes dentate LTP (31). In addition, mutation of 5-HT2c receptors impairs medial perforant path-to-dentate gyrus, but not CA1, LTP and learning (32). It remains to be determined whether such factors induce running-enhanced synaptic plasticity and learning independently, or act indirectly, supporting survival and connectivity of newborn neurons.
Taken together, our findings demonstrate that voluntary running results in long-lasting survival of BrdU-positive cells, improved performance in a water maze, and a selective increase in dentate gyrus LTP. Further research will determine whether causal links can be found among these correlated measurements.
Acknowledgments
We thank Mary Lynn Gage and Barry Jacobs for comments on the manuscript, Linda Kitabayashi for assistance with confocal imaging, and Deborah Christie for graphic design. We also thank Alice Smith, Tony Slimp, Karen Suter, and coworkers in the Salk Institute Animal Research Facility for their support. This work was funded by the National Institute on Aging, the National Institute of Neurological Disorders and Stroke, the Lookout Fund, the Pasarow Foundation, the Holfelder Foundation, and the American Paralysis Association.
Abbreviations
- APV
d-2-amino-5-phosphonovaleric acid
- BrdU
bromodeoxyuridine
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Gage F H, Kempermann G, Palmer T D, Peterson D A, Ray J. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Goldman S A, Luskin M B. Trends Neurosci. 1998;21:107–114. doi: 10.1016/s0166-2236(97)01191-0. [DOI] [PubMed] [Google Scholar]
- 3.Clayton N S, Krebs J R. Proc Natl Acad Sci USA. 1994;91:7410–7414. doi: 10.1073/pnas.91.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 5.van Praag H, Kempermann G, Gage F H. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 6.Johansson B B, Ohlsson A. Exp Neurol. 1997;139:322–327. doi: 10.1006/exnr.1996.0106. [DOI] [PubMed] [Google Scholar]
- 7.Fordyce D E, Wehner J M. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 8.Ray J, Palmer T D, Suhonen J, Takahashi J, Gage F H. In: Isolation, Characterization, and Utilization of CNS Stem Cells. Christen Y, Gage F H, editors. Berlin: Springer; 1997. pp. 129–149. [Google Scholar]
- 9.Schuman E M. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 10.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 11.Fischer W, Wictorin K, Bjorklund A, Williams L R, Varon S, Gage F H. Nature (London) 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 12.Neeper S A, Gomez-Pinilla F, Choi J, Cotman C. Nature (London) 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Pinilla F, So V, Kesslak J P. Neuroscience. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- 14.Czurko A, Hirase H, Csicsvari J, Buzsaki G. Eur J Neurosci. 1999;11:344–352. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- 15.Morris R G M. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 16.McNaughton B L. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- 17.Colino A, Malenka R C. J Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- 18.Tharp G D. Med Sci Sports Exercise. 1975;7:6–11. [PubMed] [Google Scholar]
- 19.Shors T J, Seib T B, Levine S, Thompson R F. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 20.McEwen B S. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Wigstrom H, Gustafsson B. J Physiol. 1986;81:228–236. [PubMed] [Google Scholar]
- 22.Gould E, Beylin A, Tanapat P, Reeves A, Shors T J. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 23.Tanapat P, Hastings N B, Reeves A J, Gould E. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luine V N, Richards S T, Wu V Y, Beck K D. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 25.Krugers H J, Douma B R, Andringa G, Bohus B, Korf J, Luiten P G. Hippocampus. 1997;7:427–436. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 26.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 27.Gould E, Tanapat P, Hastings N B, Shors T J. Trends Cogn Sci. 1999;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- 28.Bronzino J D, Abu-Hasballah K, Austin La France R J, Morgane P J. Hippocampus. 1994;4:439–446. doi: 10.1002/hipo.450040406. [DOI] [PubMed] [Google Scholar]
- 29.Isaacs K R, Anderson B J, Alcantara A A, Black J E, Greenough W T. J Cereb Blood Flow Metab. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 30.Chaouloff F. Med Sci Sports Exercise. 1997;29:58–62. doi: 10.1097/00005768-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Bliss T V, Goddard G V, Riives M. J Physiol. 1983;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tecott L H, Logue S F, Wehner J M, Kauer J A. Proc Natl Acad Sci USA. 1998;95:15026–15031. doi: 10.1073/pnas.95.25.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]