Abstract
Phenotypic diversity associated with pathogenic mutations of the human mitochondrial genome (mtDNA) has often been explained by unequal segregation of the mutated and wild-type genomes (heteroplasmy). However, this simple hypothesis cannot explain the tissue specificity of disorders caused by homoplasmic mtDNA mutations. We have previously associated a homoplasmic point mutation (1624C>T) in MTTV with a profound metabolic disorder that resulted in the neonatal deaths of numerous siblings. Affected tissues harboured a marked biochemical defect in components of the mitochondrial respiratory chain, presumably due to the extremely low (<1%) steady-state levels of mt-tRNAVal. In primary myoblasts and transmitochondrial cybrids established from the proband (index case) and offspring, the marked respiratory deficiency was lost and steady-state levels of the mutated mt-tRNAVal were greater than in the biopsy material, but were still an order of magnitude lower than in control myoblasts. We present evidence that the generalized decrease in steady-state mt-tRNAVal observed in the homoplasmic 1624C>T-cell lines is caused by a rapid degradation of the deacylated form of the abnormal mt-tRNAVal. By both establishing the identity of the human mitochondrial valyl-tRNA synthetase then inducing its overexpression in transmitochondrial cell lines, we have been able to partially restore steady-state levels of the mutated mt-tRNAVal, consistent with an increased stability of the charged mt-tRNA. These data indicate that variations in the levels of VARS2L between tissue types and patients could underlie the difference in clinical presentation between individuals homoplasmic for the 1624C>T mutation.
INTRODUCTION
Diseases due to mtDNA mutation are a significant cause of human morbidity and mortality, but our understanding of the factors that influence the presentation and course of these diseases is far from complete (1,2). In particular, the generally accepted explanation for phenotypic diversity of mtDNA mutations is one of uneven segregation of mutated and wild-type mtDNA to particular tissues, with the resulting phenotype being dependent on this ratio or total amount of wild-type mtDNA in these tissues. This explanation is, however, often inadequate for heteroplasmic mutations and inapplicable to homoplasmic mutations, where the same mtDNA genotype is shared between virtually all molecules in all tissues. Homoplasmic mtDNA mutations had generally been considered as having low pathogenicity, with organ-specific phenotypes (3–5). These mutations show incomplete penetrance and are believed to cause disease in association with unknown environmental or other genetic factors. Exceptions to this rule are the homoplasmic mt-tRNA mutations, which have now been identified in several individuals and families (6–9).
We have previously described a family in whom the homoplasmic 1624C>T MTTV (equivalent to mt-tRNAVal C25U; http://mamit-trna.u-strasbg.fr Figure 1A) mutation underlies a devastating multi-system disease (8). Briefly, the proband in this family (II-1) has histochemical, biochemical and molecular biological evidence of mitochondrial disease but has only mild disease with muscle weakness, fatigue and recurrent migraines. She has one surviving affected child (with Leigh's Disease) from 10 pregnancies by four different unrelated partners. Following her most recent pregnancy, she gave birth to a female infant (III-10) who died in the neonatal period from cardiomyopathy and lactic acidosis. This infant had the same pathological findings in muscle as her mother and the extremely low steady-state levels of mt-tRNAVal in cardiac and skeletal muscle confirmed that the 1624C>T mutation was pathogenic. This mutation, although homoplasmic in all maternally related family members, is clearly neither of ‘low pathogenicity’ nor is it organ specific, but probably is dependent on polygenic factors for its phenotypic expression. One candidate for such a factor is the cognate aminoacyl synthetase. Elegant studies have shown that the phenotypic effects of various pathogenic mt-tRNA mutations in the yeast Saccharomyces cerevisiae can be suppressed by over-expression of the cognate synthetase (10,11). Indeed, the respiratory-deficient phenotype caused by three separate mutations in mt-tRNALeu were all suppressed by over-expression of leucyl-tRNA synthetase, with different degrees of severity requiring different levels of expression for respiratory compensation (11).
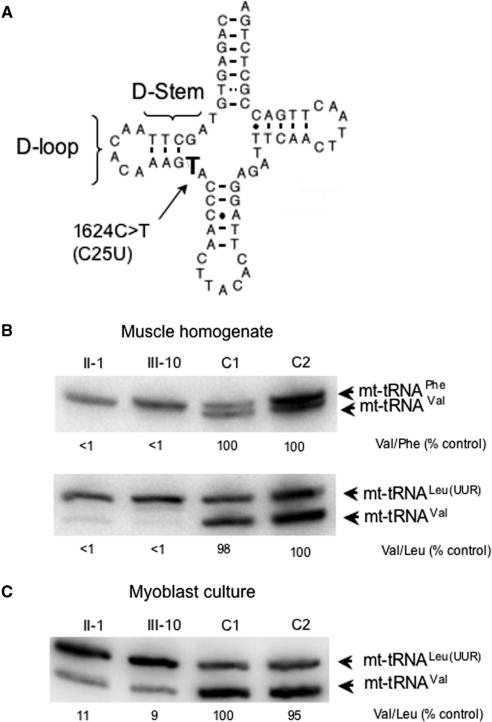
Figure 1.
Steady-state levels of mt-tRNAVal are decreased in primary cell lines from the patient and proband but do not show the dramatically low levels evident in tissues. (A) Schematic representation of the human MTTV gene sequence, highlighting the 1624C>T mutation, corresponding to C25U substitution in the tRNA (http://mamit-trna.u-strasbg.fr.) This mutation occurs in the D-stem of the tRNA secondary structure that is predicted to weaken the interaction with G10, a base pairing that has previously been shown to modulate interaction with class I-type synthetases (27,28). (B and C) High-resolution northern blots were performed on RNA isolated from muscle homogenate (B) or myoblasts (C) of the proband (II-1), offspring (III-10) and two unaffected independent controls C1 and C2 from which myoblast cultures were. Differences in specific activity of the various probes are normalized by calculating ratios of signals for the mt-tRNAVal and -tRNAPhe species (B, upper panel) or –tRNALeu(UUR) (B, lower panel; C) in each sample and comparing these ratios between samples on the same blot. Ratios of mt-tRNA levels in homogenate and myoblast sample are compared to C2 (B) or C1 (C) that is set to 100% and are reported under each lane. Representative experiments are shown. RNA was isolated from cell lines on at least five separate occasions over a 2-year period. Variance in ratios did not differ by >2%.
Here, we report the establishment of homoplasmic primary myoblasts and fibroblasts plus a variety of transmitochondrial cybrid cell lines all carrying the 1624C>T transition. Although the steady-state level of the mutated mt-tRNAVal was not depleted to the vanishingly low levels measured in mature skeletal muscle from the patients (<1% of control values), decreases of ∼90% were detected when normalized to other mt-tRNA species and compared to controls. This variability was unexpected, but may give an important clue to why the mutation can lead to such variability in clinical presentation between patients. Hence, an investigation into the underlying cause of this variability was undertaken. The decrease was found to be due greatly to the reduced stability of the deacylated mt-tRNAVal C25U. Over-expression of the cognate valyl synthetase, or inhibition of mitochondrial protein synthesis, processes that would be likely to result in the increased aminoacylation of the mutated tRNA, resulted in partial restoration of the mt-tRNAVal levels. We suggest that relative expression levels of VARS2L may underlie the tissue and patient variability of this homoplasmic mt-tRNA disease. Further, as the data show, levels of the aminoacylated form can be artificially modulated, thus a potential method for treating patients with this, or similar, disorders could be considered.
METHODS
Tissue culture manipulations
All cells were grown at 37°C in 5% CO2 under humidified conditions. Primary myoblast and fibroblast cultures were established from muscle or skin biopsy (II-1) or post-mortem biopsy (III-10) using techniques described previously (12). Myoblasts were grown in Hams F10 (Invitrogen Ltd, Paisley UK) supplemented with 20% (v:v) fetal calf serum, 1% (v:v) chick-embryo extract (ICN Flow Biomedicals, High Wycombe, UK), 2 mM l-glutamine, 1 × non-essential amino acids (NEAA), 100 μg/ml streptomycin and 100 U/ml penicillin. Fibroblasts were routinely grown in Eagle's Minimum Essential Medium (EMEM, Sigma) supplemented with 2 mM l-glutamine, 1 × NEAA, 10% (v:v) fetal calf serum with streptomycin and penicillin as described above. In one experiment, fibroblasts were also grown in valine-deficient EMEM (Sigma-Aldrich Co.Ltd, Dorset, UK) supplemented with 4 mM l-valine.
Transmitochondrial cybrids were grown in Dulbecco's Modified Essential Media (DMEM, Sigma) supplemented with 5% (v:v) fetal calf serum, 2 mM l-glutamine with streptomycin and penicillin. Cells devoid or depleted of mtDNA were further supplemented by 50 μg/ml uridine and 1 mM pyruvate.
Isolation of RNA and high-resolution northern blot analysis
TRIZOL Reagent (Invitrogen) was used to extract total RNA from ∼200 mg of skeletal and cardiac muscle and from 1 to 2 × 106 cultured myoblasts or fibroblasts. Large RNA species were removed by precipitation with an equal volume of 10 M LiCl permitting subsequent precipitation of smaller RNA molecules (5S and tRNAs) from the resulting supernatant. The precipitated small RNAs were re-suspended in a minimal volume of DEPC-treated dH2O and 1 μg aliquots were then electrophoresed through a 13% (w:v) 8 M urea polyacrylamide gel and transferred all as previously described (12). Separated RNA was electroblotted onto a GeneScreen Plus membrane (PerkinElmer, Beaconsfield, UK) and fixed by UV irradiation. Regions of human mtDNA encoding the genes for mt-tRNALeu[UUR], mt-tRNAPhe and mt-tRNAVal were PCR amplified and purified (QIAquick PCR purification columns, Qiagen) to produce probes for the blots. The human mt-tRNALeu[UUR] probe was generated using the forward primer L3200 (positions 3200-3219) 5′-TATACCCACACCCACCCAAG-3′ and reverse primer H3353 (positions 3353–3334) 5′-GCGATTAGAATGGGTACAAT-3′. The human mt-tRNAPhe probe was generated using the forward primer L552 (positions 552–570) 5′-CCAAACCCCAAAGACACCC-3′ and reverse primer H712 (positions 712–694) 5′-GAACGGGGATGCTTGCATG-3′. The human mt-tRNAVal probe was generated using the forward primer L1579 (positions 1579–1598) 5′-CTGGAAAGTGCACTTGGACG-3′and reverse primer H1734 (positions 1734–1714) 5′-GGGTAAATGGTTTGGCTAAGG-3′. Purified amplicons were radio-labelled with [α-32P] dCTP (3000 Ci/mmol, GE Healthcare) using the random primer method and unincorporated nucleotides removed by gel filtration through a Sephadex G-50 DNA grade column (GE Healthcare, Amersham, UK). Membranes were probed at 42°C in hybridization solution [5 × SSPE, 1% SDS, 10% (w:v) dextran sulphate, 50% (v:v) formamide and 5 × Denhardt's solution] for 16 h with ∼ 1–2 × 106 c.p.m. of each radiolabelled probe. Membranes were then incubated at 42°C for 16 h. Where appropriate, unlabelled amplicon was added to dilute the specific activity of the probe. Post-hybridization, non-specifically bound radiolabelled probe was removed by a series of washes: 2 × 15 min with 2 × SSPE at room temperature, 1 × 15 min with 2 × SSPE/2% (w:v) SDS. Membranes were then placed in PhosphorImage cassettes and subjected to ImageQuant (Molecular Dynamics, GE Healthcare) analysis after a 24 h exposure.
In vivo mitochondrial protein synthesis
Myoblasts were grown under the same conditions described above on 35 mm plates. Growth medium was removed, the myoblasts rinsed with methionine-free DMEM (Sigma) and placed in methionine-free medium supplemented with 10% (v:v) dialysed FBS (1000 MWCO; Sigma) containing 100 μg/ml emetine hydrochloride (Sigma) and incubated for 6 min at 37°C. Radiolabelled [35S] EasyTag methionine/cysteine (500 μCi, ∼1000 Ci/mmol; Perkin Elmer), was then added and the myoblasts incubated for 2 h before washing, re-suspension in PBS and dissolution in 50% (v:v) sample buffer [0.125 M Tris–HCl pH 6.8, 50% (v:v) glycerol, 10% (w:v) SDS, 0.1% (w:v) bromophenol blue, 5% (v:v) β-mercaptoethanol]. Samples (20 μg protein) were loaded onto a 4–20% or 10–20% Tris–glycine pre-cast gel (Novex; Invitrogen) and electrophoresed (20 mA) in standard Tris–glycine buffer. Following electrophoresis, the gel was fixed in 3% (v:v) glycerol, 10% (v:v) glacial acetic acid, 30% (v:v) methanol for 1 h, before drying at 60°C. The gel was then placed in a PhosphorImage cassette and ImageQuant analysis performed after a 16 h exposure.
mt-tRNA stability experiments
Six 75 cm2 flasks of primary myoblasts were grown to 75–80% confluency and fed with 20 ml of culture medium 16–24 h prior to the experiment. At time 0, ethidium bromide was added directly to the medium to a final concentration of 250 ng/ml. Cells were harvested at the indicated time points and low molecular weight RNA was extracted. Aliquots of the RNA were then electrophoresed through a 4% (w:v) agarose gel for assessment of quality and relative quantification. Samples were fractionated through a 13% polyacrylamide, 8 M urea gel as described earlier.
Production and visualization of the VARS2L-GFP fusion protein
A 735-bp amplicon encoding the initiation codon and N-terminal 232 residues of VARS2L flanked by BamH1 restriction sites was generated by PCR with the KOD Hot Start high-fidelity polymerase (Novagen, Merck Biosciences Ltd, Nottingham, UK), using the following primers: 5′ CACACAGGATCCTAATGTGCTCT CTCTC 3′ and 5′-CTCTCTGGATCCTGCTCACAGATCTCTCC-3′ and cDNA clone KIAA1885 (kindly provided by the Kasuza DNA Research Institute, Chiba, Japan) as template. This amplicon was confirmed by DNA sequence analysis and ligated in frame and upstream of GFP in pGFP3 (pcDNA3.1 Invitrogen, containing the GFP open reading frame and multiple cloning site). HeLa cells were seeded onto coverslips and transiently transfected with this fusion construct (5 μg; SuperFect, Qiagen) following the manufacturer's recommendations. After 24 h, 0.4 μM Mitotracker Red CM-H2XRos (Molecular Probes) was added to the growth media for 45 min, prior to washing and fixing in 1% (v:v) paraformaldehyde for 10 min. The coverslips were mounted with Vectashield (H-1200 containing DAPI for nuclear visualization, Vector Laboratories Ltd, Peterborough, UK). Fluorescence was visualized with a Leica (Nussloch, Germany) DMRA. Images were recorded using a cooled CCD camera (Spot-2, Diagnostics Instruments) and imaging system (MetaMorph 4.5, Universal Imaging Corporation, Pennsylvania, USA).
Over-expression and purification of human VARS2L
Various GST-fusion constructs were made by producing and cloning the relevant amplicons derived from KIAA1885 template cDNA into SalI–NotI linearized pGEX-6P-1 (GE Healthcare) using the following forward primers:
Δ64 5′-GCACCAGTCGACGCAAGTCACCTGCAGA AT-3′
Δ78 5′-GCACCAGTCGACCTACGAAACCCGGTG-3′
Δ92 5′-GCACCAGTCGACTGCCTCCTGCATACAG C-3′
Δ102 5′-GCACCAGTCGACGATATGTTGAGGCTG C-3′
Amplicons were all generated by standard 35-cycle PCR and KOD Hot Start polymerase (Novagen) using the same reverse primer
5′-CACACAGCGGCCGCTCAAAGACAGGTCTGAG-3′ and were NotI–SalI digested prior to ligation. The resultant constructs were used to transform Escherichia coli Rosetta(DE3)pLysS and fusion protein was expressed (1 mM IPTG) at 16°C overnight. Soluble GST-VARS2L fusion protein was affinity purified using glutathione–Sepharose 4B before release of the VARS2L by PreScission protease (GE Healthcare) following manufacturer's recommendations. Yield of soluble protein differed dependent on truncation size, but specific activities were similar.
Production of anti-VARS2L antibodies
As the cytosolic and predicted mitochondrial valyl synthetase share common sequence elements, the use of full-length VARS2L as antigen could not be considered. Rabbit anti-sera were raised against two synthetic peptides MVRDRQGRKMSKSLG and TARTPSEGEAGTQRQ representing regions of primary sequence unique to the putative mitochondrial protein and affinity purified by Eurogentec, Belgium.
Production of an mtDNA depleted HEK293 Flip-In T-Rex cell line
HEK293 Flip-In T-Rex cells (Invitrogen) were seeded into 6-well plates in DMEM supplemented with 10% (w:v) FBS, 50 μg/ml uridine in the presence of 10 μg/ml blasticidin and 100 μg/ml zeocin. Upon reaching 50% confluency, cells were treated with 100 ng/ml ethidium bromide and maintained in growth medium with ethidium bromide for 4–6 weeks.
Generation of HEK293 Flip-In T-REx transmitochondrial cybrids
Cytoplasts for fusion with HEK293 Flip-In T-REx cells were produced from 143B.206 transmitochondrial cybrids homoplasmic for the 1624C>T mutation by centrifugation on a Percoll isopycnic gradient as described by (13). In short, 2 × 107 143B cybrids were re-suspended in 20 ml 1:1 mixture of Percoll:DMEM containing 20 μg/ml cytochalasin B and centrifuged at 44 000g for 70 min at 20°C. All cytoplasts were recovered, diluted with 10 vol of DMEM, washed and re-suspended in DMEM. For fusion, cytoplasts were mixed with mtDNA-depleted HEK293 cells in a 1:1 ratio and centrifuged at 1100g for 5 min to form a pellet. DMEM was removed and the cells re-suspended in 0.2 ml of 50% (w:v) polyethylene glycol 1500 (BDH Limited). After 60 s, DMEM (10 ml) was added to the cells that were then gently re-suspended and left to recover at 37°C for 45 min. Cells were then plated in DMEM supplemented with 10% (v:v) FBS, 50 μg/ml of uridine and 1 mM Na pyruvate at a variety of cell densities (5 × 103 –2 × 104 ρ0/ml). After 24 h, the medium was replaced with DMEM supplemented with 10% dialysed FBS. To select against any non-enucleated 143B donors, cybrids were grown in 100 μg/ml zeocin, 10 μg/ml blasticidin S.
Production of VARS2L-inducible cell lines
For inducible over-expression, a 3653 bp EcoRV–NotI containing the entire VARS2L open reading frame was generated from the original Kazusa clone (KIAA1885) and inserted into pcDNA5/FRT/TO (Invitrogen). Constructs were isolated from transfectants and confirmed by restriction digest mapping and sequence analysis. Stable and inducible transfectants were generated in HEK293 Flip-In T-REx cells (Invitrogen) following company recommendations and selected for growth in hygromycin B (100 μg/ml). Crucially, the standard calcium phosphate method of transfection was necessary, as the use of other transfection reagents resulted in the loss of the mutated mtDNA for unknown reasons. Individual clones were expanded and inducible expression of VARS2L (48 h; 1 μg/ml tetracycline) confirmed by western analysis.
RESULTS
Primary cell lines homoplasmic for the 1624C>T mutation harbour low mt-tRNAVal levels
The level of mt-tRNAVal in patient-derived muscle or cardiac tissue homoplasmic for the 1624C>T mutation is vanishingly low (<1% of control) when normalized to mt-tRNAPhe [Figure 1A, or –tRNALeu(UUR) (Figure 1B and C, and (8)], consistent with this mutation contributing to the respiratory defect. Primary cell lines (myoblasts and fibroblasts) were established from the proband (II-1) and from one of her affected offspring (III-10). As shown in Figure 1, the steady-state level of mt-tRNAVal was lower than the comparable control lines, albeit that the dramatic loss demonstrated in the patients’ tissue was not recapitulated.
To determine whether this was simply a consequence of the culture conditions, we assessed whether varying the concentration of valine in the culture medium could modulate the steady-state level of mt-tRNAVal in fibroblasts. Susceptibility to limiting valine concentrations (data not shown) or to 10-fold higher valine concentrations than standard (4 mM, Figure S1) did not differ between control and patient fibroblasts.
The profound biochemical defects associated with the 1624C>T mutation in tissue are not found in primary cell lines
The observation that the very low level of mt-tRNAVal is not recapitulated in the patient-derived cell lines prompted the investigation of mitochondrial protein synthesis and associated functions in the cultured cells. We first assessed the aminoacylation (charged) status of the remaining mt-tRNAVal. RNA was extracted under acidic (pH < 5) conditions from cultured patient (III-10) myoblasts and fractionated through a denaturing polyacrylamide gel. High-resolution northern blots probed for specific mt-tRNAs confirmed the absolute amount of mt-tRNAVal was decreased; however, it also indicated that the proportion of aminoacylated to deacylated mt-tRNAVal was increased [92% (III-10) versus 71% (control) Figure S2]. To determine whether this level of charged mt-tRNAVal was sufficient in these cell lines to allow for normal mitochondrial protein synthesis, the incorporation of 35S-methionine into proteins synthesized in mitochondria was evaluated in patient (III-10) and control myoblasts. Although the 1624C>U mutation disrupts a highly conserved residue and base pairing in the stem of the dihydrouridine (DHU) loop of mt-tRNAVal secondary structure (http://mamit-trna.u-strasbg.fr), there was no marked difference in mitochondrial protein synthesis after either 30 min (data not shown) or 120 min (Figure 2), consistent with the rescue in the patients myoblasts of the profound decrease in complex I activity noted in tissue homogenate (Table 1). Recovery of complex IV activity also occurred, although to a lesser extent. These data show that at least in cultured myoblasts the normal steady-state level of aminoacylated mt-tRNAVal has a weak control strength in mitochondrial protein synthesis.
Figure 2.
Decreased levels of mt-tRNAVal C25U could not be shown to affect mitochondrial protein synthesis. Myoblasts from patient and control were grown in culture prior to in vivo mitochondrial protein labelling as described in Materials and methods section and (29). Following inhibition of cytosolic protein synthesis, radiolabelled methionine/cysteine was added for 2 h and whole-cell lysates (20 μg total protein) separated by SDS–PAGE. A representative PhosphorImage is shown. Attribution of products is determined by reference to 29.
Table 1.
Biochemical analysis of mitochondrial enzymes from tissue homogenates and myoblasts of patient and control samples
| Sample | Complex I/CS | Complex II/CS | Complex IV/CS |
|---|---|---|---|
| Muscle homogenate | |||
| Control (n = 15) | 0.240 ± 0.060 | 0.320 ± 0.088 | 1.340 ± 0.390 |
| Patient II-1 | 0.053 | 0.251 | 0.520 |
| Patient III-10 | 0.023 | 0.327 | 0.244 |
| Myoblasts | |||
| Control (n = 3) | 0.129 ± 0.038 | 0.262 ± 0.067 | 0.783 ± 0.187 |
| Patient III-10 | 0.089 | 0.185 | 0.295 |
Sample preparation and enzymatic assays were performed as detailed in Materials and methods section. All respiratory complex activities are ratioed to the activity of the mitochondrial matrix marker citrate synthase.
The C25U mutation results in increased turnover of the deacylated mt-tRNAVal
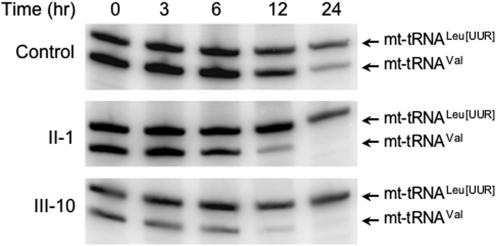
The 1624C>T mutation led to a decrease in steady-state level of mt-tRNAVal. The decrease was not as profound as in tissue homogenate from the patients and the remaining mt-tRNAVal was sufficient to facilitate normal mitochondrial protein synthesis. However, this variability in levels could be a vital clue to understanding the variable penetrance of this homoplasmic disorder. An investigation was initiated into the lowered steady-state mt-tRNAVal. The mtDNA gene encoding tRNAVal, MTTV is transcribed as part of a polycistronic unit that includes the tRNAPhe gene, MTTF, therefore both mt-tRNAVal and -tRNAPhe are encoded on the same RNA precursor (14,15). As shown in Figure 1 and when compared to other mt-tRNAs encoded on a separate polycistronic unit, it is clear that the decrease is specific to mt-tRNAVal. Thus, the decrease may be due to the mutation causing a more rapid turnover of the mutated mt-tRNAVal. We therefore compared the stability of mt-tRNAVal in control and patient-derived myoblasts following incubation with ethidium bromide, a DNA intercalator that can inhibit transcription of the mitochondrial genome. Patient and control myoblasts were treated with ethidium bromide (250 ng/ml), before harvesting after the indicated time points, RNA was extracted and the steady-state levels of mt-tRNAVal and mt-tRNALeu(UUR) were determined for each time point (Figure 3). Using this concentration of ethidium bromide, the estimated half-life of mt-tRNAVal was considerably reduced in patients III-10 and II-1 (∼3 h and 3.75 h, respectively), when compared with controls (5.75 h). The half-life of mt-tRNALeu(UUR) did not vary significantly between control and patient II-1 but appears to be longer in patient III-10 (>24 h). Whilst the mutated mt-tRNAVal is clearly being more rapidly degraded, the alteration in calculated half-life (52–65% of control value) was insufficient to explain the substantially reduced steady-state levels (<10% of control value). At steady state, tRNAs are present in both aminoacylated and deacylated forms. To our knowledge, differences in the rates of tRNA decay have never been reported to be dependent on the charged state of the tRNA. However, it is possible that the mutation may cause an alteration in tertiary structure, leading to an increased susceptibility to decay. This may be reduced both by interaction with the cognate synthetase and by aminoacylation. Consequently, one possibility to explain this discrepancy may be that mutated mt-tRNAVal is unstable until aminoacylated. This is supported by the subtle increase in the aminoacylation level of the mutated mt-tRNAVal population (Figure S2).
Figure 3.
Stability of mt-tRNAVal C25U is impaired. To compare the stability of mt-tRNAVal in patients (II-1 and III-10) and control, mitochondrial RNA synthesis was inhibited by the addition of ethidium bromide (250 ng/ml) and the remaining RNA was isolated at the indicated time points. High-resolution blots were then performed with probes specific for mt-tRNAVal and –tRNALeu(UUR). Estimates for t1/2 were calculated by measuring pixel intensity for each mt-tRNA at each time point. For each set of experiments, 5 μg of low molecular weight RNA was separated and equal loading confirmed by reference to the nuclear encoded 5S RNA (data not shown). For ease of comparison, the specific activity of mt-tRNALeu(UUR) probe was decreased to allow both signals to be visualized on the same blot and time course. A representative time course is shown for RNA isolated from the three cell lines.
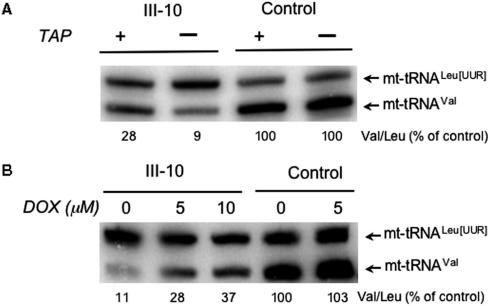
To test this hypothesis, we treated the cells with thiamphenicol, an inhibitor of mitochondrial peptidyl transferase activity (16). As translation elongation is inhibited, aminoacylated mt-tRNAs will be unable to donate their amino acids to the growing peptide, resulting in an increase in the amount of aminoacylated tRNA, as has previously been described (17,18). Thus, by inhibiting mitochondrial protein synthesis there should be an increased level of all aminoacylated tRNAs and consequently an elevation of mt-tRNAVal at steady state. As can be seen in Figure 4A, inhibition with thiamphenicol (TAP) does indeed promote an increase in mt-tRNAVal steady-state levels, but only in the cell line carrying the mutation. To further interrogate this hypothesis, the cells were subjected to a second mitochondrial protein synthesis inhibitor, doxycycline (DOX, Figure 4B), a derivative of tetracycline that inhibits protein synthesis by preventing charged tRNA binding to the A site in the mitoribosome. In agreement with this hypothesis, a graded increase in tRNA levels was noted as the concentration of DOX was increased to 10 μM.
Figure 4.
Inhibition of mitochondrial protein synthesis partially restores the levels of mt-tRNAVal. Myoblast cell lines from the patient (III-10) and control were treated with 50 μg/ml of the mitochondrial protein synthesis inhibitor thiamphenicol (+TAP, A), various concentrations of doxycycline (5 or 10 μM, B) or untreated (–TAP, A or 0, B) and RNA were isolated after 2 days. High-resolution northern blots were probed for mt-tRNAVal or –tRNALeu(UUR) and the relative level of –tRNAVal estimated by normalizing the ratio of the two signals for the untreated controls.
Verification that VARS2L is the human mitochondrial valyl-tRNA synthetase
If the deacylated form of mutated mt-tRNAVal is indeed more susceptible to degradation than either its aminoacylated form or its wild-type counterpart, it may be possible to increase the steady-state level of mt-tRNAVal by increasing aminoacylation activity. As already described in the Introduction section, over-expression of cognate synthetases has been shown to rescue various mutated mt-tRNAs in the yeast S. cerevisiae (10,11). Although appealing, this approach was complicated at the outset of these experiments by the absence of any reported mitochondrial valyl synthetase. A simple bioinformatic search revealed a strong candidate protein (KIAA1885/VARS2L) containing the HIGH and KMSKS signature motifs of the class I aminoacyl synthetases. The original UNIPROT/TrEMBL entry (Q96QO2) failed to identify an N-terminal methionine but a facile search revealed a candidate methionine 36 residues within the sequence. Strong mitochondrial import predictions were made from MitoProt (www.ihg.gsf.de/ihg/mitoprot) and TargetP (www.cbs.dtu.dk/services/TargetP), 82.2 and 87.9%, respectively. To confirm mitochondrial localization, a C-terminal GFP fusion construct was produced and transient transfection revealed good co-localization with the mitochondrial-specific marker Mitotracker Red CM-H2XRos (Figure 5A). To purify the protein and determine the aminoacylation activity of VARS2L, various fusion constructs were made each with a short, predicted N-terminal pre-sequence deletion. In all cases irrespective of the background strain or conditions of expression, only aggregated VARS2L could be made (data not shown). To resolve these problems with aggregation, more extensive N-terminal truncations of VARS2L fused to GST were designed, ensuring that all predicted functional domains were retained in the protein. All constructs were expressed and soluble VARS2L purified following removal of GST as described in Materials and methods section (Figure S3). As initial template, human mt-tRNAVal was generated in vitro following the ribozyme method outlined in Supplementary Methods and ref. 19. Unfortunately, only minimal aminoacylation activity above background activity could be detected using tRNA produced from several independent assays (data not shown). The majority of previous reports detailing the function of mammalian mitochondrial synthetases have used E. coli tRNA as substrates (20–23). Using the soluble VARS2L carrying a 102 residue N-terminal deletion, tritiated valine was successfully transferred to E. coli tRNAVal, albeit with a low aminoacylation activity (1.2 ± 0.2 × 10−3 pmol aminoacylated tRNA/min/pmol for VARS2LΔ102, Supplementary Methods). Similarly, low-level activity was noted for all other N-terminal deletions (data not shown). To confirm the activity was indeed due to VARS2L and not to any remnant of contaminating E. coli protein, activity was also noted with enzyme immunoprecipitated from the reaction mix with the anti-VARS2L antibodies prior to initiation of the assay (data not shown).
Figure 5.
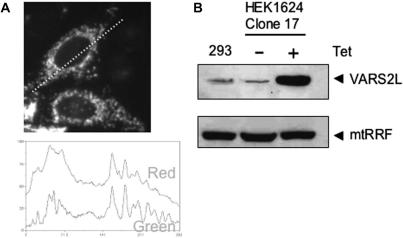
VARS2L is a mitochondrial protein. (A) HeLa cells were transiently transfected with a construct expressing the N-terminal 232 residues of VARS2L fused to GFP. Following transfection (24 h), cells were stained with the mitochondriotropic dye Mitotracker Red CM-H2XROS. Transfected cells were visualized by fluorescence microscopy. Images were captured and mitochondrial co-localization of the fusion protein was confirmed by superimposition of the green and red signals of a linescan of the image (dashed line visible on image). An image typical of three independent transfections is shown. (B) Transmitochondrial HEK293T Flip-in T-REx cybrids inducibly expressing full-length VARS2L were produced as detailed in Materials and methods section. Mitochondrial protein was isolated from control HEK293T Flip-in T-REx cells (293) and the cybrid transfectants (HEK1624 Clone 17) with (+) or without (−) 48 h of induction as described in the Materials and methods section. Aliquots (100 μg) were subjected to western analysis using anti-VARS2L antibodies. To confirm equal loading of mitochondrial protein, an antibody to mitochondrial ribosome recycling factor (mtRRF) was used.
Induction of VARS2L results in increased steady-state levels of cognate mt-tRNAVal 1624C>T
Recipient ρ0 cell lines that have been used to generate transmitochondrial cybrids are notoriously aneuploid and data produced by uncontrolled over-expression of exogenous proteins in individual clones has to be interpreted with great caution. Consequently, to show unequivocally that over-expression of the synthetase could increase aminoacylation of the mt-tRNAVal and restore steady-state levels, it was necessary to control expression of the synthetase in any cybrid. To date, such an inducible recipient ρ0 cell line is not available. To tackle this problem, the commercially available human cell line with a defined integration site that allows inducible expression (HEK293 Flip-In T-REx) was subjected to long-term culture in low-dose ethidium bromide to deplete cells of endogenous mtDNA. Complete removal of mtDNA was found not to be possible, although copy number was depleted to <1% of untreated controls (data not shown). Cybrids were generated by fusion of these mtDNA-depleted cells with cytoplasts derived from patient fibroblasts and selected as detailed in Materials and methods section. DNA was isolated from the cybrids and following RFLP analysis, three clones (clone 5, 11 and 17) containing >94% levels of 1624C>T were retained. Similar to the cell lines homoplasmic for the mutation, biochemical analysis of clone 17 revealed no profound respiratory defect (data not shown). These three cell lines were transfected with the full-length VARS2L-encoding cDNA and stable, tetracycline inducible cell lines were selected as detailed. To confirm expression, transfectants were expanded, induced and mitochondrial extracts subjected to western analysis with anti-VARS2L specific antibodies. As shown in Figure 5B, although the endogenous levels of VARS2L were only just detectable, even in large amounts of mitochondrial protein (100 μg, lane a), good induction was noted for each clone.
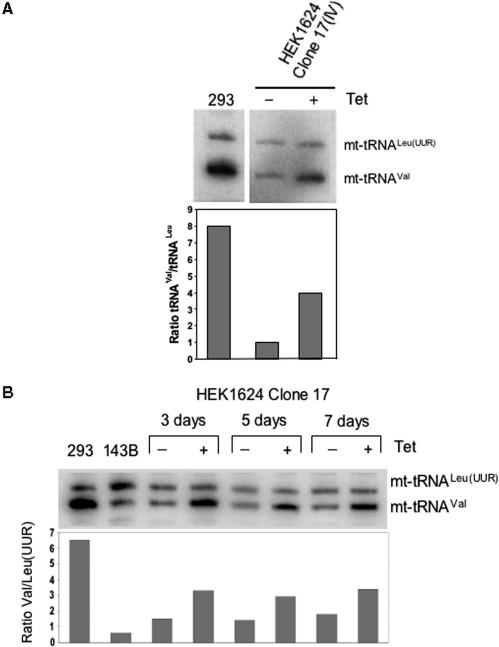
Following confirmation of transfection, clone 17 was expanded. The high level of heteroplasmy for the 1624C>T mutation was retained. Further, the steady-state level of mutated C25U mt-tRNAVal was confirmed to be lower than the wild type in HEK293 Flip-In T-REx control cells as measured by high-resolution northern blots (Figure 6). To determine whether the over-expression of VARS2L could promote increased mt-tRNAVal, cells were initially induced for 3 days prior to harvesting, RNA isolation and analysis by northern blot. As shown in Figure 6A, ∼4-fold increase in mutated mt-tRNAVal was apparent, increasing levels to almost 50% of the control HEK293 Flip-In T-REx line carrying wild-type mtDNA. The control fusion of HEK293/1624C>T that carried the mutation but were untransfected and so could not express the cognate synthetase, showed no increase in mt-tRNAVal levels under identical induction conditions (Figure S4), consistent with growth of HEK293 Flip-In T-REx cells in 1 μg/ml inducer for up to 2 weeks having no measurable effect on the rates of mitochondrial protein synthesis (data not shown). Further, over-expression of VARS2L in cybrid clones retaining the 1624C genotype resulted in no apparent increase of mt-tRNAVal steady-state levels (Figure S5). A more detailed time course also confirmed an increase at 3, 5 and 7 day post-induction (Figure 6B), consistent with the over-expression of the mitochondrial valyl synthetase partially suppressing the depletion of mt-tRNAVal associated with the 1624C>T mutation.
Figure 6.
Over-expression of human VARS2L can increase the steady-state level of mutated mt-tRNAVal. (A) HEK293T1624C>T clone 17 transfected cybrids, uninduced (−) or induced (+) were grown for 3 days prior to isolation of RNA. High-resolution northern blots were performed with probes to mt-tRNAVal and –tRNALeu(UUR) as indicated. Natural levels for both tRNA species are shown in the control untransfected HEK293 cell line (293). Signal was quantified by ImageQuant after PhosphorImager exposure and the ratio of mt-tRNAVal to –tRNALeu(UUR) given for each sample. (B) Extended over-expression of VARS2L maintains the increased steady-state level of mutated mt-tRNAVal. A similar experiment to (A) was performed, with RNA isolated after the indicated induction time in days. Ratios of the mt-tRNAs are shown for control untreated (293) and the 143B1624C>T homoplasmic cybrids (143B).
DISCUSSION
In this report, we show data that is consistent with the pathogenic mt-tRNAVal mutation causing a defect in stability, which is partially protected by charging of the mt-tRNA. We confirm the identity of a human mitochondrial valyl synthetase, VARS2L, that we show is localized to mitochondria and can charge E. coli tRNA with valine. Finally, we show that the depleted steady-state level of the C25U mt-tRNAVal can be partially recovered by over-expression of the cognate mitochondrial synthetase. At the onset of these studies, there had been no report of any candidate mitochondrial valyl synthetase. However, in 2005, Florentz and colleagues (20) reported a list of candidates for the remainder of the uncharacterized human mitochondrial synthetases, which they identified by database mining. This included reference to a different form of VARS2L. Amongst the aims of this current research, we wished to demonstrate and confirm mitochondrial localization of our version of VARS2L and to determine whether it was a bona fide mitochondrial valyl synthetase, not to complete a full biochemical characterization of this protein. Indeed, our initial studies confirmed co-localization and valyl synthetase activity of this enzyme when using total E. coli tRNA as substrate, albeit with very low specific activity. We were unable to show any great efficiency of aminoacylation by VARS2L with in vitro transcribed human mt-tRNAVal as substrate. However, it has been well documented that mammalian mitochondrial synthetases are less efficient than their cytosolic or prokaryotic counterparts (21,22,24). Further, full optimization of conditions had not been performed for this enzyme. Lower activity with mammalian in vitro transcribed mt-tRNA is also not unusual (20–23). There are clearly cases where post-transcriptional modifications are essential for robust aminoacylation and in the absence of correct modifications tRNAs have been shown occasionally to mis-fold (25,26). Although not unexpected, the poor aminoacylation of the synthetic mt-tRNAVal meant it was not possible to compare the aminoacylation efficiencies of the wild-type and mutated tRNAVal substrates.
We conclude that it is the rapid decay rate of the deacylated form of C25U mt-tRNAVal, which is responsible for its low steady-state level in all the primary and transmitochondrial cybrid cell lines that we have generated and studied. This conclusion is based on the following criteria: (i) shorter half-life of mt-tRNAVal was observed following inhibition of mitochondrial transcription, (ii) inhibition of mitochondrial protein synthesis, a condition likely to increase aminoacylation states of all mt-tRNAs, led to increased steady-state levels of C25U mt-tRNAVal, (iii) the G10-C25 pairing in the D-stem of tRNAs has been implicated in contacting the cognate class I synthetases, suggesting mutations in this pairing may result in decreased association/protection (27,28) and (iv) over-expression of VARS2L promoted an increase in C25U mt-tRNAVal levels. Partial restoration of mt-tRNAVal levels by over-expression of VARS2L was impressive but as no cell line containing the 1624C>T substitution showed a profound defect in biochemical activity of any mitochondrial respiratory enzyme, we have limited this study to understanding the mechanisms underlying the decreased stability of the mutated mt-tRNAVal. However, with reference to this difficulty in recapitulating respiratory defects due to the 1624C>T mutation, it is interesting to note that we are currently characterizing several independent 143B-based homoplasmic 1624C>T transmitochondrial cybrid clones that are unable to grow on galactose, consistent with these clones being compromised for OXPHOS. This defect is due to the aneuploid nature of the cybrids and amongst several possibilities, may be caused by differing steady-state levels of the mt-tRNAVal.
We have previously reported that this unique homoplasmic mutation in MTTV underlies a multi-system disease affecting three generations of a family. Despite its homoplasmic nature, this mutation has an extremely diverse clinical phenotype with at least one individual remaining only mildly affected. This diversity is not reflected in the biochemical deficiency in muscle as in the profound (III-10) and mildly affected (II-10) individuals, both showed severe defects in respiratory complex activities. However, there may be variability in mt-tRNAVal levels in neuronal or cardiac tissue, which may cause differences in mitochondrial protein synthesis and consequently respiratory complex defects.
In summary, on the basis of the data provided in this article, we propose the following hypothesis for the variable effects of the 1624C>T MTTV mutation. Steady-state levels are a result of the rate of tRNA production (synthesis, processing, maturation) minus the rate of decay. This mutation causes an increased decay rate of the deacylated form, which is offset in part by aminoacylation. We predict that the dramatic differences in steady-state levels is due to natural variabilities in the rates of synthesis and decay of the deacylated mutant form in a given cell type and the relative amounts of valyl synthetase available to promote increased aminoacylation and therefore increased stability. This hypothesis predicts that although these rates will vary between tissues, over-expression of VARS2L within a tissue will always increase the level of charged mt-tRNAVal steady-state levels, potentially restoring mitochondrial protein synthesis. Testing this hypothesis may lead to a greater understanding of the pathophysiology of mitochondrial disease and to formulating novel therapeutic approaches for these devastating disorders.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
R.N.L., Z.M.A.C.L, R.W.T. and D.M.T. wish to thank the Wellcome Trust for supporting this work. R.M. is funded by a Fellowship from the Medical Research Council, J.R. by an MCEST Fellowship from the EU (Contract MEST-CT-FP6-503684) and A.Y. and D.A.K. by the Malaysian Government. We gratefully acknowledge assistance with respiratory chain (LangPing He) and aminoacylation assays (André Dietrich and Anne Cosset, IBMP Strasbourg). Funding to pay the Open Access publication charges for this article was provided by The Wellcome Trust.
Conflict of interest statement. None declared.
REFERENCES
- 1.Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeviani M, Carelli V. Mitochondrial disorders. Curr. Opin. Neurol. 2007;20:564–571. doi: 10.1097/WCO.0b013e3282ef58cd. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza A.MS, Elsas L.J., II., Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- 4.Carelli V, Giordano C, d’Amati G. Pathogenic expression of homoplasmic mtDNA mutations needs a complex nuclear-mitochondrial interaction. Trends Genet. 2003;19:257–262. doi: 10.1016/S0168-9525(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Al E. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat. Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 6.Limongelli A, Schaefer J, Jackson S, Invernizzi F, Kirino Y, Suzuki T, Reichmann H, Zeviani M. Variable penetrance of a familial progressive necrotising encephalopathy due to a novel tRNA(Ile) homoplasmic mutation in the mitochondrial genome. J. Med. Genet. 2004;41:342–349. doi: 10.1136/jmg.2003.016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland R, Chinnery PF, Blakely EL, Schaefer AM, Morris AA, Foster SM, Tuppen HA, Ramesh V, Dorman PJ, Turnbull DM, et al. Homoplasmy, heteroplasmy, and mitochondrial dystonia. Neurology. 2007;69:911–916. doi: 10.1212/01.wnl.0000267843.10977.4a. [DOI] [PubMed] [Google Scholar]
- 8.McFarland R, Clark KM, Morris AA, Taylor RW, Macphail S, Lightowlers RN, Turnbull DM. Multiple neonatal deaths due to a homoplasmic mitochondrial DNA mutation. Nat. Genet. 2002;30:145–146. doi: 10.1038/ng819. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RW, Giordano C, Davidson MM, d’Amati G, Bain H, Hayes CM, Leonard H, Barron MJ, Casali C, Santorelli FM, et al. A homoplasmic mitochondrial transfer ribonucleic acid mutation as a cause of maternally inherited hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003;41:1786–1796. doi: 10.1016/s0735-1097(03)00300-0. [DOI] [PubMed] [Google Scholar]
- 10.Chiang CS, Liaw GJ. A missense mutation in the nuclear gene coding for the mitochondrial aspartyl-tRNA synthetase suppresses a mitochondrial tRNA(Asp) mutation. Nucleic Acids Res. 2000;28:1542–1547. doi: 10.1093/nar/28.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Luca C, Besagni C, Frontali L, Bolotin-Fukuhara M, Francisci S. Mutations in yeast mt tRNAs: specific and general suppression by nuclear encoded tRNA interactors. Gene. 2006;377:169–176. doi: 10.1016/j.gene.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Clark KM, Bindoff LA, Lightowlers RN, Andrews RM, Griffiths PG, Johnson MA, Brierley EJ, Turnbull DM. Reversal of a mitochondrial DNA defect in human skeletal muscle. Nat. Genet. 1997;16:222–224. doi: 10.1038/ng0797-222. [DOI] [PubMed] [Google Scholar]
- 13.Trounce I, Wallace DC. Production of transmitochondrial mouse cell lines by cybrid rescue of rhodamine-6G pre-treated L-cells. Somat. Cell Mol. Genet. 1996;22:81–85. doi: 10.1007/BF02374379. [DOI] [PubMed] [Google Scholar]
- 14.King MP, Attardi G. Post-transcriptional regulation of the steady-state levels of mitochondrisl tRNAs in HeLa cells. J. Biol. Chem. 1993;268:10228–10237. [PubMed] [Google Scholar]
- 15.Montoya J, Gaines GL, Attardi G. The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell. 1983;34:151–159. doi: 10.1016/0092-8674(83)90145-9. [DOI] [PubMed] [Google Scholar]
- 16.Nijhof W, Kroon AM. The interference of chloramphenicol and thiamphenicol with the biogenesis of mitochondria in animal tissues. A possible clue to the toxic action. Postgrad. Med. J. 1974;50:53–59. [PubMed] [Google Scholar]
- 17.Pollard JW, Galpine AR, Clemens MJ. A novel role for aminoacyl-tRNA synthetases in the regulation of polypeptide chain initiation. Eur. J. Biochem. 1989;182:1–9. doi: 10.1111/j.1432-1033.1989.tb14793.x. [DOI] [PubMed] [Google Scholar]
- 18.Stanners CP, Wightman TM, Harkins JL. Effect of extreme amino acid starvation on the protein synthetic machinery of CHO cells. J. Cell Physiol. 1978;95:125–137. doi: 10.1002/jcp.1040950202. [DOI] [PubMed] [Google Scholar]
- 19.Korencic D, Soll D, Ambrogelly A. A one-step method for in vitro production of tRNA transcripts. Nucleic Acids Res. 2002;30:e105. doi: 10.1093/nar/gnf104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnefond L, Fender A, Rudinger-Thirion J, Giege R, Florentz C, Sissler M. Toward the full set of human mitochondrial aminoacyl-tRNA synthetases: characterization of AspRS and TyrRS. Biochemistry. 2005;44:4805–4816. doi: 10.1021/bi047527z. [DOI] [PubMed] [Google Scholar]
- 21.Bullard JM, Cai YC, Demeler B, Spremulli LL. Expression and characterization of a human mitochondrial phenylalanyl-tRNA synthetase. J. Mol. Biol. 1999;288:567–577. doi: 10.1006/jmbi.1999.2708. [DOI] [PubMed] [Google Scholar]
- 22.Bullard JM, Cai YC, Spremulli LL. Expression and characterization of the human mitochondrial leucyl-tRNA synthetase. Biochim. Biophys. Acta. 2000;1490:245–258. doi: 10.1016/s0167-4781(99)00240-7. [DOI] [PubMed] [Google Scholar]
- 23.Spencer AC, Heck A, Takeuchi N, Watanabe K, Spremulli LL. Characterization of the human mitochondrial methionyl-tRNA synthetase. Biochemistry. 2004;43:9743–9754. doi: 10.1021/bi049639w. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen R, Sogaard TM, Rossing AB, Martensen PM, Justesen J. Identification and characterization of human mitochondrial tryptophanyl-tRNA synthetase. J. Biol. Chem. 2000;275:16820–16826. doi: 10.1074/jbc.275.22.16820. [DOI] [PubMed] [Google Scholar]
- 25.Helm M, Brule H, Degoul F, Cepanec C, Leroux JP, Giege R, Florentz C. The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA. Nucleic Acids Res. 1998;26:1636–1643. doi: 10.1093/nar/26.7.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohm B, Sissler M, Park H, King MP, Florentz C. Recognition of human mitochondrial tRNALeu(UUR) by its cognate leucyl-tRNA synthetase. J. Mol. Biol. 2004;339:17–29. doi: 10.1016/j.jmb.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 27.Rould MA, Perona JJ, Soll D, Steitz TA. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989;246:1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- 28.Sugiura I, Nureki O, Ugaji-Yoshikawa Y, Kuwabara S, Shimada A, Tateno M, Lorber B, Giege R, Moras D, Yokoyama S, et al. The 2.0 A crystal structure of Thermus thermophilus methionyl-tRNA synthetase reveals two RNA-binding modules. Structure. 2000;8:197–208. doi: 10.1016/s0969-2126(00)00095-2. [DOI] [PubMed] [Google Scholar]
- 29.Chomyn A. In vivo labeling and analysis of human mitochondrial translation products. Methods Enzymol. 1996;264:197–211. doi: 10.1016/s0076-6879(96)64020-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.