Abstract
Schizophrenia is characterized by deficits in cognition as well as visual perception. There have, however, been few magnetic resonance imaging (MRI) studies of the occipital lobe as an anatomically defined region of interest in schizophrenia. To examine whether or not patients with chronic schizophrenia show occipital lobe volume abnormalities, we measured gray matter volumes for both the primary visual area (PVA) and the visual association areas (VAA) using MRI based neuroanatomical landmarks and three-dimensional information. PVA and VAA gray matter volumes were measured using high-spatial resolution MRI in 25 male patients diagnosed with chronic schizophrenia and in 28 male normal controls. Chronic schizophrenia patients showed reduced bilateral VAA gray matter volume (11%), compared with normal controls, whereas patients showed no group difference in PVA gray matter volume. These results suggest that reduced bilateral VAA may be a neurobiological substrate of some of the deficits observed in early visual processing in schizophrenia.
Keywords: high-spatial resolution MRI, schizophrenia, primary visual area, visual association areas, quantitative MRI
Introduction
Schizophrenia is characterized by deficits in cognition as well as visual perception. (O’Donnell et al., 1996; Tek et al., 2002). Deficits in early visual processing have been repeatedly reported (Doniger et al., 2002; Keri et al., 2004; Butler et al., 2001, 2005), and recent electroencephalographic (EEG) studies provide further evidence for deficits in early visual processing in schizophrenia (Doniger et al., 2002; Butler et al., 2001, 2005). With respect to clinical symptoms, patients with schizophrenia sometimes evince visual hallucinations, which are associated with activity in the visual association cortex (Silbersweig et al., 1995). Of note here, Weiss and Heckers (1999) noted that the neural systems involved in the perception of hallucinations appears to involve the same modality-specific cerebral structures as are involved in normal perception. Recently, Molina et al. (2005) reported that clozapine increased occipital lobe metabolism including primary and association visual areas, and that the metabolic increase was associated with improvement of positive symptoms. Our own laboratory has documented that visual gestalt stimuli lead to abnormal gamma band EEG activity over the occipital lobe in schizophrenia, and that this abnormal gamma activity is associated with visual hallucinations (Spencer et al., 2003, 2004). It thus seems likely that the occipital lobe is involved in some aspects of the pathophysiology of schizophrenia.
Magnetic resonance imaging (MRI) has been helpful in revealing subtle structural brain abnormalities in schizophrenia (see reviews in McCarley et al., 1999; Shenton et al., 2001) although the small number of studies that have measured occipital lobe have shown mixed findings. Five studies reported volume reduction in the occipital lobe in schizophrenia (Zipursky et al., 1992; Andreasen et al., 1994; Bilder et al., 1994, 1999; Davatzikos et al., 2005). However, differences in methodology and Regions of Interest (ROI) definition may have accounted for some of the disparate results. In terms of neuroanatomically defined studies of occipital lobe, Zipursky et al. (1992) used seven axial MRI sections for segmentation and found significant gray matter volume reduction in the occipital lobe in patients. On the other hand, Goldstein et al. (1999). reported no group differences between patients with schizophrenia and controls in the occipital lobe. For voxel-based morphometric (VBM) studies, Davatzikos et al. (2005) reported reduced gray matter in occipital association areas in patients with schizophrenia, although there have also been negative findings reported using VBM (e.g., Kubicki et al., 2002; Giuliania et al., 2005).
Based on direct comparisons of VBM and ROI, we (Kubicki et al., 2002) and Giuliania et al. (2005) concluded that VBM is useful for hypothesis generation, but that its anatomical warping and density-based methodology often lead to failure to detect abnormalities seen using ROI analysis. In the present study we adopted a neuroanatomically defined and manually delineated ROI method to evaluate occipital lobe ROI.
One possible reason for the small number of neuroanatomically defined ROI studies may be due to difficulties in defining occipital lobe boundaries. The boundary between the occipital lobe and parietal lobe, the parietooccipital sulcus (POS), clearly separates the two lobes on the medial surface (Duvernoy, 1991). However, on the lateral surface, these two lobes are usually divided with a (theoretical) line starting at the parietooccipital fissure and extending to the temporo-occipital incisure (Duvernoy, 1991). In the present study, we used three-dimensional information to provide reliable measures of the occipital lobe with a software package for medical image analysis [3D slicer, http://www.slicer.org] on a workstation.
For ROI studies, it is important to separate gray and white matter in the analysis. This approach can improve the detection of subtle gray matter volume differences between groups, since white matter volume may be relatively intact as reported in several MRI studies of frontal and temporal lobes in schizophrenia (e.g., Gur et al., 2000; Hirayasu et al., 2001). Thus, in the present study, we used a fully-automated segmentation program (Wells et al., 1996) to classify tissue into gray matter, white matter, and cerebral spinal fluid (CSF). Finally, consideration must be given to linking neuroanatomically defined ROI to functional divisions whenever possible (e.g., delineation for Heschl’s gyrus, the locus of primary auditory cortex [Hirayasu et al., 2000]). For the occipital lobe, the cytoarchitecture changes abruptly at the border between Brodmann areas 17 and 18, with especially prominent changes in layer IV, which becomes thinner and less differentiated in area 18 than in area 17 (Amunts et al., 2000). Although structural MRI cannot identify this border, functional neuroimaging studies have reported that the primary visual area in humans is distributed approximately one gyrus above and below the calcarine fissure (Tootell et al., 1996; Van Essen &Drury 1997). We therefore decided to use this ROI definition to delineate these regions; for convenience we will refer to the (mainly) primary visual area as PVA and the (mainly) visual association area as VAA, comprising the non-PVA portions of the occipital lobe. Our using designations other than Brodmann areas and those in use in anatomical studies will also serve to indicate the tentative functional identification of PVA and VAA.
The principal aim of this study was to measure PVA and VAA gray matter volumes in chronic schizophrenia and normal comparison subjects, using high spatial resolution MRI (0.9375-mm3 voxels in resampled slices) and three-dimensional information, in order to provide more reliable measurement of these brain regions, as well as to determine whether or not there are differences between groups. We also investigated associations between clinical symptoms and gray matter volumes of PVA and VAA in the patient sample, where we predicted that increased severity of visual hallucinations would be significantly associated with reduced occipital gray matter volumes.
Subjects and Methods
Subjects
Twenty-five male patients with chronic schizophrenia and 28 male normal control subjects participated in this study. Subjects included 2 new subjects and 49 subjects common (23 patients and 28 normal controls) to our most recently published ROI study in chronic schizophrenia (Onitsuka et al., 2004) (the study reported reduced left middle inferior temporal and bilateral inferior temporal gyri gray matter volumes in schizophrenia). After a complete description of the study, written informed consent was obtained from all participants. The age range for inclusion was 20 to 55 years. Subjects were included if they had no history of: 1) neurologic illness or major head trauma; 2) electroconvulsive therapy; 3) alcohol or drug dependence; or 4) alcohol and drug abuse within the past five years. Table 1 shows demographic and clinical characteristics of study groups.
Table 1.
Demographic and Clinical Characteristics of Study Groups
| Patients With Schizophrenia (N=25) | Normal Contols (N=28) | d.f. | t | p | |
|---|---|---|---|---|---|
| Total ICC (ml) | 1513.8 ± 105.6 | 1566.4 ± 140.7 | 51 | 1.52 | 0.134 |
| Age (range) | 42.6 ± 8.4 (29–55) | 42.0 ± 7.5 (28–55) | 51 | −0.27 | 0.787 |
| Handedness | 0.78 ± 0.16 | 0.80 ± 0.18 | 51 | 0.52 | 0.603 |
| SESa | 4.1 ± 0.7 | 2.0 ± 1.1 | 51 | −7.82 | <0.001 |
| Parental SES | 2.9 ± 1.4 | 2.5 ± 1.0 | 51 | −1.14 | 0.262 |
| WAIS-R, information subscale | 10.1 ± 2.6 | 11.0 ± 1.8 | 51 | 1.56 | 0.126 |
| Medication doseb (CPZ equiv., mg) | 497 ± 258 | ||||
| Symptom onset (years) | 22.7 ± 4.1 | ||||
| Duration of illness (years) | 19.9 ± 9.6 | ||||
| SAPS total | 9.2 ± 3.7 | ||||
| SANS total | 12.3 ± 3.9 |
Higher scores indicating lower SES.
Patients with schizophrenia showed significantly lower SES than controls.
Patients were administered the following medications: [N=5 risperidone, N=4 haloperidol, N=3 ziprasidone; N=3 fluphenazine; N=3 clozapine, N=2 olanzapine; N=1 perphenazine; N=1 chlorpromazine; N=1 olanzapine & ziprasidone; N=1 quetiapine & olanzapine; N=1 risperidone & chlorpromazine].
Normal control subjects were recruited through newspaper advertisement and screened using the Structured Clinical Interview (SCID non-patient edition) by trained interviewers (MES, MF). No control subjects had an Axis-I psychiatric disorder or a first-degree relative with Axis-I psychiatric disorder. The mean age of the normal control group was 42.0 ± 7.5 (mean ± SD) years (range: 28-55).
All patients were diagnosed with schizophrenia based on DSM-IV criteria, using information from the Structured Clinical Interview for DSM-III-R by the same trained interviewers. Patients were recruited from the VA Boston Healthcare System, Brockton Division. All patients were receiving neuroleptic medication, with a mean daily dose equivalent to 497 ± 258 mg of chlorpromazine [typical (9 of the 25 patients), atypical (15), or both (1)]. The mean age of patients was 42.6 ± 8.4 years (range: 29–55), their mean age at symptom onset was 22.7 ± 4.1 years, and their mean duration of illness was 19.9 ± 9.6 years. The Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1984) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1981) were administered to patients. Mean ± SD SAPS and SANS scores were 8.8 ± 4.1 and 12.3 ± 3.8, respectively. For the SAPS, visual hallucination scores (0=none, 5=severe) were available in sixteen of the twenty-five patients. Mean ± SD score was 1.3 ± 1.5 (range 0–4).
Handedness was assessed using the Edinburgh inventory. Socioeconomic status (SES) of subjects and parental SES were measured by the Hollingshead two-factor index (1=highest, 5=lowest). All subjects were given the WAIS-R information subscale as an estimate of gross fund of information. T-tests were used to assess group differences in age, handedness score, SES, parental SES and WAIS-R information subscale scores. There were no significant group differences in age, handedness score, parental SES or WAIS-R information subscale (see Table 1). Patients with schizophrenia showed significantly lower SES than normal control subjects (t[51]=−7.82, p<0.001), consistent with reduced functioning due to the disorder.
MRI Procedures and Definition of Occipital Lobe
The acquisition was done with a 1.5-Tesla General Electric scanner (GE Medical Systems, Milwaukee) at the Brigham and Women’s Hospital in Boston. The protocol followed that of our previous publication (Onitsuka et al., 2004).
Figure 1 shows the medial and lateral view of the three-dimensional reconstruction of the occipital lobe. The following steps were used to define the occipital lobe. First, the parietooccipital sulcus (POS) was identified on the midsagittal plane for each hemisphere. Second, the anterior tip of the POS was identified, as well as the posterior tip of the POS that corresponds to the parietooccipital fissure (see figure 1). The occipital lobe was defined as beginning at one slice posterior to the plane that contains the anterior tip of the POS, identified on the midsagittal plane, and ending in the last slice in the coronal plane, including the posterior tip of occipital lobe. For the medial surface, the boundary between the parietal and occipital lobe was the POS. For the lateral surface, the rater (TO) drew a guideline connecting the parietooccipital fissure and the superior temporal sulcus, or anterior occipital sulcus, on the first, beginning slice of occipital lobe. This guideline was defined as the boundary between the parietal and occipital lobe for the lateral surface. This guideline was seen as a point on each coronal image (see Figure 2c). The parietal and occipital lobe were divided operationally by extending the guideline across the tissue bridge of white matter, horizontally and medially up to the intraparietal sulcus (see Figure 2 c&d).
Figure 1.
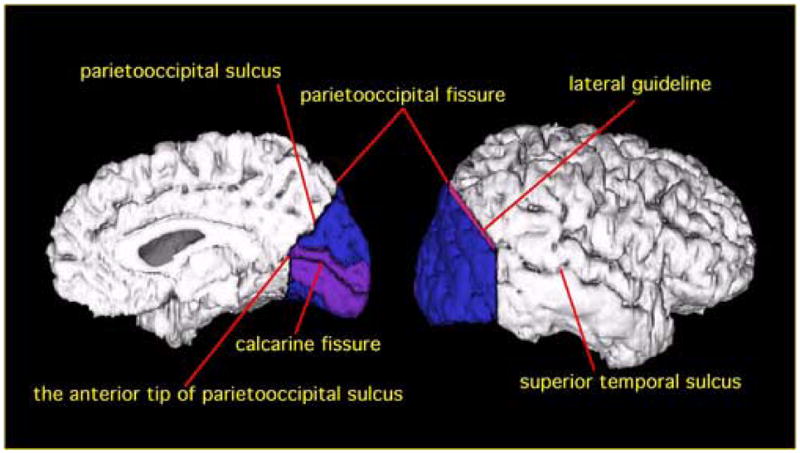
Delineation of PVA and VAA in occipital lobe. PVA is shown in purple, and VAA is shown in blue. For the medial surface (left), the border between parietal lobe and occipital lobe is the parietooccipital sulcus. PVA is defined as the area including one gyrus above and one gyrus below the calcarine fissure. For the lateral surface (right), the border between the two lobes is delineated by a guideline connecting the parietooccipital fissure and the superior temporal sulcus on the most anterior slice of occipital lobe (The guideline is shown in red).
Figure 2.
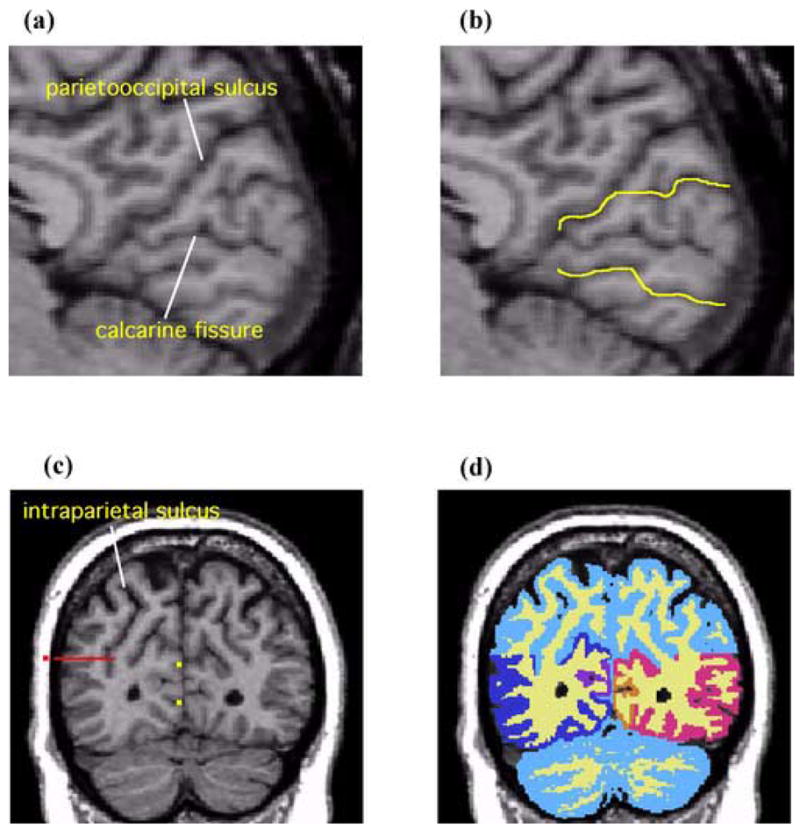
Sagittal and coronal MR images showing delineation of PVA and VAA. (a) The rater identifies the parietooccipital sulcus and the calcarine fissure on the midsaggital plane. (b) The yellow lines are the guidelines extending the sulcal courses used to delineate PVA and VAA. (c) On a coronal slice PVA and VAA are delineated by referring to the guidelines (yellow dots). In part B, here shown as yellow dots. On the lateral surface, the parietal lobe and occipital lobe are operationally separated by extending the guideline (the red dot) horizontally and medially across the tissue bridge of white matter horizontally and medially up to the intraparietal sulcus. (d) A coronal view of PVA and VAA delineation. White matter and gray matter were shown in light yellow and light blue respectively. The gray matter of PVA is shown in orange (subject left) and purple (subject right). The gray matter of VAA is shown in red (subject left) and blue (subject right).
The primary visual area (PVA) was defined as the area between one gyrus above the calcarine fissure and one gyrus below the sulcus on each coronal image. The rater drew two guidelines at 3–5 slices laterally from the medial surface to determine the gyri above and below calcarine fissure (see Figure 2b). The lines were drawn extending the sulcal course across the tissue bridge of white matter. These guidelines were seen as points on each coronal image and the rater delineated the primary visual area referring to the lines (see Figure 2c). Manual drawings of the ROIs were then performed on the realigned and resampled coronal slices (see Figure 2d).
Interrater reliability was computed for the ROIs by 3 independent raters (TO, NK, and SSD), who were blinded to diagnostic group membership. Six cases were selected randomly for interrater reliability. Three raters measured the occipital lobe on every third slice. An intraclass correlation coefficient was used to compute interrater reliability. For the three raters, the intraclass correlations were: 0.93 for the left PVA, 0.90 for the right PVA, 0.98 for the left VAA, 0.98 for the right VAA.
Statistical Analyses
A T-test was used to assess group differences in Total ICC. There was no significant group difference in total ICC (t[51]=1.52, p=0.13). However, to correct for variations in brain size, we used relative volumes of PVA and VAA computed as [(absolute volume)/(ICC)] × 100 for ROI analysis. To examine whether or not the ROI volumes were normally distributed, Shipiro-Wilk tests (Shapiro&Wilk, 1965) were performed for each ROI in both groups. To determine whether or not certain ROIs were more affected than other ROIs, the ROI volumes were first converted to Z-scores so that all the ROIs would be on the same scale. The mean and standard deviation of the control group were used to calculate the Z-scores. For ROI analysis, the standardized scores were submitted to a mixed model repeated measure ANOVA with group (schizophrenia or controls) as a between-subjects factor, and hemisphere (left or right) and subdivision (PVA or VAA) as within-subjects factors.
Exploratory analyses were performed, using Spearman’s rho, to explore the relationship between volumes for each ROI, and demographic data, the SAPS and SANS total scores, as well as for SAPS visual hallucination scores. Here, all correlations were considered significant only if they reached p≤0.05 (two-tailed), for both relative and absolute volumes.
Results
Volume of occipital lobe
Table 2 shows absolute and relative occipital lobe volumes of patients with schizophrenia and normal controls. For the 3-factor (2 groups × 2 sides × 2 subdivisions) ANOVA of standardized ROI values (Z-scores), there was a significant main effect of subdivision (F[1,51]=4.08, p=0.05, while there was no significant main effect of hemisphere (F[1,51]=0.06, p=0.81), or group (F[1,51]=2.16, p=0.15). There was also no significant hemisphere-by-subdivision-by-group interaction (F[1,51]=0.06, p=0.94). However, there was a significant subdivision-by-group interaction (F[1,51]=4.08, p=0.05) but no significant hemisphere-by-group (F[1,51]=0.06, p=0.81) or hemisphere-by-subdivision (F[1,51]=0.06, p=0.94) interactions.
Table 2.
Mean ± SD absolute (ml) and relative volume (%) for occipital lobe gray matter in chronic schizophrenia and normal controls
| Patients with Schizophrenia (N=25) | Normal Controls (N=28) | t | d.f. | p | |
|---|---|---|---|---|---|
| Total ICC (ml) | 1513.8 ± 105.6 | 1566.4 ± 140.7 | 1.52 | 51 | 0.134 |
| Left primary visual area | 5.65 ± 1.00 (0.37 ± 0.06) | 5.93 ± 1.03 (0.38 ± 0.07) | 0.98
0.37 |
51
51 |
0.330
0.717 |
| Left visual association area | 19.06 ± 4.40 (1.26 ± 0.28) | 22.26 ± 5.16 (1.42 ± 0.31) | 2.42
2.01 |
51
51 |
0.019
0.049 |
| Right primary visual area | 5.57 ± 0.91 (0.37 ± 0.05) | 5.79 ± 1.23 (0.37 ± 0.08) | 0.72
0.16 |
51
51 |
0.477
0.874 |
| Right visual association area | 18.96 ± 4.37 (1.25 ± 0.27) | 21.94 ± 5.18 (1.40 ± 0.31) | 2.24
1.90 |
51
51 |
0.029
0.064 |
Relative volumes (% total ICC) are in parentheses. Statistical significance levels are computed using t-tests.
To further delineate the subdivision-by-group significant interaction, follow up ANOVA was performed for each subdivision. For PVA, there was no significant main effect of group (F[1,51]=0.09, p=0.77) or hemisphere (F[1,51]=0.07, p=0.79), and no significant hemisphere-by-group interaction (F[1,51]=0.07, p=0.79). These results suggest that there is no group difference in PVA bilaterally.
For VAA, there was a significant main effect of group (F[1,51]=5.05, p=0.03) without a significant main effect of hemisphere (F[1,51]=0.02, p=0.89) or a significant hemisphere-by-group interaction (F[1,51]=0.02, p=0.89), indicating that patients showed bilateral VAA reduction compared with normal controls. The percentages of relative VAA reduction of patients were 11.4 % for the left and 10.7 % for the right.
Correlations between volume of occipital lobe and demographic/clinical measurements
In normal controls, age, parental SES, SES, handedness and WAIS-R information subscale scores did not correlate significantly with occipital lobe volume (−0.351≤rho≤0.301, 0.07≤p≤0.98), with the exception of a significant negative correlation between SES and right PVA volume (rho=−0.522, p=0.004 for absolute; rho=−0.525, p=0.004 for relative). In patients with schizophrenia, age, SES, parental SES, WAIS-R information subscale scores, duration of illness, and dose (chlorpromazine equivalent) of medication did not correlate significantly with occipital lobe volume (−0.383≤rho≤0.360, 0.07≤p≤0.98), with the exception of a significant positive correlation between WAIS-R information subscale score and left PVA volume (rho=0.484, p=0.01 for absolute; rho=0.464, p=0.02 for relative)
In examining the relationships between occipital lobe volume and psychopathology measures, there were no significant correlations between ROIs and SAPS or SANS total scores (−0.386≤rho≤0.097, 0.07≤p≤0.93). For SAPS visual hallucination scores, we found no significant correlations between the scores and left PVA volumes or left or right VAA volumes (−0.251≤rho≤0.074, 0.35≤p≤0.97). However there was a significant negative correlation between right PVA volumes and visual hallucination scores (rho=−0.632, p=0.009 for absolute; rho=−0.617, p=0.011 for relative). Because of the results of Spencer et al. (2003, 2004), t-tests were used for additional (exploratory) analyses of ROI volume differences between the 9 patients with and the 7 patients without visual hallucinations. Of note here, there was a significant group difference in right PVA gray matter volume (t[14]= −2.32, p=0.04), although there were no significant differences in the other ROIs (−1.15≤t[14] ≤ −0.08, 0.27≤p≤0.94). For differences in demographic/clinical measurements between patients with and without visual hallucinations, there were no significant subgroup differences in chlorpromazine equivalent (t[14]= −0.12, p=0.90), age (t[14]=−0.03, p=0.97), age at symptom onset (t[14]=1.48, p=0.16), duration of illness (t[14]=−0.80, p=0.44), SES (t[14]= −0.38, p=0.71), parental SES (t[14]=0.63, p=0.54) or handedness (t[14]=1.23, p=0.24).
Discussion
The current study examined PVA and VAA gray matter volumes and investigated correlations between ROI volumes and clinical symptoms. For PVA gray matter volume, there was no significant group difference, while patients with schizophrenia showed significant VAA gray matter volume reduction, bilaterally, compared with healthy controls.
There are a small number of previous structural MRI studies of occipital lobe in schizophrenia. Goldstein et al. (1999), for example, used a semi-automated method of cortical parcellation to measure 48 topography defined brain regions of the entire neocortex and found no difference in occipital lobe gray matter volume between 29 patients (12 females) and 26 control subjects (14 females), although they reported the results of both genders as one group. In contrast, Andreasen et al. (1994) used an automated atlas-based dissection of specific regions without separation for gray and white matter. They reported a significant difference in occipital brain tissue volume between 36 male patients and 48 male controls, while there was no group difference in female subjects; thus gender differences in the manifestation of schizophrenia may account for the reported discrepancies in findings. In addition, differences in ROI definition and methodology may also account for the reported discrepancies. For example, Zipursky et al. (1992) used seven axial MRI sections for segmentation and found significant gray matter volume reduction in the occipital lobe in patients. More recently, Quarantelli et al. (2002) used their own automated segmentation method of major brain structures according to the Talairach atlas and found no group differences in occipital lobe between controls and patients with schizophrenia. However a recent VBM study (Davatzikos et al., 2005) reported reduced gray matter in occipital lobe areas in patients with schizophrenia
Another possible reason for inconsistent findings is that occipital lobe volume reduction may be just at the threshold for MRI detection, and hence whether statistical significance is found may depend heavily on the subject group evaluated. As reviewed by Shenton et al. (2001), the left superior temporal gyrus (STG) gray matter reduction is the most frequently reported MRI finding in patients with schizophrenia; thus the left STG reduction is thought to be most common affected region across individuals in schizophrenia, while occipital lobe reduction is relatively less common across individuals. Random variation in subjects may therefore account for inconsistent findings in the occipital lobe in schizophrenia.
The current study suggests that the primary visual area was relatively intact in schizophrenia compared to the association area. Pearlson et al. (1996) highlights the importance of structural abnormalities in schizophrenia in heteromodal association cortex. Our current finding that PVA did not differ between groups may partially support this hypothesis since our PVA is mainly unimodal primary sensory cortex. In a postmortem study by Selemon et al. (1995), these investigators found neuronal density increase in pyramidal layers III and V, with a trend-level cortical thickness reduction in Brodmann area 17 in patients with schizophrenia. Of note here, our current finding may not conflict completely with their finding since cortical thickness reduction was trend-level and it remains unclear how cortical thickness relates to cortical gray matter volume.
With respect to clinical correlations, although exploratory, increased severity of visual hallucinations was significantly associated with smaller right PVA volumes in patients with schizophrenia. Of further note, a recent case report of a patient with brain infarction in the right medial occipital lesion showed visual hallucinations (Beniczky et al., 2002). Moreover, there was a case report that a patient with brain infarction in the region of the calcarine fissure showed visual hallucinations (Merabet et al., 2003). Although there were no significant group differences observed for PVA gray matter volume, t-tests comparing the 9 patients with and the 7 patients without visual hallucinations showed a significant group difference in right PVA gray matter volume. To the best of our knowledge, our study is the first structural MRI study that indicates a significant association between the occipital lobe and visual hallucinations in patients with schizophrenia. In conjunction with findings of case reports and our results, the PVA might be crucial for visual hallucinations. However, this exploratory finding warrants confirmation in a larger sample.
In reviewing the current study, it is important to point out several possible limitations. First, the current study cannot answer the question of whether the volume reduction observed is associated with progressive in the peri- and/or post-onset course of illness, or whether it is neurodevelopmental in origin, or perhaps a combination of both. It will thus be important to investigate whether VAA gray matter volume undergoes a progressive decrease over a period of 1.5 years following first hospitalization, as we have found for superior temporal gyrus (Kasai et al., 2002). Second, the current study also did not allow us to exclude the effect of chronic treatment with neuroleptic medication on VAA gray matter abnormalities in patients (although no volume measure correlated with neuroleptic dosage), nor did we demonstrate specificity to schizophrenic psychosis, as we did not include another psychosis group. It will thus be important to investigate whether VAA abnormality is observed or not in patients with schizophrenia and affective psychosis at their first hospitalization, with minimal or no medication history. Third, this study does not provide the answer as to whether female patients show gray matter volume abnormality of VAA or not, since this study did not include any female subjects. Gender effects thus remain to be examined. Finally, an association between the occipital lobe and visual hallucinations should be confirmed in a larger sample, since visual hallucination scores were available in a part of the patients with schizophrenia of the present study.
In summary, the present study used consistent neuroanatomical boundaries for defining PVA and VAA, using three-dimensional information from MRI scans. These results suggest that patients with schizophrenia have relatively intact PVA and reduced bilateral VAA, which may be the substrate of some of the deficits observed in early visual processing.
Figure 3.
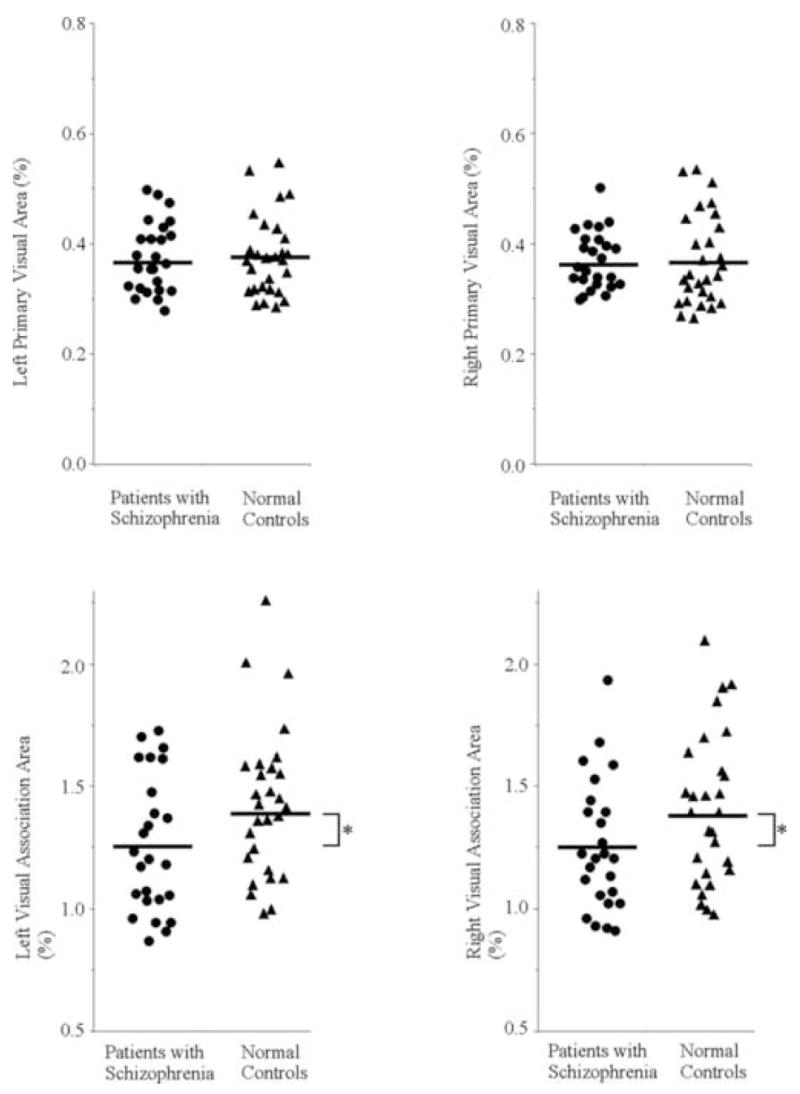
Scattergram of PVA and VAA relative volumes on the left and right in patients with schizophrenia (circles) and normal control subjects (triangles). Means are indicated by horizontal lines. * Patients showed bilateral VAA reduction compared with normal controls (F[1,51]=5.05, p=0.03).
Acknowledgments
This study was supported, in part, by the Department of Veterans Affairs Merit Awards and Research Enhancement Award Program (Drs. McCarley & Shenton), grants from the National Institute of Health (R01 MH 40799 to Dr. McCarley & K02 MH 01110 and R01 MH 50747 to Dr. Shenton), the MIND Institute (Albuquerque, NM, Dr. McCarley) a VA Career Award (Dr. Frumin), in part from the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149 (Dr. Kikinis), and the Welfide Medicinal Research Foundation, Osaka, Japan (Dr. Onitsuka). The authors gratefully acknowledge the administrative support of Marie M. Fairbanks and Nancy Maxwell, and the research assistant support of Lisa C. Lucia, B.S., Meredith C. Klump, B.A., and Sarah M. Rabbitt, B.A.
Footnotes
Contributors
Authors: Toshiaki Onitsuka, M.D., Ph.D., Robert W. McCarley, M.D., Noriomi Kuroki, M.D., Chandlee C. Dickey, M.D., Marek Kubicki, M.D., Ph.D., Susan S. Demeo, B.A., Melissa Frumin, M.D., Ron Kikinis, M.D., Ferenc A. Jolesz, M.D., Martha E. Shenton, Ph.D.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann’s areas 17 and 18 brought into stereotaxic space- where and how variable? Neuroimage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC. Scale for the assessment of negative symptoms (SANS) Department of psychiatry, University of Iowa College of medicine; Iowa City, IA: 1981. [Google Scholar]
- 3.Andreasen NC. Scale for the assessment of positive symptoms (SAPS) Department of psychiatry, University of Iowa College of medicine; Iowa City, IA: 1984. [Google Scholar]
- 4.Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze V, 2nd, O’Leary DS, Ehrhardt JC, Yuh WT. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA. 1994;272:1763–1769. [PubMed] [Google Scholar]
- 5.Beniczky S, Keri S, Voros E, Ungurean A, Benedek G, Janka Z, Vecsei L. Complex hallucinations following occipital lobe damage. Eur J Neurol. 2002;9:175–176. doi: 10.1046/j.1468-1331.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 6.Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, Snyder PJ, Lieberman JA. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- 7.Bilder RM, Wu H, Bogerts B, Ashtari M, Robinson D, Woerner M, Lieberman JA, Degreef G. Cerebral volume asymmetries in schizophrenia and mood disorders: a quantitative magnetic resonance imaging study. Int J Psychophysiol. 1999;34:197–205. doi: 10.1016/s0167-8760(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 8.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 9.Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davatzkikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 11.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 12.Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. Springer-Verlag; Wien New York: 1991. [Google Scholar]
- 13.Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res. 2005;74:135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Jr, Faraone SV, Tsuang MT. Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry. 1999;56:537–547. doi: 10.1001/archpsyc.56.6.537. [DOI] [PubMed] [Google Scholar]
- 15.Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- 16.Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11:374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- 18.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee C-U, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in first-episode schizophrenia. Am J Psychiatry. 2002;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–542. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- 20.Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. NeuroImage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 23.Merabet LB, Kobayashi M, Barton J, Pascual-Leone A. Suppression of complex visual hallucinatory experiences by occipital transcranial magnetic stimulation: a case report. Neurocase. 2003;9:436–440. doi: 10.1076/neur.9.5.436.16557. [DOI] [PubMed] [Google Scholar]
- 24.Molina V, Gispert JD, Reig S, Sanz J, Pascau J, Santos A, Desco M, Palomo T. Cerebral metabolic changes induced by Clozapine in schizophrenia and related clinical improvement. Psychopharmacology. 2005;178:17–26. doi: 10.1007/s00213-004-1981-9. [DOI] [PubMed] [Google Scholar]
- 25.Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: An MRI study. Am J Psychiatry. 2004;161:1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 27.Quarantelli M, Larobina M, Volpe U, Amati G, Tedeschi E, Ciarmiello A, Brunetti A, Galderisi S, Alfano B. Stereotaxy-based regional brain volumetry applied to segmented MRI: validation and results in deficit and nondeficit schizophrenia. Neuroimage. 2002;17:373–384. doi: 10.1006/nimg.2002.1157. [DOI] [PubMed] [Google Scholar]
- 28.Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 30.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- 32.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- 35.Tootell RB, Dale AM, Sereno MI, Malach R. New image from human visual cortex. Trends Neurosci. 1996;19:481–489. doi: 10.1016/S0166-2236(96)10053-9. [DOI] [PubMed] [Google Scholar]
- 36.Van Essen DC, Drury HA. Structural and functional analyses of human cerebral cortex using a surface-based atlas. J Neurosci. 1997;17:7079–7102. doi: 10.1523/JNEUROSCI.17-18-07079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss AP, Heckers S. Neuroimaing of hallucinations: a review of the literature. Psychiatry Res. 1999;92:61–74. doi: 10.1016/s0925-4927(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 38.Wells W, Grimson W, Kikinis R, Jolesz FA. Adaptive segmentation of MRI data. IEEE Trans Med Imag. 1996;15:429–442. doi: 10.1109/42.511747. [DOI] [PubMed] [Google Scholar]
- 39.Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49:195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]


