Abstract
Management of asthma requires attention to environmental exposures both indoors and outdoors. Americans spend most of their time indoors, where they have a greater ability to modify their environment. The indoor environment contains both pollutants (eg, particulate matter, nitrogen dioxide, secondhand smoke, and ozone) and allergens from furred pets, dust mites, cockroaches, rodents, and molds. Indoor particulate matter consists of particles generated from indoor sources such as cooking and cleaning activities, and particles that penetrate from the outdoors. Nitrogen dioxide sources include gas stoves, furnaces, and fireplaces. Indoor particulate matter and nitrogen dioxide are linked to asthma morbidity. The indoor ozone concentration is mainly influenced by the outdoor ozone concentration. The health effects of indoor ozone exposure have not been well studied. In contrast, there is substantial evidence of detrimental health effects from secondhand smoke. Guideline recommendations are not specific for optimizing indoor air quality. The 2007 National Asthma Education and Prevention Program asthma guidelines recommend eliminating indoor smoking and improving the ventilation. Though the guidelines state that there is insufficient evidence to recommend air cleaners, air cleaners and reducing activities that generate indoor pollutants may be sound practical approaches for improving the health of individuals with asthma. The guidelines are more specific about allergen avoidance; they recommend identifying allergens to which the individual is immunoglobin E sensitized and employing a multifaceted, comprehensive strategy to reduce exposure. Outdoor air pollutants that impact asthma include particulate matter, ozone, nitrogen dioxide, and sulfur dioxide, and guidelines recommend that individuals with asthma avoid exertion outdoors when these pollutants are elevated. Outdoor allergens include tree, grass, and weed pollens, which vary in concentration by season. Recommendations to reduce exposure include staying indoors, keeping windows and doors closed, using air conditioning and perhaps high-efficiency particulate arrestor (HEPA) air filters, and thorough daily washing to remove allergens from one’s person.
Keywords: asthma, pollutants, particulate matter, nitrogen dioxide, sulfur dioxide, secondhand smoke, ozone, allergens
Introduction
Asthma is a common, complex respiratory disease that is characterized by recurrent symptoms of wheeze, cough, chest tightness, and dyspnea. There is growing evidence of environmental causes of the disease, and substantial evidence of the role of the environment, especially inhaled agents such as allergens, pollutants, and viruses, in provoking asthma attacks. Asthma is best thought of as an immunologically mediated disease, in which an abnormal immune response to inhaled agents provokes a cascade of events that lead to mucus hypersecretion, airways constriction and hyperresponsiveness, and, ultimately, symptoms. Because so much evidence points to environmental factors as triggers of the exuberant immune response, there has been much attention to identifying specific environmental factors that are most responsible for provoking asthma and developing strategies to minimize relevant exposures. Indeed, avoidance of environmental factors that provoke asthma, where feasible, is a logical way to improve asthma-related health and to minimize the need for long-term use of asthma medications.
In this paper we will summarize available evidence on the current practices for managing environmental issues with patients who have asthma. In addition, given the recent release of the latest version of the National Asthma Education and Prevention Program’s Expert Panel Report 3, Guidelines for the Diagnosis and Management of Asthma (2007 NAEPP guidelines),1 we will highlight many of the newest and most prominent recommendations of that guideline committee, especially because this latest version of these guidelines supports its statements with a formal process of grading the available evidence. Though the potential scope of this paper on the environment and asthma could be quite broad and include topics such as primary prevention and occupational exposures, for the sake of brevity we will focus the discussion on common indoor and outdoor exposures that are most relevant to people in the United States, regardless of occupation. Also, though diet and infections may be conceptualized as part of the environment, discussion of those factors is beyond the scope of this paper.
Indoor and Outdoor Environments
Management of asthma requires attention to environmental exposures that originate from both the outdoor and indoor environment, though, arguably, those originating indoors may be more relevant for certain patients with asthma. Americans spend nearly 90% of their time indoors,2 such as the home, workplace, and school, which underscores the substantial contribution of indoor exposures to an individual’s total exposure. In some cases the risk of encountering certain environmental factors known to exacerbate asthma (eg, dust mite) is only relevant in the indoor environment, whereas in other cases the indoor environment accounts for the bulk of most individuals’ exposure time, although the inciting factor could be found outdoors (eg, particulate matter). Also, in contrast to the outdoors, people may have a greater ability to modify indoor environmental exposures. For example, most individuals do not have direct control over outdoor pollutant concentrations, but they may be able to decrease concentrations of specific pollutants in their homes. Because the sources of exposure differ in the indoor and outdoor environments and because interventions to limit exposure also differ, we will consider the indoor and outdoor environments separately in the following discussion.
Indoor Environmental Factors
Air Pollution
Indoor air pollution is a complex mixture of pollutants migrating indoors from outdoor air and pollutants generated by indoor sources. Since studies have shown that indoor air pollution concentrations can greatly exceed outdoor air concentrations,3 indoor sources can be very important contributors to total indoor air pollution. Table 1 lists some indoor pollutant sources relevant to asthma. Although the link between asthma and some indoor air pollutants has not yet been thoroughly studied, research to date suggests that they may play an important role in asthma morbidity.
Table 1.
Major Indoor Environment Factors That Affect Asthma
| Pollutants |
| Particulate matter |
| Nitrogen oxides |
| Secondhand smoke |
| Ozone |
| Allergens |
| Furred pets |
| Cats |
| Dogs |
| Others |
| Dust mites |
| Cockroaches |
| Rodents |
| Mice |
| Rats |
| Molds |
Particulate Matter
Particulate matter consists of solid and liquid particles suspended in the air. Particulate matter originates from a variety of natural and man-made sources. Natural sources include pollen, spores, bacteria, plant and animal debris, sea salt, and dust from the earth’s crust. Man-made sources consist mostly of combustion by-products from factories, motor vehicles, and power plants. Smoking is a major contributor to indoor particulate matter. Additional sources include cooking exhaust, wood-burning stoves and fireplaces, cleaning activities that re-suspend dust particles (eg, sweeping), and penetration of outdoor particles into the indoor environment.4,5
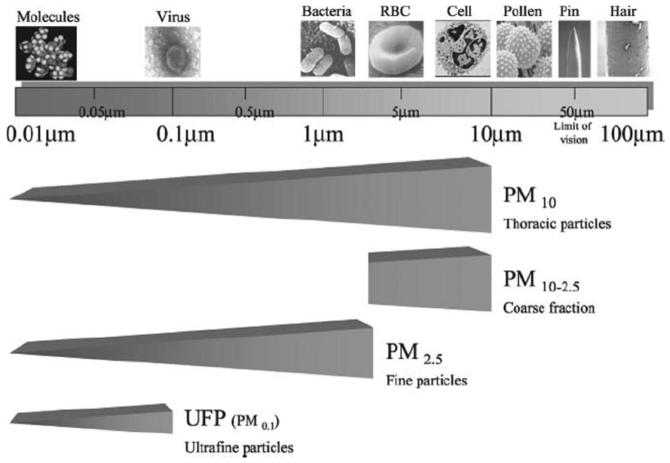
Particulate matter is also characterized and regulated on the basis of particle size (Fig. 1).6 Particles < 10 μm in diameter (PM10) are capable of entering the respiratory system, and particles < 2.5 μm (PM2.5) are capable of reaching the alveoli. The so-called “coarse fraction” particles (PM2.5-10) are too large to reach the alveoli and therefore deposit in the proximal airways. The Environmental Protection Agency regulates particulate-matter concentrations in outdoor air, but currently there are no standards for indoor air.
Fig. 1.
Characterization of particulate matter (PM) based on size. Particulate matter < 10 μm (PM10) can enter the human thorax, whereas PM < 2.5 μm (PM2.5) can reach the alveoli. RBC = red blood cell. (From Reference 6, with permission.)
Studies that have examined the effects of indoor particulate matter have found an association with asthma morbidity. For example, among children with asthma, higher indoor particulate-matter concentration is associated with decreased lung function,7,8 greater respiratory symptoms,9 and more frequent use of rescue medications. Particulate matter exposure may provoke inflammation in asthma, some evidence of which is the elevated exhaled nitric oxide concentration (a marker of airway inflammation) associated with an elevated PM2.5 concentration.8 Of particular concern is that particulate-matter concentrations in some indoor settings are even higher than those outdoors, and the particulate matter that originates indoors may, in some cases, be more harmful than that found outdoors. For example, in one study from inner-city Baltimore, investigators found that indoor PM2.5 and PM10 concentrations were more than twice as high as the simultaneously measured outdoor concentrations.5 Koenig et al8 found that PM2.5 originating from indoor sources was more potent in decreasing lung function than was outdoor-derived particulate matter.
Management of Indoor Particulate Matter
The evidence regarding what factors can be modified to reduce particulate matter and improve asthma health comes from observational studies and clinical trials. For example, in cohort studies of inner-city children with asthma, higher particulate-matter concentration was found with tobacco smoking and greater frequency of sweeping and stove use in the home.4,5 Increased ventilation with more open windows led to lower in-home particulate-matter concentration. Intervention studies have shown that high-efficiency particulate arrestor (HEPA) air filters are effective in lowering indoor particulate matter (Fig. 2).10,11 Though these studies did demonstrate a health benefit, the particulate-matter reduction was part of an overall allergen and pollutant intervention strategy, which makes it impossible to isolate the independent health effect of particulate-matter reduction with air cleaners.
Fig. 2.
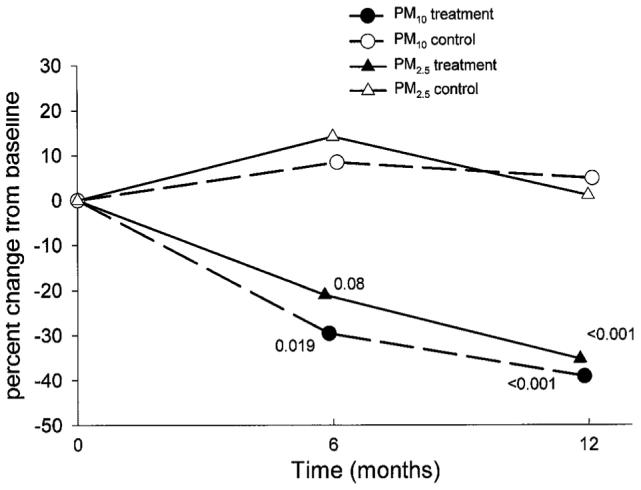
Change in particulate matter (PM) concentration in the bedrooms of children with asthma. The rooms of the intervention subjects had high-efficiency particulate arrestor (HEPA) air cleaners. The study was conducted in inner-city Baltimore, Maryland. There was substantial and sustained reduction in both of the particulate-matter size ranges (< 10 μm [PM10] and < 2.5 μm [PM2.5]) in the filtered-air rooms. (From Reference 10, with permission.)
The 2007 NAEPP guidelines1 concluded that there is insufficient evidence to recommend indoor air-cleaning devices, and make no specific recommendations for indoor particulate-matter reduction. However, we believe that the best way to apply the existing evidence regarding indoor particulate matter to asthma management is to inform individual patients about what is currently known. Patients should be warned of the potential health effects of indoor particulate matter and advised to reduce or eliminate activities (such as smoking) that raise indoor particulate matter. Furthermore, for patients with a keen interest in making all possible changes to optimize their indoor environment, use of air cleaners and improved ventilation can be recommended, if financially feasible.
Nitrogen Dioxide
Nitrogen dioxide (NO2) is a gaseous product of high-temperature combustion. It has many indoor sources, including gas stoves, space heaters, furnaces, and fireplaces, and has been linked to respiratory health effects.12 Although some studies13-18 have found adverse respiratory health effects from indoor NO2, other studies have failed to confirm that association.19-22 For example, data from the National Health and Nutrition Examination Survey III did not suggest any impact from gas stoves (the major source of indoor NO2) on pulmonary function or respiratory symptoms in adults with asthma.23 To the extent that gas appliances may confer a risk of respiratory symptoms, the exposure may be more complex than simply NO2 (Fig. 3). For example, gas stoves may also increase other indoor air pollutants, such as nitrous acid, that may also adversely affect respiratory health.13
Fig. 3.

A gas stove in the home, without proper venting. (Photograph courtesy of Karen A Callahan RN MSc, Department of Pediatrics, Johns Hopkins University, Baltimore, Maryland.)
Studies that have specifically investigated the effect of indoor NO2 on asthma morbidity have also had inconsistent results. Some found no association between indoor NO2 level and respiratory symptoms,20 but an increasing number have found a positive association with asthma morbidity. Two recent studies of asthmatic children found that higher indoor NO2 increased the likelihood and frequency of asthma symptoms, including wheeze, chest tightness, breathlessness, and daytime and nighttime asthma attacks.16,18 In a study of inner-city children with sthma there was a strong and significant association between higher indoor NO2 and respiratory morbidity, including wheeze, chest tightness, breathlessness, and daytime and nighttime asthma attacks.24,25 NO2 exposure has also been found to impair host resistance to respiratory viruses and bacteria, by reducing bacterial clearance and impairing innate immunity.26-28 Higher personal NO2 exposure increased the severity of virus-induced asthma exacerbations, as measured by symptom severity and peak-flow reduction.29
Management of Indoor NO2
Given the uncertainty about the association of NO2 and respiratory health, additional studies are needed to determine whether NO2 increases risk in people with asthma. General control strategies include source modification and ventilation. A randomized controlled trial on the effects of reducing NO2 in schools, by replacing unflued gas heaters with flued gas or electric heaters, found that a substantial reduction in mean indoor NO2 concentration was associated with a concordant decrease in asthma symptoms in school children.30 The uncertainty about the utility of NO2 reduction is apparent in the 2007 NAEPP guidelines, which found only modest support for NO2 reduction, and this recommendation is in the context of general recommendations to reduce exposure to common indoor irritants.1 We believe that the best way to apply the existing evidence regarding indoor NO2 to asthma management is to take a common-sense approach. If a patient reports symptoms in the presence of an NO2-producing device (such as a stove), we recommend that they assure that it is properly vented to the outside. And if the patient has the option to choose, we suggest they select electric rather than gas appliances. This approach to NO2, as with most pollutants, becomes especially important for the patient who has poor asthma control or requires a high dose of medication to control symptoms.
Secondhand Smoke
Secondhand smoke is involuntarily inhaled tobacco smoke that contains particles and gases generated by the combustion of the tobacco, paper, and additives of cigarettes.31 Secondhand smoke exposure is common in the United States, and there is sufficient evidence to suggest a causal relationship between secondhand smoke and incidence of wheezy illnesses in infants and pre-school-age children, though the causal link to asthma incidence has not been fully established. Exposure to secondhand smoke is convincingly linked to greater disease severity among children and adults with asthma.1,31 Secondhand smoke is associated with worse lung function and greater airway inflammation, daytime and nocturnal symptoms, exacerbations, health care utilization, and intubation.32-37
Management of Indoor Secondhand Smoke
The surgeon general’s report states that “eliminating smoking in indoor spaces fully protects nonsmokers from exposure to secondhand smoke. Separating smokers from nonsmokers, cleaning the air, and ventilating buildings cannot eliminate exposure of nonsmokers to secondhand smoke.”31 There is substantial evidence that secondhand smoke avoidance should improve asthma outcomes, but intervention studies that attempted to reduce secondhand smoke exposure showed relatively small effects on the smoking patterns of those who smoke near children with asthma.38,39 There have not, to our knowledge, been clinical trials of secondhand smoke reduction in adults with asthma.31 The 2007 NAEPP guidelines recommend that patients who are active smokers be referred to smoking cessation programs and that all patients with asthma be counseled concerning the negative effects of smoking and secondhand smoke.1 We recognize that not all patients can avoid secondhand smoke, and, because secondhand smoke contributes to air-borne particulate matter, which air cleaners can reduce, patients may want to consider air cleaners if secondhand smoke cannot be avoided.
Ozone
The indoor ozone (O3) level tends be high only in warmer months of the year, because the level is mainly influenced by ozone penetration from outdoors.40 Indoor ozone sources are uncommon, but include ionizers and ozone generators, which are sold as air-freshening or air-cleaning devices, and xerographic copy machines, found in offices and schools.41 Epidemiologic studies of ambient ozone and experimental studies show a significant association with asthma-related morbidity, including symptoms, health care utilization, airway inflammation, and decreases in lung function.42-46 However, the effect of indoor ozone on asthma morbidity has not been well studied, and the benefits of indoor ozone reduction on asthma morbidity are unknown.
Management of Indoor O3
Since O3 is a highly reactive gas, the O3 concentration is generally much lower indoors than outdoors, even in peak ozone season. Evidence suggests that indoor ozone can be reduced by keeping windows and doors closed.47 The 2007 NAEPP guidelines make no specific recommendations on indoor O3 reduction.1 We recommend a common-sense approach to indoor ozone, including avoidance of ozone-generating “air purifiers” and other ozone-generating devices in the homes of patients with asthma.
Indoor Allergens
Allergens are water-soluble glycoproteins that induce an immunoglobin E response in susceptible individuals. The allergens adhere to particles that range in size from 1 μm to > 100 μm.48,49 Particles of that size range deposit in the upper and lower respiratory tracts, where the allergen can directly elicit an inflammatory response and respiratory symptoms.
Both indoor and outdoor allergens can cause asthma symptoms in patients who are sensitized to the allergen. Sensitization is defined as having evidence of immunoglobin E specific to the particular allergen, which can be assessed by allergy skin testing in an allergist’s office, or via a serum test known as a radioallergosorbent test, the blood for which can be collected by a primary care provider or other health care provider, and the test is obtained from a commercial laboratory.
Indoor allergens are generally present year-round, so they are associated with perennial symptoms. Outdoor allergens are generally present during certain seasons and thus trigger asthma only during those seasons.
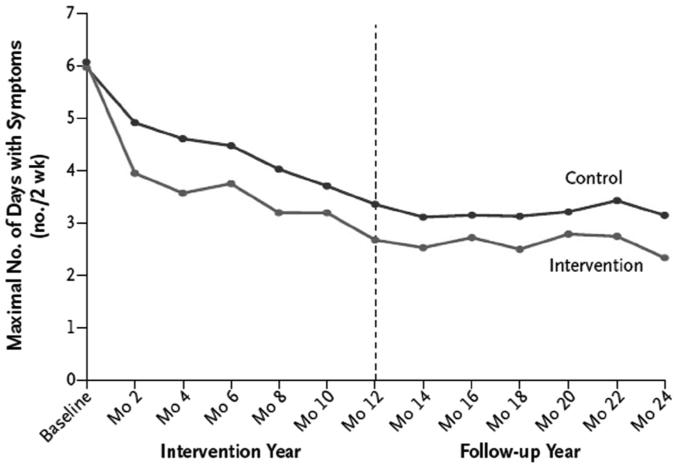
Patients may have perennial allergen-induced asthma symptoms, seasonal allergen-induced symptoms, or both. For example, a patient who presents with persistent asthma with a history of springtime exacerbations may have asthma symptoms that are triggered by both perennial and seasonal allergens. There are 5 general groups of indoor aeroallergens: furred pet, dust mite, cockroach, rodent, and mold allergens (see Table 1). The 2007 NAEPP guidelines recommend that patients be advised to reduce exposure to allergens to which they are sensitive, but particularly to note that individual steps alone are generally ineffective.1 Avoidance requires a multifaceted, comprehensive approach, and this often requires targeting several allergens simultaneously, in some cases employing multiple proven approaches for each allergen (Fig. 4).
Fig. 4.
Effect of a multi-faceted intervention on the number days with asthma symptoms. There was a modest reduction in symptoms over the 12 months of the active intervention, and the reduction persisted during the 12 follow-up months after the intervention. (From Reference 11, with permission.)
Furred Pet Allergens
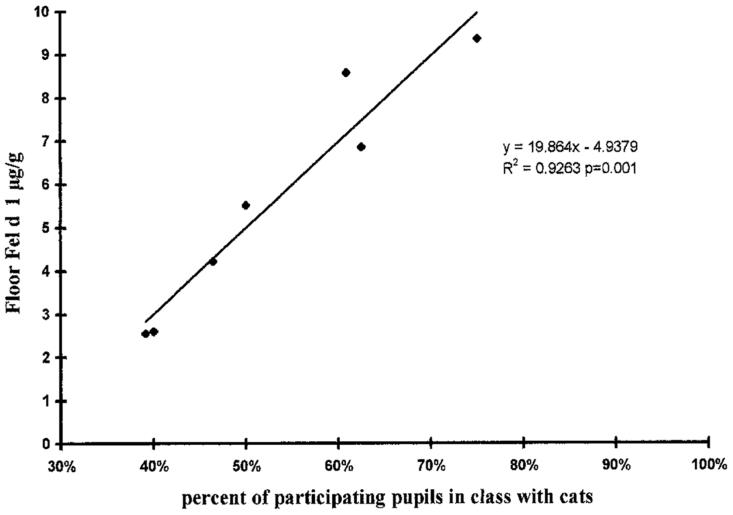
Cats and dogs are common furred pets, although families often keep other species of furred pets as well. Cat and dog allergens can be found in virtually all homes, but, not surprisingly, homes with pets contain much higher levels of the allergens than homes without pets.50 Furred pet allergens are also found in other settings, such as schools and other public buildings (Fig. 5),51 and are passively transferred from one environment to another.52 Passive transfer of cat allergens to school by children who have cats at home causes asthma symptoms in cat-sensitized classmates.53 Both cat and dog allergens can be passively transferred, because they are carried on small particles that remain airborne and adhere to surfaces and clothing.53-55
Fig. 5.
Results from a study in New Zealand schools. There was a strong correlation between the level of cat allergen (Fel d 1) on the classroom floor and the percent of students who had cats in their homes. (From Reference 51, with permission.)
Allergic sensitization to furred pet allergens is quite common, and in some populations more than 60% of children with asthma are sensitized to cat or dog allergens.56,57 The combination of widespread exposure to pet allergens and high prevalence of allergic sensitization to these allergens suggests that a substantial proportion of patients with asthma are at risk for cat or dog allergen-induced asthma symptoms. In fact, several studies have directly linked animal allergen exposure to poorer asthma outcomes among animal-sensitized patients with asthma.52,58
Assessing pet allergen exposure in patients is fairly straightforward and can be accomplished by taking a history focused on pet ownership, recent relocation into a home where pets had been living, and for children in particular, pet exposure at daycare. Most, but not all, relevant sources of exposure can be identified with this approach.
Because furred pet allergens are airborne and adhere to clothing, it is impossible to eliminate exposure entirely. Pet removal is the only method of substantially reducing the animal allergen level, but it will not decline significantly for 4-6 months,59 so clinical benefit may be slow to realize. The rate of decline may be improved by vigorous cleaning to remove allergens remaining in reservoirs, including accumulated dust, carpeting, and bedding. Allergen-proof mattress and pillow encasings sequester the allergen in the mattress and pillow and are a helpful adjunct to pet removal.
Because some families are very reluctant to give up a pet, alternative methods of furred animal allergen control have been studied and have largely proven ineffective. HEPA room-air filters can modestly reduce airborne animal allergens, but most studies have found that the allergen reduction is not sufficient to improve asthma symptoms.59,60 Other methods, such as washing the pet, are also ineffective.61 Citing the general lack of positive controlled trials, the 2007 NAEPP guidelines offer an expert-opinion basis for a general recommendation of either removal of pets from the home or isolation of the pet from the asthmatic individual if removal is not feasible.1
Although the effect of pet removal on asthma symptoms has not been well studied, because of the practical difficulties of conducting a randomized controlled trial of pet removal, one recently published study found a substantial reduction in inhaled corticosteroid requirements among furred-pet-sensitized adults with asthma who chose to remove their pets from their homes, compared to those who kept their pets.62 These findings underscore that pet removal is a critical part of the treatment plan for pet-sensitized patients with asthma.
Dust Mite Allergens
Dust mites are arachnids that infest bedding, carpet, upholstered furniture, and fabric. Their main food source is human skin scales, and they grow best in warm, humid environments,63 so they are rarely found in arid regions, such as the desert Southwestern United States, but are common in more humid regions such as the Northwestern and Southeastern United States. The predominant dust mites in the United States that have been implicated in allergy are Dermatophagoides farinae and Dermatophagoides pteronyssinus.63,64 The allergens are predominantly found on larger particles, in the range of 10-20 μm, which rapidly settle on dependent surfaces after disturbance.49
The prevalence of allergic sensitization to dust mites varies from region to region and depends on the regional prevalence of dust mites. For example, the sensitization rate among children with asthma is estimated to be 5% in the arid setting of Los Alamos, New Mexico, in contrast to as high as 66% in the more humid setting of Atlanta, Georgia.65,66
Like many other allergens, exposure to dust mite allergen in sensitized patients is associated with poorer lung function, greater medication requirements, and more asthma symptoms.65,67,68 In contrast to other allergens, there is evidence that dust mite allergen leads to the development of asthma, in addition to exacerbating pre-existing asthma in dust mite-sensitized patients. A prospective cohort study found that infants exposed to high levels of dust mite allergen were significantly more likely to have asthma later in childhood than were infants who were not exposed to high levels.69 Thus, reducing dust mite allergen exposure should both improve asthma control in sensitized patients and prevent the development of asthma in children.
Since dust mites are microscopic organisms, it is difficult to determine a patient’s exposure status by history. The most practical approach is to assume that patients living in arid climates are very unlikely to have substantial exposure to dust mites, and that patients in more humid regions are at risk for substantial exposure. However, there are commercially available home test kits for dust mite allergens that can provide semi-quantitative results so the patient can assess his/her degree of exposure.
First-line dust mite control measures include installing allergen-proof mattress and pillow encasements, washing all bedding every 1-2 weeks in hot water, removing stuffed toys, vacuuming and dusting regularly, and reducing indoor humidity. All of these measures are recommended by the 2007 NAEPP guidelines.1 These strategies reduce asthma symptoms, reduce medication requirements, and improve lung function.66,70,71
Removal of carpet and upholstered furniture may also be helpful, but is more expensive. Because dust mite allergens are predominantly found on dependent surfaces and are not airborne, air filters are not likely to offer any benefit. Carpet treatments have been developed that include agents that kill the mites (acaricides) and denature the allergen (denaturing agents). Although laboratory studies indicate that both of these reagents are efficacious, field studies indicate that neither agent is very effective in practice.72,73
A recently published study found that mattress encasements, as a single intervention, provided no health benefit to adults with asthma.74 However, the study population included many people who would not be expected to benefit from the intervention, including people who were not sensitized to dust mite, as well as persons with other underlying lung disease, such as chronic obstructive pulmonary disease. The study provided little evidence to suggest that patients with asthma and dust mite sensitization would not benefit from a multi-pronged dust mite intervention. In fact, the evidence accumulated thus far suggests that concerted efforts to control dust mite exposure are only of clinical benefit in mite-sensitized persons with asthma and are a critical component of the treatment plan for these patients.
Cockroach Allergen
The 2 most common cockroaches found in United States homes are the German cockroach (Blatella germanica) and the American cockroach (Periplaneta americana). Cockroach allergen is the predominant allergen in the inner city, where most homes contain detectable levels.75 At least half of inner-city homes have a clinically relevant level of cockroach allergen. Although as many as 30% of suburban, middle class homes also contain detectable levels of cockroach allergen, the levels in suburban homes are much lower than levels in inner-city homes.76,77
In inner-city populations, 30-40% of children with asthma are sensitized to cockroach,78 and in suburban populations, the sensitization rate is about 21%.76 Cockroach allergen has also been directly linked to poorer asthma outcomes in inner-city children with asthma, including asthma-related health-care utilization.75,79
In light of these findings, it is important to assess risk of exposure in sensitized patients and to make recommendations for reducing cockroach allergen exposure. Although patient report of cockroach infestation is a good indicator of substantial cockroach allergen exposure,77 one cannot be sure that a patient who denies cockroach infestation is not exposed to substantial cockroach allergen. Patients may be reluctant to admit pest infestation, and homes without evidence of active infestation often contain substantial levels of the allergen.
Substantial reduction in cockroach allergen can be achieved with an integrated pest management approach. The 2007 NAEPP guidelines recommend such an approach, though they caution to avoid use of volatile chemical agents, which can be irritating when inhaled by persons with asthma.1 Several studies have demonstrated that a combination of extermination, vigorous cleaning aimed at reducing reservoir allergen, and meticulous care in disposing of food remains can reduce cockroach allergen by 80-90%.80,81 A more recent study found that an entomologistled integrated pest management intervention reduced cockroach allergen more than did the efforts of commercial pest management companies.82 It is unknown if this degree of allergen reduction will have a clinical impact. However, the strong evidence that cockroach allergen exposure is linked with asthma morbidity supports recommending an integrated pest management approach to reduce exposure in cockroach-sensitized patients with asthma.
Rodent Allergens
Mice and rats excrete urinary allergens that are carried on small particles that readily become airborne, like allergens from other furred animals such as cats and dogs.83-86 The allergens are pheromone-binding proteins that are thought to have a role in mating practices87 and are excreted in very large quantities in the urine. Although these allergens have long been known to cause occupational asthma, their role in nonoccupational asthma was only recently described.88,89
The domestic house mouse is very common, particularly in urban multi-family dwellings and poorly maintained housing.90 Mouse allergen can be found in virtually all inner-city homes, and one study found detectable airborne mouse allergen in 84% of bedrooms of inner-city children with asthma.91 The mouse allergen level in some homes is similar to that in occupational settings, where mouse allergen is a known cause of asthma symptoms.
Mouse allergen is surprisingly prevalent in suburban communities as well. As many as 75% of middle-class, suburban households have detectable mouse allergen in settled dust samples,92,93 though the levels in these suburban homes are 100-fold to 1,000-fold lower than in inner-city settings. Recent studies linked exposure to mouse allergen to poorer asthma control and higher risk of asthma-related health-care utilization among mouse-sensitized inner-city children with asthma.88,94
Assessing exposure to mouse allergen can be fairly straightforward. Any patient who reports mouse sightings or evidence of mice, such as droppings, is very likely to be exposed to substantial levels of mouse allergen in the home.90-92 However, as with cockroach allergen, patients and families may be reluctant to admit a rodent infestation, so a negative history is not a good predictor of lack of exposure. In addition, substantial levels of mouse allergen can be found in homes with little or no evidence of infestation, because mice nest in hidden spaces and are active at night when they are less likely to be seen.
Reducing exposure to mouse allergen is feasible, but it can be difficult. Integrated pest management is the best approach; it includes a combination of extermination, vigorous cleaning, meticulous disposal of food remains, and sealing of holes and cracks in walls, doors, and ceilings. Using this approach, the allergen source is eliminated, the allergen reservoirs are cleaned up, and re-infestation is discouraged.
In one study, integrated pest management reduced the mouse allergen level by 75% in settled dust, while the level increased in the control group.95 Although no studies have been conducted to determine the impact of mouse allergen reduction on asthma, the evidence to date indicates that reducing exposure should improve asthma control and prevent asthma-related morbidity. Noting this lack of clinical-trial evidence to date, the 2007 NAEPP guidelines offer an expert-opinion recommendation to use measures to reduce infestation, as part of a general recommendation about reducing exposure to relevant furred animal allergens.1
Rats are also common in urban areas, and although they typically do not venture indoors, rat allergen has been found in 33% of inner-city homes. In one multicenter study, 21% of inner-city children with asthma were sensitized to rat, and the children who were sensitized and exposed to rat allergen in their homes were at greater risk for asthma-related hospitalization and unscheduled medical visits than were children who were not sensitized or exposed.96 No published studies have examined methods of reducing household rat allergen, but a reasonable approach would include sealing holes and cracks in the home’s structure, vigorous cleaning to remove reservoir allergen, and extermination.
Mold Allergens
There is a very large number of mold species, and very few have been well studied with regard to their effects on asthma. Molds can be found in both indoor and outdoor environments. Aspergillus and Penicillium species are among the most common indoor molds, and Alternaria can be found in both indoor and outdoor environments.
Molds have been extremely difficult to study because of their complexity, and a full discussion of their impact on asthma is beyond the scope of this article. However, major allergens have been isolated from a few molds, and sensitization to Alternaria has been linked to the development of asthma, as well as to asthma severity in Midwestern and Southwestern United States populations.97,98 It is important to note, however, that Alternaria is found in all regions of the country.99 In a nationwide study, higher dustborne Alternaria allergen levels were associated with higher risk of wheeze.100
Because molds prefer warm, moist environments, mold growth can be decreased by interventions that reduce moisture and humidity, such as dehumidification, air conditioning, and increased ventilation. Humidifiers and vaporizers increase indoor humidity and can become colonized with mold, so they should not be used in homes of people with asthma. For mold that is already present, thorough cleaning with fungicides is recommended. If cleaning is not possible, the item should be disposed of.
Keeping windows closed and running the air conditioner may help reduce exposure to outdoor sources of mold. A HEPA room-air filter may also help, but this intervention has not been studied.
Water leaks can contribute to the growth of indoor molds, so it is sensible to prevent and eliminate water leaks wherever possible. When outdoors, patients should avoid heavy exposure to moldy vegetation and use a properly fitting particulate mask when working with moldy material. Although there have been no randomized trials of mold abatement in persons with asthma, there is adequate evidence to recommend these basic interventions to reduce exposure for mold-sensitized patients with asthma. Given the association of indoor fungi and respiratory symptoms in some studies, the 2007 NAEPP guidelines recommend considering measures to control indoor dampness and mold.1
Outdoor Environmental Factors
Air Pollution
There are several outdoor air pollutants that can affect asthma, including particulate matter, O3, NO2, and SO2 (Table 2). Except for SO2, the general sources and characteristics of these pollutants are the same as those described above for indoor air pollution. Additional relevant information is contained in this section, as it pertains to outdoor sources and the effects of these pollutants. Generally, individuals have little control over the outdoor environment, so the following recommendations center mostly on avoiding the outdoor environment on days when specific pollutant levels are high. Patients should be cautioned to avoid exertion on those days and to stay indoors, and, when feasible, in an air-conditioned environment.
Table 2.
Major Outdoor Environment Factors That Affect Asthma
| Pollutants |
| Particulate matter |
| Ozone |
| Nitrogen oxides |
| Sulfur dioxide |
| Allergens |
| Pollens |
| Trees |
| Grasses |
| Weeds |
| Molds |
Particulate Matter
Particulate matter exposure in outdoor air is linked to higher mortality in the general population.101 Those with underlying cardiopulmonary disease may be even more susceptible to the effects of inhaled particulate matter, as evidenced by increased hospitalizations after short-term increases in particulate-matter concentration.57 Among asthmatics, ambient particulate matter has been linked to exacerbations, chronic symptoms, and decline in lung function.49,82,83 Though most studies have focused on the fine-particulate-matter fraction, outdoor coarse particulate matter is associated with a greater risk of hospitalization for childhood asthma than outdoor fine particulate matter.77
Ozone
Ozone is a highly reactive gas that is generated at ground level by a chemical reaction of sunlight on mixtures of NO2 and hydrocarbons from fossil fuel combustion.102 Major sources include vehicular traffic, power plants, and industrial operations. Sunlight and hot weather create ground-level ozone in a harmful concentration in the air, and ozone is the primary constituent of “summertime smog.” Ozone can increase airway inflammation and hyperresponsiveness.103,104 Ozone exposure is associated with reduced lung function, increased symptoms, increased rescue medication use, and increased risk of asthma exacerbation.105,106 Among people with allergic asthma, ozone exposure may interact with allergens to cause a greater asthmatic response.107,108 The 2007 NAEPP guidelines recommend, as with other outdoor pollutants, that individuals should avoid exercise or exertion outdoors when the O3 level is high.1
Nitrogen Dioxide
NO2 is formed from primary emissions of oxides of nitrogen. Automobile exhaust is the main source of ambient NO2 in most urban environments. Other sources include local industry, power plants, and forest fires. There is growing evidence that elevated ambient NO2 is associated with increased asthma symptoms, exacerbations, and hospitalizations, and with lower lung function, particularly in vulnerable populations, including young children and the elderly.109-112 A reduction of traffic density in a geographic region was associated with reduced asthma morbidity,113,114 which may be partially mediated by the lowering of NO2 exposure.
Sulfur Dioxide
Sulfur dioxide (SO2) is an ambient air pollutant mainly formed by the combustion of high-sulfur coal or oil. Indoor SO2 sources are not common, and when present indoors SO2 is rapidly adsorbed onto household surfaces, so personal SO2 exposure is mainly due to ambient air pollution.115 Experimental studies suggest that SO2 can decrease lung function in exercising adults with asthma.116-118 The 2007 NAEPP guidelines recommend that patients with asthma avoid exercise outdoors on days when air pollutants, including SO2, are high.1
Pollen Allergens
Pollen and mold allergens are the predominant outdoor allergens. Mold allergens were addressed above, in the section on indoor allergen, so this section will focus on pollen allergens. Tree, grass, and weed pollen are present in all regions of the United States and cause seasonal asthma exacerbations (see Table 2). Tree pollen is produced primarily in the spring, though the levels peak at slightly different times, depending on the regional climate. Grass pollen peaks in the summer, and weed pollen peaks in the fall. Again, the peak of these seasons varies by region, so understanding the timing of pollen seasons in one’s region is helpful when evaluating a patient with seasonal asthma exacerbations.
Pollen allergen sensitization is common. For example, a population-based study found that more than 25% of the United States population is sensitized to ragweed, and a similar proportion is sensitized to grass pollen.119 The well-known phenomenon of pollen-induced asthma is characterized by a predictable increase in asthma symptoms during the relevant pollen season in a sensitized patient.
Unfortunately, it is very difficult to reduce pollen allergen exposure during the pollen season. Recommendations for exposure reduction include staying indoors, keeping windows and doors closed, using air conditioning, and perhaps HEPA room-air filters. After spending time outdoors, pollen-allergic patients should wash their hands and face, and should wash their hair daily to remove allergens. Many of these measures are also suggested by the 2007 NAEPP guidelines.1
Summary
Environmental control practices are a sensible, evidence-based component of the overall management of asthma. Control of the environment requires attention to exposures that originate from both the outdoor and indoor environments. The indoor environment notably contains particulate matter, NO2, secondhand smoke, and O3, and allergens from furred pets, dust mites, cockroach, rodents, and molds. The 2007 NAEPP guidelines recommend eliminating indoor smoking and improving ventilation. Though the guidelines state that there is insufficient evidence to recommend air cleaners, these devices, along with reducing activities that generate indoor pollutants, may be sound practical approaches to improving the health of individuals with asthma. The guidelines are more specific about allergen avoidance; they recommend identifying allergens to which an individual is immunoglobin E sensitized. Further, the guidelines emphasize multifaceted, comprehensive strategies to reduce exposure.
Outdoor air pollutants can impact asthma, and guidelines recommend that individuals with asthma avoid exertion outdoors when concentrations of certain pollutants are elevated. Outdoor allergens include tree, grass, and weed pollens, which vary in concentration by season. Recommendations to reduce exposure include staying indoors, keeping windows and doors closed, using air conditioning, and perhaps HEPA room-air filters, as well as thorough daily washing to remove allergens from one’s person.
Though there are still considerable research opportunities to identify additional means to improve the environment for the sake of asthma health, research to date has shown that careful attention to these specific environmental controls can improve the health of people with asthma.
Acknowledgments
This research was partly supported by National Institutes of Health grants R18 HL73833 and 1P50ES015903-01, and by Environmental Protection Agency grant R8267240.
Footnotes
The authors report no conflicts of interest related to the content of this paper.
Dr Diette presented a version of this paper at the 41st Respiratory Care Journal Conference, “Meeting the Challenges of Asthma,” held September 28-30, 2007, in Scottsdale, Arizona.
REFERENCES
- 1.Expert panel report 3: guidelines for the diagnosis and management of asthma. National Institutes of Health, National Asthma Education and Prevention Program; Bethesda MD: 2007. [Accessed February 12, 2008]. NIH Publication No. 08-4051. Available from http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. [Google Scholar]
- 2.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 3.Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, et al. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115(11):1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace LA, Mitchell H, O’Connor GT, Neas L, Lippmann M, Kattan M, Koenig J, et al. Particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111(9):1265–1272. doi: 10.1289/ehp.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106(2):148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease. Circulation. 2004;109(21):2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 7.Delfino RJ, Quintana PJ, Floro J, Gastañaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perpspect. 2004;112(8):932–941. doi: 10.1289/ehp.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig JQ, Mar TF, Allen RW, Jansen K, Lumley T, Sullivan JH, et al. Pulmonary effects of indoor- and outdoor-generated particles in children with asthma. Environ Health Perpspect. 2005;113(4):499–503. doi: 10.1289/ehp.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCormack MC, Hansel NN, Matsui E, Breysse P, Tonorezos E, Diette GB. Domestic particulate matter exposure and asthma morbidity in inner-city pre-school children (abstract); American Thoracic Society International Conference; San Francisco, California. May 18-23, 2007.p. A272. [Google Scholar]
- 10.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 11.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 12.US Census Bureau 2000 census of population and housing. [Accessed February 20, 2008];Summary File 3; Table H40: House Heating Fuel. http://www.census.gov/prod/cen2000/doc/sf3.pdf.
- 13.Garrett MH, Hooper MA, Hooper BM, Abramson MJ. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Am J Respir Crit Care Med. 1998;158(3):891–895. doi: 10.1164/ajrccm.158.3.9701084. [DOI] [PubMed] [Google Scholar]
- 14.Hasselblad V, Eddy DM, Kotchmar DJ. Synthesis of environmental evidence: nitrogen dioxide epidemiology studies. J Air Waste Manage Assoc. 1992;42(5):662–671. doi: 10.1080/10473289.1992.10467018. [DOI] [PubMed] [Google Scholar]
- 15.Shima M, Adachi M. Effect of outdoor and indoor nitrogen dioxide on respiratory symptoms in schoolchildren. Int J Epidemiol. 2000;29(5):862–870. doi: 10.1093/ije/29.5.862. [DOI] [PubMed] [Google Scholar]
- 16.Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am J Respir Crit Care Med. 2006;173(3):297–303. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitschke M, Pilotto LS, Attewell RG, Smith BJ, Pisaniello D, Martin J, et al. A cohort study of indoor nitrogen dioxide and house dust mite exposure in asthmatic children. J Occup Environ Med. 2006;48(5):462–469. doi: 10.1097/01.jom.0000215802.43229.62. [DOI] [PubMed] [Google Scholar]
- 18.Smith BJ, Nitschke M, Pilotto LS, Ruggin RE, Pisaniello DL, Willson KJ. Health effects of daily indoor nitrogen dioxide exposure in people with asthma. Eur Respir J. 2000;16(5):879–885. doi: 10.1183/09031936.00.16587900. [DOI] [PubMed] [Google Scholar]
- 19.Florey CV, Melia RJ, Chinn S, Goldstein BD, Brooks AG, John HH, et al. The relation between respiratory illness in primary schoolchildren and the use of gas for cooking. Nitrogen dioxide, respiratory illness and lung inflection. Int J Epidemiol. 1979;8(4):347–353. doi: 10.1093/ije/8.4.347. [DOI] [PubMed] [Google Scholar]
- 20.Hoek G, Brunekreef B, Meijer R, Scholten A, Boleij J. Indoor nitrogen dioxide pollution and respiratory symptoms of schoolchildren. Int Arch Occup Environ Health. 1984;55(1):79–86. doi: 10.1007/BF00378070. [DOI] [PubMed] [Google Scholar]
- 21.Samet JM, Lambert WE, Skipper BJ, Cushing AH, Hunt WC, Young SA, et al. Nitrogen dioxide and respiratory illnesses in infants. Am Rev Respir Dis. 1993;148(5):1258–1265. doi: 10.1164/ajrccm/148.5.1258. [DOI] [PubMed] [Google Scholar]
- 22.Sunyer J, Puig C, Torrent M, Garcia-Algar O, Calico I, Munoz-Ortiz L, et al. Nitrogen dioxide is not associated with respiratory infection during the first year of life. Int J Epidemiol. 2004;33(1):116–120. doi: 10.1093/ije/dyh037. [DOI] [PubMed] [Google Scholar]
- 23.Eisner MD, Blanc PD. Gas stove use and respiratory health among adults with asthma in NHANES III. Occup Environ Med. 2003;60(10):759–764. doi: 10.1136/oem.60.10.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansel NN, McCormack MC, Breysse PN, Matsui EC, Curtin-Brosnan J, Rusher R, Williams D, Diette GB. Indoor NO2 concentrations are associated with increased asthma morbidity in preschool inner city children. Am J Respir Crit Care Med. 2008 in press. [Google Scholar]
- 25.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immunol. 2007;120(3):618–624. doi: 10.1016/j.jaci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Jakab GJ. Modulation of pulmonary defense mechanisms by acute exposures to nitrogen dioxide. Environ Res. 1987;42(1):215–228. doi: 10.1016/s0013-9351(87)80023-3. [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich R. Effect of nitrogen dioxide on resistance to respiratory infection. Bacteriol Rev. 1966;30(3):604–614. doi: 10.1128/br.30.3.604-614.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frampton MW, Smeglin AM, Roberts NJ, Jr, Finkelstein JN, Morrow PE, Utell MJ. Nitrogen dioxide exposure in vivo and human alveolar macrophage inactivation of influenza virus in vitro. Environ Res. 1989;48(2):179–192. doi: 10.1016/s0013-9351(89)80033-7. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, Holgate ST. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet. 2003;361(9373):1939–1944. doi: 10.1016/S0140-6736(03)13582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilotto LS, Nitschke M, Smith BJ, Pisaniello D, Ruffin RE, McElroy HJ, Martin J, Hiller JE. Randomized controlled trial of unflued gas heater replacement on respiratory health of asthmatic schoolchildren. Int J Epidemiol. 2004;33(1):208–214. doi: 10.1093/ije/dyh018. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services . The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon General. Office on Smoking and Health; Atlanta GA: 2006. [Google Scholar]
- 32.Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics. 1992;89(1):21–26. [PubMed] [Google Scholar]
- 33.Evans D, Levison MJ, Feldman CH, Clark NM, Wasilewski Y, Levin B, Mellins RB. The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis. 1987;135(3):567–572. doi: 10.1164/arrd.1987.135.3.567. [DOI] [PubMed] [Google Scholar]
- 34.LeSon S, Gershwin ME. Risk factors for asthmatic patients requiring intubation. I. Observations in children. J Asthma. 1995;32(4):285–294. doi: 10.3109/02770909509044836. [DOI] [PubMed] [Google Scholar]
- 35.Cook DG, Strachan DP. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax. 1997;52(12):1081–1094. doi: 10.1136/thx.52.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morkjaroenpong V, Rand CS, Butz AM, Huss K, Eggleston P, Malveaux FJ, Bartlett SJ. Environmental tobacco smoke exposure and nocturnal symptoms among inner-city children with asthma. J Allergy Clin Immunol. 2002;110(1):147–153. doi: 10.1067/mai.2002.125832. [DOI] [PubMed] [Google Scholar]
- 37.Feleszko W, Zawadzka-Krajewska A, Matysiak K, Lewandowska D, Peradzyńska J, Dinh QT, et al. Parental tobacco smoking is associated with augmented IL-13 secretion in children with allergic asthma. J Allergy Clin Immunol. 2006;117(1):97–102. doi: 10.1016/j.jaci.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Wilson SR, Yamada EG, Sudhakar R, Roberto L, Mannino D, Mejia C, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120(5):1709–1722. doi: 10.1378/chest.120.5.1709. [DOI] [PubMed] [Google Scholar]
- 39.Klerman L. Protecting children: reducing their environmental tobacco smoke exposure. Nicotine Tob Res. 2004;6(Suppl 2):S239–S253. doi: 10.1080/14622200410001669213. [DOI] [PubMed] [Google Scholar]
- 40.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, et al. Indoor exposures to air pollutants and allergents in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98(2):167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Medicine Committee on the Assessment of Asthma and Indoor Air . Clearing the air: asthma and indoor air exposures. National Academy Press; Washington, DC: 2000. [Google Scholar]
- 42.Kim JJ. American Academy of Pediatrics Committee on Environmental Health. Ambient air pollution: health hazards to children. Pediatrics. 2004;114(6):1699–1707. doi: 10.1542/peds.2004-2166. [DOI] [PubMed] [Google Scholar]
- 43.Mortimer KM, Neas LM, Dockery DW, Redline S, Tager IB. The effect of air pollution on inner-city children with asthma. Eur Respir J. 2002;19(4):699–705. doi: 10.1183/09031936.02.00247102. [DOI] [PubMed] [Google Scholar]
- 44.Graham DE, Koren HS. Biomarkers of inflammation in ozone-exposed humans. Comparison of the nasal and bronchoalveolar lavage. Am Rev Respir Dis. 1990;142(1):152–156. doi: 10.1164/ajrccm/142.1.152. [DOI] [PubMed] [Google Scholar]
- 45.Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. Effect of short-term exposure to gaseous pollution on asthma hospitalisation in children: a bi-directional case-crossover analysis. J Epidemiol Community Health. 2003;57(1):50–55. doi: 10.1136/jech.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross MA, Persky VW, Scheff PA, Chung J, Curtis L, Ramakrishnan V, et al. Effect of ozone and aeroallergens on the respiratory health of asthmatics. Arch Environ Health. 2002;57(6):568–578. doi: 10.1080/00039890209602090. [DOI] [PubMed] [Google Scholar]
- 47.Gold DR, Allen G, Damokosh A, Serrano P, Hayes C, Castillejos M. Comparison of outdoor and classroom ozone exposures for school children in Mexico City. J Air Waste Manag Assoc. 1996;46(4):335–342. [PubMed] [Google Scholar]
- 48.Wood RA, Laheri AN, Eggleston PA. The aerodynamic characteristics of cat allergen. Clin Exp Allergy. 1993;23(9):733–739. doi: 10.1111/j.1365-2222.1993.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 49.Platts-Mills TA, Heymann PW, Longbottom JL, Wilkins SR. Airborne allergens associated with asthma: particle sizes carrying dust mite and rat allergens measured with a cascade impactor. J Allergy Clin Immunol. 1986;77(6):850–857. doi: 10.1016/0091-6749(86)90383-0. [DOI] [PubMed] [Google Scholar]
- 50.Bollinger ME, Eggleston PA, Flanagan E, Wood RA. Cat antigen in homes with and without cats may induce allergic symptoms. J Allergy Clin Immunol. 1996;97(4):907–914. doi: 10.1016/s0091-6749(96)80064-9. [DOI] [PubMed] [Google Scholar]
- 51.Patchett K, Lewis S, Crane J, Fitzharris P. Cat allergent (Fel d 1) levels on school children’s clothing and in primary school classrooms in Wellington, New Zealand. J Allergy Clin Immunol. 1997;100(6 Pt 1):755–759. doi: 10.1016/s0091-6749(97)70269-0. [DOI] [PubMed] [Google Scholar]
- 52.Almqvist C, Wickman M, Perfetti L, Berglind N, Renström A, Hedrén M, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. Am J Respir Crit Care Med. 2001;163(3 Pt 1):694–698. doi: 10.1164/ajrccm.163.3.2006114. [DOI] [PubMed] [Google Scholar]
- 53.Wood RA, Mudd KE, Eggleston PA. The distribution of cat and dust mite allergens on wall surfaces. J Allergy Clin Immunol. 1992;89(1 Pt 1):126–130. doi: 10.1016/s0091-6749(05)80049-1. [DOI] [PubMed] [Google Scholar]
- 54.Custovic A, Simpson B, Simpson A, Hallam C, Craven M, Woodcock A. Relationship between mite, cat, and dog allergens in reservoir dust and ambient air. Allergy. 1999;54(6):612–616. doi: 10.1034/j.1398-9995.1999.00062.x. [DOI] [PubMed] [Google Scholar]
- 55.Munir AK, Einarsson R, Dreborg S. Variability of airborne cat allergen, Fel d1, in a public place. Indoor Air. 2003;13(4):353–358. doi: 10.1111/j.1600-0668.2003.00181.x. [DOI] [PubMed] [Google Scholar]
- 56.Munir AK, Björkstén B, Einarsson R, Schou C, Ekstrand-Tobin A, Warner A, Kjellman NI. Cat(Fel d I), dog(Can f I), and cockroach allergens in homes of asthmatic children from three climatic zones in Sweden. Allergy. 1994;49(7):508–516. doi: 10.1111/j.1398-9995.1994.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 57.Sporik R, Ingram JM, Price W, Sussman JH, HOnsinger RW, Platts-Mills TA. Association of asthma with serum IgE and skin test reactivity to allergens among children living at high altitude. Tickling the dragon’s breath. Am J Respir Crit Care Med. 1995;151(5):1388–1392. doi: 10.1164/ajrccm.151.5.7735590. [DOI] [PubMed] [Google Scholar]
- 58.Lewis SA, Weiss ST, Platts-Mills TA, Burge H, Gold DR. The role of indoor allergen sensitization and exposure in causing morbidity in women with asthma. Am J Respir Crit Care Med. 2002;165(7):961–966. doi: 10.1164/ajrccm.165.7.2103044. [DOI] [PubMed] [Google Scholar]
- 59.Wood RA, Charpman MD, Adkinson NF, Jr, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. J Allergy Clin Immunol. 1989;83(4):730–734. doi: 10.1016/0091-6749(89)90006-7. [DOI] [PubMed] [Google Scholar]
- 60.Gore RB, Bishop S, Durrell B, Curbishley L, Woodcock A, Custovic A. Air filtration units in homes with cats: can they reduce personal exposure to cat allergen? Clin Exp Allergy. 2003;33(6):765–769. doi: 10.1046/j.1365-2222.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- 61.Nageotte C, Park M, Havstad S, Zoratti E, Ownby D. Duration of airborne Fel d 1 reduction after cat washing. J Allergy Clin Immunol. 2006;118(2):521–522. doi: 10.1016/j.jaci.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 62.Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet allergic asthma. Chest. 2005;127(5):1565–1571. doi: 10.1378/chest.127.5.1565. [DOI] [PubMed] [Google Scholar]
- 63.Arlian LG. Dust mites: update on their allergens and control. Curr Allergy Asthma Rep. 2001;1(6):581–586. doi: 10.1007/s11882-001-0069-4. [DOI] [PubMed] [Google Scholar]
- 64.Platts-Mills TA, Thomas WR, Aalberse RC, Vervloeet D, Chapman MD. Dust mite allergens and asthma: report of a second international workshop. J Allergy Clin Immunol. 1992;89(5):1046–1060. doi: 10.1016/0091-6749(92)90228-t. [DOI] [PubMed] [Google Scholar]
- 65.Call RS, Smith TF, Morris E, Chapman MD, Platts-Mills TA. Risk factors for asthma in inner city children. J Pediatr. 1992;121(6):862–866. doi: 10.1016/s0022-3476(05)80329-4. [DOI] [PubMed] [Google Scholar]
- 66.Ingram JM, Sporik R, Rose G, Honsinger R, Chapman MD, Platts-Mills TA. Quantitative assessment of exposure to dog (Can f 1) and cat (Fel d 1) allergens: relation to sensitization and asthma among children living in Los Alamos, New Mexico. J Allergy Clin Immunol. 1995;9(4):449–456. doi: 10.1016/s0091-6749(95)70286-5. 6. [DOI] [PubMed] [Google Scholar]
- 67.Ehnert B, Lau-Schadendorf S, Weber A, Buettner P, Schou C, Wahn U. Reducing domestic exposure to dust mite allergen reduces bronchial hyperreactivity in sensitive children with asthma. J Allergy Clin Immunol. 1992;90(1):135–138. doi: 10.1016/s0091-6749(06)80024-2. [DOI] [PubMed] [Google Scholar]
- 68.Custovic A, Taggart SC, Francis HC, Chapman MD, Woodcock A. Exposure to house dust mite allergens and the clinical activity of asthma. J Allergy Clin Immunol. 1996;98(1):64–72. doi: 10.1016/s0091-6749(96)70227-0. [DOI] [PubMed] [Google Scholar]
- 69.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323(8):502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 70.Carswell F, Birmingham K, Oliver J, Crewes A, Weeks J. The respiratory effects of reduction of mite allergen in the bedrooms of asthmatic children: a double-blind controlled trial. Clin Exp Allergy. 1996;26(4):386–396. [PubMed] [Google Scholar]
- 71.Halken S, Høst A, Niklassen U, Hansen LG, Nielsen F, Pedersen S, et al. Effect of mattress and pillow encasings on children with asthma and house dust mite allergy. J Allergy Clin Immunol. 2003;111(1):169–176. doi: 10.1067/mai.2003.5. [DOI] [PubMed] [Google Scholar]
- 72.Woodfolk JA, Hayden ML, Couture N, Platts-Mills TA. Chemical treatment of carpets to reduce allergen: comparison of the effects of tannic acid and other treatments on proteins derived from dust mites and cats. J Allergy Clin Immunol. 1995;96(3):325–333. doi: 10.1016/s0091-6749(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 73.Hayden ML, Rose G, Diduch KB, Domson P, Chapman MD, Heymann PW, Platts-Mills TA. Benzyl benzoate moist powder: investigation of acaricidal activity in cultures and reduction of dust mite allergens in carpets. J Allergy Clin Immunol. 1992;89(2):536–545. doi: 10.1016/0091-6749(92)90320-2. [DOI] [PubMed] [Google Scholar]
- 74.Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med. 2003;349(3):225–236. doi: 10.1056/NEJMoa023175. [DOI] [PubMed] [Google Scholar]
- 75.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 76.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Curtin-Brosnan J, Eggleston PA. Cockroach allergen exposure and sensitization in suburban middle-class children with asthma. J Allergy Clin Immunol. 2003;112(1):87–92. doi: 10.1067/mai.2003.1588. [DOI] [PubMed] [Google Scholar]
- 77.Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in US households. Environ Health Perspect. 2006;114(4):522–526. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, et al. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J Allergy Clin Immunol. 1998;102(4 Pt 1):563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- 79.Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115(3):478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Eggleston PA, Wood RA, Rand C, Nixon WJ, Chen PH, Lukk P. Removal of cockroach allergen from inner-city homes. J Allergy Clin Immunol. 1999;104(4 Pt 1):842–846. doi: 10.1016/s0091-6749(99)70296-4. [DOI] [PubMed] [Google Scholar]
- 81.Arbes SJ, Jr, Sever M, Mehta J, Gore JC, Schal C, Vaughn B, et al. Abatement of cockroach allergens (Bla g 1 and Bla g 2) in low-income, urban housing: month 12 continuation results. J Allergy Clin Immunol. 2004;113(1):109–114. doi: 10.1016/j.jaci.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 82.Sever ML, Arbes SJ, Jr, Gore JC, Santangelo RG, Vaughn B, Mitchell H, et al. Cockroach allergen reduction by cockroach control alone in low-income urban homes: a randomized control trial. J Allergy Clin Immunol. 2007;120(4):849–855. doi: 10.1016/j.jaci.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schumacher MJ, Tait BD, Holmes MC. Allergy to murine antigens in a biological research institute. J Allergy Clin Immunol. 1981;68(4):310–318. doi: 10.1016/0091-6749(81)90157-3. [DOI] [PubMed] [Google Scholar]
- 84.Platts-Mills TA, Longbottom J, Edwards J, Cockroft A, Wilkins S. Occupational asthma and rhinitis related to laboratory rats: serum IgG and IgE antibodies to the rat urinary allergen. J Allergy Clin Immunol. 1987;79(3):505–515. doi: 10.1016/0091-6749(87)90369-1. [DOI] [PubMed] [Google Scholar]
- 85.Ohman JL, Jr, Hagberg K, MacDonald MR, Jones RR, Jr, Paigen BJ, Kacergis JB. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94(5):810–817. doi: 10.1016/0091-6749(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 86.Eggleston PA, Ansari AA, Ziemann B, Adkinson NF, Jr, Corn M. Occupational challenge studies with laboratory workers allergic to rats. J Allergy Clin Immunol. 1990;86(1):63–72. doi: 10.1016/s0091-6749(05)80124-1. [DOI] [PubMed] [Google Scholar]
- 87.Sharrow SD, Vaughn JL, Zídek L, Novotny MV, Stone MJ. Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci. 2002;11(9):2247–2256. doi: 10.1110/ps.0204202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, Diette GB. Household mouse allergen exposure and asthma morbidity in inner-city pre-school children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 89.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. National Cooperative Inner-City Asthma Study. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106(6):1075–1080. [Google Scholar]
- 90.Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111(10):1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsui EC, Simons E, Rand C, Butz A, Buckley TJ, Breysse P, Eggleston PA. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115(2):358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Eggleston PA. Mouse allergen exposure and mouse skin test sensitivity in suburban, middle-class children with asthma. J Allergy Clin Immunol. 2004;113(5):910–915. doi: 10.1016/j.jaci.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 93.Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. J Allergy Clin Immunol. 2004;113(6):1167–1171. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- 94.Phipatanakul W, Celédon JC, Sredl DL, Weiss ST, Gold DR. Mouse exposure and wheeze in the first year of life. Ann Allergy Asthma Immunol. 2005;94(5):593–599. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phipatanakul W, Cronin B, Wood RA, Eggleston PA, Shih M, Song L, et al. Effect of environmental intervention on mouse allergent levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92(4):420–425. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perry T, Matsui E, Merriman B, Duong T, Eggleston PA. The prevalence of rat allergen in inner-city homes and its relationship to sensitization and asthma morbidity. J Allergy Clin Immunol. 2003;112(2):346–352. doi: 10.1067/mai.2003.1640. [DOI] [PubMed] [Google Scholar]
- 97.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113(2):227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 98.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med. 1997;155(4):1356–1361. doi: 10.1164/ajrccm.155.4.9105079. [DOI] [PubMed] [Google Scholar]
- 99.Salo PM, Yin M, Arbes SJ, Jr, Cohn RD, Sever M, Muilenberg M, et al. Dustborne Alternaria alternata antigens in US homes: results from the National Survey of Lead and Allergens in Housing. J Allergy Clin Immunol. 2005;116(3):623–629. doi: 10.1016/j.jaci.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salo PM, Arbes SJ, Jr, Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118(4):892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 US cities, 1987-1994. N Engl J Med. 2000;343(24):1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 102.US Environmental Protection Agency [Accessed February 21, 2008];Ground-level ozone. Last updated January 31, 2007. http://www.epa.gov/air/ozonepollution/index.html.
- 103.Kehrl HR, Peden DB, Ball B, Folinsbee LJ, Horstman D. Increased specific airway reactivity of persons with mild allergic asthma after 7.6 hours of exposure to 0.16 ppm ozone. J Allergy Clin Immunol. 1999;104(6):1198–1204. doi: 10.1016/s0091-6749(99)70013-8. [DOI] [PubMed] [Google Scholar]
- 104.Bayram H, Sapsford RJ, Abdelaziz MM, Khair OA. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J Allergy Clin Immunol. 2001;107(2):287–294. doi: 10.1067/mai.2001.111141. [DOI] [PubMed] [Google Scholar]
- 105.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, et al. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA. 2003;290(14):1859–1867. doi: 10.1001/jama.290.14.1859. [DOI] [PubMed] [Google Scholar]
- 106.Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol. 2005;115(4):689–699. doi: 10.1016/j.jaci.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 107.Peden DB, Setzer RW, Jr, Devlin RB. Ozone exposure has both a priming effect on allergen-induced responses and an intrinsic inflammatory action in the nasal airways of perennially allergic asthmatics. Am J Respir Crit Care Med. 1995;151(5):1336–1345. doi: 10.1164/ajrccm.151.5.7735583. [DOI] [PubMed] [Google Scholar]
- 108.Molfino NA, Slutsky AS, Zamel N. The effects of air pollution on allergic bronchial responsiveness. Clin Exp Allergy. 1992;22(7):667–672. doi: 10.1111/j.1365-2222.1992.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 109.Villeneuve PJ, Chen L, Rowe BH, Coates F. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. Environ Health. 2007;6(1):40. doi: 10.1186/1476-069X-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peel JL, Tolbert PE, Klein M, Metzger KB, Flanders WD, Todd K, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology. 2005;16(2):164–174. doi: 10.1097/01.ede.0000152905.42113.db. [DOI] [PubMed] [Google Scholar]
- 111.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164(6):505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 112.Nitschke M, Smith BJ, Pilotto LS, Pisaniello D, Abramson MJ, Ruffin RE. Respiratory health effects of nitrogen dioxide exposure and current guidelines. Int J Environ Health Res. 1999;9:39–53. [Google Scholar]
- 113.Friedman MS, Powell KE, Hutwagner L, Graham LM, Teague WG. Impact of changes in transportation and commuting behaviors during the 1996 Summer Olympic Games in Atlanta on air quality and childhood asthma. JAMA. 2001;285(7):897–905. doi: 10.1001/jama.285.7.897. [DOI] [PubMed] [Google Scholar]
- 114.Lee JT, Son JY, Cho YS. Benefits of mitigated ambient air quality due to transportation control on childhood asthma hospitalization during the 2002 summer Asian games in Busan, Korea. J Air Waste Manag Assoc. 2007;57(8):968–973. doi: 10.3155/1047-3289.57.8.968. [DOI] [PubMed] [Google Scholar]
- 115.Lee K, Bartell SM, Paek D. Interpersonal and daily variability of personal exposures to nitrogen dioxide and sulfur dioxide. J Expo Anal Environ Epidemiol. 2004;14(2):137–143. doi: 10.1038/sj.jea.7500304. [DOI] [PubMed] [Google Scholar]
- 116.Linn WS, Venet TG, Shamoo DA, Valencia LM, Anzar UT, Spier CE, et al. Respiratory effects of sulfur dioxide in heavily exercising asthmatics. A dose-response study. Am Rev Respir Dis. 1983;127(3):278–283. doi: 10.1164/arrd.1983.127.3.278. [DOI] [PubMed] [Google Scholar]
- 117.Roger LJ, Kehrl HR, Hazucha M, Horstman DH. Bronchoconstriction in asthmatics exposed to sulfur dioxide during repeated exercise. J Appl Physiol. 1985;59(3):784–791. doi: 10.1152/jappl.1985.59.3.784. [DOI] [PubMed] [Google Scholar]
- 118.Schachter EN, Witek TJ, Jr, Beck GJ, Hosein HR, Colice G, Leaderer BP, et al. Airway effects of low concentrations of sulfur dioxide: dose-response characteristics. Arch Environ Health. 1984;39(1):34–42. doi: 10.1080/00039896.1984.10545831. [DOI] [PubMed] [Google Scholar]
- 119.Arbes SJ, Jr, Gergen PJ, Elliot L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]