Abstract
Objective
In addition to being prevalent and bothersome, urologic and sexual symptoms may be related to chronic medical illnesses. We investigate the relationship between ten urologic and sexual symptoms and four major illnesses (type II diabetes, cardiac disease, hypertension, and depression).
Methods
We analyze data from the Boston Area Community Health (BACH) Survey, a community-based epidemiologic study of urologic symptoms and risk factors. BACH used a two-stage stratified cluster sampling design to recruit 5,506 adults aged 30–79 (2,301 men, 3,205 women; 1,770 Black, 1,877 Hispanic, and 1,859 White respondents).
Results
In bivariate analyses, most urologic and sexual symptoms are associated with type II diabetes, cardiac disease, hypertension, and depression. However, in multivariate models adjusting for all four illnesses, gender, race/ethnicity, age, alcohol intake, smoking, physical activity, and body mass index there were fewer significant associations. We found that all urologic symptoms were significantly related to at least one illness, with depression increasing the odds of all urologic and sexual symptoms studied.
Conclusions
Urinary tract specialists may need to give greater weight to consideration of factors outside the urinary tract that may be contributing to urologic symptoms. It remains unknown whether treatment of medical and psychological illnesses can result in meaningful improvement in urologic symptoms, or conversely, whether urinary tract symptoms can provide valuable insight into an individual’s overall health status.
Keywords: epidemiology, diabetes, hypertension, cardiac disease, depression, lower urinary tract symptoms, painful bladder syndrome, urinary incontinence, prostatitis, overactive bladder, frequency, urgency, nocturia, erectile dysfunction, female sexual dysfunction
1. Introduction
Decades of clinical practice and scientific enquiry suggest that abnormalities of the lower urinary tract and prostate can give rise to urologic symptoms that include lower urinary tract symptoms and sexual dysfunction in men and women. Urinary symptoms are also known to be influenced by many medical and lifestyle factors. For example, even patients with an entirely normal lower urinary tract may experience urinary frequency and nocturia after taking a diuretic medication, and medical illnesses that are linked with polyuria can be expected to give rise to some level of lower urinary tract symptoms or to exacerbate existing symptoms [1, 2].
Less obvious, but perhaps of clinical importance, are possible links between urologic symptoms and disorders that affect sympathetic/parasympathetic balance, endothelial pathways, or metabolic pathways involved in signal transduction and neurotransmission [3]. Such a relationship is exemplified by the suggested link between erectile dysfunction (ED) and cardiovascular disease [4–6], and by the recently highlighted relationship between ED and lower urinary tract symptoms [7, 8]. ED is increasingly considered a sentinel symptom of cardiovascular risk in men [9]. Though the results of several studies are somewhat conflicting, there is some evidence that links lower urinary tract symptoms to chronic medical illnesses [10, 11]. Broadly, the topic of whether there are also links between common urologic symptoms and medical and psychological illness remains understudied. Our objective is to determine whether there is an association between urologic and sexual symptoms and medical and psychological illness in a large, cross-sectional study of American adults. We want to contrast the association with organic and psychological illness.
2. Methods
The Boston Area Community Health (BACH) survey is a community-based epidemiologic survey of a broad range of urologic symptoms and risk factors conducted from 2002–2005. BACH uses a cross-sectional random sample, not a convenience sample. Detailed methods are given in a separate paper [12]. In brief, BACH used a two-stage stratified cluster sample design to recruit 5506 adults aged 30–79 in three racial/ethnic groups from the city of Boston (2301 men, 3205 women, 1770 Black, 1877 Hispanic, 1859 White respondents). Information about urologic symptoms, co-morbidities, lifestyle, anthropometrics, and psycho-social attributes were collected via an interviewer-administered questionnaire, while respondents used a self-administered questionnaire to answer questions about sexual function and abuse. All protocols and informed consent procedures were approved by New England Research Institutes’ Institutional review Board. Signed informed consent was obtained prior to commencing the interview.
2.1 Urologic and Sexual Symptoms
Using validated instruments in widespread use, we obtained information on ten urologic or sexual symptoms. Lower urinary tract symptoms (LUTS) was defined by an American Urological Association Symptom Index score of eight or greater [13]. This symptom scale is identical to the International Prostate Symptom Score (IPSS) (without the quality of life question)[14]. Painful bladder syndrome (PBS) was considered to be present if the respondent had its cardinal symptoms [15] of pain increasing as the bladder fills and/or pain relieved by urination (fairly often, usually, almost always) lasting for at least three months. Urinary incontinence (UI) was said to be present if the respondent reported involuntary loss of urine at least weekly [16]. Prostatitis was said to be present if the respondent reported perineal and/or ejaculatory pain and had a chronic prostatitis symptom index (CPSI) score of four or greater [17, 18]. Frequency was considered to be present if respondents reported urinating more frequently than every two hours or had frequent urination during the day (fairly often, usually, almost always), or those who reported urinating eight or more times per day. Urgency was considered to be present if respondents reported having difficulty postponing urination or had a strong urge to urinate (fairly often, usually, almost always) in the last month, or those who experienced a strong urge to urinate in the last seven days (four or more times). Nocturia was considered to be present if the respondent got up to urinate more than once at night (fairly often, usually, almost always) or reported two or more urinations at night after falling asleep. Note that one of the two or three symptoms for frequency, urgency, and nocturia are contained in the AUA Symptom Index/IPSS. The source(s) of the other questions are given in [19]. We have found that the different questions for frequency, urgency, and nocturia may not give similar responses, therefore we have constructed composite measures which do not necessarily match the International Continence Society definitions [20]. Overactive bladder (OAB) was considered to be present if a respondent had frequency and urgency as defined above [21]. OAB was further characterized as OAB-wet if the respondent also reported leaking urine when they had the strong feeling of needing to empty the bladder, but couldn’t get to the toilet soon enough, or as OAB-dry otherwise. No urodynamic testing was performed. Erectile dysfunction (ED) was considered to be present if the respondent scored 16 or below on the abridged International Index of Erectile Function (IIEF-5) [22]. Female sexual dysfunction (FSD) was considered to be present if the respondent scored 26.2 or less on a modified Female Sexual Function Index scale [23]. For FSD, we only considered women reporting sexual activity with a partner during the prior month as many of the questions were not asked of sexually inactive women.
2.2 Illnesses
There were four co-morbid illnesses considered. Respondents were asked if they had ever been told by a health care provider that they have or had the following medical conditions: 1) type II diabetes, 2) cardiac disease (including myocardial infarction, angina, congestive heart failure, coronary artery bypass or angioplasty stent), or 3) hypertension. A fourth psychological condition (depression) was also considered: respondents reporting five or more depressive symptoms based on the abbreviated eight question Center for Epidemiologic Studies-Depression (CES-D) scale [24] were considered to be depressed.
Additional covariates considered included age (30–39, 40–49, 50–59, 60–69, 70–79), race/ethnicity (Black, Hispanic, White), gender (men, women), alcohol consumption (0, <1, 1–3, 3+ drinks per day), smoking (never, current, previous), physical activity as measured by the Physical Activity Scale for the Elderly (PASE) [25] divided into three categories: <100, 100–250, 250+ with the 250+ category indicating the highest level of activity and body mass index (BMI) calculated as measured weight in kilograms divided by measured height in meters squared categorized as <25, 25–29, 30+ kg/m2.
To examine the relationships between urologic symptoms and co-morbid illnesses/conditions, chi-square tests for the association between the two were performed overall and by gender. To assess the joint effect of these conditions we fit multiple logistic regression models overall and by gender including the four illnesses, as well as age, (gender), race/ethnicity, alcohol consumption, smoking, physical activity, and body mass index. Missing values were replaced by plausible values using multiple imputation [26]. To be representative of the city of Boston, observations were weighted inversely proportional to their probability of selection [27]. Weights were further post-stratified to the Boston population according to the 2000 census. Analyses were conducted in version 9.1 of SAS (SAS Institute, Cary, NC, USA) and version 9.0.1 of SUDAAN (Research Triangle Institute, Research Triangle Park, NC, USA).
3. Results
In total, 5506 people (2301 men, 3205 women, 1770 Black, 1877 Hispanic, 1859 White respondents) completed the BACH survey. Table 1 details demographic characteristics of the analytic sample and prevalences of the co-morbidities, covariates, and urologic conditions overall and by gender. Figure 1 summarizes the bivariate associations of the urologic and sexual symptoms with the four illnesses. It is clear that most symptoms are significantly more prevalent in the presence of any of the illnesses than when the conditions are not present. For example, 35.4 percent of respondents with Type II diabetes also have LUTS, whereas only 17.2 percent of respondents without Type II diabetes also have LUTS (p<.0001). We note that all urologic and sexual symptoms studied are significantly associated with depression, while some other associations such as PBS and prostatitis with hypertension are non-existent. The relative strength of each association can be noted, such as the effect of cardiac disease is greater than the effect of depression on the prevalence of ED.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF THE ANALYSIS SAMPLE AND PREVALENCES OF COVARIATES, ILLNESSES, UROLOGIC AND SEXUAL SYMPTOMS BY GENDER. Figures represent weighted percents.
| Overall | Men | Women | |
|---|---|---|---|
| Race/Ethnicity | |||
| Black | 27.6 | 25.0 | 29.9 |
| Hispanic | 13.2 | 13.0 | 13.3 |
| White | 59.2 | 61.9 | 56.8 |
| Age | |||
| 30–39 | 35.2 | 37.2 | 33.5 |
| 40–49 | 25.1 | 25.8 | 24.4 |
| 50–59 | 18.1 | 17.8 | 18.4 |
| 60–69 | 13.3 | 11.3 | 15.1 |
| 70–79 | 8.2 | 7.8 | 8.6 |
| Type II Diabetes | 7.9 | 8.0 | 7.8 |
| Cardiac disease | 9.0 | 10.2 | 7.9 |
| Hypertension | 27.3 | 26.2 | 28.3 |
| Depression | 17.2 | 14.0 | 20.1 |
| Alcoholic drinks per day | |||
| 0 | 34.9 | 27.5 | 41.6 |
| <1 | 41.2 | 38.9 | 43.3 |
| 1–3 | 18.2 | 24.0 | 12.9 |
| 3+ | 5.7 | 9.6 | 2.2 |
| Smoking status | |||
| never | 44.6 | 38.9 | 49.8 |
| former | 27.9 | 28.9 | 27.1 |
| current | 27.4 | 32.2 | 23.1 |
| Physical activity (PASE) | |||
| 0–100 | 27.4 | 26.8 | 27.8 |
| 101–250 | 50.6 | 47.4 | 53.6 |
| 251+ | 22.0 | 25.8 | 18.6 |
| Body Mass Index (kg/m2) | |||
| <25 | 30.1 | 26.6 | 33.3 |
| 25–30 | 34.4 | 40.7 | 28.6 |
| 30+ | 35.5 | 32.7 | 38.1 |
| LUTS | 18.7 | 18.7 | 18.6 |
| Urinary Incontinence | 8.0 | 5.3 | 10.4 |
| Painful Bladder Syndrome | 2.0 | 1.3 | 2.6 |
| Prostatitis | - | 6.5 | - |
| Frequency | 32.6 | 27.8 | 36.9 |
| Urgency | 11.9 | 9.3 | 14.2 |
| Nocturia | 28.4 | 25.3 | 31.3 |
| Overactive Bladder | 9.6 | 7.3 | 11.7 |
| Overactive Bladder – Wet | 4.9 | 2.8 | 6.8 |
| Overactive Bladder – Dry | 4.7 | 4.5 | 5.0 |
| Erectile Dysfunction | - | 20.7 | - |
| Female Sexual Dysfunction | - | - | 34.3 |
FIGURE 1. THE CONSISTENT RELATIONSHIPS BETWEEN TEN UROLOGIC AND SEXUAL SYMPTOMS AND FOUR ILLNESSES (TYPE II DIABETES, CARDIAC DISEASE, HYPERTENSION AND DEPRESSION) BY GENDER.
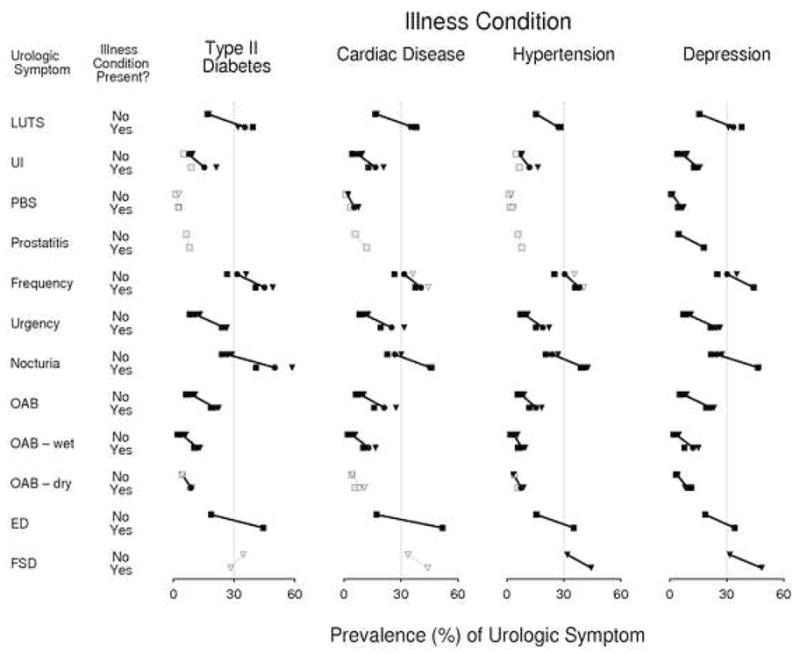
LEGEND: SQUARE (MEN), TRIANGLE (WOMEN), CIRCLE (OVERALL), BLACK (GRAY) FIGURES INDICATE THAT A P VALUE FROM A CHI-SQUARE TEST OF INDEPENDENCE IS LESS THAN (GREATER THAN) 0.05
EXPLANATION: The prevalence of twelve urologic symptoms is plotted as a function of the presence or absence of four illnesses (type-II diabetes, cardiac disease, hypertension, depression). Each symptom’s estimates are given in two horizontal rows, the first row (marked “No”) applying to subjects who do not exhibit the illness denoted in the relevant column heading, and the second (marked “Yes”) applying to those subjects who do suffer from that illness. Numeric values corresponding to the symptom prevalence estimates are given in the horizontal axis presented at the bottom of each column. Estimates for men and women combined are denoted by circular symbols, for women alone by triangles, and for men alone by squares. For each urologic symptom/illness combination, the two estimates for men and women combined are connected with a line segment, the length of which indicates the increase in prevalence of the symptom that is associated with the presence of the illness. Where this increase is statistically significant, the corresponding prevalence estimates are presented with black symbols, while statistically insignificant associates are indicated by estimates presented in grey. With the exception of the FSD/diabetes combination, each symptom is positively, though not always significantly, associated with every illness, as evidenced by the fact that all line segments (save that corresponding to the FSD/diabetes combination) exhibit a negative slope. That every symptom is significantly associated with depression, both within each gender and overall, is indicated by the fact that all symbols in the last column of the figure are drawn in black.
After adjusting for age (the prevalence of Type II diabetes, cardiac disease, and hypertension increase with age – data not shown), gender, race/ethnicity, and covariates, the associations of urologic and sexual symptoms with these illnesses are less significant, (Figure 2). Frequency (p=.0368), urgency in men (p=.0420), and nocturia in women (p=.0023) remain significantly associated with type II diabetes. LUTS (p=.0098), UI (p=.0118), PBS (p=.0001), urgency in women (p=.0140), OAB in women (p=.0050), OAB wet (p=.0022), and ED in men (p=.0042) remain significantly associated with cardiac disease. Nocturia in men (p=.0218) remains significantly associated with hypertension. All urologic and sexual symptoms studied remain significantly associated with depression (p values range from .01 to <.0001).
FIGURE 2. ODDS RATIOS (WITH 95% CONFIDENCE INTERVALS) DEPICTING THE ASSOCIATIONS BETWEEN TEN UROLOGIC AND SEXUAL SYMPTOMS AND FOUR ILLNESSES (TYPE II DIABETES, CARDIAC DISEASE, HYPERTENSION AND DEPRESSION).
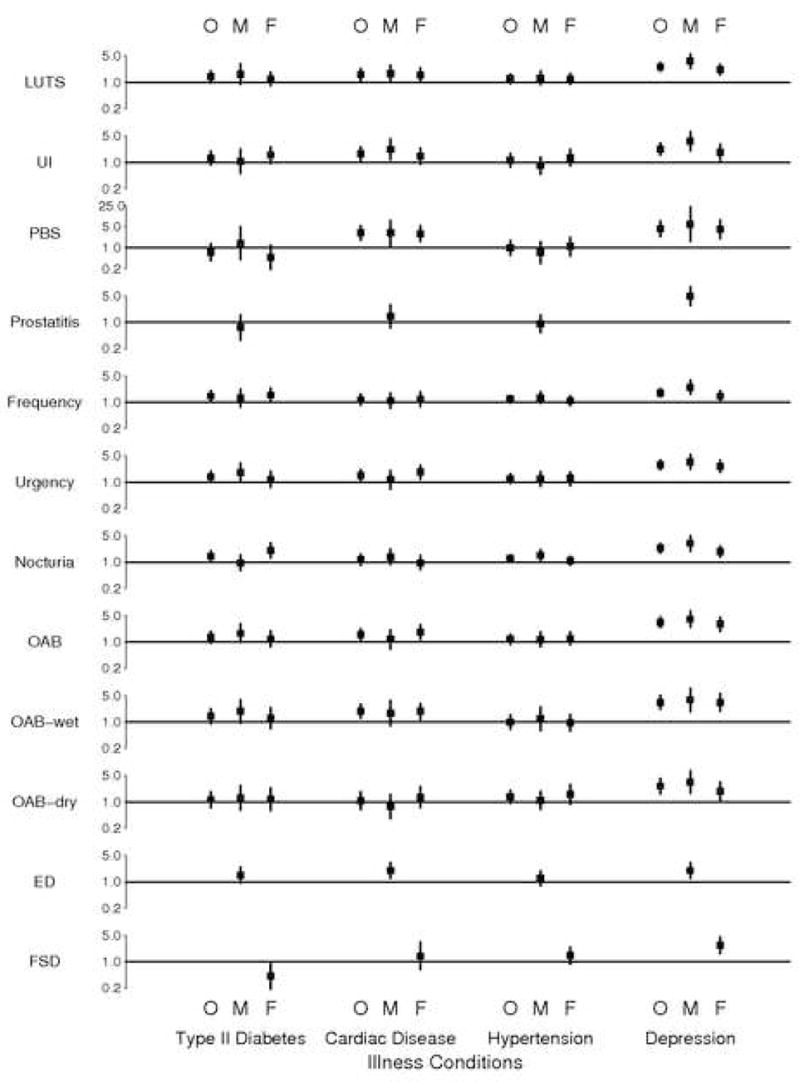
LEGEND: O (OVERALL), M (MEN), F (WOMEN). THE ODDS RATIOS ARE ESTIMATED FROM A MULTIVARIATE MODEL INCLUDING TYPE II DIABETES,CARDIAC DISEASE, HYPERTENSION, DEPRESSION, AGE, RACE/ETHNICITY, GENDER, ALCOHOL CONSUMPTION, SMOKING, PHYSICAL ACTIVITY AND BODY MASS INDEX. NOTE THAT THE ODDS RATIO SCALE GOES TO 25 FOR PBS ONLY.
EXPLANATION: Point (squares) and 95% confidence interval (line segments) estimates for the multiplicative increase in odds of subjects’ experiencing individual urologic symptoms that is associated with the presence of specific illnesses. The illnesses are organized into columns as indicated at the bottom of the figure. Estimates are given for men and women together ( “O”), men only (“M”), and women subjects only (“F”). For each symptom, a horizontal line has been drawn to indicate the location of a hypothetical odds ratio of 1.0, which would denote no association between the relevant symptom and illness. Confidence intervals that do not cross these horizontal axes indicate statistically significant evidence of association between symptom and illness.
To determine if the effect of a given illness is the same in both genders, we considered the interaction of the illness and gender. The significant interactions (the confidence interval for one gender does not include the point estimate for the other gender) include diabetes and gender for frequency, and depression and gender for LUTS, UI, frequency, and nocturia. It is interesting to note that the odds ratios for each non-gender specific urologic symptom with depression are higher in men (some significantly higher) than in women.
There were some significant two-way interactions between the medical illnesses that apparently increase the risk of urologic symptoms to a degree greater than that which arises when the illness occurred alone. We found (data not shown) that (a) women with both type II diabetes and cardiac disease had odds of UI that were greater than the products of the individual odds, and (b) respondents with both cardiac disease and hypertension had odds of frequency and urgency, that were greater than the product of the individual odds.
Table 2 summarizes the multivariate results. In summary, only depression is consistently associated with all the urologic and sexual symptoms considered after adjusting for other co-morbid illnesses and in the presence of other covariates including age, race/ethnicity, gender, alcohol consumption, smoking, physical activity and body mass index. To a lesser extent, cardiac disease is also associated with many urologic symptoms even after adjusting for other co-morbid illnesses and other covariates.
TABLE 2.
SUMMARY OF RESULTS OF THE ASSOCIATION OF UROLOGIC AND SEXUAL SYMPTOMS WITH FOUR ILNESSES (TYPE II DIABETES, CARDIAC DISEASE, HYPERTENSION, AND DEPRESSION).
| Type II Diabetes | Cardiac Disease | Hypertension | Depression | |
|---|---|---|---|---|
| LUTS | X | X | ||
| UI | X | X | ||
| PBS | X | X | ||
| Prostatitis | X (M) | |||
| Frequency | X | X | ||
| Urgency | X (M) | X (F) | X | |
| Nocturia | X (F) | X (M) | X | |
| OAB | X (F) | X | ||
| OAB – wet | X | X | ||
| OAB – dry | X | |||
| ED | X (M) | X (M) | ||
| FSD | X (F) |
Note: An X indicates a significant association which may be modified by gender: (M) men only, (F) women only
4. Discussion
Using cross-sectional data from a large sample (n=5506) of adults from Boston, we examined the relationship between urologic and sexual symptoms and four illnesses (three medical and one psychological). After adjusting for covariates (age, gender, race/ethnicity, alcohol consumption, smoking, physical activity, and BMI), we found that all urologic symptoms studied were significantly associated with depression. We also found that several urologic symptoms were related to cardiac disease and diabetes, while only nocturia in men was related to hypertension. Some of these relationships have been previously noted, but several of our findings are novel, possibly revealed because of the unique design of the BACH survey, which asked about many urologic and sexual symptoms instead of one at a time. Our results add to the growing literature that suggests underlying medical and psychological illnesses contribute to or are reflected in urologic symptoms. Our analysis cannot clarify whether the association of medical illness and urologic symptoms may arise because those urologic symptoms have brought a patient to medical attention, during which other medical illnesses were diagnosed i.e. those with more urologic symptoms become more likely to carry medical diagnoses.
Our findings with respect to the relationship between UI, OAB and illness are consistent with those other researchers who found a link with diabetes [28–30], with depression [31], and with general poor health [30]. In contrast, our finding of an association of cardiac disease and UI, LUTS, PBS, urgency, OAB, and OAB wet has not been previously reported. The pathophysiology of this association is unexplored, but common sense suggests that the fluid shifts associated with congestive heart failure may exacerbate urinary symptoms, and vasculopathy that affects the heart may similarly affect the bladder. Other studies of the relationship between LUTS and medical illnesses have usually focused on men, and have found varying relationship with aging, hypertension, dyslipidemia, and general poor health [10, 11, 32–36] but were not positioned to explore an association with cardiac disease.
Also novel is the finding that all of these urologic and sexual symptoms are associated with depression. Prior studies have noted that depression approximately double the odds of ED [37–39] and depression is certainly known to be linked with incontinence [40, 41] but the directionality of the association of urologic and sexual symptoms with depression has not been explored in those analyses. Until we have more information about this relationship, we cannot simply assume that depression has arisen because urologic symptoms are present. For example, it is plausible that the same neurotransmitter abnormalities that lead to depression are causing urologic symptoms. This possibility is supported by the recent success of duloxetine, an antidepressant that is also used for treatment of incontinence in Europe [42]. Other antidepressants have been used to treat voiding disorders [43]. It has also been shown that the treatment of OAB improves depression scores [44].
Our finding that ED is associated with cardiac disease is consistent with findings from the Massachusetts Male Aging Study [4] and others [5, 6, 37, 45–48] which find that the odds of ED are approximately doubled in the presence of cardiovascular disease and its risk factors include hypertension, insulin resistance, smoking, diabetes, lack of exercise and hyperlipidemia. ED is increasingly viewed as a manifestation of endothelial dysfunction and a potential harbinger of cardiovascular end points [46] including angina pectoris and myocardial infarction [45, 49], and is understood to have important pathophysiologic, pharmacologic and clinical implications. Importantly, a recent randomized controlled trial [50] demonstrated that lifestyle modification is of moderate clinical benefit in ED (about a third of 110 obese men with moderate ED in an exercise group recovered normal erectile function during a two-year study period). Derby and colleagues have also shown that reductions in smoking, obesity and alcohol consumption and increases in physical activity are associated with lower incidence of ED [51].
The notion that urologic symptoms relate only to urologic structures is overly simplistic. Our findings may explain some of the lack of response to usual urologic therapies in some patients with urologic symptoms, since an associated or underlying contributory medical or psychological condition may remain unrecognized and untreated. The clinical importance of our findings is this: clinicians should remain sensitive to the possibility that co-morbidities may be partially or even entirely responsible for urologic symptoms. When planning treatment in the setting of type II diabetes, cardiac disease, hypertension, and depression, it may be reasonable to delay empiric treatment of urologic symptoms until formal testing (e.g. with urodynamics, ultrasound or biopsy) and, possibly, correction of associated medical conditions, confirms symptoms are of lower urinary tract origin. Even after the nature of these relationships are clarified, clinicians should employ a global, collaborative medical approach to treatment plans for patients with urologic symptoms.
We recommend that just as ED is increasingly considered a sentinel sign of certain medical illnesses, so too should other common urologic symptoms be viewed as possible sentinel symptoms for major illness. Further research is needed however, to determine whether the relationship between other urologic symptoms and medical and psychological illness will have the same clinical utility as the suggested relationship between ED and cardiovascular risk. Beyond that, we will also need information about whether treatment of the associated conditions improves urologic symptoms. The current study was not positioned to comment on this possibility. We do hope that our planned longitudinal studies will provide further clinical guidance on this topic.
5. Conclusion
We have shown that urologic and sexual symptoms are associated with depression, and to a lesser extent with cardiac disease, type II diabetes, and hypertension. With the recognition that urologic symptoms are related to major diseases, clinical providers may need to give greater weight to consideration of contributory factors that lie outside the urinary tract. It remains unknown whether treatment of medical and psychological illnesses can result in meaningful improvement in urologic symptoms, or conversely, whether several urologic symptoms can provide valuable insight into an individual’s overall health status. We may need to revise current evaluation and treatment approaches for urologic symptoms [52] that tend to be focused on the urinary tract, with relatively little attention to the contributions that co-morbid illnesses may make to symptoms.
Acknowledgments
Funded by NIH NIDDK, U01 DK56842. We would like to acknowledge the contributions of Thomas G. Travison, Ph.D. and Gretchen R. Esche, M.S. to this paper.
Footnotes
Take-home message
Urological and sexual symptoms are significantly associated with depression, and to a lesser extent with cardiac disease, type II diabetes, and hypertension. Providers may need to look beyond the bladder and lower urinary tract to understand and/or treat presenting symptoms.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wagg A, Andersson KE, Cardozo L, et al. Nocturia: morbidity and management in adults. Int J Clin Pract. 2005;59(8):938–45. doi: 10.1111/j.1368-5031.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 2.Hirayama A, Fujimoto K, Matsumoto Y, et al. Nocturia in men with lower urinary tract symptoms is associated with both nocturnal polyuria and detrusor overactivity with positive response to ice water test. Urology. 2005;65(6):1064–9. doi: 10.1016/j.urology.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan SA. Male pelvic health: a urological call to arms. J Urol. 2006;176(6 Pt 1):2351–2. doi: 10.1016/j.juro.2006.08.125. [DOI] [PubMed] [Google Scholar]
- 4.Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 5.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. Jama. 1999;281(6):537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 6.Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139(3):161–8. doi: 10.7326/0003-4819-139-3-200308050-00005. [DOI] [PubMed] [Google Scholar]
- 7.McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005;47(6):838–45. doi: 10.1016/j.eururo.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Rosen RC, Giuliano F, Carson CC. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) Eur Urol. 2005;47(6):824–37. doi: 10.1016/j.eururo.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. Jama. 2005;294(23):2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 10.Burke JP, Jacobson DJ, McGree ME, et al. Diabetes and benign prostatic hyperplasia progression in Olmsted County, Minnesota. Urology. 2006;67(1):22–5. doi: 10.1016/j.urology.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A, Gupta S, Pavuk M, et al. Anthropometric and metabolic factors and risk of benign prostatic hyperplasia: A prospective cohort study of Air Force veterns. Urology. 2006;68(6):1198–1205. doi: 10.1016/j.urology.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 12.McKinlay JB, Link CL. Measuring the Urologic Iceberg: Design and Implementation of the Boston Area Community Health (BACH) Survey. European Urology. 2007 doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549–57. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 14.Mebust W, Roizo R, Schroider F, et al. Internation Consultation on Benign Prostatic Hyperplasia. Paris: World Health Organization; 1991. Correlations between pathology, clinical symptoms, and the course of the disease. [Google Scholar]
- 15.Hanno PM, Landis JR, Matthews-Cook Y, et al. The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161(2):553–7. doi: 10.1016/s0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 16.Sandvik H, Seim A, Vanvik A, et al. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19(2):137–45. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Nickel JC, Downey J, Hunter D, et al. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001;165(3):842–5. [PubMed] [Google Scholar]
- 18.Roberts RO, Jacobson DJ, Girman CJ, et al. Prevalence of prostatitis-like symptoms in a community based cohort of older men. J Urol. 2002;168(6):2467–71. doi: 10.1016/S0022-5347(05)64170-5. [DOI] [PubMed] [Google Scholar]
- 19.Link CL, Lutfey KE, Steers WD, et al. Is abuse causally related to urologic symptoms? Results from the Boston Area Community Health (BACH) Survey. European Urology. 2007 doi: 10.1016/j.eururo.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 21.Wein AJ. In: Pathophysiology and categorization of voiding dysfunction, in Campbell’s Urology. Walsh PC, et al., editors. 2002; Saunders; [Google Scholar]
- 22.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–26. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 23.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 24.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11(2):139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 25.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 26.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman Hall; 1997. [Google Scholar]
- 27.Cochran WG. Sampling Techniques. New York: John Wiley and Sons, Inc; 1977. [Google Scholar]
- 28.Lifford KL, Curhan GC, Hu FB, et al. Type 2 diabetes mellitus and risk of developing urinary incontinence. J Am Geriatr Soc. 2005;53(11):1851–7. doi: 10.1111/j.1532-5415.2005.53565.x. [DOI] [PubMed] [Google Scholar]
- 29.Jackson RA, Vittinghoff E, Kanaya AM, et al. Urinary incontinence in elderly women: findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004;104(2):301–7. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 30.McGrother CW, Donaldson MMK, Hayward T, et al. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age and Aging. 2006;35(1):16–24. doi: 10.1093/ageing/afi205. [DOI] [PubMed] [Google Scholar]
- 31.Irwin DE, Milsom I, Kopp Z, et al. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int. 2006;97(1):96–100. doi: 10.1111/j.1464-410X.2005.05889.x. [DOI] [PubMed] [Google Scholar]
- 32.Prezioso D, Catuogno C, Galassi P, et al. Life-style in patients with LUTS suggestive of BPH. Eur Urol. 2001;40(Suppl 1):9–12. doi: 10.1159/000049871. [DOI] [PubMed] [Google Scholar]
- 33.Rohrmann S, Crespo CJ, Weber JR, et al. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third National Health And Nutrition Examination Survey. BJU Int. 2005;96(1):77–82. doi: 10.1111/j.1464-410X.2005.05571.x. [DOI] [PubMed] [Google Scholar]
- 34.Joseph MA, Harlow SD, Wei JT, et al. Risk factors for lower urinary tract symptoms in a population-based sample of African-American men. Am J Epidemiol. 2003;157(10):906–14. doi: 10.1093/aje/kwg051. [DOI] [PubMed] [Google Scholar]
- 35.Glynn RJ, Campion EW, Bouchard GR, et al. The development of benign prostatic hyperplasia among volunteers in the Normative Aging Study. Am J Epidemiol. 1985;121(1):78–90. [PubMed] [Google Scholar]
- 36.Koskimaki J, Hakama M, Huhtala H, et al. Association of non-urological diseases with lower urinary tract symptoms. Scand J Urol Nephrol. 2001;35(5):377–81. doi: 10.1080/003655901753224431. [DOI] [PubMed] [Google Scholar]
- 37.Nicolosi A, Glasser DB, Moreira ED, et al. Prevalence of erectile dysfunction and associated factors among men without concomitant diseases: a population study. Int J Impot Res. 2003;15(4):253–7. doi: 10.1038/sj.ijir.3901010. [DOI] [PubMed] [Google Scholar]
- 38.Holden CA, McLachlan RI, Pitts M, et al. Men in Australia Telephone Survey (MATeS): a national survey of the reproductive health and concerns of middle-aged and older Australian men. Lancet. 2005;366(9481):218–24. doi: 10.1016/S0140-6736(05)66911-5. [DOI] [PubMed] [Google Scholar]
- 39.Wong SY, Chan D, Hong A, et al. Depression and lower urinary tract symptoms: Two important correlates of erectile dysfunction in middle-aged men in Hong Kong, China. Int J Urol. 2006;13(10):1304–10. doi: 10.1111/j.1442-2042.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- 40.Melville JL, Delaney K, Newton K, et al. Incontinence severity and major depression in incontinent women. Obstet Gynecol. 2005;106(3):585–92. doi: 10.1097/01.AOG.0000173985.39533.37. [DOI] [PubMed] [Google Scholar]
- 41.Nygaard I, Turvey C, Burns TL, et al. Urinary incontinence and depression in middle-aged United States women. Obstet Gynecol. 2003;101(1):149–56. doi: 10.1016/s0029-7844(02)02519-x. [DOI] [PubMed] [Google Scholar]
- 42.Mariappan P, Ballantyne Z, N’Dow JM, et al. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst Rev. 2005;3:CD004742. doi: 10.1002/14651858.CD004742.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Andersson KE, Pehrson R. CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs. 2003;63(23):2595–611. doi: 10.2165/00003495-200363230-00003. [DOI] [PubMed] [Google Scholar]
- 44.Sand P, Zinner N, Newman D, et al. Oxybutynin transdermal system improves the quality of life in adults with overactive bladder: a multicentre, community-based, randomized study. BJU Int. 2006 doi: 10.1111/j.1464-410X.2006.06658.x. [DOI] [PubMed] [Google Scholar]
- 45.Billups KL, Bank AJ, Padma-Nathan H, et al. Erectile dysfunction is a marker for cardiovascular disease: results of the minority health institute expert advisory panel. J Sex Med. 2005;2(1):40–50. doi: 10.1111/j.1743-6109.2005.20104_1.x. discussion 50–2. [DOI] [PubMed] [Google Scholar]
- 46.Cheitlin MD. Erectile dysfunction: the earliest sign of generalized vascular disease? J Am Coll Cardiol. 2004;43(2):185–6. doi: 10.1016/j.jacc.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Riedner CE, Rhoden EL, Ribeiro EP, et al. Central obesity is an independent predictor of erectile dysfunction in older men. J Urol. 2006;176(4 Pt 1):1519–23. doi: 10.1016/j.juro.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 48.Blanker MH, Bohnen AM, Groeneveld FP, et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community-based study. J Am Geriatr Soc. 2001;49(4):436–42. doi: 10.1046/j.1532-5415.2001.49088.x. [DOI] [PubMed] [Google Scholar]
- 49.Solomon H, Man JW, Jackson G. Erectile dysfunction and the cardiovascular patient: endothelial dysfunction is the common denominator. Heart. 2003;89(3):251–3. doi: 10.1136/heart.89.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. Jama. 2004;291(24):2978–84. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 51.Derby CA, Mohr BA, Goldstein I, et al. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56(2):302–6. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]
- 52.Wein AJ, Rackley RR. Overactive bladder: a better understanding of pathophysiology, diagnosis and management. J Urol. 2006;175(3 Pt 2):S5–10. doi: 10.1016/S0022-5347(05)00313-7. [DOI] [PubMed] [Google Scholar]


