Abstract
Despite decades of intense research, malaria remains a deadly disease worldwide and new antimalarials are urgently needed due to increasing drug resistance of Plasmodium falciparum to existing drugs. This article reports the evaluation of four Indian Diospyros species viz., Diospyros melanoxylon, D. peregrina, D. sylvatica, D. tomentosa for antiplasmodial activities against chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains of P. falciparum. Six of eight methanolic extracts were found to have significant activity, (IC50 = 16.5–92.9 µg ml−1), against strain 3D7 and five of these showed similar activities against strain K1 (IC50 = 20.5–121.6 µg ml−1). Diospyros sylvatica was found to be the most active species (IC50 = 16.5–29.4 µg ml−1) and is worthy of further investigation.
Keywords: Diospyros melanoxylon, Diospyros peregrina, Diospyros sylvatica, Diospyros tomentosa, Ebenaceae, Malaria, Plasmodium falciparum
Introduction
Malaria is one of the most devastating diseases in the world responsible for more than 2.5 million deaths (1) and at least 300 million clinical cases per annum (2). Resistance of Plasmodium falciparum to chloroquine and some other antimalarial drugs continuous to increase. Efforts are now being directed towards the discovery and development of new chemically diverse antimalarial agents. Diospyros is one of the most important genera of the Ebenaceae and Diospyros species are well-known for their medicinal uses since ancient times in many traditional medicinal systems such as Ayurveda, Traditional Chinese Medicine and the African folklore (3–9). About 41 Diospyros species occur in India of more than 350 species identified (10,11). These are mostly trees and rarely shrubs. The literature revealed that these plants contain pentacyclic triterpenes and juglone-based 1,4-naphthoquinones and about 130 species have undergone some phytochemical and/or pharmacological investigations (8).
Two in-vivo studies by different groups revealed that the methanolic extracts of Diospyros mespiliformis (bark) and D. variegata (stem) possessed potent antipyretic properties supporting their folkloric use in relieving fevers (12,13) suggesting that species of Diospyros may be worth screening for antimalarial properties. Diospyros melanoxylon, D. peregrina, D. sylvatica, D. tomentosa have been used extensively in Indian traditional medicine to treat for a variety of diseases including diarrhea, cholera, dysentery, intermittent fevers, bleeding gums, bronchitis, carbuncles, cough, cramps, pneumonia, syphilis, tumors, etc. (8,14) and are widely available in the forests of Orissa (North East India). Therefore, these species have been collected and used for antiplasmodial screening.
Methods
Plant Collection
Plant materials were collected from the local forest, Bhubaneswar (20°15′ N, 85°50′ E), Orissa, India during June–August 2004. The identity of these plants was confirmed by taxonomist, Mr Murty K.S. (Ayurvedic Research Institute, Bhubaneswar, India) and the voucher specimens (040511–040518) are kept available in The School of Pharmacy, University of Bradford, UK.
Extraction
Method 1. Dried pulverized plant material (10 g) was extracted with methanol at room temperature; the methanol was decanted after 24 h and the extraction repeated three times. The pooled extracts were filtered and then concentrated under vacuum using a rotary evaporator at 40°C.
Method 2. Powdered stem bark (10 g) of D. peregrina, D. sylvatica and D. tomentosa were extracted (separately) in a Soxhlet apparatus successively with n-hexane, ethyl acetate and methanol and the extracts concentrated under vacuum.
Sample Preparation
Crude extracts and control drugs stock solutions (1 mg ml−1) were prepared by weighing 1 mg accurately and dissolving in 50 μl of dimethyl sulphoxide (DMSO) or ethanol and made up to 1 ml with double distilled water. Later these solutions were diluted in complete medium.
Chemicals and Reagents
All the materials for the antiplasmodial assays were purchased from Sigma Chemical Co, Poole, UK, except for artemether which was a gift from Aventis Pharma.
Cultivation of P. falciparum
Chloroquine sensitive (3D7) and resistant (K1) strains were kindly supplied by Professor D. C. Warhurst (London School of Hygiene and Tropical Medicine). The strains were maintained in human A + erythrocytes suspended in RPMI 1640 medium supplemented with A + serum and D-glucose according methods of Trager and Jensen, 1976 (15) and Fairlamb et al. 1985 (16).
Antiplasmodial Assay
Cultures containing predominantly early ring stages of P. falciparum were used for testing. After adding sample solutions to 96-well microtiter plates in duplicate, 2-fold serial dilutions were made with RPMI 1640 medium and infected erythrocytes were added to give a final volume of 100 µl with 2.5% hematocrit and 1% parasitemia. Chloroquine diphosphate and artemether were used as positive controls, and uninfected and infected erythrocytes without sample solutions were incubated in each test. Plates were placed into a modular incubator gassed with 93% nitrogen, 3% oxygen and 4% carbon dioxide and incubated at 37°C for 48 h. Parasite growth was assessed by measuring parasite lactate dehydrogenase activity (pLDH) as described by Makler et al. 1993 (17). The reagent used contained the following in each micro-litre acetylpyridine adenine dinucleotide (APAD), 0.74 mg; lithium lactate, 19.2 mg; diaphorase, 0.1 mg; Triton X-100, 2 µl; nitroblue tetrazolium, 1 mg; and phenazine ethosulfate, 0.5 mg. Fifty microliters of this reagent was added to each well and mixed, and plates were read at 550 nm using a Dynatech Laboratories MRX microplate reader, and per cent inhibition of growth was calculated by comparison with control values. Optical densities were read at 550 nm using a Dynatech Laboratories MRX microplate reader and percentage inhibition of growth was calculated by comparison with control values. IC50 values were determined using linear regression analysis (Microsoft Excel). A minimum of three separate determinations was carried out for each sample.
Results
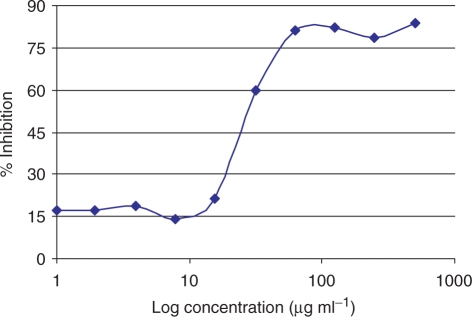
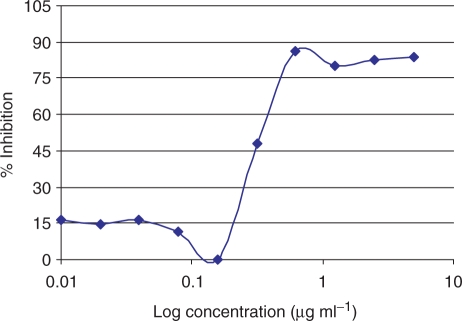
The antiplasmodial activities of the extracts and control drugs are shown in Tables 1 and 2. Data are expressed as the mean ± standard deviation of the IC50 of three independent experiments on different days for each strain. The IC50 values ranged from 16.5 to >500 µg ml−1. The effect of the methanolic extract of root, D. sylvatica on growth of P. falciparum strain K1 is shown in Fig. 1. No significant difference was found in IC50 values against strains 3D7 and K1 in the case of artemether (0.008 and 0.014 µg ml−1, respectively), but ∼20-fold difference was found in CQ between the strains 3D7 and K1 (0.02 and 0.38 µg ml−1, respectively) showing that, as expected strain K1 is resistant to CQ, but not to artemether. The effect of CQ on growth of P. falciparum strain K1 is shown in Fig. 2.
Table 1.
In vitro activities of MeOH extracts and antimalarial drugs against P. falciparum
| S. No. | Species and control | Ext. Part | IC50 μg ml−1 against Pf [Mean ± SD] | |
|---|---|---|---|---|
| Strain 3D7 | Strain K1 | |||
| 1. | D. melanoxylon | Root | 78.4 ± 1.2 | 121.6 ± 0.9 |
| 2. | St. heartwood | 55.6 ± 1.7 | 201.7 ± 2.1 | |
| 3. | St. bark | >500 | >500 | |
| 4. | D. peregina | St. bark | 92.9 ± 1.8 | 120.2 ± 2.3 |
| 5. | D. sylvatica | Root | 27.3 ± 0.6 | 29.4 ± 1.3 |
| 6. | St. heartwood | 16.6 ± 0.4 | 20.5 ± 0.7 | |
| 7. | St. bark | 16.5 ± 0.2 | 21.9 ± 0.3 | |
| 8. | D. tomentosa | St. bark | >500 | >500 |
| 9. | Positive controls | Chloroquine | 0.02 ± 0.001 | 0.38 ± 0.03 |
| 10. | Artemether | 0.008 ± 0.002 | 0.014 ± 0.01 | |
Note. Pf : P. falciparum; SD: Standard deviation; St.: Stem; Ext.: Extract; n = 3.
Table 2.
In vitro activities of successive extracts against P. falciparum
| S. No. | Species and control | Ext. of St. bark | IC50 μg ml−1 against Pf [Mean ± SD] | |
|---|---|---|---|---|
| Strain 3D7 | Strain K1 | |||
| 1. | D. peregina | Hexane | >500 | >500 |
| 2. | Ethyl acetate | >500 | >500 | |
| 3. | Methanol | 44.7 ± 0.7 | 66.7 ± 1.6 | |
| 4. | D. sylvatica | Hexane | >500 | >500 |
| 5. | Ethyl acetate | 19.4 ± 1.1 | 29.3 ± 0.8 | |
| 6. | Methanol | 51.5 ± 1.6 | 81.5 ± 1.3 | |
| 7. | D. tomentosa | Hexane | >500 | >500 |
| 8. | Ethyl acetate | >500 | >500 | |
| 9. | Methanol | >500 | >500 | |
Note. Pf : P. falciparum; SD: Standard deviation; St.: Stem; Ext.: Extract; n = 3.
Figure 1.
Effect of extract of D. sylvatica on growth of P. falciparum strain K1.
Figure 2.
Effect of chloroquine diphosphate on growth of P. falciparum strain K1.
Discussion
Six of the eight methanolic extracts from the four Diospyros species were found to have significant activities against strain 3D7 (IC50 ≤ 93 µg ml−1; see Table 1) and five extracts were active in case of strain K1 (IC50 ≤ 122 µg ml−1; see Table 1) suggesting that the latter do not exhibit cross-resistance with chloroquine. Interestingly, the extracts of D. sylvatica possessed very good activity (IC50 = 16.5–29.4 µg ml−1; see Table 1) against both strains 3D7 and K1 and were the most active of these Diospyros extracts. Diospyros melanoxylon, stem heartwood and root possessed significant activities against strain 3D7 (IC50 = 55.6 and 78.4 µg ml−1, respectively), but the heartwood extract was 2-fold less active against strain K1 compared with strain 3D7 (IC50 = 201.7 and 121.6 µg ml−1, respectively). Diospyros peregrina stem bark possessed activity against both strains 3D7 & K1 (IC50 = 92.9 and 120.2 µg ml−1, respectively). Methanolic extracts of the stem barks of D. melanoxylon and D. tomentosa were inactive (IC50 > 500 µg ml−1). Stem barks of D. peregrina, D. sylvatica and D. tomentosa were fractionated successively with n-hexane, ethyl acetate and methanol and screened against both parasite strains (Table 2). Only the methanolic fraction from D. peregrina was active while in the case of D. sylvatica the ethyl acetate fraction was the most active, but some activity remained in the methanol fraction. No activity was seen in the fractions from D. tomentosa, consistent with the lack of activity found in the cold methanolic extract.
A literature study of Diospyros species revealed that only the leaves of D. melanoxylon have previously been tested against P. falciparum (3D7) and the ethanolic extract was found to be active (IC50 = 97 µg ml−1) (18). Diospyros peregrina, D. sylvatica, D. tomentosa and the root, heartwood and stem bark of D. melanoxylon have been screened for the first time in this study.
A large number of compounds have been reported from D. melanoxylon and D. peregrina including lupeol, betulin, betulinic acid, ursolic acid, oleanolic acid, β-sitosterol, etc. (8). A few studies have been carried out with D. sylvatica. Lupeol, betulin and betulinic acid have been isolated from the bark (19) and 2-methylanthraquinone, plumbagin, diosindigo, diospyrin, iso-diospyrin and microphyllone reported from roots by Ganapaty et al. 2004 (20). The antiplasmodial activities of some of these compounds have been reported (21–27); plumbagin (25) and diospyrin (26) have been shown to have potent activities against P. falciparum in vitro (IC50 = 0.27 and 0.21 µg ml−1, respectively) while betulinic acid and lupeol, ursolic acid and oleanolic acid were only weakly active. The presence of these compounds, especially plumbagin and diospyrin may explain the antiplasmodial activities of the extracts tested in this study but further studies will be needed in order to confirm this.
Conclusion
Extracts of three of the four species of Indian Diospyros tested in this study showed antiplasmodial activities, with the best activity shown by D. sylvatica. This species especially is worthy of further investigation to determine which of its constituents are responsible for the activity.
Acknowledgments
V.S.S.K. expresses his gratitude to the Association of Commonwealth Universities, UK for financial support and to Dr U. V. Mallavadhani and Mr K. R. Kaushal from the Regional Research Laboratory, Bhubaneswar for their scientific support.
References
- 1.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–5. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 2.WHO. WHO information. 2003. World Health Organization Fact Sheet No. 94. < http://www.int/inf~fs/en/fact094.html>. [Google Scholar]
- 3.Laloo RC, Kharlukhi L, Jeeva S, Mishra BP. Status of medicinal plants in the disturbed and the undisturbed sacred forests of Meghalaya, northeast India: population structure and regeneration efficacy of some important species. Current Science. 2006;90:225–232. [Google Scholar]
- 4.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi, India: CSIR; 1956. pp. 98–9. [Google Scholar]
- 5.Kirtikar KR, Basu BD. In: Indian Medicinal Plants. Blatter E, Cains JF, Mhaskar KS, editors. Allahabad, India: Lalit Mohan Basu; 1933. p. 1498. [Google Scholar]
- 6.Lewis WH, Elvin-Lewis MPF. Medical Botany: Plants Affecting Man's Health. New York: Wiley Interscience; 1977. [Google Scholar]
- 7.Phongboonrod S. Medicinal Plants of Thailand. Bangkok: Kasem Banakit; 1979. [Google Scholar]
- 8.Mallavadhani UV, Panda AK, Rao YR. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry. 1998;49:901–51. doi: 10.1016/s0031-9422(97)01020-0. [DOI] [PubMed] [Google Scholar]
- 9.Bhushan P, Dnyaneshwar W, Pushpangadan P, Narendra B. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternative Med. 2005;2:465–73. doi: 10.1093/ecam/neh140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George Watt MB. A Dictionary of the Economic Products of India. New Delhi, India: Cosmo Publication; 1952. p. 136. [Google Scholar]
- 11.Sastry BN. The Wealth of India: Raw Materials. New Delhi, India: CSIR; 1952. p. 76. [Google Scholar]
- 12.Adzu B, Amos S, Dzarma S, Muazzam I, Gamaniel KS. Pharmacological evidence favouring the folkloric use of Diospyros mespiliformis Hochst in the relief of pain and fever. J Ethnopharmacol. 2002;82:191–5. doi: 10.1016/s0378-8741(02)00179-4. [DOI] [PubMed] [Google Scholar]
- 13.Trongsakul S, Panthong A, Kanjanapothi D, Taesotikul T. The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegata Kruz. J Ethnopharmacol. 2003;85:221–5. doi: 10.1016/s0378-8741(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, DeFilipps R. Medicinal Plants of India. Algonac, Michigan: Reference Publications, Inc.; 1991. p. 286. [Google Scholar]
- 15.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 16.Fairlamb AH, Warhurst DC, Peters W. An improved technique for the cultivation of Plasmodium falciparum in vitro without daily medium change. Ann Trop Med Parasitol. 1985;79:379–84. doi: 10.1080/00034983.1985.11811935. [DOI] [PubMed] [Google Scholar]
- 17.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–41. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 18.Simonsen HT, Nordskjold JB, Smitt UW, Nyman U, Palpu P, Joshi P, et al. In vitro screening of Indian medicinal plants for antiplasmodial activity. J Ethnopharmacol. 2001;74:195. doi: 10.1016/s0378-8741(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 19.Row L, Sankara R, Ramaiah T. Chemical examination of Diospyros species. IV. Triterpenes of the leaves of D. sylvatica and D. melanoxylon. Current Science. 1966;35:485. [Google Scholar]
- 20.Ganapaty S, Thomas PS, Fotso S, Laatsch H. Antitermitic quinones from Diospyros sylvatica. Phytochemistry. 2004;65:1265–71. doi: 10.1016/j.phytochem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Steele JC, Warhurst DC, Kirby GC, Simmonds MS. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res. 1999;13:115–9. doi: 10.1002/(SICI)1099-1573(199903)13:2<115::AID-PTR404>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Bringmann G, Saeb W, Assi LA, Francois G, Sankara Narayanan AS, Peters K, et al. Betulinic acid: isolation from Triphyophyllum peltatum and Ancistrocladus heyneanus, antimalarial activity, and crystal structure of the benzyl ester. Planta Med. 1997;63:255–7. doi: 10.1055/s-2006-957666. [DOI] [PubMed] [Google Scholar]
- 23.Alves TM, Nagem TJ, de Carvalho LH, Krettli AU, Zani CL. Antiplasmodial triterpene from Vernonia brasiliana. Planta Med. 1997;63:554–5. doi: 10.1055/s-2006-957764. [DOI] [PubMed] [Google Scholar]
- 24.Amusan OOG. Antimalarial active principles of Spathodea campanulata stem bark. Phytother Res. 1996;10:692–3. [Google Scholar]
- 25.Likhitwitayawuid K, Kaewamatawong R, Ruangrungsi N, Krungkrai J. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Med. 1998;64:237–41. doi: 10.1055/s-2006-957417. [DOI] [PubMed] [Google Scholar]
- 26.Hazra B, Ghosh R, Banerjee A, Kirby GC, Warhurst DC, Phillipson JD. In-Vitro antiplasmodial effects of Diospyrin, a plant-derived Naphthoquinoid, and a novel series of derivatives. Phytother Res. 1995;9:72–4. [Google Scholar]
- 27.Selma R, Shirley da S, Maria RF M. Plumbagine: a pharmacological approach. Floresta e Ambiente. 2003;10:98–105. [Google Scholar]