Abstract
OBJECTIVE
Interferon (IFN)-γ inducible protein, CXCL10/IP-10, is a member of the CXC chemokine family with pro-inflammatory and anti-angiogenic properties. This chemokine has been proposed to be a key link between inflammation and angiogenesis. The aim of this study was to determine whether preeclampsia and delivery of a small for gestational age (SGA) neonate are associated with changes in maternal serum concentration of CXCL10/IP-10.
STUDY DESIGN
This cross-sectional study included patients in the following groups: (1) non pregnant women (N=49); (2) women with normal pregnancies (N=89); (3) patients with preeclampsia (N=100); and (4) patients who delivered an SGA neonate (N=78). SGA was defined as birth weight below the 10th percentile. Maternal serum concentrations of CXCL10/IP-10 were measured by sensitive immunoassay. Non-parametric statistics were used for analysis.
RESULTS
(1) Patients with normal pregnancies had a significantly higher median serum concentration of CXCL10/IP-10 than non-pregnant women (median: 116.1 pg/mL, range: 40.7-1314.3 vs. median: 90.3 pg/mL, range: 49.2-214.7, respectively; p=0.002); (2) no significant correlation was found between maternal serum concentration of CXCL10/IP-10 and gestational age (between 19 and 38 weeks); (3) there were no differences in median serum CXCL10/IP-10 concentrations between patients who delivered an SGA neonate and those with normal pregnancies (median: 122.4 pg/mL, range: 37.3-693.5 vs. median: 116.1 pg/mL, range: 40.7-1314.3, respectively; p>0.05); (4) patients with preeclampsia had a higher median serum concentration of CXCL10/IP-10 than normal pregnant women (median: 156.4 pg/mL, range: 47.4-645.9 vs. median: 116.1 pg/mL, range: 40.7-1314.3, respectively; p<0.05); (5) patients with preeclampsia had a higher median concentration of CXCL10/IP-10 than those who delivered an SGA neonate (median: 156.4 pg/mL, range: 47.4-645.9 vs. median: 122.4 pg/mL, range: 37.3-693.5, respectively; p<0.05).
CONCLUSIONS
Patients with preeclampsia have significantly higher serum concentrations of CXCL10/IP-10 than both normal pregnant women and mothers who have SGA neonates. These results are likely to reflect an anti-angiogenic state as well as an enhanced systemic inflammatory response in patients with preeclampsia. Alternatively, since preeclampsia and SGA share several mechanisms of disease, it is possible that a higher concentration of this chemokine may contribute to the clinical presentation of preeclampsia in patients with a similar intrauterine insult.
Keywords: Pregnancy, CXCL10, IP-10, chemokine, chemotactic cytokine, small for gestational age, SGA, angiogenesis
Introduction
Patients with preeclampsia and those with a small for gestational age (SGA) neonate share a number of pathophysiological characteristics including: (1) abnormal physiologic transformation of the spiral arteries [1-8]; (2) chronic uteroplacental ischemia [9-16]; (3) endothelial cell dysfunction [17-24]; (4) an anti-angiogenic state [25-32]; and (5) intravascular inflammation [18,33-37]. Furthermore, a biased Th1/Th2 (T-helper 1/T-helper 2) balance towards a Th-1 response has been reported in preeclampsia [38-46] and SGA [47].
The human interferon-inducible protein 10 (IP-10 or CXCL10) is a chemokine of the CXC family [48]. A unique feature of members of this chemokine family is that they have pro-inflammatory properties and act as modulators of angiogenesis in conditions such as wound healing, ischemia and neoplasia. These dual properties are related to the shared expression of specific chemokine receptors by leukocytes and endothelial cells [49-60].
IP-10 is inducible by pro-inflammatory stimuli such as interferfon-γ (IFN-γ) [61-72], tumor necrosis factor-α (TNF-α) [70,73-79], viruses, and microbial products [66,70,80-85], directly or through activation of nuclear factor-kappaB (NF-kB) [81,82,86-89]. It has been proposed that this chemokine is also involved in recruitment and potentiation of Th1 responses as well as in the pathogenesis of allograft rejection [90-103], multiple sclerosis [104-108], diabetes mellitus type 1 [109,110], Graves’ disease [111-114], autoimmune thyroiditis [115,116], pulmonary fibrosis [117-119], and cardiovascular diseases such as atherosclerosis [120], and coronary syndromes [121,122]. Importantly, IP-10 has potent anti-angiogenic activity, in vitro and in vivo [123-126].
The balance between angiogenic and anti-angiogenic (angiostatic) factors controlled by molecules involved in inflammatory processes may have impact on the pathogenesis of many diseases [54,58].
We propose that IP-10 is involved in the pathophysiology of preeclampsia because this condition is characterized by intravascular inflammation and an anti-angiogenic state. The objective of this study was to compare the maternal serum IP-10 concentrations in normal pregnancy, preeclampsia, and SGA.
Methods
Study design
This retrospective cross-sectional study included patients with preeclampsia (N=100), women who delivered a SGA neonate (N=78), normal pregnant women (N=89) and non-pregnant women (N=49). All patients were enrolled at Hutzel Hospital, Detroit, MI. All women provided written informed consent for the collection of clinical data and biological materials under protocols approved by the Institutional Review Boards of both Wayne State University and the National Institute of Child Health and Human Development of the National Institute of Health (NIH/DHHS). Many of these samples have been employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in non-pregnant women, normal pregnant women and those with pregnancy complications.
Preeclampsia was defined in the presence of hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, four hours to one week apart, after the 20th week of gestation) and proteinuria (≥ 300 mg in a 24-hour urine collection, or two random urine specimens obtained four hours to one week apart containing ≥1+ protein by dipstick [127,128], or one dipstick measurement ≥2+) [129]. Severe preeclampsia was diagnosed according to the criteria proposed by the American College of Obstetricians and Gynecologists (ACOG) committee [128]. Patients with preeclampsia were sub-classified as either early-onset (<34 weeks) or late onset (≥34 weeks) disease according to the gestational age at which preeclampsia was diagnosed. A neonate was defined as SGA when the birth weight was below the 10th percentile for gestational age according to the reference range proposed by Alexander et al.[130]. Patients were considered to have a normal pregnancy if they met the following criteria: (1) no medical, obstetrical or surgical complications; (2) absence of labor at the time of venipuncture; and (3) delivery of a normal term (≥37 weeks) infant whose birth weight was between the 10th and 90th percentile for gestational age [130]. The non-pregnant group consisted of healthy volunteers not taking oral contraceptives whose blood was withdrawn in the secretory phase of the menstrual cycle.
IP-10 (CXCL10) determinations
Specific and sensitive enzyme-linked immunoassays were used to determine concentrations of IP-10 in human maternal serum. Immunoassays for IP-10 were obtained from R&D Systems (Minneapolis, MN, USA). Briefly, maternal serum samples were incubated in duplicate wells of the microtiter plates, pre-coated with a monoclonal antibody specific for IP-10. During this incubation step, any IP-10 present in the standards or maternal serum is bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for IP-10 was added to the wells. Following a wash to remove excess and unbound materials, a substrate solution was added to the wells and color developed in proportion to the amount of IP-10 bound in the initial step. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of IP-10 in serum samples were determined by interpolation from individual standard curves composed of recombinant human IP-10. The calculated inter and intra-assay coefficients of variation for IP-10 immunoassays in our laboratory were 7.99% and 4.12% respectively. The lower limit of detection (sensitivity) was calculated to be 5.01pg/mL.
Statistical analysis
The Kolmogorov-Smirnov test was used to determine whether the data were normally distributed. The Spearman’s correlation test was used in assessing the relationship between maternal serum concentration of IP-10 and gestational age at blood draw in patients with normal pregnancies. Comparisons among groups were performed using Kruskal-Wallis tests with post-hoc analysis for continuous variables, and Chi-square or Fisher’s exact test for categorical variables. A p value <0.05 was considered statistically significant. The statistical package used was SPSS v.12.0 (SPSS Inc., Chicago, IL, USA).
Results
Three hundred sixteen patients were included in this study. The demographic and clinical characteristics of the study groups are displayed in Table I.
TABLE I.
Clinical and obstetrical characteristics of the study groups
| Normal pregnancy (N=89) | Preeclamps ia (N=100) | p* | SGA (N=78) | p | |
|---|---|---|---|---|---|
| Maternal age (years) † | 23 (17 - 34) | 25 (14 - 43) | NS | 23.5 (15 - 43) | NS |
| Race | |||||
| African-American | 83.1 (74/89) | 80.8 (80/99) | NS | 84.6 (66/78) | NS |
| Caucasian | 12.4 (11/89) | 13.1 (13/99) | NS | 10.3 (8/78) | NS |
| Others | 4.5 (4/89) | 6.1 (6/99) | NS | 5.1 (4/78) | NS |
| BMI (kg/m2) † | 25.5 (16.3 - 51.6) | 26.3 (18.3 - 44.5) | NS | 24.9 (14 - 36) | NS |
| Nulliparity | 21.3 (19/89) | 29.3 (29/99) | NS | 23.1 (18/78) | NS |
| Smoking | 20.5 (17/83) | 13.2 (12/91) | NS | 28.6 (20/70) | NS |
| Gestational age at blood draw (weeks) † | 31.1 (19.4 - 38.3) | 32.6 (20.0 - 40.9) | <0.05 | 36.6 (24.4-40.3) | <0.05‡ |
| Gestational age at delivery weeks) † | 39.6 (37 - 42) | 33.3 (20.1 - 40.9) | <0.05 | 37.2 (24.9 - 41.7) | <0.05‡ |
| Birth weight (g) † | 3342 (2550 - 4050) | 1700 (220 - 4460) | <0.05 | 2130 (300 - 2895) | <0.05§ |
Values are expressed as percentage (number) or median (range).
SGA: small for gestational age neonate; BMI: body mass index; NS: not significant.
comparison between normal pregnancy and PE.
Kruskal-Wallis with post-hoc analysis.
<0.05 between SGA and normal pregnancy, as well as SGA and PE.
<0.05 between SGA and normal pregnancy.
Among patients with preeclampsia, 63% (63/100) were classified as early-onset and 88% (88/100) as severe preeclampsia. In 75.6% (59/78) of patients who delivered an SGA neonate, the birth weight was below the 5th percentile. IP-10 was detectable in the serum of all subjects.
Serum concentration of IP-10 in pregnancy
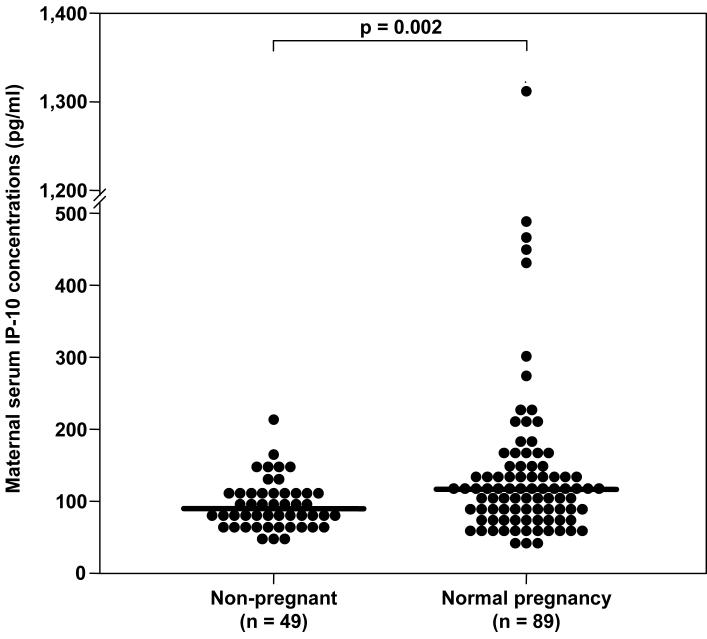
Patients with normal pregnancies had a significantly higher median serum concentration of IP-10 than non-pregnant women (median 116.1 pg/mL, range 40.7-1314.3 vs. median 90.3 pg/mL, range 49.2-214.7, respectively; p=0.002) (see Figure 1). There was no significant correlation between maternal serum IP-10 concentration and gestational age at blood draw (r=0.039; p=0.7).
Figure 1.
Serum concentrations of CXCL10/IP-10 in non-pregnant women and in patients with normal pregnancies. Patients with normal pregnancies had a significantly higher median serum concentration of IP-10 than non-pregnant women (median: 116.1 pg/mL, range: 40.7-1314.3 vs. median: 90.3 pg/mL, range: 49.2-214.7, respectively; p=0.002).
IP-10 in preeclampsia
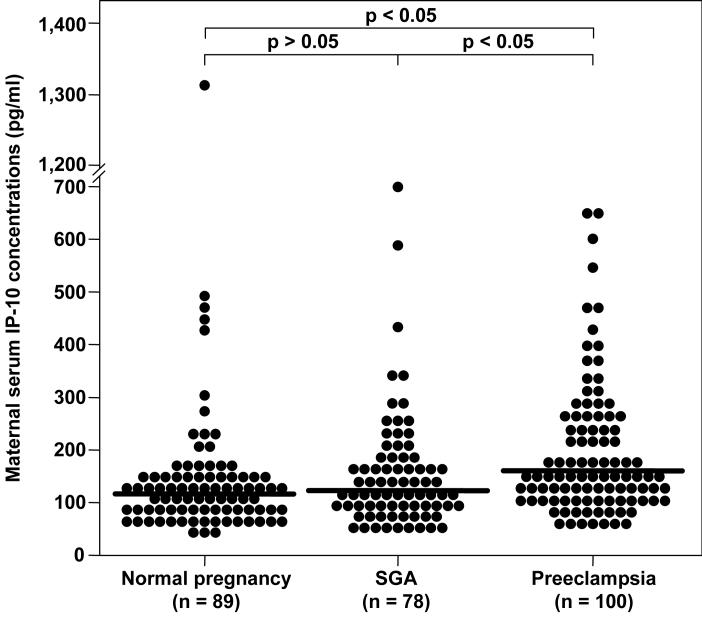
Patients with preeclampsia had a higher median serum concentration of IP-10 than normal pregnant women and those who delivered a SGA neonate (preeclampsia: median 156.4 pg/mLl, range 47.4-645.9; normal pregnancy: median 116.1 pg/mL, range 40.7-1314.3; SGA: median 122.4 pg/mLl, range 37.3-693.5; p<0.05 for both comparisons). In contrast, there were no significant differences in the maternal serum median IP-10 concentrations between patients who delivered an SGA neonate and those with normal pregnancies (SGA: median 122.4 pg/mll, range 37.3-693.5 vs. normal pregnancy: median 116.1 pg/mL, range 40.7-1314.3; p>0.05) (see Figure 2).
Figure 2.
Maternal serum concentrations of CXCL10/IP-10 among the study groups. Patients with preeclampsia had a significantly higher median serum concentration of IP-10 than normal pregnant women (median: 156.4 pg/mL, range: 47.4-645.9 vs. median: 116.1 pg/mL, range: 40.7-1314.3, respectively; p<0.05) and than patients who delivered a SGA neonate (median: 156.4 pg/mL, range: 47.4-645.9 vs. median: 122.4 pg/mL, range: 37.3-693.5, respectively; p<0.05). No significant differences were found in maternal serum median IP-10 concentrations between patients who delivered a SGA neonate and those with normal pregnancies (median: 122.4 pg/mL, range: 37.3-693.5 vs. median: 116.1 pg/mL, range: 40.7-1314.3, respectively; p>0.05).
Among patients with preeclampsia, no significant differences in serum concentrations of IP-10 were observed between patients with early onset and late onset disease (early onset preeclampsia: median 155.6 pg/mL, range 47.5-645.9 vs. late onset preeclampsia: median 165.2 pg/mL, range 64.7-401.1; p=0.4). Similarly, there were no significant differences between mild and severe preeclampsia (mild preeclampsia: median 177.7 pg/mL, range 79.4-401.1 vs. severe preeclampsia: median 155.5 pg/mL, range 47.5-645.9; p=0.56).
Among patients who delivered an SGA neonate, there were no significant differences in serum concentrations of IP-10 between patients who delivered a neonate with a birth weight at < 5th percentile and those who delivered a neonate with a birth weight between 5th and 9th percentile (SGA <5th percentile, median: 126.7 pg/ml, range: 37.3-693.5 vs. SGA 5th-9th percentile, median: 108.7 pg/ml, range: 40.7-349.5; p=0.2).
Using analysis of covariance (ANCOVA), only diagnostic groups but not storage time had a significant effect on the serum concentrations of CXCL10 (storage time: p=0.5; diagnostic groups: p < 0.001). Storage time did not have a significant effect on the serum concentrations of CXCL10 even when gestational age at blood draw was included in the analysis (storage time: p=0.72; diagnostic groups: p=0.02; gestational age at blood draw: p=0.5).
Discussion
Principal findings of this study
(1) The median serum concentration of IP-10 in normal pregnancy was higher than that of non-pregnant women; (2) there was no relationship between gestational age and the maternal serum concentration of IP-10; (3) preeclampsia, but not SGA, was associated with a higher median concentration of maternal serum IP-10. These results are novel and suggest that IP-10 may participate in the pathophysiology of preeclampsia.
What is CXCL10/IP-10?
IP-10 (CXCL10) is a chemokine of the CXC family [48], which was first described as the product of a gene induced in response to recombinant IFN-γ in several cell populations, including U937 histiocitic lymphoma, human fibroblasts, mononuclear and endothelial cells [61]. The principal biological activity of chemotactic cytokines, such as IP-10, is regulation and control of the basal homeostatic and inflammatory leukocyte movement [57].
In addition, IP-10 has potent anti-angiogenic properties [54,56,57,59], promotes adhesion, migration and invasion of trophoblast cell [131,132], has an inhibitory effect on early hematopoietic progenitors [133], and regulates intestinal crypt cell renewal in both physiologic conditions and during mucosal regeneration following injury [134].
The biological properties of IP-10 are mediated through the interaction with a transmembrane G protein-coupled receptor, CXCR3 [135,136], shared by two other IFN-γ inducible CXC chemokines—CXCL9 (MIG) and CXCX11 (I-TAC)—whose distinct biological activities are related to different transduction pathways [137-140].
Factors controlling the expression of IP-10 and cell sources
IP-10 gene and protein expression is modulated by pro-inflammatory stimuli. Indeed, IFN-γ is an inducer of the gene, and protein expression of this chemokine by mononuclear cells [61], neutrophils [69,70], eosinophils,[70,141] keratinocytes [61,64,65,67], fibroblasts [61], endothelial cells [61-63], pancreatic β cells [71,72], and animal astrocytes/microglia [66,68], TNFα [70,73-79], IL-1β [70,71], as well as viral [66,81,82], and microbial products [70,80,83-85] can also stimulate the production of IL-10.
Activation of the nuclear factor-kappa B (NF-kB) pathway [81,82,86-89] through ligation of pattern recognition receptors (TLR4 [83] and TLR3 [89]) can also upregulate gene and protein expression of IP-10. Interestingly, IP-10 is considered an ‘NF-kB responsive gene’[142].
IP-10 in inflammation
The pro-inflammatory activity of IP-10 includes: chemotaxis and endothelial adhesion of activated T cells [143] as well as chemotaxis [144] and enhancement of natural killer (NK) cell-mediated cytolysis [144]. However, this chemokine is a poor neutrophil activator [143,145] and there is controversy about its effects on both monocytes [135,143] and B cells [146,147].
CXCR3 receptor expression on T lymphocytes is selective for activated cells [135,143,146,148-151]. Interestingly, analysis of polarized T lymphocytes using specific monoclonal antibodies has demonstrated high CXCR3 expression (mRNA and protein) on Th1 cells and low on Th2 cells [149,152], and this receptor has, therefore, been proposed as a useful clinical maker of circulating Th1-type cells [149,153]. Further evidence that IP-10 promotes a Th1-like dominance is the observation that, following stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin, the production of IFN-γ (a Th1-type chemokine) resides exclusively in CXCR3-expressing memory CD4+ T cells, whereas the production of Th2-type cytokines is mainly observed in those CXCR3-negative [153]. In addition, in vitro assays show that, in polyclonally stimulated T cells, recombinant IP-10 selectively enhances IFN-γ protein synthesis, while having no effect on IL-4 production [154]. The capability of IP-10 to enhance its own inducer, IFN-γ, supports the hypothesis that a positive amplification loop between IP-10 and IFN-γ also exists in vivo[154].
IP-10 in pathologic states
IP-10 has been implicated in states characterized by prominent T cell response [155,156], particularly when a Th1/Th2 imbalance is involved, including: (1) multiple sclerosis, where the serum and cerebrospinal fluid concentrations of IP10 correlate with the disease activity and CXCR3 expression is detectable on the majority of CNS-infiltrating lymphocytes [104-108]; (2) herpetic encephalitis in mice [157,158]; (3) experimental autoimmune encephalomyelitis [159-162]; (4) inflammatory bowel/colon disease [134,163,164]; (5) chronic hepatitis C, in which IP-10 serum concentrations are related to the inflammatory activity and the response to therapy [165]; (6) Sjogren’s syndrome, where the expression of IP-10 mRNA is significantly up-regulated in salivary glands (p<0.01) and IP-10 has a potential role in the accumulation of T cells infiltrates [166]; (7) type 1 diabetes, whose immunopathogenesis is likely to be linked to the IP-10 [109,110] property of enhancing the traffic of auto-aggressive cells to the pancreas [167] and imprinting a pattern for the subsequent development of the autoimmune disease [109]; (8) Graves’ disease [111-114] and autoimmune thyroiditis [115,116], where high concentrations of IP-10 have been detected in the serum of affected individuals; and (9) systemic lupus erythematosus, where IP-10 plasma concentrations not only are higher than in non-diseased individuals but also correlate with the disease activity [168].
IP-10 as an inhibitor of angiogenesis
IP-10 has potent anti-angiogenic properties [54,56,57,59]. Several mechanisms have been proposed to mediate these activities: (1) interaction with the CXCR3 receptor [169,170]; (2) binding to a cell surface heparan sulfate site shared with platelet factor 4 [124]; (3) interference with the pro-angiogenic activity of basic fibroblast growth factor (bFGF) and IL-8 [124]; and (4) through another high affinity receptor (different from CXCR3 and glycosaminoglycans) [171].
The IP-10 receptor CXCR3 (mRNA and protein) has been localized on the endothelial cells of several human tissues (kidney, gut, liver, thyroid and thymus) [170,172]. Interestingly, the endothelial cell expression is cell-cycle dependent [170]. Indeed, staining is remarkably more frequent in conditions of activation and in the presence of a high proliferative rate (such as in inflamed and neoplastic tissues rather than in normal tissues), particularly during the S/G2-M phase of the endothelial cell cycle [170]. Romagnani et al.[170] suggested that these results represent evidence that CXCR3 is mediating the angiostatic activity of IP-10, thus controlling endothelial cell proliferation. In addition, two distinct isoforms of this receptor, CXCR3-A and CXCR3-B (both products of alternative splicing) are likely to mediate opposite effects on cellular proliferation [173,174].
A specific region of the IP-10 molecule has been linked to its anti-angiogenic effect. Indeed, an aminoterminal truncated form of IP-10, resulting from a post-translational processing, has impaired receptor signaling and lymphocyte chemotaxis, but retains anti-angiogenic properties [175].
Evidence in support of the anti-angiogenic role of IP-10 includes: (1) IP-10, added to human umbilical cord vein endothelial cells (HUVECs), cultured on a matrigel substrate, inhibits their differentiation into tube-like structures in a dose-dependent fashion [125] and reduces the extent of the neo-vascular network [126]; (2) IP-10, both in vitro[123,124] and in vivo [123,125] inhibits in a dose-dependent manner the pro-angiogenic effects of IL-8 and bFGF, including endothelial cells chemotaxis and proliferation as well as neovascularization in animal models of angiogenesis (rat cornea [123] or matrigel injected in the subcutaneous tissue of nude mice [125]); (3) recombinant IP-10 can inhibit [3H]-thymidine incorporation into HUVECs cultured with bFGF [124]. Further support of the anti-angiogenic properties of IP-10 is the role attributed to this chemokine in the pathogenesis of pulmonary fibrosis. Indeed, the lower production of IP-10 in lung tissue of patients with pulmonary fibrosis, as determined by ELISA, has been proposed to contribute to the greater angiogenic activity observed in these patients [117,119]. Additionally, when IP-10 is administered systemically to bleomycin pre-treated mice, it inhibits fibroplasia and deposition of extracellular matrix through the regulation of the local angiogenesis, resulting in a significant attenuation of the severity of the pulmonary fibrosis induced by this chemical [118]. This experimental evidence indicates that IP-10 has an important anti-angiogenic effect.
Evidence that the anti-angiogenic properties of IP-10 may have therapeutic value is the observation that it inhibits tumor growth. Observations in support of this include: (1) Burkitt’s tumors implanted into athymic mice, once injected or transfected with IP-10, show histological evidence of tissue necrosis, capillary damage, intimal thickening and vascular trombosis [176]; (2) high serum concentrations of IP-10 have been reported in patients with lymphoproliferative disorders [177]; (3) tumors derived from IP-10 transduced melanoma cells have a reduced in vivo growth compared to those originating from parental or null-transduced cells (p=0.0002) and the growth inhibition is associated with a marked reduction in microvessel density [178]; (4) the angiostatic activity of IP-10 in human melanoma cell line appears to depend on binding to CXCR3 [169]; (5) tumors originating from human fibrosarcoma cell lines, secreting IP-10 upon vector transduction, and implanted into mice, have histological evidence of a significantly lower number of microvessels than controls (p=0.01) [126]; (6) recombinant IP-10 injection into non-small cell lung cancers grown in SCID (severe combined immunodeficiency) mice reduces their angiogenesis, growth, and incidence of spontaneous metastasis, whilst IP-10 neutralization results in enhanced tumor-derived angiogenic activity. Furthermore, plasma or tumor-associated IP-10 concentrations are inversely correlated to tumor growth. Extracts from these tumors decrease the angiogenic activity in the corneal micropocket assay [179]; and (7) IP-10 contributes to the anti-angiogenic and anti-tumor properties of IL-12 [180-183].
In summary, this evidence indicates that IP-10 plays an important anti-angiogenic role.
IP-10 concentration in serum during normal pregnancy
The observation that normal pregnant women have a significantly higher median serum concentration of IP-10 than non-pregnant women is novel. This observation is consistent with the findings that normal pregnancy is associated with systemic intravascular inflammation [35,184-187]. Additional evidence in support of this view is the observation that pregnancy is associated with higher (serum or plasma) concentrations of IL12 [186] TNF-α [188] than the non-pregnant state.
IP-10, inflammation and preeclampsia
The observation that preeclampsia is associated with a higher maternal serum concentration of IP-10 than SGA and normal pregnancy is novel, as well as being consistent with the view that preeclampsia is characterized by an exaggerated intravascular pro-inflammatory state [18,33-37].
Furthermore, accumulating evidence indicates that preeclampsia is associated with a predominant Th1 immune response, including: (1) high maternal plasma or serum concentrations of IL-2 [38], TNF-α [40,41,189-191], IFN-γ [192], and IL-12 [42,193]; (2) low maternal plasma or serum concentrations of IL-10 [194], and of IL-4 [192] (although this topic is subject to some controversy) [195]; and (3) up-regulation of mRNA and protein expression of IL-1β [196] and TNF-α [196,197] in the placenta. Thus, the high maternal plasma concentration of this chemokine in patients with preeclampsia may represent yet another feature of a pro-inflammatory state or intravascular inflammation or even contribute to the generation of such state. Some evidence also indicates that SGA may be associated with intravascular inflammation [47]. However, the results presented herein do not implicate IP-10 in such process.
IP-10, preeclampsia and anti-angiogenesis
An imbalance between pro-angiogenic and anti-angiogenic factors is involved in the pathophysiology of preeclampsia [25-27,198-218]. The group of Maynard and Karumanchi has recently made a major set of observations that favor this hypothesis [219]. Evidence in support of this includes: (1) over-expression of s-VEGFR-1 mRNA and protein in placenta of patients with preeclampsia [27,219]; (2) higher median plasma/serum concentration of sVEGFR-1 in preeclampsia at the time of diagnosis than in patients with normal pregnancies [27,219,220], and the plasma concentration correlates with the severity of the disease [28]; (3) preeclampsia is associated with decreased plasma/serum concentrations of VEGF and PlGF [25,211,219,221]; (4) serum of pregnant women with preeclampsia has anti-angiogenic effects in the endothelial cell tube formation bioassay and these effects can be restored by the addition of VEGF and PlGF [219]; and (5) administration of sVEGFR-1 to pregnant animals can induce the clinical manifestation of preeclampsia, including hypertension and proteinuria [219]. Moreover, these animals develop the pathologic finding of glomerular endotheliosis but not pathognomonic of preeclampsia [222,223]. (6) preeclampsia is associated with a higher maternal serum concentration of endoglin at the time of the diagnosis or before the recognition of the disease [31,32].
Given the evidence that IP-10 has anti-angiogenic properties and that this is a feature of preeclampsia, we propose that elevated IP-10 maternal serum concentrations may contribute to generating an anti-angiogenic state along with sVEGF-R1 and endoglin.
IP-10, preeclampsia and allograft rejection
Allograft rejection has also been proposed as a mechanism of disease in preeclampsia [224]. Of note, there is growing evidence that IP-10 is also involved in the process of graft rejection, including: (1) CXCR3 deficient (-/-) mice are resistant to both acute and chronic allograft rejection [91]; (2) grafts from IP-10 (-/-) donors are less likely to undergo graft injury [90]; (3) treatment with anti-CXCR3 [91] or anti CXCL10 [96] monoclonal antibodies results in prolongation of cardiac and small bowel allograft survival in mice; (4) clinical rejection of human renal [92,100], lung [93], cardiac [94,95], small bowel [99], and arteries [102] transplants is associated with intra-graft over expression of IP-10 and/or with intra-graft recruitment of CXCR3 positive T cells; and (5) pre-transplant serum [97,103] and urine [98,101] CXCL10 cellular mRNA or protein concentrations identify patients at risk for the development of acute rejection and/or chronic allograft nephropaty. Collectively, this evidence indicates that IP-10 is involved in the pathogenesis of allograph rejection. Whether or not this mechanism of disease operates in preeclampsia remains an interesting concept. Our observation that IP-10 is elevated in preeclampsia provides a possible link between preeclampsia and this potential pathologic process.
IP-10, preeclampsia and atherosclerosis
Striking parallels exist between preeclampsia and atherosclerosis (see bibliography for details). Patients who develop preterm preeclampsia are at increased risk of death from coronary artery disease later on in life [225]. Moreover, atherosis of the spiral arteries, a lesion observed in patients with preeclampsia, has striking similarities with that of coronary artery disease [226-228].
The current view of atherosclerosis is that it is an inflammatory mediated process [229-231]. Th1 biased responses are thought to be pro-atherogenic, while Th2 biased immunoresponses confer atheroprotection [232]. Chemokine-dependent migration of cells through the inflamed endothelium into the arterial intima has been proposed to be critical in atherosclerosis [233-237]. There is evidence of a specific involvement of IP-10 in the pathogenesis of atherosclerosis and cardiovascular diseases. Such evidence includes: (1) patients with stable angiographically confirmed coronary heart disease have a higher mean IP-10 serum concentration than control patients, and the magnitude of the elevation correlates with the concentration of several acute-phase proteins (C-reactive proteins) or cytokines known to be central in the pathogenesis of atherosclerosis [122]; (2) serum IP-10 baseline concentrations are significantly higher in individuals who develop coronary heart disease (CHD) than in those who do not (follow-up of 11 years). After adjustment for cardiovascular and immunological risk factors, however, the observed relationship with IP-10 disappeared, suggesting that the association may be explained by other markers of inflammation and not be specific to IP-10. Nonetheless, this observation is important because it suggests that an elevation of IP-10 precedes the development of CHD, thus strengthening the case that inflammation has a causal role rather being a consequence of atherosclerosis [121]; (3) CXCR3-bearing T cells and chemokines IP-10, I-TAC and MIG (all three IFN-γ induced) are present in atherosclerotic plaques [120].
Conclusion
Preeclampsia is associated with higher maternal plasma concentrations of IP-10 than normal pregnancy and SGA. These results suggest that this chemokine may contribute to both the exaggerated systemic inflammation and the anti-angiogenic state that characterize preeclampsia.
Acknowledgment
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J.Pathol. 1970;101:vi. [PubMed] [Google Scholar]
- 2.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br.J.Obstet.Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 3.De WF, Brosens I, Renaer M. Fetal growth retardation and the maternal arterial supply of the human placenta in the absence of sustained hypertension. Br.J.Obstet.Gynaecol. 1980;87:678–685. doi: 10.1111/j.1471-0528.1980.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 4.Khong TY, De WF, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br.J.Obstet.Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van AA. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br.J.Obstet.Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 6.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van AA. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br.J.Obstet.Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 7.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am.J.Obstet.Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 8.Espinoza J, Romero R, Mee KY, Kusanovic JP, Hassan S, Erez O, Gotsch F, Than NG, Papp Z, Jai KC. Normal and abnormal transformation of the spiral arteries during pregnancy. J.Perinat.Med. 2006;34:447–458. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell S, az-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K, Teague MJ. New doppler technique for assessing uteroplacental blood flow. Lancet. 1983;1:675–677. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- 10.Harrington KF, Campbell S, Bewley S, Bower S. Doppler velocimetry studies of the uterine artery in the early prediction of pre-eclampsia and intra-uterine growth retardation. Eur.J.Obstet.Gynecol.Reprod.Biol. 1991;42(Suppl):S14–S20. [PubMed] [Google Scholar]
- 11.Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br.J.Obstet.Gynaecol. 1993;100:989–994. doi: 10.1111/j.1471-0528.1993.tb15139.x. [DOI] [PubMed] [Google Scholar]
- 12.Harrington K, Cooper D, Lees C, Hecher K, Campbell S. Doppler ultrasound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound Obstet.Gynecol. 1996;7:182–188. doi: 10.1046/j.1469-0705.1996.07030182.x. [DOI] [PubMed] [Google Scholar]
- 13.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am.J.Obstet.Gynecol. 1998;179:1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 14.Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet.Gynecol. 2000;96:559–564. doi: 10.1016/s0029-7844(00)00946-7. [DOI] [PubMed] [Google Scholar]
- 15.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet.Gynecol. 2001;18:441–449. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 16.Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best.Pract.Res.Clin.Obstet.Gynaecol. 2004;18:383–396. doi: 10.1016/j.bpobgyn.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am.J.Obstet.Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 18.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am.J.Obstet.Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 19.Bretelle F, Sabatier F, Blann A, D’Ercole C, Boutiere B, Mutin M, Boubli L, Sampol J, gnat-George F. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG. 2001;108:1277–1282. doi: 10.1111/j.1471-0528.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 20.Poston L, Chappell LC. Is oxidative stress involved in the aetiology of pre-eclampsia? Acta Paediatr.Suppl. 2001;90:3–5. doi: 10.1111/j.1651-2227.2001.tb01619.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson MR, nim-Nyame N, Johnson P, Sooranna SR, Steer PJ. Does endothelial cell activation occur with intrauterine growth restriction? BJOG. 2002;109:836–839. doi: 10.1111/j.1471-0528.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JM, Lain KY. Recent Insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 23.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 24.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am.J.Obstet.Gynecol. 2006;195:40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 25.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am.J.Obstet.Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am.J.Obstet.Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 27.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J.Clin.Endocrinol.Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am.J.Obstet.Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N.Engl.J.Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 30.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J.Clin.Endocrinol.Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 31.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N.Engl.J.Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat.Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 33.Barden A, Graham D, Beilin LJ, Ritchie J, Baker R, Walters BN, Michael CA. Neutrophil CD11B expression and neutrophil activation in pre-eclampsia. Clin.Sci.(Lond) 1997;92:37–44. doi: 10.1042/cs0920037. [DOI] [PubMed] [Google Scholar]
- 34.Haller H, Ziegler EM, Homuth V, Drab M, Eichhorn J, Nagy Z, Busjahn A, Vetter K, Luft FC. Endothelial adhesion molecules and leukocyte integrins in preeclamptic patients. Hypertension. 1997;29:291–296. doi: 10.1161/01.hyp.29.1.291. [DOI] [PubMed] [Google Scholar]
- 35.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am.J.Obstet.Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 36.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am.J.Obstet.Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 37.Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, Romero R. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am.J.Obstet.Gynecol. 2002;187:889–893. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- 38.Sunder-Plassmann G, Derfler K, Wagner L, Stockenhuber F, Endler M, Nowotny C, Balcke P. Increased serum activity of interleukin-2 in patients with pre-eclampsia. J.Autoimmun. 1989;2:203–205. doi: 10.1016/0896-8411(89)90156-x. [DOI] [PubMed] [Google Scholar]
- 39.Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet.Gynecol. 1994;84:937–940. [PubMed] [Google Scholar]
- 40.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br.J.Obstet.Gynaecol. 1995;102:20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 41.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am.J.Reprod.Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 42.Daniel Y, Kupferminc MJ, Baram A, Jaffa AJ, Fait G, Wolman I, Lessing JB. Plasma interleukin-12 is elevated in patients with preeclampsia. Am.J.Reprod.Immunol. 1998;39:376–380. doi: 10.1111/j.1600-0897.1998.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 43.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, Morikawa H. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am.J.Reprod.Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 44.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin.Exp.Immunol. 1999;117:550–555. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur.J.Obstet.Gynecol.Reprod.Biol. 1999;86:165–170. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 46.Sakai M, Ogawa K, Shiozaki A, Yoneda S, Sasaki Y, Nagata K, Saito S. Serum granulysin is a marker for Th1 type immunity in pre-eclampsia. Clin.Exp.Immunol. 2004;136:114–119. doi: 10.1111/j.1365-2249.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet.Gynecol.Scand. 2003;82:1099–1102. doi: 10.1046/j.1600-0412.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 48.Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207–219. doi: 10.1016/s1359-6101(97)00015-4. [DOI] [PubMed] [Google Scholar]
- 49.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van DJ, Walz A, Marriott D. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J.Biol.Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 50.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Strieter RM, Polverini PJ, Arenberg DA, Walz A, Opdenakker G, Van DJ, Kunkel SL. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J.Leukoc.Biol. 1995;57:752–762. doi: 10.1002/jlb.57.5.752. [DOI] [PubMed] [Google Scholar]
- 52.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 53.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N.Engl.J.Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 54.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J.Leukoc.Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 55.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu.Rev.Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 56.Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. Analysis of the role of chemokines in angiogenesis. J.Immunol.Methods. 2003;273:83–101. doi: 10.1016/s0022-1759(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 57.Rosenkilde MM, Schwartz TW. The chemokine system -- a major regulator of angiogenesis in health and disease. APMIS. 2004;112:481–495. doi: 10.1111/j.1600-0463.2004.apm11207-0808.x. [DOI] [PubMed] [Google Scholar]
- 58.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N.Engl.J.Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 61.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 62.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) J.Exp.Med. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luster AD, Ravetch JV. Genomic characterization of a gamma-interferon-inducible gene (IP-10) and identification of an interferon-inducible hypersensitive site. Mol.Cell Biol. 1987;7:3723–3731. doi: 10.1128/mcb.7.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan G, Luster AD, Hancock G, Cohn ZA. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J.Exp.Med. 1987;166:1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottlieb AB, Luster AD, Posnett DN, Carter DM. Detection of a gamma interferon-induced protein IP-10 in psoriatic plaques. J.Exp.Med. 1988;168:941–948. doi: 10.1084/jem.168.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanguri P, Farber JM. IFN and virus-inducible expression of an immediate early gene, crg-2/IP-10, and a delayed gene, I-A alpha in astrocytes and microglia. J.Immunol. 1994;152:1411–1418. [PubMed] [Google Scholar]
- 67.Sarris AH, Esgleyes-Ribot T, Crow M, Broxmeyer HE, Karasavvas N, Pugh W, Grossman D, Deisseroth A, Duvic M. Cytokine loops involving interferon-gamma and IP-10, a cytokine chemotactic for CD4+ lymphocytes: an explanation for the epidermotropism of cutaneous T-cell lymphoma? Blood. 1995;86:651–658. [PubMed] [Google Scholar]
- 68.Vanguri P. Interferon-gamma-inducible genes in primary glial cells of the central nervous system: comparisons of astrocytes with microglia and Lewis with brown Norway rats. J.Neuroimmunol. 1995;56:35–43. doi: 10.1016/0165-5728(94)00131-7. [DOI] [PubMed] [Google Scholar]
- 69.Cassatella MA, Gasperini S, Calzetti F, Bertagnin A, Luster AD, McDonald PP. Regulated production of the interferon-gamma-inducible protein-10 (IP-10) chemokine by human neutrophils. Eur.J.Immunol. 1997;27:111–115. doi: 10.1002/eji.1830270117. [DOI] [PubMed] [Google Scholar]
- 70.Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J.Immunol. 1999;162:4928–4937. [PubMed] [Google Scholar]
- 71.Frigerio S, Junt T, Lu B, Gerard C, Zumsteg U, Hollander GA, Piali L. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat.Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 72.Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia. 2003;46:255–266. doi: 10.1007/s00125-002-1017-0. [DOI] [PubMed] [Google Scholar]
- 73.Narumi S, Yoneyama H, Inadera H, Nishioji K, Itoh Y, Okanoue T, Matsushima K. TNF-alpha is a potent inducer for IFN-inducible protein-10 in hepatocytes and unaffected by GM-CSF in vivo, in contrast to IL-1beta and IFN-gamma. Cytokine. 2000;12:1007–1016. doi: 10.1006/cyto.1999.0672. [DOI] [PubMed] [Google Scholar]
- 74.Kraft M, Riedel S, Maaser C, Kucharzik T, Steinbuechel A, Domschke W, Luegering N. IFN-gamma synergizes with TNF-alpha but not with viable H. pylori in up-regulating CXC chemokine secretion in gastric epithelial cells. Clin.Exp.Immunol. 2001;126:474–481. doi: 10.1046/j.1365-2249.2001.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Algood HM, Lin PL, Yankura D, Jones A, Chan J, Flynn JL. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J.Immunol. 2004;172:6846–6857. doi: 10.4049/jimmunol.172.11.6846. [DOI] [PubMed] [Google Scholar]
- 76.Villagomez MT, Bae SJ, Ogawa I, Takenaka M, Katayama I. Tumour necrosis factor-alpha but not interferon-gamma is the main inducer of inducible protein-10 in skin fibroblasts from patients with atopic dermatitis. Br.J.Dermatol. 2004;150:910–916. doi: 10.1111/j.1365-2133.2004.05937.x. [DOI] [PubMed] [Google Scholar]
- 77.Hardaker EL, Bacon AM, Carlson K, Roshak AK, Foley JJ, Schmidt DB, Buckley PT, Comegys M, Panettieri RA, Jr., Sarau HM, Belmonte KE. Regulation of TNF-alpha- and IFN-gamma-induced CXCL10 expression: participation of the airway smooth muscle in the pulmonary inflammatory response in chronic obstructive pulmonary disease. FASEB J. 2004;18:191–193. doi: 10.1096/fj.03-0170fje. [DOI] [PubMed] [Google Scholar]
- 78.Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J.Leukoc.Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- 79.Berthier-Vergnes O, Bermond F, Flacher V, Massacrier C, Schmitt D, Peguet-Navarro J. TNF-alpha enhances phenotypic and functional maturation of human epidermal Langerhans cells and induces IL-12 p40 and IP-10/CXCL-10 production. FEBS Lett. 2005;579:3660–3668. doi: 10.1016/j.febslet.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 80.Shin HS, Drysdale BE, Shin ML, Noble PW, Fisher SN, Paznekas WA. Definition of a lipopolysaccharide-responsive element in the 5′-flanking regions of MuRantes and crg-2. Mol.Cell Biol. 1994;14:2914–2925. doi: 10.1128/mcb.14.5.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nazar AS, Cheng G, Shin HS, Brothers PN, Dhib-Jalbut S, Shin ML, Vanguri P. Induction of IP-10 chemokine promoter by measles virus: comparison with interferon-gamma shows the use of the same response element but with differential DNA-protein binding profiles. J.Neuroimmunol. 1997;77:116–127. doi: 10.1016/s0165-5728(97)00070-2. [DOI] [PubMed] [Google Scholar]
- 82.Cheng G, Nazar AS, Shin HS, Vanguri P, Shin ML. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent kappaB site but not IRF-1 or viral transcription. J.Interferon Cytokine Res. 1998;18:987–997. doi: 10.1089/jir.1998.18.987. [DOI] [PubMed] [Google Scholar]
- 83.Gasper NA, Petty CC, Schrum LW, Marriott I, Bost KL. Bacterium-induced CXCL10 secretion by osteoblasts can be mediated in part through toll-like receptor 4. Infect.Immun. 2002;70:4075–4082. doi: 10.1128/IAI.70.8.4075-4082.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen Q, Zhang R, Bhat NR. MAP kinase regulation of IP10/CXCL10 chemokine gene expression in microglial cells. Brain Res. 2006;1086:9–16. doi: 10.1016/j.brainres.2006.02.116. [DOI] [PubMed] [Google Scholar]
- 85.Durand SH, Flacher V, Romeas A, Carrouel F, Colomb E, Vincent C, Magloire H, Couble ML, Bleicher F, Staquet MJ, Lebecque S, Farges JC. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J.Immunol. 2006;176:2880–2887. doi: 10.4049/jimmunol.176.5.2880. [DOI] [PubMed] [Google Scholar]
- 86.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J.Biol.Chem. 1993;268:6677–6688. [PubMed] [Google Scholar]
- 87.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J.Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 88.Osawa Y, Iho S, Takauji R, Takatsuka H, Yamamoto S, Takahashi T, Horiguchi S, Urasaki Y, Matsuki T, Fujieda S. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J.Immunol. 2006;177:4841–4852. doi: 10.4049/jimmunol.177.7.4841. [DOI] [PubMed] [Google Scholar]
- 89.Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 90.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J.Exp.Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J.Exp.Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segerer S, Cui Y, Eitner F, Goodpaster T, Hudkins KL, Mack M, Cartron JP, Colin Y, Schlondorff D, Alpers CE. Expression of chemokines and chemokine receptors during human renal transplant rejection. Am.J.Kidney Dis. 2001;37:518–531. [PubMed] [Google Scholar]
- 93.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am.J.Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melter M, Exeni A, Reinders ME, Fang JC, McMahon G, Ganz P, Hancock WW, Briscoe DM. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 95.Zhao DX, Hu Y, Miller GG, Luster AD, Mitchell RN, Libby P. Differential expression of the IFN-gamma-inducible CXCR3-binding chemokines, IFN-inducible protein 10, monokine induced by IFN, and IFN-inducible T cell alpha chemoattractant in human cardiac allografts: association with cardiac allograft vasculopathy and acute rejection. J.Immunol. 2002;169:1556–1560. doi: 10.4049/jimmunol.169.3.1556. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z, Kaptanoglu L, Haddad W, Ivancic D, Alnadjim Z, Hurst S, Tishler D, Luster AD, Barrett TA, Fryer J. Donor T cell activation initiates small bowel allograft rejection through an IFN-gamma-inducible protein-10-dependent mechanism. J.Immunol. 2002;168:3205–3212. doi: 10.4049/jimmunol.168.7.3205. [DOI] [PubMed] [Google Scholar]
- 97.Rotondi M, Rosati A, Buonamano A, Lasagni L, Lazzeri E, Pradella F, Fossombroni V, Cirami C, Liotta F, La VG, Serio M, Bertoni E, Salvadori M, Romagnani P. High pretransplant serum levels of CXCL10/IP-10 are related to increased risk of renal allograft failure. Am.J.Transplant. 2004;4:1466–1474. doi: 10.1111/j.1600-6143.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 98.Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am.J.Transplant. 2004;4:432–437. doi: 10.1111/j.1600-6143.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Z, Kaptanoglu L, Tang Y, Ivancic D, Rao SM, Luster A, Barrett TA, Fryer J. IP-10-induced recruitment of CXCR3 host T cells is required for small bowel allograft rejection. Gastroenterology. 2004;126:809–818. doi: 10.1053/j.gastro.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 100.Panzer U, Reinking RR, Steinmetz OM, Zahner G, Sudbeck U, Fehr S, Pfalzer B, Schneider A, Thaiss F, Mack M, Conrad S, Huland H, Helmchen U, Stahl RA. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–1350. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 101.Tatapudi RR, Muthukumar T, Dadhania D, Ding R, Li B, Sharma VK, Lozada-Pastorio E, Seetharamu N, Hartono C, Serur D, Seshan SV, Kapur S, Hancock WW, Suthanthiran M. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 2004;65:2390–2397. doi: 10.1111/j.1523-1755.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 102.Burns WR, Wang Y, Tang PC, Ranjbaran H, Iakimov A, Kim J, Cuffy M, Bai Y, Pober JS, Tellides G. Recruitment of CXCR3+ and CCR5+ T cells and production of interferon-gamma-inducible chemokines in rejecting human arteries. Am.J.Transplant. 2005;5:1226–1236. doi: 10.1111/j.1600-6143.2005.00892.x. [DOI] [PubMed] [Google Scholar]
- 103.Lazzeri E, Rotondi M, Mazzinghi B, Lasagni L, Buonamano A, Rosati A, Pradella F, Fossombroni V, La VG, Gacci M, Bertoni E, Serio M, Salvadori M, Romagnani P. High CXCL10 expression in rejected kidneys and predictive role of pretransplant serum CXCL10 for acute rejection and chronic allograft nephropathy. Transplantation. 2005;79:1215–1220. doi: 10.1097/01.tp.0000160759.85080.2e. [DOI] [PubMed] [Google Scholar]
- 104.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM, Frederiksen JL, Ransohoff RM. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J.Clin.Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc.Natl.Acad.Sci.U.S.A. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol.Appl.Neurobiol. 2000;26:133–142. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- 107.Franciotta D, Martino G, Zardini E, Furlan R, Bergamaschi R, Andreoni L, Cosi V. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J.Neuroimmunol. 2001;115:192–198. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 108.Trebst C, Ransohoff RM. Investigating chemokines and chemokine receptors in patients with multiple sclerosis: opportunities and challenges. Arch.Neurol. 2001;58:1975–1980. doi: 10.1001/archneur.58.12.1975. [DOI] [PubMed] [Google Scholar]
- 109.Christen U, McGavern DB, Luster AD, Von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J.Immunol. 2003;171:6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 110.Christen U, Von Herrath MG. IP-10 and type 1 diabetes: a question of time and location. Autoimmunity. 2004;37:273–282. [PubMed] [Google Scholar]
- 111.Romagnani P, Rotondi M, Lazzeri E, Lasagni L, Francalanci M, Buonamano A, Milani S, Vitti P, Chiovato L, Tonacchera M, Bellastella A, Serio M. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves’ disease. Am.J.Pathol. 2002;161:195–206. doi: 10.1016/S0002-9440(10)64171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Antonelli A, Fallahi P, Rotondi M, Ferrari SM, Serio M, Miccoli P. Serum levels of the interferon-gamma-inducible alpha chemokine CXCL10 in patients with active Graves’ disease, and modulation by methimazole therapy and thyroidectomy. Br.J.Surg. 2006;93:1226–1231. doi: 10.1002/bjs.5401. [DOI] [PubMed] [Google Scholar]
- 113.Antonelli A, Fallahi P, Rotondi M, Ferrari SM, Romagnani P, Grosso M, Ferrannini E, Serio M. Increased serum CXCL10 in Graves’ disease or autoimmune thyroiditis is not associated with hyper- or hypothyroidism per se, but is specifically sustained by the autoimmune, inflammatory process. Eur.J.Endocrinol. 2006;154:651–658. doi: 10.1530/eje.1.02137. [DOI] [PubMed] [Google Scholar]
- 114.Antonelli A, Rotondi M, Fallahi P, Romagnani P, Ferrari SM, Barani L, Ferrannini E, Serio M. Increase of interferon-gamma-inducible CXC chemokine CXCL10 serum levels in patients with active Graves’ disease, and modulation by methimazole therapy. Clin.Endocrinol.(Oxf) 2006;64:189–195. doi: 10.1111/j.1365-2265.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 115.Antonelli A, Rotondi M, Fallahi P, Romagnani P, Ferrari SM, Buonamano A, Ferrannini E, Serio M. High levels of circulating CXC chemokine ligand 10 are associated with chronic autoimmune thyroiditis and hypothyroidism. J.Clin.Endocrinol.Metab. 2004;89:5496–5499. doi: 10.1210/jc.2004-0977. [DOI] [PubMed] [Google Scholar]
- 116.Antonelli A, Rotondi M, Fallahi P, Romagnani P, Ferrari SM, Paolicchi A, Ferrannini E, Serio M. Increase of interferon-gamma inducible alpha chemokine CXCL10 but not beta chemokine CCL2 serum levels in chronic autoimmune thyroiditis. Eur.J.Endocrinol. 2005;152:171–177. doi: 10.1530/eje.1.01847. [DOI] [PubMed] [Google Scholar]
- 117.Keane MP, Arenberg DA, Lynch JP, III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J.Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- 118.Keane MP, Belperio JA, Arenberg DA, Burdick MD, Xu ZJ, Xue YY, Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J.Immunol. 1999;163:5686–5692. [PubMed] [Google Scholar]
- 119.Strieter RM, Belperio JA, Keane MP. CXC chemokines in angiogenesis related to pulmonary fibrosis. Chest. 2002;122:298S–301S. doi: 10.1378/chest.122.6_suppl.298s. [DOI] [PubMed] [Google Scholar]
- 120.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J.Clin.Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herder C, Baumert J, Thorand B, Martin S, Lowel H, Kolb H, Koenig W. Chemokines and incident coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984-2002. Arterioscler.Thromb.Vasc.Biol. 2006;26:2147–2152. doi: 10.1161/01.ATV.0000235691.84430.86. [DOI] [PubMed] [Google Scholar]
- 122.Rothenbacher D, Muller-Scholze S, Herder C, Koenig W, Kolb H. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler.Thromb.Vasc.Biol. 2006;26:194–199. doi: 10.1161/01.ATV.0000191633.52585.14. [DOI] [PubMed] [Google Scholar]
- 123.Strieter RM, Kunkel SL, Arenberg DA, Burdick MD, Polverini PJ. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem.Biophys.Res.Commun. 1995;210:51–57. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 124.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J.Exp.Med. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J.Exp.Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sun Y, Finger C, varez-Vallina L, Cichutek K, Buchholz CJ. Chronic gene delivery of interferon-inducible protein 10 through replication-competent retrovirus vectors suppresses tumor growth. Cancer Gene Ther. 2005;12:900–912. doi: 10.1038/sj.cgt.7700854. [DOI] [PubMed] [Google Scholar]
- 127.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am.J.Obstet.Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 128.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet.Gynecol. 2002 January;99(33):159–167. doi: 10.1016/s0029-7844(01)01747-1. 2002. [DOI] [PubMed] [Google Scholar]
- 129.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, Goldenberg RL, Joffe G, The Calcium for Preeclampsia Prevention (CPEP) Study Group Risk factors associated with preeclampsia in healthy nulliparous women. Am.J.Obstet.Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 130.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 131.Nagaoka K, Nojima H, Watanabe F, Chang KT, Christenson RK, Sakai S, Imakawa K. Regulation of blastocyst migration, apposition, and initial adhesion by a chemokine, interferon gamma-inducible protein 10 kDa (IP-10), during early gestation. J.Biol.Chem. 2003;278:29048–29056. doi: 10.1074/jbc.M300470200. [DOI] [PubMed] [Google Scholar]
- 132.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat.Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 133.Sarris AH, Broxmeyer HE, Wirthmueller U, Karasavvas N, Cooper S, Lu L, Krueger J, Ravetch JV. Human interferon-inducible protein 10: expression and purification of recombinant protein demonstrate inhibition of early human hematopoietic progenitors. J.Exp.Med. 1993;178:1127–1132. doi: 10.1084/jem.178.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sasaki S, Yoneyama H, Suzuki K, Suriki H, Aiba T, Watanabe S, Kawauchi Y, Kawachi H, Shimizu F, Matsushima K, Asakura H, Narumi S. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur.J.Immunol. 2002;32:3197–3205. doi: 10.1002/1521-4141(200211)32:11<3197::AID-IMMU3197>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 135.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J.Exp.Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Curr.Top.Dev.Biol. 2005;68:149–181. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- 137.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J.Exp.Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cox MA, Jenh CH, Gonsiorek W, Fine J, Narula SK, Zavodny PJ, Hipkin RW. Human interferon-inducible 10-kDa protein and human interferon-inducible T cell alpha chemoattractant are allotopic ligands for human CXCR3: differential binding to receptor states. Mol.Pharmacol. 2001;59:707–715. doi: 10.1124/mol.59.4.707. [DOI] [PubMed] [Google Scholar]
- 139.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11) J.Immunol. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 140.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J.Biol.Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 141.Dajotoy T, Andersson P, Bjartell A, Lofdahl CG, Tapper H, Egesten A. Human eosinophils produce the T cell-attracting chemokines MIG and IP-10 upon stimulation with IFN-gamma. J.Leukoc.Biol. 2004;76:685–691. doi: 10.1189/jlb.0803379. [DOI] [PubMed] [Google Scholar]
- 142.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 143.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J.Exp.Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J.Immunol. 1995;155:3877–3888. [PubMed] [Google Scholar]
- 145.Dewald B, Moser B, Barella L, Schumacher C, Baggiolini M, Clark-Lewis I. IP-10, a gamma-interferon-inducible protein related to interleukin-8, lacks neutrophil activating properties. Immunol.Lett. 1992;32:81–84. doi: 10.1016/0165-2478(92)90203-z. [DOI] [PubMed] [Google Scholar]
- 146.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch AE, Moser B, Mackay CR. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J.Clin.Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, Hiepe F, Radbruch A, Arce S, Manz RA. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–3971. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 148.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J.Leukoc.Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 149.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J.Exp.Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Piali L, Weber C, LaRosa G, Mackay CR, Springer TA, Clark-Lewis I, Moser B. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur.J.Immunol. 1998;28:961–972. doi: 10.1002/(SICI)1521-4141(199803)28:03<961::AID-IMMU961>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 151.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur.J.Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 152.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J.Exp.Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yamamoto J, Adachi Y, Onoue Y, Adachi YS, Okabe Y, Itazawa T, Toyoda M, Seki T, Morohashi M, Matsushima K, Miyawaki T. Differential expression of the chemokine receptors by the Th1- and Th2-type effector populations within circulating CD4+ T cells. J.Leukoc.Biol. 2000;68:568–574. [PubMed] [Google Scholar]
- 154.Gangur V, Simons FE, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. FASEB J. 1998;12:705–713. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- 155.Goldberg SH, van der MP, Hesselgesser J, Jaffer S, Kolson DL, Albright AV, Gonzalez-Scarano F, Lavi E. CXCR3 expression in human central nervous system diseases. Neuropathol.Appl.Neurobiol. 2001;27:127–138. doi: 10.1046/j.1365-2990.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 156.Christensen JE, Nansen A, Moos T, Lu B, Gerard C, Christensen JP, Thomsen AR. Efficient T-cell surveillance of the CNS requires expression of the CXC chemokine receptor 3. J.Neurosci. 2004;24:4849–4858. doi: 10.1523/JNEUROSCI.0123-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wickham S, Lu B, Ash J, Carr DJ. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J.Neuroimmunol. 2005;162:51–59. doi: 10.1016/j.jneuroim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 158.Sellner J, Dvorak F, Zhou Y, Haas J, Kehm R, Wildemann B, Meyding-Lamade U. Acute and long-term alteration of chemokine mRNA expression after anti-viral and anti-inflammatory treatment in herpes simplex virus encephalitis. Neurosci.Lett. 2005;374:197–202. doi: 10.1016/j.neulet.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 159.Narumi S, Kaburaki T, Yoneyama H, Iwamura H, Kobayashi Y, Matsushima K. Neutralization of IFN-inducible protein 10/CXCL10 exacerbates experimental autoimmune encephalomyelitis. Eur.J.Immunol. 2002;32:1784–1791. doi: 10.1002/1521-4141(200206)32:6<1784::AID-IMMU1784>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 160.Wildbaum G, Netzer N, Karin N. Plasmid DNA encoding IFN-gamma-inducible protein 10 redirects antigen-specific T cell polarization and suppresses experimental autoimmune encephalomyelitis. J.Immunol. 2002;168:5885–5892. doi: 10.4049/jimmunol.168.11.5885. [DOI] [PubMed] [Google Scholar]
- 161.Klein RS. Regulation of neuroinflammation: the role of CXCL10 in lymphocyte infiltration during autoimmune encephalomyelitis. J.Cell Biochem. 2004;92:213–222. doi: 10.1002/jcb.20052. [DOI] [PubMed] [Google Scholar]
- 162.Klein RS, Izikson L, Means T, Gibson HD, Lin E, Sobel RA, Weiner HL, Luster AD. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J.Immunol. 2004;172:550–559. doi: 10.4049/jimmunol.172.1.550. [DOI] [PubMed] [Google Scholar]
- 163.Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am.J.Pathol. 1999;155:331–336. doi: 10.1016/S0002-9440(10)65128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Singh UP, Singh S, Taub DD, Lillard JW., Jr. Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10-/- mice. J.Immunol. 2003;171:1401–1406. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 165.Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J.Immunol. 1997;158:5536–5544. [PubMed] [Google Scholar]
- 166.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis Rheum. 2002;46:2730–2741. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 167.Rhode A, Pauza ME, Barral AM, Rodrigo E, Oldstone MB, Von Herrath MG, Christen U. Islet-specific expression of CXCL10 causes spontaneous islet infiltration and accelerates diabetes development. J.Immunol. 2005;175:3516–3524. doi: 10.4049/jimmunol.175.6.3516. [DOI] [PubMed] [Google Scholar]
- 168.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann.Rheum.Dis. 2006;65:209–215. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang J, Richmond A. The angiostatic activity of interferon-inducible protein-10/CXCL10 in human melanoma depends on binding to CXCR3 but not to glycosaminoglycan. Mol.Ther. 2004;9:846–855. doi: 10.1016/j.ymthe.2004.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J.Clin.Invest. 2001;107:53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Soejima K, Rollins BJ. A functional IFN-gamma-inducible protein-10/CXCL10-specific receptor expressed by epithelial and endothelial cells that is neither CXCR3 nor glycosaminoglycan. J.Immunol. 2001;167:6576–6582. doi: 10.4049/jimmunol.167.11.6576. [DOI] [PubMed] [Google Scholar]
- 172.Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, Wasserman K, Oppenheim JJ. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 2000;14:2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 173.Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Ras-induced Modulation of CXCL10 and Its Receptor Splice Variant CXCR3-B in MDA-MB-435 and MCF-7 Cells: Relevance for the Development of Human Breast Cancer. Cancer Res. 2006;66:9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- 174.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J.Exp.Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Proost P, Schutyser E, Menten P, Struyf S, Wuyts A, Opdenakker G, Detheux M, Parmentier M, Durinx C, Lambeir AM, Neyts J, Liekens S, Maudgal PC, Billiau A, Van DJ. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98:3554–3561. doi: 10.1182/blood.v98.13.3554. [DOI] [PubMed] [Google Scholar]
- 176.Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, Vanguri P, Burd PR, Sheikh N, Gupta G, Teruya-Feldstein J, Tosato G. Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc.Natl.Acad.Sci.U.S.A. 1996;93:13791–13796. doi: 10.1073/pnas.93.24.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Teruya-Feldstein J, Jaffe ES, Burd PR, Kanegane H, Kingma DW, Wilson WH, Longo DL, Tosato G. The role of Mig, the monokine induced by interferon-gamma, and IP-10, the interferon-gamma-inducible protein-10, in tissue necrosis and vascular damage associated with Epstein-Barr virus-positive lymphoproliferative disease. Blood. 1997;90:4099–4105. [PubMed] [Google Scholar]
- 178.Feldman AL, Friedl J, Lans TE, Libutti SK, Lorang D, Miller MS, Turner EM, Hewitt SM, Alexander HR. Retroviral gene transfer of interferon-inducible protein 10 inhibits growth of human melanoma xenografts. Int.J.Cancer. 2002;99:149–153. doi: 10.1002/ijc.10292. [DOI] [PubMed] [Google Scholar]
- 179.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J.Exp.Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- 181.Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G, Farber JM, Liao F, Liu L, Tosato G. Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12. J.Leukoc.Biol. 1998;64:384–392. doi: 10.1002/jlb.64.3.384. [DOI] [PubMed] [Google Scholar]
- 182.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J.Immunol. 1998;161:927–932. [PubMed] [Google Scholar]
- 183.Pertl U, Luster AD, Varki NM, Homann D, Gaedicke G, Reisfeld RA, Lode HN. IFN-gamma-inducible protein-10 is essential for the generation of a protective tumor-specific CD8 T cell response induced by single-chain IL-12 gene therapy. J.Immunol. 2001;166:6944–6951. doi: 10.4049/jimmunol.166.11.6944. [DOI] [PubMed] [Google Scholar]
- 184.Koumandakis E, Koumandaki I, Kaklamani E, Sparos L, Aravantinos D, Trichopoulos D. Enhanced phagocytosis of mononuclear phagocytes in pregnancy. Br.J.Obstet.Gynaecol. 1986;93:1150–1154. doi: 10.1111/j.1471-0528.1986.tb08636.x. [DOI] [PubMed] [Google Scholar]
- 185.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am.J.Obstet.Gynecol. 2001;185:1118–1123. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 186.Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin.Exp.Immunol. 2003;131:490–497. doi: 10.1046/j.1365-2249.2003.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Smarason AK, Gunnarsson A, Alfredsson JH, Valdimarsson H. Monocytosis and monocytic infiltration of decidua in early pregnancy. J.Clin.Lab Immunol. 1986;21:1–5. [PubMed] [Google Scholar]
- 188.Melczer Z, Banhidy F, Csomor S, Toth P, Kovacs M, Winkler G, Cseh K. Influence of leptin and the TNF system on insulin resistance in pregnancy and their effect on anthropometric parameters of newborns. Acta Obstet.Gynecol.Scand. 2003;82:432–438. [PubMed] [Google Scholar]
- 189.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am.J.Obstet.Gynecol. 1994;170:1752–1757. [PubMed] [Google Scholar]
- 190.Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-alpha) and the development of pre-eclampsia. Clin.Exp.Immunol. 1994;98:110–114. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int.J.Gynaecol.Obstet. 2001;75:243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 192.Arriaga-Pizano L, Jimenez-Zamudio L, Vadillo-Ortega F, Martinez-Flores A, Herrerias-Canedo T, Hernandez-Guerrero C. The predominant Th1 cytokine profile in maternal plasma of preeclamptic women is not reflected in the choriodecidual and fetal compartments. J.Soc.Gynecol.Investig. 2005;12:335–342. doi: 10.1016/j.jsgi.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 193.Dudley DJ, Hunter C, Mitchell MD, Varner MW, Gately M. Elevations of serum interleukin-12 concentrations in women with severe pre-eclampsia and HELLP syndrome. J.Reprod.Immunol. 1996;31:97–107. doi: 10.1016/0165-0378(96)00976-x. [DOI] [PubMed] [Google Scholar]
- 194.Hennessy A, Pilmore HL, Simmons LA, Painter DM. A deficiency of placental IL-10 in preeclampsia. J.Immunol. 1999;163:3491–3495. [PubMed] [Google Scholar]
- 195.Omu AE, Al-Qattan F, Diejomaoh ME, Al-Yatama M. Differential levels of T helper cytokines in preeclampsia: pregnancy, labor and puerperium. Acta Obstet.Gynecol.Scand. 1999;78:675–680. [PubMed] [Google Scholar]
- 196.Rinehart BK, Terrone DA, Lagoo-Deenadayalan S, Barber WH, Hale EA, Martin JN, Jr., Bennett WA. Expression of the placental cytokines tumor necrosis factor alpha, interleukin 1beta, and interleukin 10 is increased in preeclampsia. Am.J.Obstet.Gynecol. 1999;181:915–920. doi: 10.1016/s0002-9378(99)70325-x. [DOI] [PubMed] [Google Scholar]
- 197.Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J.Reprod.Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 198.Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J.Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- 199.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem.Biophys.Res.Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 200.Lyall F, Greer IA. The vascular endothelium in normal pregnancy and pre-eclampsia. Rev.Reprod. 1996;1:107–116. doi: 10.1530/ror.0.0010107. [DOI] [PubMed] [Google Scholar]
- 201.Cheung CY. Vascular endothelial growth factor: possible role in fetal development and placental function. J.Soc.Gynecol.Investig. 1997;4:169–177. [PubMed] [Google Scholar]
- 202.Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, Gull I, Lessing JB. Vascular endothelial growth factor is increased in patients with preeclampsia. Am.J.Reprod.Immunol. 1997;38:302–306. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 203.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br.J.Obstet.Gynaecol. 1997;104:223–228. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 204.Vuorela P, Hatva E, Lymboussaki A, Kaipainen A, Joukov V, Persico MG, Alitalo K, Halmesmaki E. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol.Reprod. 1997;56:489–494. doi: 10.1095/biolreprod56.2.489. [DOI] [PubMed] [Google Scholar]
- 205.Athanassiades A, Lala PK. Role of placenta growth factor (PIGF) in human extravillous trophoblast proliferation, migration and invasiveness. Placenta. 1998;19:465–473. doi: 10.1016/s0143-4004(98)91039-6. [DOI] [PubMed] [Google Scholar]
- 206.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol.Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 207.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin.Reprod.Endocrinol. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 208.Desai J, Holt-Shore V, Torry RJ, Caudle MR, Torry DS. Signal transduction and biological function of placenta growth factor in primary human trophoblast. Biol.Reprod. 1999;60:887–892. doi: 10.1095/biolreprod60.4.887. [DOI] [PubMed] [Google Scholar]
- 209.Hayman R, Brockelsby J, Kenny L, Baker P. Preeclampsia: the endothelium, circulating factor(s) and vascular endothelial growth factor. J.Soc.Gynecol.Investig. 1999;6:3–10. [PubMed] [Google Scholar]
- 210.Lash GE, Cartwright JE, Whitley GS, Trew AJ, Baker PN. The effects of angiogenic growth factors on extravillous trophoblast invasion and motility. Placenta. 1999;20:661–667. doi: 10.1053/plac.1999.0427. [DOI] [PubMed] [Google Scholar]
- 211.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br.J.Obstet.Gynaecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 212.Torry DS, Ahn H, Barnes EL, Torry RJ. Placenta growth factor: potential role in pregnancy. Am.J.Reprod.Immunol. 1999;41:79–85. doi: 10.1111/j.1600-0897.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 213.Charnock-Jones DS, Burton GJ. Placental vascular morphogenesis. Baillieres Best.Pract.Res.Clin.Obstet.Gynaecol. 2000;14:953–968. doi: 10.1053/beog.2000.0137. [DOI] [PubMed] [Google Scholar]
- 214.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest. 2000;80:443–454. doi: 10.1038/labinvest.3780050. [DOI] [PubMed] [Google Scholar]
- 215.Ong S, Lash G, Baker PN. Angiogenesis and placental growth in normal and compromised pregnancies. Baillieres Best.Pract.Res.Clin.Obstet.Gynaecol. 2000;14:969–980. doi: 10.1053/beog.2000.0138. [DOI] [PubMed] [Google Scholar]
- 216.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am.J.Obstet.Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 217.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am.J.Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Torry DS, Mukherjea D, Arroyo J, Torry RJ. Expression and function of placenta growth factor: implications for abnormal placentation. J.Soc.Gynecol.Investig. 2003;10:178–188. doi: 10.1016/s1071-5576(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 219.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J.Clin.Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin.Endocrinol.Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 221.Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am.J.Obstet.Gynecol. 2000;183:1554–1557. doi: 10.1067/mob.2000.108022. [DOI] [PubMed] [Google Scholar]
- 222.Gaber LW, Spargo BH, Lindheimer MD. Renal pathology in pre-eclampsia. Baillieres Clin.Obstet.Gynaecol. 1987;1:971–995. doi: 10.1016/s0950-3552(87)80045-7. [DOI] [PubMed] [Google Scholar]
- 223.Kincaid-Smith P. The renal lesion of preeclampsia revisited. Am.J.Kidney Dis. 1991;17:144–148. doi: 10.1016/s0272-6386(12)81119-x. [DOI] [PubMed] [Google Scholar]
- 224.Wilczynski JR. Immunological analogy between allograft rejection, recurrent abortion and pre-eclampsia - the same basic mechanism? Hum.Immunol. 2006;67:492–511. doi: 10.1016/j.humimm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 225.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meekins JW, Pijnenborg R, Hanssens M, van AA, McFadyen IR. Immunohistochemical detection of lipoprotein(a) in the wall of placental bed spiral arteries in normal and severe preeclamptic pregnancies. Placenta. 1994;15:511–524. doi: 10.1016/s0143-4004(05)80420-5. [DOI] [PubMed] [Google Scholar]
- 227.Katabuchi H, Yih S, Ohba T, Matsui K, Takahashi K, Takeya M, Okamura H. Characterization of macrophages in the decidual atherotic spiral artery with special reference to the cytology of foam cells. Med.Electron Microsc. 2003;36:253–262. doi: 10.1007/s00795-003-0223-2. [DOI] [PubMed] [Google Scholar]
- 228.Harsem NK, Roald B, Braekke K, Staff AC. Acute Atherosis in Decidual Tissue: Not Associated with Systemic Oxidative Stress in Preeclampsia. Placenta. 2007 doi: 10.1016/j.placenta.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 229.Plutzky J. Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am.J.Cardiol. 2001;88:10K–15K. doi: 10.1016/s0002-9149(01)01924-5. [DOI] [PubMed] [Google Scholar]
- 230.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 231.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N.Engl.J.Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 232.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat.Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 233.Reape TJ, Groot PH. Chemokines and atherosclerosis. Atherosclerosis. 1999;147:213–225. doi: 10.1016/s0021-9150(99)00346-9. [DOI] [PubMed] [Google Scholar]
- 234.Mach F. The role of chemokines in atherosclerosis. Curr.Atheroscler.Rep. 2001;3:243–251. doi: 10.1007/s11883-001-0067-y. [DOI] [PubMed] [Google Scholar]
- 235.Burke-Gaffney A, Brooks AV, Bogle RG. Regulation of chemokine expression in atherosclerosis. Vascul.Pharmacol. 2002;38:283–292. doi: 10.1016/s1537-1891(02)00253-7. [DOI] [PubMed] [Google Scholar]
- 236.Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis. 2002;160:91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 237.Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann.Med. 2004;36:98–118. doi: 10.1080/07853890310019961. [DOI] [PubMed] [Google Scholar]