Abstract
Long-lived animals have evolved a robust set of defenses to maintain genomic integrity over their entire lifespan. The DNA damage response and DNA repair pathways are critical pillars of organismal defenses, minimizing somatic mutations in both post-mitotic and mitotic cells. These genomic maintenance systems not only prevent the premature emergence of cancers, but may also maintain normal tissue function and organismal longevity. Genetic defects in a number of DNA repair and DNA damage response genes often leads to a dramatic increase in cancer incidence; in other cases, premature aging or progeroid syndromes may be induced. In this review, we discuss two recent reports of two nucleotide excision repair deficient models that exhibit dramatic premature aging and shortened longevity. The DNA repair defects were also associated with a significant inhibition of the growth hormone/insulin-like growth factor 1 (GH/IGF-1) axis, an endocrine signaling pathway shown to influence aging and longevity in both vertebrates and invertebrates. Potential mechanisms of how DNA damage might affect IGF-1 signaling and aging are discussed, with a particular emphasis on the role of such signaling alterations in the adult tissue stem cell compartments.
Keywords: DNA damage, IGF-1, aging, cancer, p53, stem cells, p16INK4A
1. Introduction
The study of aging has undergone a renaissance with the discovery that manipulation of specific genes and signaling pathways can dramatically increase lifespan of model organisms (Guarente and Kenyon, 2000). Until the discovery of such genes, dietary restriction was the only intervention shown to produce a significant effect on enhancing organismal longevity (Masoro, 2005). Comparison of longevity-associated genetic alterations in multiple model organisms has frequently implicated the insulin-like growth factor-1 (IGF-1) signaling pathway as a critical player in the control of longevity (Kenyon, 2001; Longo and Finch, 2003). Nematodes and fruit flies with mutations in insulin/IGF-1 signaling pathway members often show dramatically increased longevity (Giannakou and Partridge, 2007; Kenyon, 2001; Tatar, Bartke, and Antebi, 2003). Moreover, mice with defects in growth hormone (GH)/IGF-1 pathway genes exhibit enhanced median and maximal lifespans (Bartke and Brown-Borg, 2004; Kopchick, 2008). While the mechanisms by which reduced IGF-1 signaling augments survival are not entirely clear, the phenotypes of the IGF-1 signaling deficient worms, flies and mice and downstream transcriptional targets of the IGF-1 pathway provide some important clues. Mice with GH/IGF-1 signaling defects generally display reduced body mass, reduced IGF-1 and insulin levels, improved stressed resistance, and reduced incidence of cancer (Bartke and Brown-Borg, 2004; Bluher, Kahn, and Kahn, 2003; Holzenberger et al., 2003; Kopchick, 2008). The FOXO transcription factors upregulated by decreased IGF-1 signaling activate genes important for carbohydrate metabolism, DNA repair, anti-oxidant resistance, apoptosis, and cell cycle arrest (Greer and Brunet, 2005). Given the central role of DNA damaging free radicals in many models of aging (Finkel and Holbrook, 2000; Sohal and Weindruch, 1996), the activation of DNA repair and anti-oxidant genes under reduced IGF-1 conditions provides a potential mechanism for longevity enhancement in the IGF-1 deficient models.
There is now a robust literature derived from multiple models associating reduced IGF-1 signaling with enhanced longevity. Yet, whether GH/IGF-1 reduction is uniquely longevity enhancing and anti-aging in mammalian organisms is somewhat controversial (Anversa, 2005; Bartke et al., 2003; Ceda et al., 2005; Giordano et al., 2005; Janssen and Lamberts, 2004; Laron, 2005; Rincon et al., 2004). Normal mammalian aging is characterized by reduced potency in the GH/IGF-1 axis. Reduction in IGF-1 levels with age is directly linked to atrophy of bone, skeletal muscle, cardiac muscle, and other tissues displaying pathological aging (Adamo and Farrar, 2006; Anversa, 2005; Ceda et al., 2005; Geusens and Boonen, 2002). Exogenous administration of GH to aged individuals improves body composition, fitness, bone density, and other biological markers, albeit with some side effects (Liu et al., 2007). However, this anti-aging effect of excess GH/IGF-1 is counterbalanced by the finding that transgenic mice expressing a GH transgene exhibit decreased longevity (Bartke, 2003).
To further complicate this picture, two seminal papers by Niedernhofer et al. (Niedernhofer et al., 2006) and van der Pluijm et al. (van der Pluijm et al., 2007) describe nucleotide excision repair (NER) deficient mice with progeroid phenotypes and very short life spans. Interestingly, both repair-deficient models exhibited greatly reduced serum IGF-1. The tissues of the mutant mice exhibited gene expression patterns confirming reduced IGF-1 signaling, reduced oxidative metabolism, and increased anti-oxidant defenses. The authors concluded that the unrepaired DNA damage in these mice induces an IGF-1/insulin mediated metabolic response that reallocates resources from growth to somatic preservation. The association of DNA damage-induced progeria with attenuation of IGF-1 signaling is profoundly interesting and raises a number of questions. In this review, we will briefly describe the authors' key findings, outline their model relating DNA damage to IGF-1 signaling and longevity, and provide some additional interpretations based on our own perspectives studying aspects of the DNA damage response and mouse aging models.
2. Suppression of IGF-1 signaling by DNA damage
The first paper, by Niedernhofer et al. (Niedernhofer et al., 2006), examined an established NER-deficient progeroid model, the Ercc1-/- mouse. ERCC1, together with its binding partner XPF, form a key NER endonuclease important for both NER and DNA cross-link repair pathways (Niedernhofer et al., 2004; Sijbers et al., 1996). In addition, ERCC1 may be important for maintenance of telomere integrity by removing the 3′ overhang of uncapped telomeres and preventing telomere fusions (Zhu et al., 2003). Ercc1-/- mice are only mildly developmentally retarded, but their growth decreases dramatically by the second week of age and they have an average life span of about three weeks (McWhir et al., 1993; Weeda et al., 1997). These mutant mice exhibit early aging associated phenotypes in the skin, liver, and bone marrow, as well as ataxia, renal insufficiency, and sarcopenia. Fibroblasts from the Ercc1-/- embryos showed profound sensitivity to interstrand crosslink damage induced by mitomycin C (Niedernhofer et al., 2004).
To gain further insights into the Ercc1-/- model, the authors performed microarray expression analyses on livers of 15 day old mutant and wild type mice (Niedernhofer et al., 2006). Gene ontology analyses of the differentially regulated genes indicated a reduction in the somatotroph, lactotroph, thyrotroph hormonal axes, consistent with a reduction in GH/IGF-1 signaling. In addition, increases in anti-oxidant genes, DNA repair genes, and pro-apoptotic genes were noted, also associated with reduced IGF-1 signaling and increased FOXO transcription factor function. This particular signature of reduced IGF-1 signaling in the liver led the authors to examine serum IGF-1 levels. Indeed, mean IGF-1 levels were dramatically decreased in 15 day old Ercc1-/- mice compared to their wild type counterparts. Blood glucose and insulin levels were also reduced in the mutant mice.
To show that the low IGF-1 levels in the Ercc1-/- mice were not due to some developmental defect in the pituitary (leading to reduced GH production), but to increased DNA damage, the authors showed that growth hormone production in the pituitary gland of the mutant mice appeared to be normal. Moreover, they induced reduced IGF-1 in the serum of normal mice by treatment with mitomycin C, confirming that induction of DNA damage alone was sufficient to suppress IGF-1 levels.
The gene expression patterns in the progeroid Ercc1-/- mice led the authors to compare gene expression patterns from young (8 and 16 week) with old (130 week) wild type mice. Gene ontology analyses indicated a similarity between categories of genes differentially regulated in the old wild type mice with those of the Ercc1-/- mice analyzed previously. In particular, somatotroph axis and carbohydrate and oxidative metabolism genes were similarly affected in old wildtype and Ercc1-/- mice. An important caveat in this comparison was that increased apoptotic and anti-oxidant responses were limited to the Ercc1-/- mice. Overall, statistical analyses revealed that the expression patterns of Ercc1-/- mice had significant correlation with those of old wild type mice, but not those of young wild type mice.
The second related paper, by van der Pluijm et al. (van der Pluijm et al., 2007), focused on a different NER-deficient model, the Csbm/m/Xpa-/- mouse. In humans, defects in the Cockayne syndrome (CS) A or B genes result in a transcriptional coupled NER defect, and these individuals exhibit growth failure, progressive neurological defects, along with kyphosis, osteoporosis, and a dramatically reduced mean life span of 12.5 years (Cleaver, 2005; Nance and Berry, 1992). Mice mutant for CS-B show some phenotypes of the human syndrome (van der Horst et al., 1997), but when crossed to NER-deficient Xpa-/- mice, the NER defect is complete and defective phenotypes are exacerbated, resembling those of the Ercc1-/- mice (van der Pluijm et al., 2007). The Csbm/m/Xpa-/- mice were growth retarded, exhibited multiple tissue defects, and had progeroid phenotypes, ultimately dying by 22 days of age. Tissue gene expression analyses of the Csbm/m/Xpa-/- and wild type mice revealed similar differential gene expression profiles as were observed when Ercc1-/- and wild type littermates were compared. The profiles emblematic of GH/IGF-1 downregulation were observed in the Csbm/m/Xpa-/- tissues with upregulation of anti-oxidant genes and downregulation of GH/IGF-1 signaling genes. Serum IGF-1 and glucose levels were significantly lower in the Csbmm/Xpa-/- mice compared to their wild type counterparts. Again, GH levels and pituitary function appeared to be normal, suggesting that IGF-1 downregulation was not due to reduced GH function. Finally, chronic exposure of 4 week old wild type mice to a mutagen generating oxidative damage resulted in reduced GH/IGF-1 axis gene expression, similar to the effects observed in mitomycin C treated normal mice by Niedernhofer et al. (Niedernhofer et al., 2006).
Monnat, in a recent review of these two papers (Monnat, 2007), rightly praises the authors' contributions for the wealth of data and the new concepts they introduce. In particular, he indicates that “these experiments provide one of the most persuasive arguments to date that senescence and the appearance of progeroid features result from active recognition and response to DNA damage, rather than passive damage accumulation”.
The nature of the DNA damage response in each model included a dramatic increase in apoptosis in some tissues in response to various DNA damaging agents. The high apoptosis in the Ercc1-/- mice was thought to be a result of the severe defect in both NER and DNA interstrand crosslink (ICL) repair. Milder NER deficiencies in XPF patients and other NER-deficient models result in increased cancer rates, but not accelerated aging. Similarly, the progeroid Csbm/m/Xpa-/- mice exhibited increased apoptotic rates compared to their singly deficient Csbm/m and Xpa-/- counterparts, which had milder progeroid phenotypes and increased cancer rates, respectively. Thus, accumulation of DNA damage in cells with less severe NER/ICL repair defects are likely to result in survival with increased mutation rates due to a less robust apoptosis and/or senescence response. Ultimately, the increased genomic instability in these cells would lead to early cancers. In contrast, damage accumulation in cells with severe NER/ICL repair defects would result in numerous unrepaired lesions that would evoke a strong apoptosis/senescence response. Rapid clearance of these damaged cells would prevent cancer, but might result in accelerated aging phenotypes due to loss of tissue homeostasis. Thus, the authors indicate that aging phenotypes are due not to DNA damage, but rather the evoked DNA damage response that causes increased cell death or senescence. Because the Csbm/m/Xpa-/- mice are a good model for Cockayne syndrome in humans, the pathologic mechanisms investigated in the mice are likely to be highly relevant to the corresponding human syndrome.
The evidence that DNA damage suppresses IGF-1 signaling pathways in the two repair-deficient mice (as well as in mutagen-treated normal mice) is compelling. The authors did not propose a mechanism by which DNA damage affects IGF-1 signaling. However, they did advance a hypothesis that the effects of reduced IGF-1 have a protective role both in the repair-deficient mice and in normal aging mice. The very similar gene expression patterns in the tissues of the repair deficient mice and aged normal mice supports the idea that both mutant and normal mice are undergoing similar stress responses, but at greatly differing rates dependent on the rate of DNA damage. This observed similarity between the repair-deficient mice and normal aged mice with respect to reduced IGF-1 levels led the authors to propose that reduced IGF-1 signaling during aging is a protective response that reduces further damage from metabolic stress and optimizes metabolic changes to help respond to the increased stress. Energy usage is shifted from growth and proliferation to protection and maintenance of tissues. This model is consistent with the metabolic changes and increased anti-oxidant and stress responses associated with reduced IGF-1 signaling. Thus, despite the early progeroid phenotypes observed in the two repair deficient models, the authors indicate that the reduced IGF-1 provides a compensatory protective role that is largely overwhelmed by the continuous massive DNA damage response.
3. How does the DNA damage response suppress IGF-1 signaling?
In their original papers, the authors do not speculate on the mechanisms by which DNA damage could suppress the IGF-1 signaling axis. This is a profoundly important question and answering it will provide key insights into aging and longevity. Given our current extensive knowledge of DNA damage response proteins and pathways, there is no shortage of candidates that could affect aging and mediate suppression of IGF-1 signaling. Attractive candidates for this role include tumor suppressors such as p53 and p16INK4A (Sharpless and DePinho, 2007). Both are inducers of cellular senescence after cytotoxic damage. P53 in particular is a stress response protein that is activated by numerous types of DNA damage, including oxidative damage and UV-induced damage repairable by NER (Giaccia and Kastan, 1998; Ljungman, 2000). Once activated and stabilized, p53 acts as a transcription factor that regulates hundreds of genes, many of them involved in cell proliferation and survival (Levine, 1997; Vousden and Lu, 2002). In the damaged cell, activated p53 can induce either cell cycle arrest or apoptosis. The p53-induced cell cycle arrest can be either transient or permanent. The latter is usually equated with senescence. Induction of senescence or apoptosis by p53 are the major mechanisms underlying p53 tumor suppressor function, as either will prevent the emergence of a nascent cancer cell (Lowe, Cepero, and Evan, 2004).
Senescence and apoptosis are also processes likely to underlie central aspects of aging, so, unsurprisingly, increased attention has been paid to the role of p53 in regulating aging and longevity (Serrano and Blasco, 2007; Sharpless and DePinho, 2007) (Bauer and Helfand, 2006; Donehower and Levine, 2008; Gatza et al., 2005; Papazoglu and Mills, 2007). Table 1 summarizes some representative mouse models that exhibit alterations in longevity in which altered p53 levels and/or activity have been implicated. In most accelerated aging models, p53 levels are significantly increased, which is a key marker of p53 activation. Our laboratory has generated a mutant p53 mouse (p53+/m) that results in the expression of a hyperactive p53 protein that produces enhanced cancer resistance and accelerated aging (Tyner et al., 2002). Importantly, not all models of altered p53 activity exhibit accelerated aging (Table 1). In some cases, increased p53 can result in cancer resistance without apparent effects on longevity (Garcia-Cao et al., 2002; Mendrysa et al., 2006). On the other hand, in two mouse models of extended longevity, p53 activities are shown to be abrogated (Table 1, p66Shc and Klotho TG). Interestingly, the last two models in Table 1, the Ercc1-/- and the Csb-/- Xpc-/- mice, are identical or very similar to the two models discussed above and each of these has significantly elevated p53 levels. Thus, the obvious question is whether increased p53 levels in the Ercc1-/- and Csbmm/Xpa-/- mice have a causative role in their progeroid phenotypes (Laposa, Huang, and Cleaver, 2007; McWhir et al., 1993). Van der Pluijm et al. (van der Pluijm et al., 2007) briefly indicate that introduction of p53 nullizygosity into the Csbmm/Xpa-/- mice failed to rescue any of the detrimental phenotypes. It could be argued that the p53 pathway is only one of the DNA damage response pathways activated by the NER deficiency and while perhaps contributing to the aging phenotypes, its inactivation is not sufficient for phenotype rescue. Nevertheless, there are some accelerated aging models in Table 1 where inactivation of p53 results in attenuation or rescue of the accelerated aging/senescence phenotypes (Cao et al., 2003; Chin et al., 1999; Varela et al., 2005).
Table 1.
Representative Aging Models with Links to p53 Signaling
| Model/Gene | Longevity | Cancer | Phenotypes | p53 Link | Reference |
|---|---|---|---|---|---|
| Old C57BL/6 mice | Normal | Normal | Normal | Upregulation of p53 targets | Edwards et al., 2007 |
| super p53 (extra p53 TG) | Normal | Decreased | Normal, cancer resistance | Enhanced p53 response | Garcia-Cao et al., 2002 |
| Mdm2 hypomorph | Normal | Decreased | Normal, cancer resistance | Enhanced p53 response | Mendrysa et al., 2006 |
| p53+/m (truncated p53) | Shortened | Decreased | Accelerated aging | Increased p53 stability | Tyner et al., 2002 |
| p44 TG (truncated p53) | Shortened | Decreased | Accelerated aging | IGF-1 signaling increased | Maier et al., 2004 |
| mTerc-/- G4-6 | Shortened | Increased | Accelerated aging | elevated p53 expression | Chin et al., 1999 |
| Brca1-/- p53+/- | Shortened | Increased | Accelerated aging | p53-induced senescence | Cao et al., 2003 |
| ATM-/- mTerc-/- | Shortened | Reduced* | Accelerated aging | Increased p53 expression | Wong et al., 2003 |
| Ku80-/- | Shortened | Reduced | Accelerated aging | Increased p53 apoptosis | Holcomb et al., 2006 |
| p66Shc-/- | Enhanced | Normal | 30% increase in life span | Impaired p53 response | Trinei et al., 2002 |
| klotho TG | Enhanced | NR | Increased longevity | suppressed p53 signaling | de Oliveira, 2007 |
| Zmpste24-/- | Shortened | NR | Accelerated aging | Upregulation of p53 targets | Varela et al., 2005 |
| Bub3+/- Rae+/- | Shortened | Normal | Accelerated aging | Elevated p53 levels | Baker et al., 2006 |
| Csb-/- Xpc-/- | Shortened | NR | Accelerated aging | Increased p53 stabilization | Laposa et al., 2007 |
| Ercc1-/- | Shortened | NR | Accelerated aging | Elevated p53 levels | McWhir et al., 1993 |
NR – not reported; TG – transgene or transgenic
Cancers reduced in comparison to ATM-/- mice
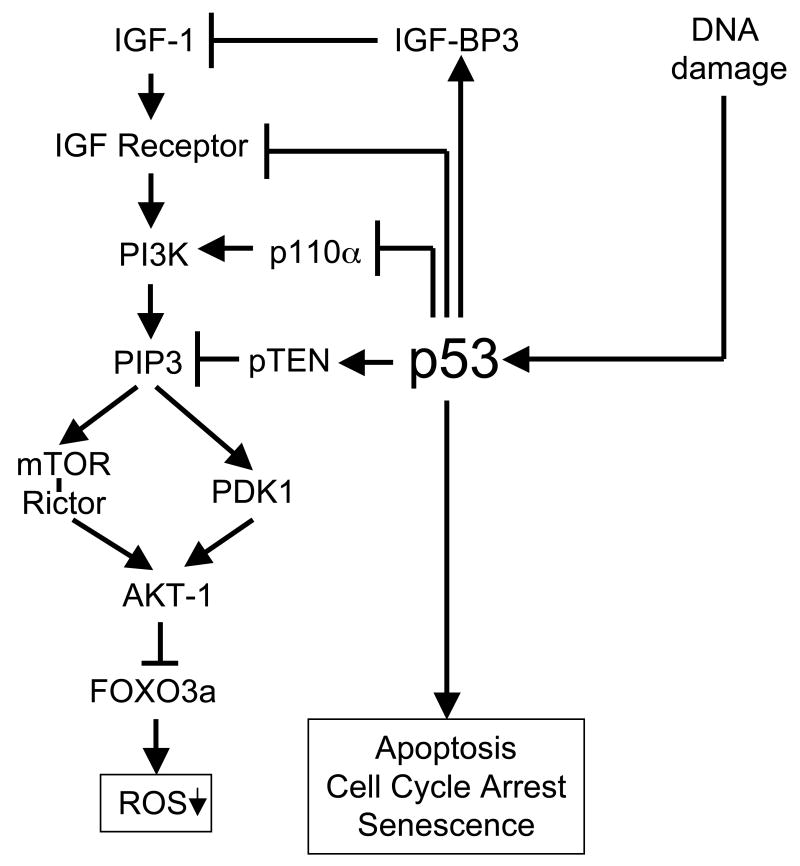
Even if it can be argued that p53 plays a role in these aging models and in normal aging (some aged tissues exhibit evidence of increased p53 activity; see normal aged mouse at top of Table 1), it remains unclear whether such a role includes suppression of IGF-1 signaling. However, there is a body of evidence indicating that in normal cells p53 suppresses the IGF-1 signaling pathway at multiple entry points (Donehower, 2008; Levine et al., 2006). For example, as shown in Figure 1, p53 is known to transcriptionally activate the insulin-like growth factor binding protein 3 (IGF-BP3) gene, encoding an IGF-1 binding molecule that suppresses IGF-1 function (Buckbinder et al., 1995). Additionally, p53 transcriptionally suppresses the insulin and IGF-1 receptors (Webster et al., 1996; Werner et al., 1996). Suppression of the IGF-1 receptor is accompanied by reduced IGF-1R and IRS-1 (a downstream modulator of IGF-1R signaling) tyrosine phosphorylation (Ohlsson et al., 1998). P53 also upregulates PTEN transcription, a tumor suppressor and lipid/tyrosine phosphatase that inhibits phosphatidylinositol 3-kinase (PI3K) function downstream of IGF-1 (Figure 1) (Stambolic et al., 2001). Finally, p53 also directly suppresses PI3K function by inhibiting p110 alpha, a subunit of the PI3K complex (Donehower, 2008).
Figure 1.
Effects of p53 on the IGF-1 signaling pathway. A simplified version of the IGF-1 signaling pathway is presented at the left. P53, shown to the right of the IGF-1 signaling pathway, is activated by DNA damage and can induce cell cycle arrest, senescence or apoptosis. In addition, four entry points of p53 into the IGF-1 signaling pathway are indicated. From top to bottom: (1) p53 transcriptionally activates the IGF-BP3 gene, whose product binds to IGF-1 and inhibits its function; (2) p53 transcriptionally downregulates the IGF-1 receptor; (3) p53 transcriptionally inhibits the p110 subunit of the PI3K subunit; and (4) p53 transcriptionally activates the pTEN gene, which encodes a phosphatase that dephosphorylates PIP3 to downregulate IGF-1 signaling. The end result of suppression of IGF-1 signaling is decreased phoshorylation of Forkhead (FOXO) transcription factors by AKT and increased activity of Forkhead in transcriptionally activating anti-oxidant and DNA repair genes.
Despite the potential of p53 to inhibit IGF-1 signaling and affect aging, is there is evidence that it does so in mammals? Our laboratory has shown that mutant mice expressing a hyperactive truncated p53 allele and displaying premature aging phenotypes have significantly reduced serum IGF-1 levels compared to their age-matched counterparts (Gatza et al., 2008). In contrast, another truncated p53 model developed by Scrable and colleagues that also exhibits accelerated aging, has modestly higher levels of IGF-1 and increased IGF-1 signaling activity (Maier et al., 2004). Thus, further more definitive experiments need to be performed to determine the role of p53 in influencing aging phenotypes and in modulating IGF-1 levels in the normal aging organism. In addition, it is likely that other members of the DNA damage response pathway play a role in aging (and possibly IGF-1 regulation), as a number of them are associated with altered aging and longevity phenotypes when mutated (Brosh and Bohr, 2007; Hasty et al., 2003; Hoeijmakers, 2007; Lombard et al., 2005).
4. Does suppressed IGF-1 signaling affect tissue homeostasis?
The underlying assumption of the models proposed by Niedernhofer et al. (Niedernhofer et al., 2006) and van der Pluijm et al. (van der Pluijm et al., 2007) is that the suppression of IGF-1 signaling by DNA damage is generally protective against the ravages produced by such damage (van de Ven et al., 2007). Ultimately, however, despite this protective effect, NER-deficient cells incur too much damage and cells are lost at too high a rate to prevent loss of tissue homeostasis. Overall, this assumption makes sense, since the various pathways upregulated by reduced IGF-1 signaling (and increased FOXO activity) largely appear to be beneficial to organismal maintenance and survival (e.g. reduced oxidative metabolism, improved anti-oxidant defenses, and enhanced DNA repair). Yet two categories of genes affected by reduced IGF-1 signaling that were noted in both papers might have deleterious effects under some circumstances. These are the genes affecting cell proliferation and cell survival. Generally, growth and proliferation genes were downregulated and pro-apoptotic genes were upregulated (while anti-apoptotic genes were downregulated). Tissues are maintained in a dynamic equilibrium of cell loss and replenishment known as tissue homeostasis. Normally, when a cell is responding to DNA damage, arrest of DNA replication and cell division will prevent further propagation of potentially deleterious mutations, a good strategy to prevent cancer. Elimination of highly damaged cells through apoptosis is also likely to be beneficial. Nevertheless, under persistent conditions of rampant DNA damage (as in the two repair-deficient models), where cells are continuously lost at a high rate, rapid replacement of those cells by stem and progenitor cells would be necessary to maintain tissue homeostasis. A low growth/high apoptosis environment in the stem/progenitor cell niches would likely be suboptimal for stem and progenitor cell self renewal. Inhibition of stem cell function would result in a cumulative failure to adequately replace cells rendered non-functional (through senescence or other mechanisms) or lost through apoptosis. The final effect of this stem cell inhibition might be tissue atrophy of the type observed in a number of progeroid syndromes and normal mammalian aging. Such effects would be irrelevant in adult worms and flies, which have only post-mitotic somatic cells, but could be of primary importance in organisms with self-renewing tissues and stem cells.
Of course, this caveat assumes that GH/IGF-1 levels are critical for stem and progenitor cell self renewal and differentiation. In fact, there is ample evidence that IGF-1 is important for this function in multiple tissues. In particular, it is well established that IGF-1 is critical for muscle satellite cell maintenance and proliferation (Adamo and Farrar, 2006). IGF-1 has been shown to rescue the aging-related or inactivity-induced loss of muscle mass through the activation of satellite cells (Machida and Booth, 2004). Other stem and progenitor cell types where IGF-1 has been shown to be important for proliferation and differentiation include cardiac muscle stem cells, neuronal stem cells, hematopoietic stem cells, mesenchymal stem cells, and adipocytes (Kelley et al., 1998; Merchav, 1998; Schulze and Spate, 2005; Spagnoli et al., 2005; Wabitsch et al., 1995).
IGF-1 and its receptor are important for embryonic and postnatal development, as shown by the phenotypes of IGF-1 knockout mice. Greater than 95% of Igf-1 null mice die perinatally with severe muscle dystrophy, and those that survive have less than 60% of the body mass of wild type mice (Allan et al., 2001). Interestingly, Igf-1-/- females that do survive exhibit defective mammary gland ductal morphogenesis during puberty, an indicator of defective mammary gland stem/progenitor cell function (Kleinberg et al., 2000). Administration of IGF-1 to the Igf-1 null females rescued mammary development. In our laboratory, we noted in the p53+/m mice that displayed hyperactive p53, reduced serum IGF-1, and accelerated aging, that the only non-aging related developmental defect was defective mammary gland ductal morphogenesis (Gatza et al., 2008). This mammary gland developmental defect was rescued by administration of recombinant IGF-1 to the p53+/m mice or by crossing the p53+/m mice to transgenic mice that overexpress serum IGF-1. Mammary gland transplantation experiments indicated functional defects in both mammary gland stem cells and the mammary gland stroma of p53+/m mice. The stroma also exhibited reduced IGF-1 signaling and evidence of increased p53 activity (Gatza et al., 2008). Such results suggest a direct link between p53, IGF-1, and stem cell/stem cell niche function, at least in one tissue compartment.
The reduction of IGF-1 levels in the serum and tissues of aged mice is associated with decreased stem cell function in these tissues. What about IGF-1 signaling in aging stem cells themselves? To address this, the Goodell laboratory showed first that aged HSCs are less functional in long term hematopoietic system reconstitution compared to their young HSC counterparts (Chambers et al., 2007). In addition, they examined gene expression profiles in purified hematopoietic stem cells (HSCs) from 2, 6, 12, and 21 month C57BL/6 mice and identified 1500 genes that were significantly age-induced and 1600 genes that were significantly age-repressed (Chambers et al., 2007). Among those genes upregulated were those involved in stress response and inflammatory response pathways. Downregulated genes included those involved in chromatin remodeling, DNA repair, and proliferation. Importantly, two downregulated gene ontology categories in the aged HSCs were insulin and IGF-1 receptor signaling. The association of reduced insulin/IGF-1 signaling with reduced activity of cell cycle, DNA replication, cell proliferation, cell migration, and ribosome biogenesis gene categories in aged HSCs provides a plausible mechanism for the reduced self renewal and reconstitution functions in these cells.
The Goodell laboratory and our laboratory also collaborated on a transcriptome analysis of HSCs purified from 12 month p53+/- mice and p53+/m mice (the accelerated aging model with elevated p53 activity) (Chambers et al., 2007). Surprisingly, after comparison of the gene expression levels in the two types of HSCs, 84 out of 87 gene ontology categories in the p53+/m HSCs exhibited a significantly “younger” expression profile compared to their p53+/- counterparts. Only inflammatory response pathways and ion transport pathways displayed on “older” profile in p53+/m HSCs, while IGF-1 signaling pathways were not significantly different between the two genotypes of HSCs. Since we had earlier shown that HSC numbers do not increase during aging in p53+/m mice, unlike in p53+/+ and p53+/- mice (Dumble et al., 2007), we interpreted these unexpected gene expression profiles to be a result of reduced self renewal capacity in the p53+/m HSCs. We hypothesized that the increased p53 activity in the p53+/m HSCs imposed a slower rate of proliferation and that the reduced number of cell divisions would result in less replication-associated damage and a consequent “younger” profile of gene expression, despite a more aged inflammatory and tissue milieu.
5. How does the DNA damage response affect tissue homeostasis?
In the section above, evidence was presented that reduced IGF-1 signaling in the NER-deficient mice might be deleterious to tissue homeostasis through anti-proliferative effects on stem/progenitor cells and their niche environments. In addition, it seems likely that the DNA damage response would have an important anti-proliferative role in tissue stem cell function. As more fully described in recent reviews, DNA damage has been linked to stem cell attrition in a number of experimental contexts (Blasco, 2007; Chambers and Goodell, 2007; Rando, 2006; Sharpless and DePinho, 2007). Perhaps most relevant to the two NER papers discussed here, Prasher et al. (Prasher et al., 2005) have shown that 3 week old Ercc1-/- mice exhibit multi-lineage cytopenia and fatty replacement of bone marrow, similar to old wild type mice. Moreover, hematopoietic progenitor numbers and proliferative reserves were dramatically decreased in the Ercc1-/- mice compared to their wild type counterparts. Defects in hematopoietic stem cell (HSC) function were also described in Fancd1, Msh2, and Rad50-deficient mice (Morales et al., 2005; Navarro et al., 2006; Reese et al., 2003). In other recent experiments, Ninjik et al. (Nijnik et al., 2007) and Rossi et al. (Rossi et al., 2007), examining various DNA repair or telomerase-deficient mouse strains (Lig4, Ku80, Xpd, and mTR), showed that hematopoietic stem cells declined rapidly in functionality with age, if not in number. Moreover, transplantation of mutant HSCs into normal recipient mice resulted in greatly decreased reconstitutive ability in their hosts compared to HSCs derived from normal mice. Interestingly, by staining young and old HSCs and progenitors for gamma H2AX, a marker for DNA damage, Rossi et al. (Rossi et al., 2007) showed that 82% of old long term HSCs had damage foci compared to a virtual absence of such foci in young HSCs. However, in an analysis of gliomas, it was shown that brain cancer stem cells (CD133 expressing, also a marker for neural stem cells) that such stem cells had a more robust DNA damage response and repair compared to their non-stem cell neighbors (Bao et al., 2006). These findings suggest that stem cells are not necessarily more protected from damage, but may repair it more efficiently than their more differentiated progeny.
The importance of the DNA damage response in tissue homeostasis is nicely illustrated by a set of experiments reported by Ruzankina et al. (Ruzankina et al., 2007), who examined a conditional Atr-deficient mouse in which the Atr gene was deleted in all tissues during adulthood. ATR is a critical sensor kinase in the DNA damage response and its loss leads to cell death. After Atr deletion, there was an acute loss of cells in rapidly proliferating tissue compartments, which resulted in a transient intestinal atrophy and bone marrow hypoplasia. However, the animals emerged from this acute phase and were overtly normal within one month after Atr inactivation. Examination of proliferating tissues revealed that they were reconstituted with rare cells that had not undergone ATR deletion. However, within several months, the mice exhibited a number of progeroid phenotypes, including osteopenia, skin atrophy, and loss of hematopoietic progenitors. The progeroid phenotypes were likely caused by stem cell exhaustion due to excess regeneration requirements of the rare ATR-competent stem cells or their niches. While the Atr model does not directly mimic the DNA repair deficient models, it suggests that excessive cellular toxicity arising from unrepaired DNA damage could overtax the abilities of the stem cells to replace lost cells and ultimately lead to loss of tissue homeostasis and progeroid phenotypes.
ATR and its related sensor kinase, ATM, are critical mediators of the DNA damage reponse (Abraham, 2001; Shiloh, 2003). It has been demonstrated that in Atm-deficient mouse HSCs, increased reactive oxygen species (ROS) levels compromise HSC function, in part through activation of p16INK4a and ARF, tumor suppressors implicated in induction of senescence (Ito et al., 2004). ROS are potent inducers of senescence in cell culture and may also produce accumulated DNA damage and senescence in stem cell compartments with aging. Both p53 and p16INK4a tumor suppressors have been shown to induce and maintain the senescent phenotype. In mouse tissues, senescence markers such as p16INK4a protein accumulation increase dramatically with age (Campisi, 2005; Sharpless and DePinho, 2004; Sharpless and DePinho, 2007). Moreover, the importance of p16INK4a in stem function during aging has recently been demonstrated by three groups. Examination of HSCs, neuronal stem cells, and pancreatic islet cells from p16INK4a null and p16INK4a overexpressing mice showed that increased p16INK4a expression was associated with reduced stem cell proliferation and function during aging (Janzen et al., 2006; Krishnamurthy et al., 2006; Molofsky et al., 2006). Likewise, p16INK4a deficiency attenuated the stem cell functional decline during aging. Our laboratory has also shown that HSCs from aged p53+/m mutant mice with increased p53 activity showed reduced hematopoietic reconstitution capability compared to their aged wildtype counterparts when transplanted into normal hosts (Dumble et al., 2007).
The above discussion points to an important role for multiple members of the DNA damage reponse pathway in affecting stem cell function during the aging process. These effects appear to be generally anti-proliferative and are likely to be beneficial in the short term by preventing the emergence of clones of stem and progenitor cells that could become cancerous. In the long term, however, sufficient numbers of stem cells (and perhaps the supporting niche cells in the stem cell environment) incurring DNA damage may be killed through apoptosis, or rendered dysfunctional through senescence or other mechanisms. Stem cell exhaustion and loss of functionality with aging may lead to loss of tissue homeostasis and the associated tissue atrophy that may ultimately result in organ failure and death.
6. DNA damage, IGF-1, stem cells, and aging: Tweaking the model
The model presented by Niedernhofer et al. (Niedernhofer et al., 2006) and van der Pluijm et al. (van der Pluijm et al., 2007) to explain the mechanisms by which NER deficiency could provoke such profound progeroid phenotypes is straightforward and is consistent with their data. Basically, they propose that the accumulation of DNA damage in the repair-deficient mice contributes to a dampening of the GH/IGF-1 axis through an unknown mechanism. The resulting reduced IGF-1 signaling leads to metabolic changes that shift energy usage from growth and proliferation to protective maintenance. This would result in reduced oxidant production and damage and would be accompanied by an increased anti-oxidant defense and DNA repair response. Despite this protective response, Ercc1-/- mice rapidly accumulate DNA damage and exhibit progeroid phenotypes. In normal animals, DNA damage accumulates more slowly, but as repair mechanisms decline, the organism eventually succumbs to age-related morbidity and mortality. The authors propose that aging is retarded (though not prevented) by the IGF-1-mediated stress response.
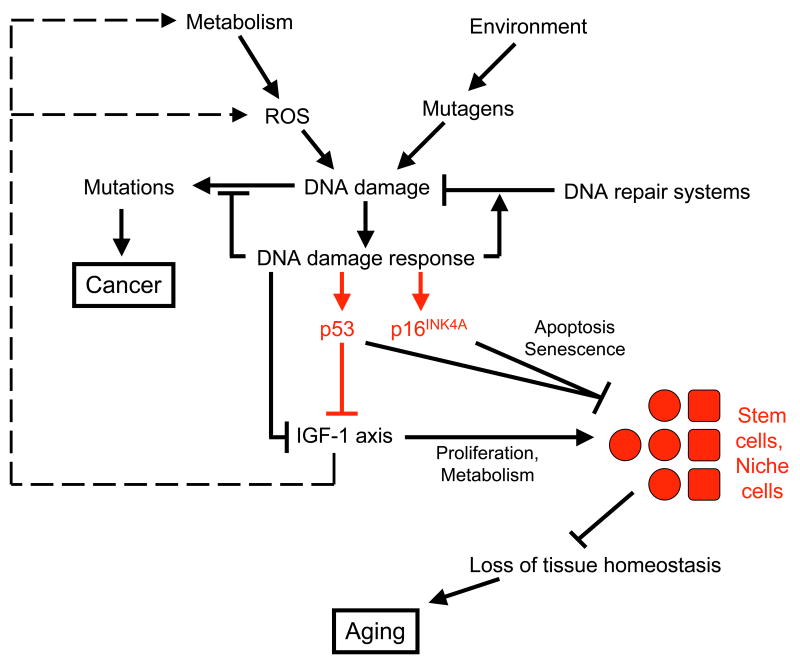
In Figure 2, we have presented a slightly modified version of the Niedernhofer/van der Pluijm model with a few embellishments. These embellishments, indicated in red, represent our own biases and we hope they do not detract from the elegance of the original model. For example, the authors did not originally specify the mechanism by which DNA damage would suppress IGF-1 signaling. P53 has been added as a potential suppressor of IGF-1 signaling based not on definitive evidence but rather on accumulated circumstantial evidence. P53 and p16INK4a, as critical mediators of apoptosis and senescence, and newly associated with stem cell functionality, have been positioned to show their potential effects on stem cells and the stem cell niche. The addition of stem cells to the figure may provide more specificity to the concept of “loss of tissue homeostasis” or “loss of physical reserves”. While such losses likely occur in part outside stem and progenitor cells (e.g. postmitotic cells), the critical events leading to loss of tissue homeostasis are most likely to occur in the stem/progenitor cells or their niches.
Figure 2.
Modified version of the Niedernhofer/van der Pluijm model showing the link between DNA damage, IGF-1 signaling suppression, and aging. Components in black are from the original model and components in red are those added by this author. DNA damage induced by endogenous or exogenous stresses evoke a DNA damage response that suppresses IGF-1 signaling through unknown mechanisms and perhaps in part through p53. Lowered IGF-1 signaling reduces ROS production and enhances anti-oxidant defenses, perhaps enhancing stem cell survival. However, DNA damage may also inhibit stem cell function, partly through apoptosis or senescence mediated by p53 and p16INK4a. Reduction of stem cell function, either directly, or through effects on the stem cell niche, results in loss of tissue homeostasis, and ultimately, tissue atrophy and aging.
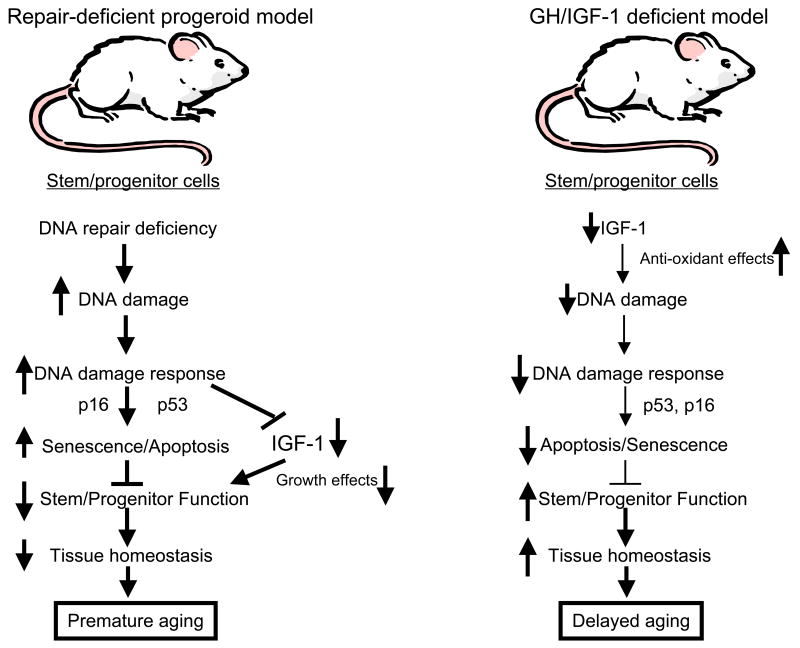
In addition to the changes indicated above, we would like to propose one final “tweak”. In their model, Niedernhofer et al. (Niedernhofer et al., 2006) and van der Pluijm et al. (van der Pluijm et al., 2007) hypothesize that suppression of IGF-1 signaling by DNA damage is likely to be uniformly protective both in NER-deficient mice and in normal mice. Despite this protective effect, NER-deficient cells incur too much damage and cells are lost at too high a rate to prevent loss of tissue homeostasis. As indicated earlier in this review, it seems possible, that because reduced IGF-1 signaling inhibits cell proliferation and stimulates apoptosis, it might actually be deleterious to stem and progenitor cells and ultimately contribute to loss of tissue homeostasis in the NER-deficient model. One obvious counterargument is that reduced IGF-1 signaling is clearly beneficial in many mice with GH/IGF-1 axis mutations that exhibit extended longevity (Kopchick, 2008). In response, we would propose that genetic reduction of IGF-1 signaling, in a normal environment, would result in low ROS production, as well as activation of anti-oxidant and DNA repair defenses. DNA damage rates would be consequentially low, resulting in low cell loss rates and thus requiring minimal levels of stem cell activity to maintain tissue homeostasis. Thus, low IGF-1 signaling levels would be compatible with the low rate of stem cell renewal required in the IGF-1 deficient mice (Figure 3). In contrast, in NER-deficient mice with very high DNA damage rates, IGF-1 signaling is reduced, but any benefits (such as lowered ROS and improved anti-oxidant defenses) are swamped by the inability to repair DNA lesions. Cells are correspondingly lost at a very high rate and can only be replaced by a very robust stem/progenitor cell response. However, because IGF-1 levels are reduced, stem and progenitor cells may be unable to adequately replenish lost cells and loss of tissue homeostasis may actually occur sooner than if IGF-1 signaling was at normal levels. In addition, the enhanced anti-proliferative DNA damage response, in part mediated by p53, would likely inhibit stem cell self renewal. Ultimately, tissue atrophy and progeroid phenotypes would result (Figure 3). Thus, lowered IGF-1 signaling may actually contribute to the progeroid phenotypes rather than attenuate them. An alternative possibility is that Niedernhofer et al. and van der Pluijm et al. are indeed correct in their hypothesis that reduced IGF-1 signaling in their models is entirely protective and that the DNA damage response is solely responsible for the progeroid phenotypes. One possible way to test this would be to manipulate IGF-1 levels in the NER-deficient mice to see whether it could alter progeroid phenotypes in any significant way.
Figure 3.
Reduced IGF-1 signaling may have different outcomes that depend on the level of DNA damage. On the left is shown a flow diagram representing events in stem cells from NER-deficent mice with progeroid phenotypes (e.g. Ercc1-/- and Csbm/m/Xpa-/- mice). In these mice, DNA damage is extensive and often unrepairable, inducing a robust DNA damage response that induces apoptosis or senescence and suppresses IGF-1 signaling in the stem cells. The combination of increased damage, apoptosis, senescence, and reduced IGF-1 in the stem cells prevents them from adequately responding to the massive demand for self-renewal and differentiation. Tissues are inadequately replenished, leading to loss of tissue homeostasis and appearance of progeroid phenotypes. In contrast, on the right, stem cells from mice with genetic deficiencies in the GH/IGF-1 axis have low DNA damage rates as a result of enhanced anti-oxidant defenses and reduced metabolism mediated by lowered IGF-1 signaling. Low levels of apoptosis and senescence result in reduced need for stem cell turnover. Thus, demands on stem cell self renewal and differentiation are low and tissue homeostasis is maintained, despite the low proliferation ability of stem cells with reduced IGF-1 signaling. The end result is maintenance of tissue homeostasis and extended longevity.
Regardless of the “tweaks” proposed here, these two papers are extremely important in altering our perception of the relationship of DNA damage (and the DNA damage response) to aging through IGF-1 signaling. While clarifying a number of issues that were previously unresolved, like all important discoveries, their work raises even more exciting questions about the basic mechanisms of aging and cancer. We should see a number of these questions addressed soon.
Acknowledgments
This work was supported by grants from the Ellison Medical Foundation and the National Institute for Aging. We thank Margaret Goodell for her comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev. 2006;5:310–331. doi: 10.1016/j.arr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Allan GJ, Flint DJ, Patel K. Insulin-like growth factor axis during embryonic development. Reproduction. 2001;122:31–39. doi: 10.1530/rep.0.1220031. [DOI] [PubMed] [Google Scholar]
- Anversa P. Aging and longevity: the IGF-1 enigma. Circ Res. 2005;97:411–4. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Jeganathan KB, Malureanu L, Perez-Terzic C, Terzic A, van Deursen JM. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol. 2006;172:529–540. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Dominici F, Turyn D, Kinney B, Steger R, Kopchick JJ. Insulin-like growth factor 1 (IGF-1) and aging: controversies and new insights. Biogerontology. 2003;4:1–8. doi: 10.1023/a:1022448532248. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Helfand SL. New tricks of an old molecule: lifespan regulation by p53. Aging Cell. 2006;5:437–440. doi: 10.1111/j.1474-9726.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Brosh RM, Jr, Bohr VA. Human premature aging, DNA repair and RecQ helicases. Nucleic Acids Res. 2007;35:7527–7544. doi: 10.1093/nar/gkm1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckbinder L, Talbott R, Velasco-Miguel S, Takenaka I, Faha B, Seizinger BR, Kley N. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Cao L, Li W, Kim S, Brodie SG, Deng CX. Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev. 2003;17:201–213. doi: 10.1101/gad.1050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceda GP, Dall'Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, Grimaldi W, Ceresini G, Corradi F, Ferrucci L, Valenti G, Hoffman AR. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):96–100. [PubMed] [Google Scholar]
- Chambers SM, Goodell MA. Hematopoietic stem cell aging: wrinkles in stem cell potential. Stem Cell Rev. 2007;3:201–211. doi: 10.1007/s12015-007-0027-1. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Cleaver JE. Cancer in xeroderma pigmentosum and related disorders of DNA repair. Nat Rev Cancer. 2005;5:564–573. doi: 10.1038/nrc1652. [DOI] [PubMed] [Google Scholar]
- de Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 2006;580:5753–5758. doi: 10.1016/j.febslet.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Levine AJ. p53, cancer, and longevity. In: Guarente L, Partridge L, Wallace DC, editors. Molecular Biology of Aging. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. pp. 127–152. [Google Scholar]
- Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. Embo J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatza C, Hinkal G, Moore L, Dumble M, Donehower LA. p53 and Mouse Aging Models. In: Masoro EJ, A SN, editors. Handbook of the Biology of Aging, Sixth Edition. Sixth. Academic Press; San Diego, CA: 2005. pp. 147–178. [Google Scholar]
- Gatza CE, Dumble M, Kittrell F, Edwards DG, Dearth RK, Lee AV, Xu J, Medina D, Donehower LA. Altered mammary gland development in the p53+/m mouse, a model of accelerated aging. Dev Biol. 2008;313:130–141. doi: 10.1016/j.ydbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geusens PP, Boonen S. Osteoporosis and the growth hormone-insulin-like growth factor axis. Horm Res. 2002;58 3:49–55. doi: 10.1159/000066483. [DOI] [PubMed] [Google Scholar]
- Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Giordano R, Lanfranco F, Bo M, Pellegrino M, Picu A, Baldi M, Balbo M, Bonelli L, Grottoli S, Ghigo E, Arvat E. Somatopause reflects age-related changes in the neural control of GH/IGF-I axis. J Endocrinol Invest. 2005;28 3:94–98. [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. Genome maintenance mechanisms are critical for preventing cancer as well as other aging-associated diseases. Mech Ageing Dev. 2007;128:460–462. doi: 10.1016/j.mad.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Janssen JA, Lamberts SW. Igf-I and longevity. Horm Res. 2004;62 3:104–109. doi: 10.1159/000080508. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, Depinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16(INK4a) Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Meier WA, Minshall C, Schacher DH, Liu Q, VanHoy R, Burgess W, Dantzer R. Insulin growth factor-I inhibits apoptosis in hematopoietic progenitor cells. Implications in thymic aging. Ann N Y Acad Sci. 1998;840:518–524. doi: 10.1111/j.1749-6632.1998.tb09590.x. [DOI] [PubMed] [Google Scholar]
- Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Bartke A, Berryman DE. Extended life span in mice with reduction in the GH/IGF-1 axis. In: Guarente LP, Partridge L, Wallace DC, editors. Molecular Biology of Aging. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2008. pp. 347–370. [Google Scholar]
- Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. p16(INK4a) induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- Laposa RR, Huang EJ, Cleaver JE. Increased apoptosis, p53 upregulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice. Proc Natl Acad Sci USA. 2007;104:1389–1394. doi: 10.1073/pnas.0610619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laron Z. Do deficiencies in growth hormone and insulin-like growth factor-1 (IGF-1) shorten or prolong longevity? Mech Ageing Dev. 2005;126:305–307. doi: 10.1016/j.mad.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- Ljungman M. Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia. 2000;2:208–225. doi: 10.1038/sj.neo.7900073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc. 2004;63:337–340. doi: 10.1079/PNS2004354. [DOI] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet. 1993;5:217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- Mendrysa SM, O'Leary KA, McElwee MK, Michalowski J, Eisenman RN, Powell DA, Perry ME. Tumor suppression and normal aging in mice with constitutively high p53 activity. Genes Dev. 2006;20:16–21. doi: 10.1101/gad.1378506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchav S. The haematopoietic effects of growth hormone and insulin-like growth factor-I. J Pediatr Endocrinol Metab. 1998;11:677–685. doi: 10.1515/jpem.1998.11.6.677. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16(INK4a) expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat RJ., Jr From broken to old: DNA damage, IGF1 endocrine suppression and aging. DNA Repair (Amst) 2007;6:1386–1390. doi: 10.1016/j.dnarep.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Theunissen JW, Kim CF, Kitagawa R, Kastan MB, Petrini JH. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19:3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance MA, Berry SA. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Navarro S, Meza NW, Quintana-Bustamante O, Casado JA, Jacome A, McAllister K, Puerto S, Surralles J, Segovia JC, Bueren JA. Hematopoietic dysfunction in a mouse model for Fanconi anemia group D1. Mol Ther. 2006;14:525–535. doi: 10.1016/j.ymthe.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Kley N, Werner H, LeRoith D. p53 regulates insulin-like growth factor-I (IGF-I) receptor expression and IGF-I-induced tyrosine phosphorylation in an osteosarcoma cell line: interaction between p53 and Sp1. Endocrinology. 1998;139:1101–1107. doi: 10.1210/endo.139.3.5832. [DOI] [PubMed] [Google Scholar]
- Papazoglu C, Mills AA. p53: at the crossroad between cancer and ageing. J Pathol. 2007;211:124–133. doi: 10.1002/path.2086. [DOI] [PubMed] [Google Scholar]
- Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, Niedernhofer LJ. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1-/- mice. Embo J. 2005;24:861–871. doi: 10.1038/sj.emboj.7600542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102:1626–1633. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- Rincon M, Muzumdar R, Atzmon G, Barzilai N. The paradox of the insulin/IGF-1 signaling pathway in longevity. Mech Ageing Dev. 2004;125:397–403. doi: 10.1016/j.mad.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze PC, Spate U. Insulin-like growth factor-1 and muscle wasting in chronic heart failure. Int J Biochem Cell Biol. 2005;37:2023–2035. doi: 10.1016/j.biocel.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–168. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD. Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell. 1996;86:811–822. doi: 10.1016/s0092-8674(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli A, Longobardi L, O'Rear L. Cartilage disorders: potential therapeutic use of mesenchymal stem cells. Endocr Dev. 2005;9:17–30. doi: 10.1159/000085719. [DOI] [PubMed] [Google Scholar]
- Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Trinei M, Giorgio M, Cicalese A, Barozzi S, Ventura A, Migliaccio E, Milia E, Padura IM, Raker VA, Maccarana M, Petronilli V, Minucci S, Bernardi P, Lanfrancone L, Pelicci PG. A p53-p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene. 2002;21:3872–3878. doi: 10.1038/sj.onc.1205513. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- van de Ven M, Andressoo JO, Holcomb VB, Hasty P, Suh Y, van Steeg H, Garinis GA, Hoeijmakers JH, Mitchell JR. Extended longevity mechanisms in short-lived progeroid mice: identification of a preservative stress response associated with successful aging. Mech Ageing Dev. 2007;128:58–63. doi: 10.1016/j.mad.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, Morreau H, Beems RB, van Kreijl CF, de Gruijl FR, Bootsma D, Hoeijmakers JH. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- van der Pluijm I, Garinis GA, Brandt RM, Gorgels TG, Wijnhoven SW, Diderich KE, de Wit J, Mitchell JR, van Oostrom C, Beems R, Niedernhofer LJ, Velasco S, Friedberg EC, Tanaka K, van Steeg H, Hoeijmakers JH, van der Horst GT. Impaired genome maintenance suppresses the growth hormone--insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 2007;5:e2. doi: 10.1371/journal.pbio.0050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, Lopez-Otin C. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wabitsch M, Hauner H, Heinze E, Teller WM. The role of growth hormone/insulin-like growth factors in adipocyte differentiation. Metabolism. 1995;44 4:45–49. doi: 10.1016/0026-0495(95)90220-1. [DOI] [PubMed] [Google Scholar]
- Webster NJ, Resnik JL, Reichart DB, Strauss B, Haas M, Seely BL. Repression of the insulin receptor promoter by the tumor suppressor gene product p53: a possible mechanism for receptor overexpression in breast cancer. Cancer Res. 1996;56:2781–2788. [PubMed] [Google Scholar]
- Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, Hoeijmakers JH. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- Werner H, Karnieli E, Rauscher FJ, LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci USA. 1996;93:8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature. 2003;421:643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- Zhu XD, Niedernfofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol Cell. 2003;12:1489–1498. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]