Abstract
Antipsychotic drug treatment of schizophrenia may be complicated by side effects of widespread dopaminergic antagonism, including exacerbation of negative and cognitive symptoms due to frontal cortical hypodopaminergia. Atypical antipsychotics have been shown to enhance frontal dopaminergic activity in animal models. We predicted that substitution of risperidone for typical antipsychotic drugs in the treatment of schizophrenia would be associated with enhanced functional activation of frontal cortex. We measured cerebral blood oxygenation changes during periodic performance of a verbal working memory task, using functional MRI, on two occasions (baseline and 6 weeks later) in two cohorts of schizophrenic patients. One cohort (n = 10) was treated with typical antipsychotic drugs throughout the study. Risperidone was substituted for typical antipsychotics after baseline assessment in the second cohort (n = 10). A matched group of healthy volunteers (n = 10) was also studied on a single occasion. A network comprising bilateral dorsolateral prefrontal and lateral premotor cortex, the supplementary motor area, and posterior parietal cortex was activated by working memory task performance in both the patients and comparison subjects. A two-way analysis of covariance was used to estimate the effect of substituting risperidone for typical antipsychotics on power of functional response in the patient group. Substitution of risperidone increased functional activation in right prefrontal cortex, supplementary motor area, and posterior parietal cortex at both voxel and regional levels of analysis. This study provides direct evidence for significantly enhanced frontal function in schizophrenic patients after substitution of risperidone for typical antipsychotic drugs, and it indicates the potential value of functional MRI as a tool for longitudinal assessment of psychopharmacological effects on cerebral physiology.
Keywords: functional magnetic resonance imaging , hypofrontality, dopamine, psychopharmacology, supplementary motor area
The introduction of antipsychotic drugs such as chlorpromazine and haloperidol to clinical practice in the 1950s provided the first effective treatment for schizophrenia. The efficacy of these drugs in treating positive symptoms of schizophrenia (such as hallucinations and delusions) is highly correlated with their affinity for postsynaptic dopamine (D2) receptors (1), suggesting that psychotic symptoms might be caused by excessive dopaminergic effects or hyperdopaminergia (2). However, antipsychotic drug treatment has frequently been complicated by serious side effects of widespread D2 antagonism, notably an extrapyramidal or parkinsonian syndrome caused by antagonism of the dopaminergic projection from substantia nigra to corpus striatum. In the last 10 years, a new generation of antipsychotic drugs, including clozapine, olanzapine, and risperidone, has offered an effective therapeutic alternative to traditional agents with reduced nigrostriatal blockade. These newer compounds are often referred to as “atypical” antipsychotic drugs because of their lack of “typical” parkinsonian effects in both animal models and patients (3). Compared with typical antipsychotics, atypical drugs are also more effective in treating secondary negative signs of schizophrenia (such as apathy and social withdrawal) (4), [though atypicals may not have superior efficacy for primary (deficit) symptoms (5)] and have been associated with improved performance on cognitive tasks dependent on frontal lobe function (6, 7). Like the reduced incidence of parkinsonism, these therapeutic and cognitive benefits of atypical antipsychotic treatment have been attributed to the relative selectivity of D2 antagonism mediated by these drugs (8). Compared with typical antipsychotics in animal models, atypical drugs are less effective antagonists of the mesocortical dopaminergic projection from ventral tegmentum to prefrontal cortex (although they do effectively block postsynaptic effects of the mesolimbic dopaminergic projection from ventral tegmentum to limbic cortex). Indeed, atypical antipsychotics have been shown to augment prefrontal dopaminergic activity in animal models (9).
Functional integrity of prefrontal cortex (PFC) is critical for normal working memory—i.e., the short-term storage of information and its executive manipulation to guide behavior in the absence of environmental cues (10–12). Performance on working memory tasks is normally associated with prefrontal dopamine release (13, 14) and is impaired by dopamine depletion (13). Working memory deficits have been well documented in schizophrenic patients (15), and impaired working memory is central to several contemporary models of negative and disorganized symptoms in schizophrenia (11, 16, 17). Functional imaging studies of schizophrenic patients performing cognitive tasks that demand executive processing and/or working memory have frequently demonstrated reduced prefrontal blood flow, or “hypofrontality,” compared with healthy comparison subjects (18–21). Hypofrontality in schizophrenic patients can be corrected by administration of dopamine agonist drugs (22). In light of these and other observations, it has been suggested that negative signs and cognitive deficits associated with schizophrenia may reflect impaired prefrontal cortical function, because of reduced dopaminergic drive to PFC via the mesocortical projection (16, 18, 23–25). If this hypodopaminergic model of PFC function in schizophrenia is correct, one can see that typical antipsychotic drugs could exacerbate some of the symptoms they are intended to treat. It could also explain improvement in cognitive and negative symptoms after substitution of atypical antipsychotic drug treatment. However, there is as yet no direct evidence from human studies that, compared with typical antipsychotics, atypical drugs do indeed enhance prefrontal function.
We predicted that substitution of risperidone for typical antipsychotic drugs in the treatment of patients with schizophrenia would be associated with enhanced functional activation of neural systems for working memory, hypothetically due to relatively increased dopaminergic drive to frontal cortex.**
Methods
Subjects and Study Design.
Twenty right-handed male patients with chronic schizophrenia were recruited from the Bethlem Royal and Maudsley National Health Service Trust, London. All patients had been hospitalized on at least one occasion and were receiving stable doses of typical antipsychotic drugs for at least one month prior to participation; they had no history of neurological disease or alcohol or other substance abuse in the preceding 6 months. A group of 10 right-handed male volunteers, with no history of neurological or psychiatric disease, was recruited from the local community by advertisement. There was no significant difference between patient and comparison groups in age (ANOVA: F = 0.06; df = 2, 27; P = 0.93) or premorbid IQ (ANOVA: F = 1.99; df = 2, 27; P = 0.16), as measured with the National Adult Reading Test (NART) (26); see Table 1 for details.
Table 1.
Neuropsychological, clinical, and demographic characteristics of the groups
| Cohort | Working memory performance
|
Full scale IQ | Age, yr | Age at onset of illness, yr | Duration of illness, yr | Anti-psychotic medication, mg/day | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of correct responses* | Reaction time, s | PANSS subscale ratings
|
||||||||
| Positive | Negative | General psychopathology | ||||||||
| T1 | 9.1 (2.23) | 0.78 (0.21) | 108.2 (11.96) | 37.2 (8.1) | 8.4 (1.6) | 10.7 (3.5) | 20.2 (4.5) | 24.3 (6.5) | 12.9 (6.6) | 194.5 (168.8) |
| T2 | 9.9 (2.18) | 0.82 (0.19) | 10.0 (3.1) | 10.8 (4.1) | 21.8 (4.8) | 216.5 (164.2) | ||||
| R1 | 10.6 (0.53) | 0.67 (0.15) | 100.9 (14.9) | 37.6 (13.4) | 13.5 (3.9) | 15.3 (6.0) | 28.1 (7.5) | 25 (6.8) | 12.6 (10.1) | 282.9 (161.3) |
| R2 | 10.1 (1.52) | 0.6 (0.15) | 12.1 (4.4) | 12.3 (4.9) | 25.6 (7.3) | 4.6 (1.7) | ||||
| C | 10.9 (0.32) | 0.5 (0.11) | 110.9 (5.7) | 38.9 (9.7) | — | — | — | — | — | — |
T1, typically treated patient cohort at baseline; T2, typically treated patient cohort at 6 weeks; R1, risperidone-treated cohort at baseline; R2, risperidone-treated cohort at 6 weeks; C, comparison subjects. PANSS, Positive and Negative Syndrome Scale for Schizophrenia (27). Dose of antipsychotic medication is given as chlorpromazine equivalents, with the exception of R2, where the dose is of risperidone. Standard deviations are given in parentheses.
Maximum = 11.
Comparison subjects were examined by using functional magnetic resonance imaging (fMRI) on a single occasion; patients were scanned twice, once at recruitment (baseline) and again 6 weeks later. On each occasion, patients were also symptomatically assessed by using the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) (27). Within 1 week of the baseline assessment, half of the patient group started treatment with risperidone only, whereas the other half continued treatment with a variety of typical antipsychotic drugs. The assignment of patients to atypical or typical treatments for the duration of the study was decided naturalistically by the psychiatrists responsible for their clinical care at the Maudsley Hospital, London. In practice, this meant that patients were assigned to risperidone treatment because of uncontrolled psychotic symptoms or intolerable parkinsonian side effects after a preliminary trial of typical antipsychotic treatment. In accordance with the manufacturer’s guidelines for use of risperidone in the U.K., patients were switched to risperidone within 5 days of baseline assessment and were then maintained on a stable dose until the second assessment. Typically and atypically treated cohorts were well matched for duration of illness [12.9 ± (SD) 6.6 and 12.6 ± 10.1 years], age at onset of illness (24.3 ± 6.5 and 25 ± 6.9 years), and dose of typical antipsychotic drugs administered before recruitment (194.5 ± 168.8 and 282.9 ± 161.3 chlorpromazine equivalent mg/day). There were no significant between-group differences in any of these variables. The mean dose of risperidone received by the cohort of patients switched to atypical drug treatment was 4.6 ± 1.7 mg/day. Some patients were also taking adjunctive anticholinergic treatment: 6/10 of the typically treated cohort were taking procyclidine (mean dose = 10.8 ± 5.8 mg/day) at baseline and at 6 weeks; 4/10 patients in the risperidone-treated cohort were taking procyclidine (mean dose = 5 ± 0 mg/day) at baseline and 3/10 patients were doing so at 6 weeks (mean dose = 5 ± 0 mg/day). The difference between cohorts in frequency of anticholinergic treatment was not significant at baseline or 6 weeks (Fisher’s exact test: P = 0.57).
Written informed consent was obtained from all participants. The study was approved by the Bethlem Royal and Maudsley Hospital (Research) Ethical Committee.
fMRI.
Verbal working memory experiment.
We used a blocked periodic design to activate brain regions specialized for executive and active maintenance components of verbal working memory, as originally described by Cohen et al. (12). Two contrasting conditions were visually presented in 30-s epochs to subjects by means of a prismatic mirror as they lay in the scanner. During each epoch of the control condition, subjects viewed a series of 13 letters, which appeared one at a time with interstimulus interval (ISI) = 2.3 s, and were required to press a button with their right index finger when the letter X appeared. During each epoch of the working memory condition, subjects again viewed a series of 13 letters (ISI = 2.3 s) and were required to press a button with their right index finger if the currently presented letter was the same as that presented two trials previously (e.g., G-D-G, but not R-L-F-R or T-T). The two conditions were matched for number of target letters presented (2 or 3 targets per epoch, with a total of 11 targets during the experiment for each condition). Five cycles of alternation between conditions were presented in the course of each 5-min experiment; the control condition was always presented first. Subject performance on both tasks during scanning was monitored in terms of reaction time to target letters and accuracy (number of target letters correctly identified). All subjects received identical training in task performance prior to scanning.
Image acquisition.
Gradient-echo echoplanar magnetic resonance images were acquired by using a 1.5-tesla GE Signa System (General Electric, Milwaukee, WI) fitted with Advanced NMR hardware and software (ANMR, Woburn MA) at the Maudsley Hospital. In each of 14 noncontiguous planes parallel to the intercommissural (AC-PC) line, 100 T2*-weighted magnetic resonance images depicting BOLD contrast (28) were acquired: time to echo (TE) = 40 ms, repetition time (TR) = 3 s, in-plane resolution = 3.1 mm, slice thickness = 7 mm, slice skip = 0.7 mm.
Image analysis.
Head motion during scanning was estimated and corrected in each scan (29); there were no significant differences in stimulus-correlated motion between patient cohorts at baseline or 6 weeks, or between patients and controls. Experimentally determined power was estimated in each motion-corrected fMRI time series by sinusoidal regression. The regression model included a pair of sine and cosine waves at the frequency of alternation between conditions (1/60 Hz) with amplitudes γ and δ, respectively. The sign of γ indicates the timing of periodic response with respect to the experimental design: if γ > 0, maximum signal is observed during the first (control) condition; if γ < 0, maximum signal is observed during the second (working memory) condition. These parameters were estimated by an iterated least-squares procedure, modeling the residuals of a preliminary ordinary least-squares fit as a first-order autoregressive process (30). The sum of squared amplitudes, γ2 + δ2, divided by its standard error, yielded an estimate of standardized power at the experimentally determined frequency (P) at each voxel.
To identify voxels that were significantly activated over all subjects in a group, we used permutation testing. Each observed fMRI time series was randomly permuted 10 times, and P was estimated after each permutation. This resulted in 10 maps (for each subject at each plane) of P estimated under the null hypothesis. All maps of P (observed and permuted) were identically registered in standard space (31), and smoothed by a two-dimensional Gaussian filter with full width at half maximum = 7 mm. Generically activated voxels were then identified by comparing the median value of P at each voxel of the observed maps to its permutation distribution. If observed median P exceeded the 100⋅(1 − α)th percentile value of the permutation distribution, then that voxel was considered generically activated by a one-tailed test of size α < 0.005. Generically activated voxels with median γ < 0 were colored and superimposed on a spoiled gradient recalled (SPGR) template image to create a generic brain activation map (GBAM) (32).
To estimate and test the effect of substituting risperidone for typical antipsychotic drugs on the power of functional activation in the group of patients, we adopted the following approach. A two-way analysis of covariance (ANCOVA) model was specified as below, and fit by least squares to the 40 observations of power (P) available at each intracerebral voxel in standard space:
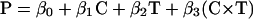 |
1 |
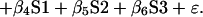 |
Here β0 denotes the overall mean power of response at a given voxel; C is a factor coding cohort membership (typically or atypically treated) for each observation; T is a factor coding time (baseline or 6 weeks) for each observation; (C × T) is a factor coding the interaction between cohort and time; S1, S2, and S3 are scores on positive, negative, and global symptom subscales of the Positive and Negative Syndrome Scale for Schizophrenia at the time of each observation; and ɛ is an error term.
To identify voxels where there was a significant effect of drug substitution, we tested the null hypothesis that β3 = 0 by permutation, with two-tailed probability of false positive error α = 0.05 (29, 33, 34). To minimize the number of tests conducted at this threshold, only the 285 voxels that were generically activated in the generic brain activation map computed from all 40 data sets in the group of patients were tested for an effect of drug substitution. Over this search volume and at this size of test, we expect 13 false positive tests.
The mean power of response in four brain regions [supplementary motor area (SMA), right prefrontal cortex, and bilateral posterior parietal cortex] was estimated for each subject at each assessment. A set of four index voxels was defined by the Talairach coordinates of the most significant cohort × time interactive effects identified by voxel-level ANCOVA, and the power of functional response was averaged over each index voxel and its eight nearest neighbors in two dimensions (total cortical volume = 0.57 cm3 for each region).
Results
Clinical and Behavioral Data.
The patients who were subsequently switched to risperidone treatment had higher scores on positive, negative, and global psychopathology scales at both assessments than did patients who continued on typical antipsychotic drug treatment. There were no significant changes on any psychopathology scale between baseline and 6 weeks in either cohort, although the risperidone-treated cohort showed a nonsignificant trend toward improvement on all three scales; see Table 1. All subjects were able to perform the task with a high degree of accuracy. There was no significant difference between cohorts in the mean change in reaction time between baseline and 6 weeks. (t test; t = 1.43; df = 18; P = 0.17); see Table 1.
fMRI Data.
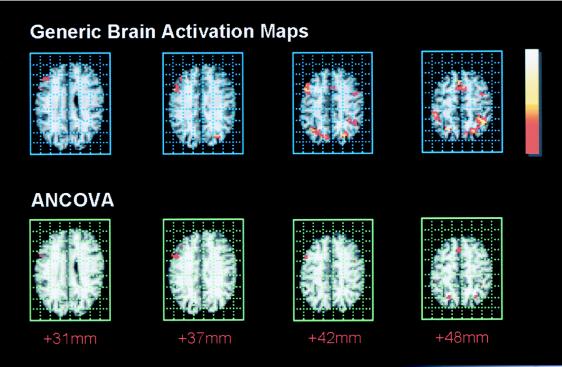
The generic brain activation maps computed from both groups of data, patients and comparison subjects, demonstrated a remarkably similar pattern of functional activation in three main brain regions: (i) the SMA, approximate Brodmann area (BA) 6, extending inferiorly to cingulate gyrus (BA 32); (ii) dorsolateral prefrontal cortex (BA 9) extending inferiorly to inferior frontal gyrus (BA 44) and superiorly to lateral premotor cortex (BA 6); and (iii) parietal cortex, extending from the angular and supramarginal gyri (BA 39, 40) to precuneus (BA 7) and dorsal BA 19 medially and superiorly. Frontal and parietal regions were activated bilaterally. Talairach coordinates and other details for these regional foci of activation are given in Table 2; selected slices of the generic brain activation map computed from the patients’ data are shown in Fig. 1.
Table 2.
Main regional foci of generic brain activation by the working memory task
| Brain region | Approximate BA | Hemisphere | Number of voxels | Talairach coordinates
|
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Posterior parietal cortex | 40/39/7/19 | Left | 96 (188) | −38 (−38) | −47 (−53) | 42 (37) |
| Right | 64 (53) | 35 (32) | −61 (−64) | 37 (37) | ||
| Dorsolateral PFC | 9/10/46 | Left | 20 (54) | 46 (−43) | 5 (3) | 37 (37) |
| Right | 26 (44) | 49 (35) | 8 (44) | 31 (15) | ||
| Inferior frontal gyrus | 44/45 | Left | 11 (15) | −55 (−35) | 20 (19) | 9 (9) |
| Right | 9 | 52 | 18 | 4 | ||
| SMA/anterior cingulate | 6/32 | Medial | 32 (61) | 0 (−3) | −6 (8) | 48 (48) |
| Lateral premotor cortex | 6 | Left | 5 (34) | −35 (−43) | −3 (3) | 42 (42) |
| Precentral gyrus | 4 | Left | 10 (29) | −26 (−40) | −11 (−14) | 48 (48) |
| Right | (67) | (32) | (−6) | (53) | ||
| Extrastriate cortex | 19 | Left | 5 (49) | −32 (−40) | −69 (−69) | −13 (−13) |
| Right | (17) | (17) | (−75) | (−13) | ||
| Cerebellum | — | Left | 5 (13) | −6 (−32) | −72 (−67) | −13 (−18) |
Data are from 20 patients with schizophrenia, each scanned twice, and 10 healthy volunteers, each scanned once. The total number of voxels and Talairach coordinates for peak response in each regional cluster are shown in parentheses for the normal healthy volunteers.
Figure 1.
Maps of generic brain activation in the patient group (upper row) and of brain regions demonstrating an enhanced power of response after substitution of risperidone (lower row). Upper row: brain regions activated by working memory in the patient group; one-tailed probability of false positive error at each voxel α < 0.005. Activated voxels are colored according to their power of functional response (P) scaled to the maximum and minimum power of response. Lower row: a two-way ANCOVA was fitted at each generically activated voxel to identify regions that showed a significant enhancement of response in the risperidone-treated cohort at 6 weeks. The two-tailed probability of false positive error at each voxel α < 0.05. The distance of each map above the intercommissural line in the standard space of Talairach and Tournoux (29) is given in millimeters below. The right side of the brain is shown on the left side of each map.
A significant interaction between cohort and time was identified by ANCOVA at 37 voxels in total. These were mainly located in right dorsolateral prefrontal cortex (BA 9, 46; 15 voxels at x, y, z coordinates 46, 8, 31), the SMA (BA 6; 7 voxels at 6, 19, 42), and bilateral precuneus (BA 19; 5 voxels at 23, −64, 42 and 3 voxels at −26, −61, 42). The largest interactive effect was located in SMA; β3 = 0.5. Selected slices of the ANCOVA map, showing voxels where there was a significant enhancement of response in the risperidone-treated cohort at 6 weeks, are shown in Fig. 1.
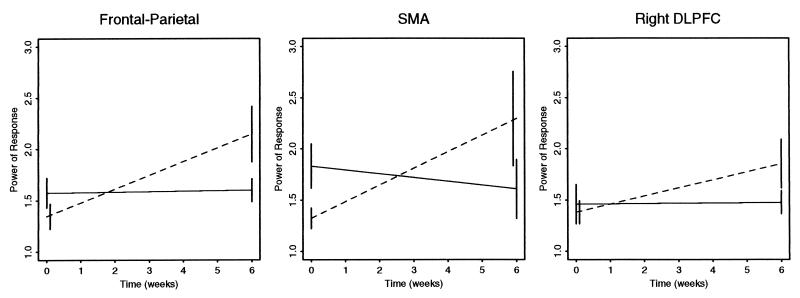
The effects of drug substitution on regional power of response in right DLPFC and SMA are graphically summarized in Fig. 2. Also shown are the effects of drug substitution on a measure of supraregional power obtained by averaging estimates of regional mean power over all four regions for each subject at each assessment. The two-way ANCOVA model, Eq. 1, was also fitted with supraregional power as the dependent variable, and the standardized coefficient β3 was tested against critical values of the t distribution. The interactive effect of cohort by time was statistically significant at this multiregional level of analysis: t = 2.6, df = 33, two-tailed α = 0.01.
Figure 2.
Interaction plots. The mean regional power of response in supplementary motor area (SMA; Middle) and right dorsolateral prefrontal cortex (DLPFC; Right) is shown for each patient cohort at each assessment: solid lines represent typically treated cohort; broken lines represent risperidone-treated cohort; error bars denote ± standard error of the mean. Also shown is mean power of response averaged over these two regions and bilateral areas of precuneus (the frontoparietal supraregional measure shown in Left).
Discussion
This study tested the prediction that substituting risperidone for typical antipsychotic drugs in the treatment of patients with schizophrenia would cause increased functional activation of frontal cortex during performance of a working memory task, hypothetically caused by enhanced mesocortical dopaminergic effects. fMRI was used to assess longitudinal changes in cerebral blood oxygenation in 10 patients with chronic schizophrenia treated for a period of 6 weeks with risperidone. This cohort was compared with another cohort of 10 patients who continued treatment with typical antipsychotics, thus controlling for effects of practice on repeated imaging measurements. The data were consistent with our prediction. We found that switching patients from typical antipsychotic drugs to risperidone was associated with increased power of functional activation by the working memory task in right dorsolateral prefrontal cortex, SMA, and precuneus. This effect was estimated by a two-way ANCOVA model, specified to control for possible confounding effects of differences between cohorts in symptom status, and was statistically significant at both voxel and regional levels of analysis.
The mechanism(s) by which substitution of risperidone might enhance fMRI measures of frontal function remain(s) hypothetical. Withdrawal of typical antipsychotics, without introduction of an alternative agent, might be expected to increase neuronal activity in frontal cortex by reducing postsynaptic D2 receptor antagonism. In support of this interpretation, Holcomb et al. (35) have reported that schizophrenic patients withdrawn from treatment with the D2 antagonist haloperidol over a 30-day period had increased metabolism in anterior cingulate and medial and inferior frontal cortices; additionally, evoked response studies by Dick et al. (36) have demonstrated reduction in amplitude of the motor readiness potential (generated by medial and lateral premotor cortex) after treatment of normal subjects with haloperidol. Reduced D2 antagonism in the nigrostriatal dopaminergic system might enhance frontal cortical activity by reducing inhibitory output from globus pallidus (37, 38). Reduced D2 antagonism in the mesoneocortical system might disinhibit pyramidal cell activity in frontal cortex by inhibiting (γ-aminobutyric acid) GABAergic interneuronal activity. GABAergic interneurons normally act to inhibit pyramidal cell activity, and they are the main cellular site of D2 receptors in frontal cortex (39); however, it is currently controversial whether dopamine has excitatory or inhibitory effects on these cells (40–43).
Alternatively, it is possible that substitution of risperidone might have modulated frontal neuronal activity by a serotonergic mechanism. Risperidone has greater antagonist affinity for postsynaptic 5HT2A receptors than typical antipsychotic drugs have, and 5HT2 receptors mediate the excitatory effects of ascending projections from the brainstem raphe nuclei on GABAergic interneurons in frontal cortex (39). Thus substitution of risperidone might act to disinhibit pyramidal cell activity, and so increase functional response, by blocking serotonin effects on inhibitory interneurons.
A third possible mechanism is based on an interaction between dopaminergic and serotonergic systems. Serotonin has been shown to have an inhibitory effect on presynaptic dopamine release (44, 45), and 5HT2 receptor antagonism has been shown to cause increased dopamine release in animal models (46). Serotonergically enhanced dopamine release might in turn affect neuronal activity by either one or both of the D2 receptor-based mechanisms already described, or by D1 receptors, which are located preferentially on pyramidal cell bodies and have a critical role in optimizing frontal cortical response to working memory tasks (47).
Thus dopaminergic and/or serotonergic effects of substituting risperidone for typical antipsychotic drugs might directly cause enhanced functional activation of prefrontal and medial premotor areas, which are known to receive a rich dopaminergic input from midbrain nuclei in the macaque monkey (48). Associated changes in functional response of posterior parietal cortex were not hypothetically predicted, and dopaminergic afferentation of this cortical area has not been so well characterized in nonhuman primates. It is possible that posterior parietal cortex independently receives a dopaminergic input which is modulated by drug substitution. However, it is well known that prefrontal and posterior parietal cortices are densely and reciprocally interconnected anatomically (49), and it may be that the parietal changes observed in these data reflect a “downstream” effect of the primary effects of drug substitution on frontal cortical areas.
All of these explanatory models have in common a neuronal site of action, but of course fMRI does not directly measure neuronal activity: it measures change in cerebral blood flow and oxygenation. Such changes as we have observed could be induced by pharmacological effects on underlying neuronal activity, but it is also conceivable that they reflect pharmacological effects on the hemodynamic response characteristics of the cortical vasculature. It is relevant to this interpretation that dopaminergic terminals have recently been demonstrated in close proximity to intracortical arterioles in brain slice preparations, and introduction of dopamine agonist drugs caused vasoconstriction (50). Gollub et al. (51) have also used perfusion-weighted magnetic resonance imaging to demonstrate a global reduction in cerebral perfusion after intravenous administration of cocaine. These observations suggest a role for dopamine in cerebral vasoregulation, but this seems an unlikely explanation for our data for three reasons: (i) dopamine has a vasoconstricting effect on cerebral blood vessels, whereas a vascular explanation for our findings would demand that it acted as a vasodilator; (ii) vasoconstricting effects of dopaminergic drugs on cerebral blood flow do not necessarily affect the magnitude of acute hemodynamic response to experimental stimulation (51); and (iii) evoked response potential effects of dopaminergic agonists and antagonists are consistent with a neuronal explanation for enhanced functional response after substitution of risperidone (36).
Although it is clearly not possible to decide definitively between these possible explanations for a pharmacological effect on these data, an entirely nonpharmacological explanation for our findings is difficult to sustain. There was no significant difference between typically and atypically treated cohorts in task performance during scanning. Nor was there any significant difference between groups in stimulus-correlated head motion (which can cause major artifacts in fMRI time series). Our experimental and analytic procedures are validated by the close correspondence between the activation pattern we observed in healthy volunteers and previous accounts of the functional anatomy of working memory in normal human subjects.
The main limitation of the study is the naturalistic assignment of patients to treatment groups. This was dictated by ethical considerations. fMRI is a relatively new technique for human brain mapping, which has not previously been used to measure treatment effects in psychiatric patients. In light of the innovative nature of this study, we considered it inappropriate to risk either denying atypical drug treatment to patients who might benefit from it, or disturbing patients satisfactorily managed on typical drugs, by a randomized design. Consequently, the patient cohorts are not well matched symptomatically; the risperidone-treated cohort had higher scores on psychopathology scales than the typically treated cohort at baseline and 6 weeks. There was also a nonsignificant difference between cohorts in the frequency of adjunctive anticholinergic treatment: more patients in the typically treated cohort were taking procyclidine than in the risperidone-treated cohort. However, we have used a two-way ANCOVA model to estimate effects of drug substitution while controlling post hoc for effects on functional response of symptom status and other consistent differences between cohorts (such as frequency of procyclidine treatment). We hope that by demonstrating the feasibility of using fMRI to map psychopharmacological effects in patients, the present study makes randomized assignment of patients to treatments ethically less problematic in the future.
Another question concerns the cognitive and clinical correlates of enhanced functional activation in the risperidone-treated group. Treatment with risperidone has previously been associated with significantly improved clinical status (52) and cognitive performance (6) in larger samples of patients. We found comparable (but nonsignificant) trends toward improvement on both these dimensions. We suggest that our inability to detect significant clinical or cognitive improvement in these data may be a type II error, reflecting our smaller sample size, the relatively low load imposed on working memory, and/or a ceiling effect on the measure of performance accuracy. The question of how antipsychotic drug effects on frontal activation might be related to, or even predictive of, their effects on behavioral measures of cognition remains an intriguing focus for further study.
In summary, we have provided direct evidence for enhanced prefrontal cortical activation in schizophrenic patients after substitution of risperidone for typical antipsychotic drug treatment. We suggest that these data are compatible with in vitro evidence for relatively reduced mesocortical D2 blockade by atypical antipsychotic drugs, and indicate the potential value of fMRI as a tool for longitudinal assessment of psychopharmacological effects on cerebral physiology.
Acknowledgments
We are grateful to the subjects who participated in this study and we thank Dr. Andy Simmons, Mr. Chris Andrew, and the neuroimaging staff at the Maudsley Hospital for technical support. E.T.B. is supported by the Wellcome Trust. This study was supported in part by funds from PsychMed Ltd.
Abbreviations
- PFC
prefrontal cortex
- fMRI
functional magnetic resonance imaging
- ANCOVA
analysis of covariance
- SMA
supplementary motor area
- BA
Brodmann area
Footnotes
Preliminary results were presented at the XXIst Congress of the Collegium Internationalle Neuro-Psychopharmacologium, July 12–16, 1998, Glasgow, U.K.
References
- 1.Seeman P, Lee T, Chau-Wong M, Wong K. Nature (London) 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 2.Crow T J. Br Med J. 1980;280:66–68. doi: 10.1136/bmj.280.6207.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerwin R W. Br J Psychiatry. 1994;164:141–148. doi: 10.1192/bjp.164.2.141. [DOI] [PubMed] [Google Scholar]
- 4.Sharma T. Br J Hosp Med. 1996;55:194–198. [PubMed] [Google Scholar]
- 5.Buchanan R W, Breier A, Kirkpatrick B, Ball P, Carpenter W T., Jr Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- 6.Green M F, Marshall B D, Wirshing M D, Ames D, Marder S R, McGurk S, Kern R S, Mintz J. Am J Psychiatry. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 7.Sharma, T. & Mockler, D. (1998) J. Clin. Psychopharmacol.18 (2 Suppl. 1), 12S–19S. [DOI] [PubMed]
- 8.Meltzer H Y. Br J Psychiatry. 1996;168,(Suppl. 29):23–31. [PubMed] [Google Scholar]
- 9.Hertel P, Nomikos G G, Iurlo M, Svensson T H. Psychopharmacology (Berlin) 1996;124:74–86. doi: 10.1007/BF02245607. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Rakic P S. In: Handbook of Physiology: The Nervous System. Plum F, editor. Vol. 5. Bethesda, MD: Am. Physiol. Soc.; 1987. pp. 373–417. [Google Scholar]
- 11.Goldman-Rakic P S. In: Psychopathology and the Brain. Carroll B J, Bartrett J E, editors. New York: Raven; 1990. pp. 1–23. [Google Scholar]
- 12.Cohen J D, Forman S D, Braver T S, Casey B J, Servan-Schreiber D, Noll D S. Hum Brain Mapp. 1994;1:293–304. doi: 10.1002/hbm.460010407. [DOI] [PubMed] [Google Scholar]
- 13.Brozoski T J, Brown R M, Rosvold H E, Goldman P S. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe M, Kodama T, Hikosaka K. J Neurophysiol. 1997;78:2795–2798. doi: 10.1152/jn.1997.78.5.2795. [DOI] [PubMed] [Google Scholar]
- 15.Keefe R S, Roitman S E, Harvey P D. Schizophr Res. 1995;17:25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- 16.Cohen J D, Servan-Schreiber D. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger D R. J Neuropsychiatry Clin Neurosci. 1993;5:241–253. doi: 10.1176/jnp.5.3.241. [DOI] [PubMed] [Google Scholar]
- 18.Weinberger D R, Berman K F, Zec R F. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 19.Weinberger D R, Berman K F, Illowsky B P. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 20.Yurgelun-Todd D A, Waternaux C M, Cohen B M, Gruber S A, English C D, Renshaw P F. Am J Psychiatry. 1996;153:200–205. doi: 10.1176/ajp.153.2.200. [DOI] [PubMed] [Google Scholar]
- 21.Andreasen N C, O’Leary D S, Flaum M, Nopoulos P, Watkins G L, Boles-Ponto L L, Hichwa R D. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 22.Daniel D G, Berman K F, Weinberger D F. J Neuropsychiatry Clin Neurosci. 1989;1:377–384. doi: 10.1176/jnp.1.4.377. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger D R, Berman K F. Schizophr Bull. 1988;14:157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- 24.Weinberger D R, Berman K F, Chase T N. Ann NY Acad Sci. 1988;537:330–338. doi: 10.1111/j.1749-6632.1988.tb42117.x. [DOI] [PubMed] [Google Scholar]
- 25.Davis K L, Kahn R S, Ko G, Davidson M. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 26.Nelson H E. National Adult Reading Test. Windsor, U.K.: NFER-Nelson; 1982. [Google Scholar]
- 27.Kay S R, Fizbein A, Opler L A. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa S, Lee T, Kay A, Tank D. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bullmore E T, Brammer M J, Rabe-Hesketh S, Curtis V A, Morris R G, Williams S C R, Sharma T, McGuire P K. Hum Brain Mapp. 1999;7:38–48. doi: 10.1002/(SICI)1097-0193(1999)7:1<38::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bullmore E, Brammer M, Williams S, Rabe-Hesketh S, Janot N, David A, Mellers J, Howard R, Sham P. Magn Res Med. 1996;35:261–277. doi: 10.1002/mrm.1910350219. [DOI] [PubMed] [Google Scholar]
- 31.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- 32.Brammer M J, Bullmore E T, Simmons A, Williams S C R, Grasby P M, Howard R J, Woodruff P W R, Rabe-Hesketh S. Magn Reson Imaging. 1997;15:763–770. doi: 10.1016/s0730-725x(97)00135-5. [DOI] [PubMed] [Google Scholar]
- 33.Edgington E S. Randomization Tests. New York: Dekker; 1980. [Google Scholar]
- 34.Bullmore E T, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer M J. IEEE Trans Med Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- 35.Holcomb H, Cascella N, Thaker G, Medoff D, Dannals R, Tamminga C. Am J Psychiatry. 1996;153:41–49. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- 36.Dick J P, Cantell R, Bruma O, Giox M, Beneck R, Day B L, Rothwell J C, Thompson P D, Marsden C D. Electroencephalogr Clin Neurophysiol. 1987;66:263–274. doi: 10.1016/0013-4694(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 37.Alexander G E, Crutcher M D, DeLong M R. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 38.Wichmann T, DeLong M R. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- 39.Goldman-Rakic P S, Selemon L D. Schizophr Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- 40.Retaux S, Besson M J, Penit-Soria J. Neuroscience. 1991;42:61–71. doi: 10.1016/0306-4522(91)90150-m. [DOI] [PubMed] [Google Scholar]
- 41.Pirot S, Godbout R, Mantz J, Tassin J P, Glowinski J, Thierry A M. Neuroscience. 1992;49:857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- 42.Law-Tho D, Hirsch J C, Crepel F. Neurosci Res. 1994;21:151–160. doi: 10.1016/0168-0102(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald L W, Deutch A Y, Gasic G, Heinemann S F, Nestler E J. J Neurosci. 1995;15:2453–2461. doi: 10.1523/JNEUROSCI.15-03-02453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meltzer H Y. Psychopharmacology (Berlin) 1989;99,(Suppl.):S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- 45.Busatto G F, Kerwin R W. J Psychopharmacol (Oxford) 1997;11:3–12. doi: 10.1177/026988119701100102. [DOI] [PubMed] [Google Scholar]
- 46.Pehek E. Synapse. 1996;24:12–18. doi: 10.1002/(SICI)1098-2396(199609)24:1<12::AID-SYN2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 47.Williams G V, Goldman-Rakic P S. Nature (London) 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 48.Williams S M, Goldman-Rakic P S. Cereb Cortex. 1993;3:199–222. doi: 10.1093/cercor/3.3.199. [DOI] [PubMed] [Google Scholar]
- 49.Selemon L D, Goldman-Rakic P S. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krimer L S, Muly E C, Williams G V, Goldman-Rakic P S. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- 51.Gollub R L, Brieiter H C, Kantor H, Kennedy D, Gastfriend D, Mathew R T, Makris N, Guimaraes A, Rioprden J, Campbell T, et al. J Cereb Blood Flow Metab. 1998;18:724–734. doi: 10.1097/00004647-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Marder S R, Meibach R C. Am J Psychiatry. 1994;151:825–835. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]