Abstract
Previous research suggests that, in reaction time (RT) measures of episodic memory retrieval, the unique effects of adult age are relatively small compared to the effects aging shares with more elementary abilities such as perceptual speed. Little is known, however, regarding the mechanisms of perceptual speed. We used diffusion tensor imaging (DTI) to test the hypothesis that white matter integrity, as indexed by fractional anisotropy (FA), serves as one mechanism of perceptual slowing in episodic memory retrieval. Results indicated that declines in FA in the pericallosal frontal region and in the genu of the corpus callosum, but not in other regions, mediated the relationship between perceptual speed and episodic retrieval RT. This relation held, though to a different degree, for both hits and correct rejections. These findings suggest that white matter integrity in prefrontal regions is one mechanism underlying the relation between individual differences in perceptual speed and episodic retrieval.
Keywords: Aging, Neuroimaging, White matter, Fractional anisotropy, Region of interest, Cognition, Memory retrieval, Episodic memory
1. Introduction
As people age, cognitive abilities such as executive functioning, attention and memory tend to decline, even in the absence of significant disease (Kramer and Madden, in press; Zacks et al., 2000; Salthouse, 1996). Regarding memory, age-related declines are most pronounced for episodic memory, which involves specific contextual detail. Semantic memory, which involves context-independent facts and linguistic structure, typically shows less decline (Burke et al., 2000; Light, 2000; Veiel and Storandt, 2003). Age-related decline in episodic memory is evident both for encoding processes (Craik, 1994; Glisky et al., 2001) and retrieval processes (Spaniol et al., 2006). There is also evidence, however, that these declines in episodic memory functioning are not unique to memory but instead share a substantial proportion of their age-related variance with other, more elementary perceptual-motor abilities, including processing speed (Park et al., 2002; Salthouse, 1993, 1996; Veiel and Storandt, 2003).
Little is known currently regarding the neurobiological substrates of perceptual speed and how they may vary as a function of adult age (Salthouse and Madden, in press). The goal of this research was to identify some of the individual differences in brain structure that may comprise a mechanism for the speed mediation of episodic memory retrieval. Previous studies established that various aspects of brain structure and functioning, particularly in the prefrontal and medial temporal lobe regions, change during adulthood in a manner that has both task-independent and task-specific influences on memory performance (Cabeza, 2001, 2002; Grady, 2005; Park and Gutchess, 2005). Further, age-related deficits in measures of fluid intelligence and processing speed have been linked to several aspects of brain structure and function, including age-related decline in cortical volume, particularly the prefrontal regions (Schretlen et al., 2000), as well as to decline in the neurotransmitter dopamine, especially in the basal ganglia (Braver and Barch, 2002; Li et al., 2001).
In this study, we investigated the potential role of cerebral white matter integrity as a mediator of the relation between perceptual speed and episodic retrieval. Because memory retrieval involves other complex cognitive processes in addition to memory (e.g., attention), it is likely to depend on neural networks that are distributed widely throughout the brain; thus, white matter pathways would be essential for maintaining the coherence of information processing within these pathways (Greenwood, 2000; Tisserand and Jolles, 2003). Anatomical studies of nonhuman primates (Peters and Sethares, 2002), as well as human neuroimaging studies (Gunning-Dixon and Raz, 2000; Leaper et al., 2001; Madden et al., 2004; O’Sullivan et al., 2001; Sullivan et al., 2006; van den Heuvel et al., 2006), suggest that decreasing white matter integrity in older adults may lead to decreases in perceptual speed. Further, in a diffusion tensor imaging (DTI) investigation using ageing Rhesus monkeys, Makris et al. (2006) found that regional decreases in fractional anisotropy (FA), one index of white matter integrity, were negatively correlated with performance on tests of executive functioning but not for recognition memory.
We assessed white matter integrity using DTI, which is an MR technique that measures the rate and direction of diffusion of water molecules in neural tissue (Beaulieu, 2002; Le Bihan, 2003). Water diffusion can be described as occurring equally in all directions (isotropic diffusion) or occurring primarily in one direction (anisotropic). For instance, water diffusion in cerebral gray matter has a relatively low degree of anisotropy. On the other hand, water diffusion in white matter has varying degrees of anisotropy depending on many factors, such as the degree of compactness of white matter tracts, their myelination and number of axons within a specific region. Fractional anisotropy is an index of the degree to which water molecules diffuse in a single direction. FA values range from 0 (isotropic diffusion) to 1 (highly anisotropic diffusion). Various white matter disease processes, as well as the normal aging process can produce loss of white matter integrity and decreases in FA values. Several previous investigations have reported an age-related decline in FA consistent with a decline in white matter integrity (Moseley, 2002). There is a general anterior to posterior gradient of the age-related decline in FA, with the prefrontal regions exhibiting the greatest change, but decline is also evident in regions (e.g., posterior periventricular) outside of the frontal lobe (Head et al., 2004; Madden et al., 2006; Pfefferbaum et al., 2005; Salat et al., 2005).
It is clear that white matter integrity supports performance on a wide variety of cognitive tasks (Madden et al., 2004; O’Sullivan et al., 2001; Sullivan et al., 2001; Ylikoski et al., 1993). Indeed, Ylikoski et al. (1993) found that as the number of white matter lesions increased, performance on neuropsychological measures of processing speed and attention decreased. Other studies have demonstrated a relationship between decreasing white matter integrity and decreasing cognitive performance, as indexed by decreasing FA (Madden et al., 2004; O’Sullivan et al., 2001; Sullivan et al., 2001) or the increased rate of diffusion (O’Sullivan et al., 2001). In view of the established relation between elementary perceptual speed and age-related cognitive change, an important unresolved issue is the degree to which white matter integrity mediates perceptual speed.
To assess the role of white matter integrity as a mediator of perceptual speed effects, we used regression analyses similar to those that have established the role of speed as a mediator of age-related differences in memory performance (Moseley, 2002; Pfefferbaum et al., 2005; Salthouse, 1996; Veiel and Storandt, 2003). These previous studies demonstrated the mediating role of perceptual speed by means of hierarchical regression models. These models compare the variance in memory performance accounted for by age, when age was the sole predictor, relative to the remaining age-related variance when a measure of perceptual speed was entered as a predictor, before age, in the regression model. The current study expands on these findings by comparing single-task performance on semantic and episodic retrieval memory tasks. An additional contribution of this study is that we divided the episodic memory trials into those on which participants correctly categorized previously presented words as “old” (hit trials) and trials on which participants correctly categorized words that had not been presented during encoding as “new (correct rejection trials). According to dual-process models of recognition memory, different processes are involved in correct responses to “old” words or hits versus correct responses to “new” words or correct rejections, with hits being influenced by both recollection and familiarity but correct rejections representing a lack of recollection along with a low level of familiarity (Yonelinas, 1994). In our experiment, during a neuroimaging session that included DTI, participants performed several behavioral tasks including memory retrieval and perceptual speed. We hypothesized that, consistent with the previous behavioral studies (Park et al., 2002; Salthouse, 1993; Salat et al., 2005; Veiel and Storandt, 2003), speed would be a significant mediator of the relation between age and episodic retrieval RT. We did not expect to find significant age differences between younger and older adults on the semantic memory task as semantic memory remains relatively well-preserved with increased age (Zacks et al., 2000). Critically, we also predicted that white matter integrity, especially in prefrontal regions, would mediate the relationship between speed and episodic memory retrieval.
2. Methods
2.1. Design
The behavioral and DTI data were both collected during a single scanning session, which also included functional magnetic resonance imaging (fMRI; to be reported separately). We acquired the memory performance measures during an encoding phase, a retention phase and a retrieval phase, which cycled in that sequence four times. During the encoding phase, participants viewed a series of to-be-remembered words, presented one at a time and performed an encoding task (pleasant/unpleasant judgments). The DTI imaging was conducted in four runs during these encoding trials. Each encoding phase was followed first by a retention interval (during which participants performed a choice RT task) and then a retrieval phase (during which a series of words was also presented visually, one at a time). Two of the retrieval trial blocks involved episodic judgments (old/new in relation to the immediately preceding encoding trials) and two of the retrieval blocks involved semantic judgments (living/nonliving). The fMRI imaging was conducted during the four retention intervals and four retrieval blocks (Fig. 1).
Fig. 1.
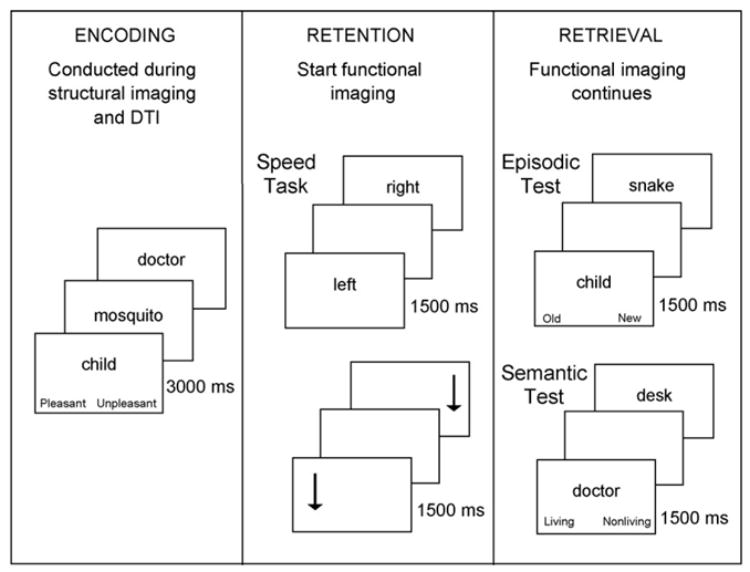
The sequence of trials during each of the three task phases. During retention and retrieval, the stimulus was followed by a blank screen of variable duration (1000, 1500 or 2000 ms).
2.2. Participants
All procedures were approved by the Internal Review Board of the Duke University Medical Center and the participants provided written, informed consent. Nineteen older adults (9 women) between the ages of 63 and 78 years of age (M = 69.59 years) and 19 younger adults (9 women) between the ages of 20 and 28 years of age (M = 23.89 years) participated. All participants had near visual acuity of at least 20/40, with a minimum score of 28 on the Mini-Mental State Exam (Folstein et al., 1975) and scored less than 10 on the Beck Depression Inventory (Beck, 1978). Participants were screened using a version of the Christensen Health Screener (Christensen et al., 1992) and excluded if they reported any major medical conditions known to affect cognition (e.g., recent stroke, Parkinson’s disease, etc.) or were currently taking antihypertensive or psychotropic medication. Participants’ T2-weighted structural brain images were reviewed by a neuroradiologist (one of the authors, J.M.P.) and determined to be within normal limits for atrophy, ventricular dilation and white matter lesions. We used the criterion that white matter lesions could not be: (a) more than five in number, (b) greater than 3 mm in diameter or (c) confluent (i.e., adjoining other white matter lesions) (Fazekas et al., 1988).
The older adults’ responses on a computer-administered digit-symbol coding test (Salthouse, 1992) were slower (M = 1770 ms) than younger adults’ responses (M = 1283 ms), t(36) = 5.34, p < 0.01, but the two age groups were comparable in their raw scores on the Vocabulary subtest of the Wechsler Adult Intelligence Scale (Wechsler, 1981); younger adults M = 64.63; older adults M = 66.68. The older adult participants (M = 18.00) had significantly more years of education than did the younger adults (M = 16.16), t(36) = 2.15, p = 0.04. Participants performed these psychometric and screening tests, as well as a practice version of the memory retrieval task, in a separate session approximately 2 weeks before the scanning session.
2.3. Stimuli and procedure
The test stimuli consisted of 312 familiar, concrete nouns, 156 that could be classified as either living (e.g., mosquito) and 156 that could be classified as nonliving (e.g., scissors). Each word was between 4 and 9 letters in length (M = 6 letters). An additional 72 words were used for practice during the separate screening session.
All phases of the task – encoding, retention and retrieval – were performed within the MR scanner during four run sequences. Participants viewed the stimuli displayed on the computer screen through liquid-crystal display goggles (Resonance Technology, Northridge, CA, USA) fitted with removable lenses that were matched to their prescription (if needed). Participants responded via two buttons on a four-button fiber optic response box (Resonance Technology), resting the thumb of each hand on a response button throughout testing.
During the encoding phase, which took place during the DTI imaging, participants viewed 39 words (18 words denoting living things, 18 words denoting nonliving things and 1 primacy and 2 recency buffer words) presented one at a time on the computer screen. The words were presented for 3 s each, in a fixed pseudorandom order for all participants. The participants’ task was to categorize each word as “pleasant” or “unpleasant.” The assignment of responses to buttons was counterbalanced over participants. Participants were instructed that each encoding block would be followed by a retrieval block, but that the task during each retrieval block could be (unpredictably) either episodic (old/new item recognition) or semantic (living/nonliving classification), without reference to the previous encoding list.
Following each encoding phase, there was a 120 s retention phase that began with a 30 s off-task period during which participants were instructed to focus on a white fixation cross. This period was in turn followed by a 90 s interval during which participants completed one of two choice RT tasks, one task involved pressing the left and right response buttons to the word “left” or right presented on each trial, whereas the other task involved pressing the buttons to an arrow located on the left or right side of the display. In these tasks the stimuli remained on the screen for 1500 ms followed by a blank screen of variable duration (1000, 1500 or 2000 ms). There was a total of 4 blocks of choice RT trials, with 2 blocks, each of 30 trials, for each type of choice RT task. These four blocks were presented in an alternating sequence, counterbalanced over participants. We constructed a composite speed score by obtaining each participant’s median RT for each choice RT task and then averaging these two values. Following each choice RT task, there was a 30 s off-task period during which participants were instructed to focus on a white fixation cross.
Immediately following the off-task period, participants completed the retrieval phase, consisting of either the episodic or semantic memory task. As mentioned previously, at the time of encoding, participants did not know which type of test would be presented during the subsequent retrieval phase. At the beginning of the retrieval tests, an instruction screen indicated whether the participants should perform old/new (episodic) or living/nonliving (semantic) judgments. Participants viewed the 39 words from the encoding phase along with 39 words that had not been presented during encoding. The words occurred in a pseudo-random order, and each word remained on the screen for 1500 ms followed by a blank screen of variable duration (1000, 1500 or 2000 ms). Each participant performed the semantic and episodic retrieval tests in one of two orders (ABBA or BAAB), which was counterbalanced across participants.
2.4. Diffusion tensor imaging
Imaging was conducted on a GE 4T scanner. Prior to the behavioral task, we acquired the following types of whole-brain images: sagittal localizer, spoiled gradient recalled (SPGR), T2-weighted and DTI. At the start of the behavioral task, we conducted four alternating runs of DTI, started at encoding and -weighted (functional) images, started at retrieval. Thus, there were five signal averages of the DTI sequence, one included with the other structural imaging and one conducted during each of the four encoding blocks. The DTI sequence included 30 near-axial slices, parallel to the plane connecting the anterior and posterior commissures. Slices were 3.8 mm thick with no interslice gap. Diffusion was measured in six directions, plus one image with no diffusion weighting, TR/TE = 30,000/138.8, NEX = 1, FOV = 24 × 24, flip angle = 90°, b = 1000 s/mm2, 128 × 128 matrix resolution, in-plane resolution = 1.875 mm.
We selected seven white matter regions of interest (ROIs) containing tracts providing pathways to a widely distributed network of cortical areas involved in memory retrieval. These areas include the prefrontal, anterior cingulate, medial temporal and parietal regions (Cabeza, 2001, 2002; Park and Gutchess, 2005). These ROIs, illustrated in Fig. 2, included: (a) the cingulum bundle (CIN) providing for associational interactions along the cingulate gyrus and between cortical areas in the cingulate gyrus and the medial temporal lobe; (b) the uncinate fasciculus (UNC) connecting the inferior frontal and anterior temporal lobes; (c) the superior longitudinal fasciculus connecting the frontal lobes and lateral portions of the temporal lobe, which we have subdivided into anterior (ASL) and posterior (PSL) portions; (d) white matter slightly lateral to the genu of the corpus callosum and bounded by the frontal sulci, insular cortex and the anterior horn of lateral ventricles (PCF; pericallosal frontal) providing connections among prefrontal and subcortical areas. We also selected the genu (GNU) and splenium (SPN) of the corpus callosum as these white matter tracts provide interhemispheric connections across anterior (GNU) and posterior (SPN) portions of the cortex. These ROIs were drawn directly on the diffusion tensor images by two trained operators, with the high resolution SPGR images as a guide, using previously established methods (Madden et al., 2004, 2006).
Fig. 2.
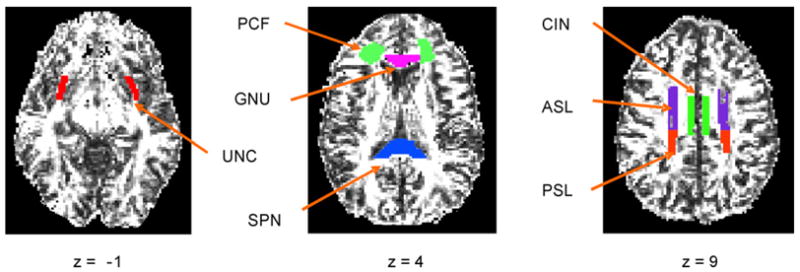
Example of white matter ROIs for a younger and older adult. The ROIs were drawn individually for each participant directly on the diffusion tensor images. GNU, Genu of the corpus callosum; SPN, splenium of the corpus callosum; PCF, pericallosal frontal white matter; ASL, white matter in the anterior portion of the superior longitudinal fasciculus; PSL, posterior portion of the superior longitudinal fasciculus; UNC, uncinate fasciculus; CIN, cingulum bundle.
Prior to drawing ROIs independently, the two operators attained at least 80% spatial overlap of voxels. The genu and splenium of the corpus callosum were clearly visible on the T1-weighted images, and thus standard anatomical landmarks were used to define these regions. The pericallosal frontal ROI was defined on image slices containing the genu of the corpus callosum. The pericallosal frontal ROI targeted the anterior centrum semiovale, where fibers of passage associated with dorsal prefrontal cortical areas and related subcortical structures would be expected to course. This ROI was bounded by gray matter at the fundi of frontal sulci anteriorly and laterally (it did not sample subcortical white matter within frontal gyri), by the anterior horn of the lateral ventricles medially and by insular cortex posteriorly. For the ASL and PSL, the ROIs were defined as beginning on the last slice where the ventricles where present and ending when the white matter tracts disappeared. For the ASL, the anterior boundary was the anterior cingulate; the posterior boundary was the central sulcus. For the PSL, the anterior boundary was the central sulcus and the posterior boundary was the precuneus. We defined the UNC as beginning on the last slice in which the brain stem was present. The anterior boundary was ten voxels beyond the rostral portion of the diencephalons and approximately three voxels wide. The posterior boundary was the top of the brain stem. We consulted the SPGR image to be certain that the ROI contained white matter only. The CIN was defined as beginning one slice above where the corpus callo-sum and ventricles were present. The CIN was medial to the superior longitudinal fascicules and above the corpus callosum.
Using custom MATLAB scripts, we calculated the average FA for each ROI. For regions outside the corpus callosum, we calculated separate FA values for the left and right hemispheres.
3. Results
3.1. Memory retrieval performance
On the semantic memory test, older adults did not differ significantly in either accuracy (M = 94% for older adults, 91% for younger adults) or mean of median RT (M = 725 ms for older adults, 633 ms for younger adults), t(36) = 1.67 p = 0.10. On the episodic test, accuracy (hits–false alarms) was also not significantly different between the two age groups (M = 65% for older adults, 73% for younger adults), t(36) = −1.62 p = 0.11. As noted in the Introduction, current dual-process models of recognition memory propose that different processes are involved in correct responses to “old” words (hits) versus correct responses to “new” words (correct rejections) during episodic retrieval (Yonelinas, 1994). Therefore, we divided the episodic retrieval data into hits and correct rejections and conducted separate analyses of RT for each type of trial. Younger adults were significantly faster than older adults for both hits (M = 659 ms for younger adults, 750 ms for older adults), t(36) = 2.31 p = 0.03 and correct rejections (M = 738 ms for younger adults, 875 ms for older adults), t(36) = 3.62 p = 0.0009. Because we did not obtain age differences in semantic retrieval RT or accuracy, or in episodic accuracy, we restricted our analyses to the RT data for hit and correct rejection trials.
3.2. Speed mediation of age effects in memory retrieval
Our goal in the mediation analyses was to determine, first, whether perceptual speed (as defined by the mean of the two left/right choice RT tasks) mediated the age-related slowing in episodic retrieval RT, and secondly, whether white matter integrity mediated perceptual-motor speed. As a first step, we determined whether the assumptions of mediation (Baron and Kenny, 1986) were met for all of our variables of interest (age group, perceptual-motor speed, FA, hit RT and correct rejection RT). We conducted the regression analyses to test for these relations, separately for RT on hit trials and correct rejection trials. Age group was coded as a categorical variable. We found a significant relationship between age group and hit RT (r = 0.36, p = 0.03), between age group and correct rejection RT (r = 0.52, p = 0.0009), between perceptual-motor speed and hit RT (r = 0.36, p = 0.02), between perceptual-motor speed and correct rejection RT (r = 0.56, p = 0.0002) and between age and perceptual-motor speed (r = 0.41, p = 0.01). Thus, our measure of perceptual speed meets the requirements for mediation.
When age group was the only predictor of hit RT, 13% of the variance was age-related (Table 1). To determine if speed was a significant mediator of the effect of age on hit RT, in a regression model predicting hit RT, we entered our perceptual-motor composite score first, followed by age. After controlling for perceptual-motor speed in this manner, age no longer accounted for significant variance in episodic hit RT. To determine the degree to which speed attenuated the amount of variance in episodic hit RT that can be explained by age, we used a procedure advocated by Salthouse (1993), in which we subtracted the amount of variance in episodic hit RT uniquely associated with age (partialled for the effect of speed) from the amount of variance in episodic hit RT associated with age as a sole predictor. We then divided this difference by the amount of age-related variance in episodic hit RT when age was the sole predictor. Using this procedure, we found that speed attenuated 59% of the age-related variance in episodic hit RT.
Table 1.
Effects of age and speed for hit and correct rejection RT
| β | r2 | Increment in r2 | F | Percentage attenuation | |
|---|---|---|---|---|---|
| Hit RT | |||||
| Model 1 | |||||
| Age | 0.359 | 0.129 | 5.32* | ||
| Model 2 | |||||
| Speed | 0.364 | 0.133 | 5.52* | ||
| Age | 0.252 | 0.186 | 0.053 | 2.28 | 58.91 |
| Correct rejection RT | |||||
| Model 1 | |||||
| Age | 0.517 | 0.267 | 13.12* | ||
| Model 2 | |||||
| Speed | 0.564 | 0.319 | 16.84* | ||
| Age | 0.343 | 0.417 | 0.099 | 5.91* | 62.92 |
p < 0.05.
We used these same steps to determine if speed was a mediator between age and correct rejection RT (Table 1). As the sole predictor, age accounted for 27% of the variance in correct rejection RT. Similar to the analysis for episodic hit RT, speed was a significant predictor when entered before age. Speed attenuated 63% of the age-related variance in correct rejection RT. However, age continued to account for significant variance in correct rejection RT even after controlling for speed.
3.3. Fractional anisotropy
Preliminary analyses revealed no significant differences in FA between hemispheres and we thus used the average FA across hemispheres for each participant and region of interest as our FA measure. The mean FA values are presented in Fig. 2. To protect against type I error (Tabachnick and Fidell, 2001), we performed a multivariate analysis of variance (MANOVA) for the set of seven ROIs using age group as the between-subjects variable and the seven ROIs as separate dependent variables. As predicted, the results revealed a significant effect of age group, F(7, 30) = 6.34, p < 0.0001. Univariate tests of the age group difference for each region revealed significant age differences in the ASL (p = 0.0060), CIN (p = 0.0015), GNU (p = 0.0073), PCF (p = 0.0002) and SPN (p = 0.0332). In each case, mean FA was significantly lower for the older adult group reflecting age-related declines in white matter integrity (Fig. 3).
Fig. 3.
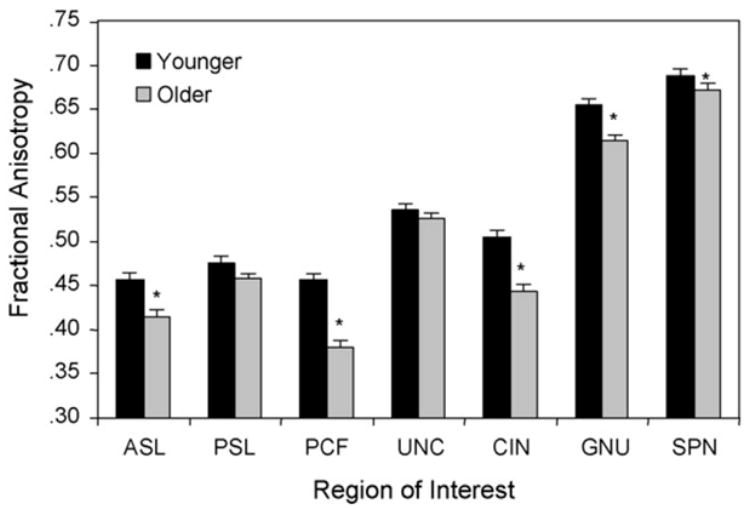
Mean FA values for each ROI by age group. For abbreviations see legend for Fig. 2.
3.4. Fractional anisotropy mediation of perceptual speed
To investigate whether white matter integrity mediates the relation between speed and episodic retrieval, we used the same regression procedures to investigate whether FA mediated the relationship between perceptual speed and episodic RT. The GNU and PCF were the only FA variables meeting the assumptions for mediation. We conducted separate regression analyses for GNU and PCF, using mean FA as a predictor variable. Additional analyses including interaction terms for left/right hemisphere differences indicated that the mediation effects did not vary significantly by hemisphere.
In the analyses of hit RT, perceptual speed accounted for 13% of the variance when it was the sole predictor (Table 2). When entered before speed, mean FA in both the GNU and PCF were significant predictors. In addition, the parameter estimates for both variables were negative, indicating that decreasing white matter integrity is associated with increasing RT (slower responses). Speed did not remain a significant predictor of hit RT after controlling for FA in the GNU and PCF. When entered before speed, mean FA in the GNU attenuated the amount of variance in hit RT associated with speed by 59%. Analogously, entering mean FA in the PCF as the first predictor attenuated the speed-related variance in hit RT by 60%.
Table 2.
Effects of speed and fractional anisotropy (FA)
| β | r2 | Increment in r2 | F | Percentage attenuation | |
|---|---|---|---|---|---|
| Hit RT | |||||
| Model 1 | |||||
| Speed | 0.364 | 0.133 | 5.52* | ||
| Model 2 | |||||
| FA-GNU | −0.427 | 0.182 | 8.02* | ||
| Speed | 0.247 | 0.236 | 0.054 | 2.46 | 59.40 |
| Model 3 | |||||
| FA-PCF | −0.463 | 0.214 | 9.82* | ||
| Speed | 0.242 | 0.267 | 0.053 | 2.54 | 60.15 |
| Correct rejection RT | |||||
| Model 1 | |||||
| Speed | 0.564 | 0.319 | 16.84* | ||
| Model 2 | |||||
| FA-GNU | −0.479 | 0.229 | 10.72* | ||
| Speed | 0.453 | 0.410 | 0.181 | 10.75* | 43.26 |
| Model 3 | |||||
| FA-PCF | −0.615 | 0.379 | 21.94* | ||
| Speed | 0.411 | 0.531 | 0.152 | 11.38* | 62.92 |
Note: FA-GNU, Fractional anisotropy in the genu of the corpus callosum; FA-PCF, fractional anisotropy in the pericallosal frontal region.
p < 0.05.
For episodic correct rejection RT (Table 2), mean FA in the GNU were also significant mediators, with negative parameter estimates. However, speed remained a significant predictor of correct rejection RT after controlling for either the GNU. Mean FA in the GNU reduced the amount of speed-related variance in correct rejection RT by speed by 43%. For mean FA in the PCF, the degree of attenuation was 52%. The bivariate correlations for all FA variables, hit RT and correct rejection RT are presented in Tables 3 and 4.
Table 3.
Correlations among fractional anisotropy and RT variables for younger adults
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Hit RT | – | |||||||||
| (2) Correct rejection RT | 0.833** | – | ||||||||
| (3) Perceptual-motor speed | 0.324 | 0.457 | – | |||||||
| (4) PCF | −0.149 | −0.276 | −0.427 | – | ||||||
| (5) ASL | 0.108 | 0.032 | 0.175 | 0.054 | – | |||||
| (6) PSL | −0.032 | −0.164 | 0.061 | 0.452 | 0.420 | – | ||||
| (7) UNC | −0.083 | −0.199 | −0.249 | 0.585** | 0.002 | 0.355 | – | |||
| (8) CIN | −0.089 | 0.058 | −0.137 | 0.332 | −0.120 | 0.261 | 0.416 | – | ||
| (9) GNU | 0.119 | 0.285 | 0.201 | −0.277 | −0.197 | −0.251 | −0.048 | 0.122 | – | |
| (10) SPN | −0.032 | 0.129 | −0.265 | 0.277 | −0.328 | −0.272 | 0.375 | 0.382 | 0.458* | – |
p < 0.05.
p < 0.01.
Table 4.
Correlations among fractional anisotropy and RT variables for older adults
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Hit RT | – | |||||||||
| (2) Correct rejection RT | 0.777** | – | ||||||||
| (3) Perceptual-motor speed | 0.205 | 0.452 | – | |||||||
| (4) PCF | −0.493* | −0.613** | 0.114 | – | ||||||
| (5) ASL | 0.210 | −0.002 | −0.092 | −0.124 | – | |||||
| (6) PSL | −0.152 | −0.087 | 0.152 | 0.237 | 0.367 | – | ||||
| (7) UNC | −0.271 | −0.270 | 0.180 | 0.370 | 0.191 | 0.251 | – | |||
| (8) CIN | 0.180 | 0.014 | 0.052 | 0.372 | −0.102 | −0.178 | 0.233 | – | ||
| (9) GNU | −0.493* | −0.565** | −0.323 | 0.405 | −0.325 | 0.169 | 0.119 | 0.005 | – | |
| (10) SPN | −0.109 | −0.151 | 0.294 | 0.410 | −0.488* | 0.010 | 0.011 | 0.505* | 0.200 | – |
p < 0.05.
p < 0.01.
4. Discussion
The results of this research lead to two important conclusions regarding individual differences in white matter integrity and the mechanisms for the speed mediation of episodic memory retrieval. First, consistent with previous research (Park et al., 2002; Salthouse, 1993, 1996; Veiel and Storandt, 2003), we found that perceptual-motor speed mediated the relationship between episodic retrieval RT and age. This was true for both hit and correct rejection trials. For correct rejection RT, however, age continued to account for significant variance in performance even after controlling for perceptual-motor speed. That is, although age-related slowing in both hits and correct rejections shares substantial variance with elementary perceptual speed, there is some additional age-related effect in correct rejection decisions. This difference in the degree to which perceptual speed mediates age-related changes in hits versus correct rejections has not been reported previously. This pattern may represent age-related variation in the decision process involved in terminating memory search on correct rejection trials, or rechecking the decision outcome on these trials prior to responding (Chun and Wolfe, 1996). Although the age group × trial type interaction was not significant, there was a trend for a greater degree of age-related slowing for correct rejections (137 ms) compared to hits (91 ms).
Second, and more importantly, we demonstrated a mechanism for the slowing in perceptual speed that is a common dimension of age-related differences in many forms of cognitive performance. As mentioned previously in the Introduction, although there is substantial evidence that speed mediates age-related declines in episodic retrieval, very little is known about the neural mechanism of speed. Consistent with other research (Head et al., 2004; Madden et al., 2006; Pfefferbaum et al., 2005; Salat et al., 2005), we found age-related declines in white matter integrity, as indexed by lower FA, in five of our seven ROIs. Only FA in the genu of the corpus callosum and in pericallosal frontal white matter, however, mediated the relation between perceptual-motor speed and episodic retrieval for both hit and correct rejection RT. This finding suggests that white matter decline as indexed by FA, particularly for prefrontal regions, serves as one of the mechanisms for perceptual-motor slowing. Because much of the age-related variance in episodic memory retrieval is shared with speed, we propose that decreasing integrity of white matter pathways contributes to the age-related deficits found in our episodic retrieval task by impairing the transmission of information among a widely distributed network of cortical regions, essentially disconnecting task-relevant regions. Although FA is a statistical mediator of this relationship, we cannot be sure of the biological changes that lead to these changes in FA. Such changes during normal aging may include an increased number of white matter lesions, age-related myelin loss, and increases in extracellular space (Moseley, 2002).
The disconnection idea is consistent with other research showing a relationship between white matter integrity and speed-based measures of cognitive performance, as defined by measures of white matter lesion volume (Gunning-Dixon and Raz, 2000; Leaper et al., 2001; van den Heuvel et al., 2006) and FA (Madden et al., 2004; O’Sullivan et al., 2001; Sullivan et al., 2006). In the current study, the mediating effects of FA varied as a function of the decision process, being stronger for hits than for correct rejections. This finding raises the possibility that for hits trials, episodic recollection and familiarity are dependent on perceptual speed as mediated by white matter pathways within the frontal lobe, whereas other processes contributing to correct rejections rely on additional input from nonfrontal mechanisms.
In conclusion, this research suggests that declines in white matter integrity of prefrontal regions contribute to the age-related slowing in episodic retrieval occurring as a function of age. It appears that, even in otherwise healthy older adults, deterioration of the white matter tracts due to normal aging slows the transmission of information across a network of cortical structures necessary for the retrieval of information from episodic long term memory.
Acknowledgments
This research was supported by grants R01 AG011622, R01 AG19731, R37 AG002163 and T32 AG00029, from the National Institute on Aging. We are grateful for assistance from Susanne Harris, Leslie Crandell Dawes and Sara Moore. We also thank Ian Dobbins for helpful comments.
Footnotes
Conflict of interest
The authors have no actual or potential conflict of interest associated with this research.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51 (6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15 (7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck Depression Inventory. Psychological Corporation; New York: 1978. [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26 (7):809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, James LE. Theoretical approaches to language and aging. In: Perfect TJ, Maylor EA, editors. Models of Cognitive Aging. Oxford University Press; New York: 2000. pp. 204–237. [Google Scholar]
- Cabeza R. Functional neuroimaging of cognitive aging. In: Cabeza R, Kingstone A, editors. Handbook of Functional Neuroimaging of Cognition. MIT Press; Cambridge, MA: 2001. pp. 331–377. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17 (1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Christensen KJ, Moye J, Armson RR, Kern TM. Health screening and random recruitment for cognitive aging research. Psychol Aging. 1992;7:204–208. doi: 10.1037//0882-7974.7.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Wolfe JM. Just say no: how are visual searches terminated when there is no target present? Cogn Psychol. 1996;30 (1):39–78. doi: 10.1006/cogp.1996.0002. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Memory changes in normal aging. Curr Dir Psychol Sci. 1994;3 (5):155–158. [Google Scholar]
- Fazekas F, Niederkor K, Schmidt R, Offenbacher H, Honner S, Bertha G, Lechner H. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonagraphy, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988;19:1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Minimental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12 (3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: an encoding or retrieval problem? J Exp Psychol Learn Mem Cogn. 2001;27 (5):1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Grady C. Functional connectivity during memory tasks in healthy aging and dementia. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; Oxford: 2005. pp. 286–308. [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6 (6):705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14 (2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14 (4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3. Erlbaum; Mahwah, NJ: in press. [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4 (6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Leaper SA, Murray AD, Lemmon HA, Staff RT, Deary IJ, Crawford JR, et al. Neuropsychologic correlates of brain white matter lesions depicted on MR images: 1921 Aberdeen birth cohort. Radiology. 2001;221 (1):51–55. doi: 10.1148/radiol.2211010086. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S. Aging cognition: from neuromodulation to representation. Trends Cogn Sci. 2001;5 (11):479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory changes in adulthood. In: Qualls SH, Abeles N, editors. Psychology and The Aging Revolution: How We Adapt to a Longer Life. American Psychological Association; Washington, DC: 2000. pp. 73–97. [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, et al. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21 (3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwec A, Kennedy DN, Hodgea SM, Dale AM, et al. Frontal connections and cognitive changes in normal aging Rhesus monkeys: a DTI study. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging—a review. NMR Biomed. 2002;15 (7–8):553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57 (4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Long-term memory and aging: a cognitive neuroscience perspective. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; New York: 2005. pp. 218–245. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17 (2):299–320. [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442 (3):277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: evidence from diffusion tensor imaging. Neuroimage. 2005;26 (3):891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26 (8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. What do adult age differences in the Digit Symbol Substitution Test reflect? J Gerontol. 1992;47 (3):P121–P128. doi: 10.1093/geronj/47.3.p121. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed mediation of adult age differences in cognition. Dev Psychol. 1993;29 (4):722–738. [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103 (3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Madden DJ. Information processing speed and aging. In: Deluca J, Kalmar J, editors. Information Processing Speed in Clinical Populations. Psychology Press; New York: in press. [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, et al. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. J Int Neuropsychol Soc. 2000;6 (1):52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, Voss A. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. J Exp Psychol Learn Mem Cogn. 2006;32 (1):101–117. doi: 10.1037/0278-7393.32.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport. 2001;12:99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16 (7):1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4. Allyn and Bacon; Needham Heights, MA: 2001. [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex. 2003;39 (4–5):1107–1128. doi: 10.1016/s0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- van den Heuvel DM, ten Dam VH, de Craen AJ, Admiraal-Behloul F, Olofsen H, Bollen EL, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77 (2):149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiel LL, Storandt M. Processing costs of semantic and episodic retrieval in younger and older adults. Aging Neuropsychol Cogn. 2003;10 (1):61–73. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50 (8):818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20 (6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2. Erlbaum; Mahwah, NJ: 2000. pp. 293–357. [Google Scholar]


