Abstract
Clinical and basic studies have indicated that upper cervical spinal cord stimulation (cSCS) significant increases cerebral blood flow (CBF), but the mechanisms are incompletely understood. This investigation was conducted to differentiate between stimulation of dorsal column fibers and upper cervical spinal cord cell bodies in cSCS-induced increases in CBF and decreases in cerebral vascular resistance (CVR). cSCS (50 Hz, 0.2 ms, 1 min) was applied on the left C1-C2 dorsal column n pentobarbital anesthetized, ventilated and paralyzed male rats. Laser Doppler flowmetry probes were placed bilaterally over the parietal cortex, and arterial pressure was monitored. cSCS at 30%, 60%, and 90% of motor threshold (MT) produced vasodilation bilaterally in cerebral cortices. Subsequently, cSCS was applied at 90% MT, and ipsilateral responses were recorded. Ibotenic acid (0.3mg/ml, 0.1ml) placed on dorsal surface of C1-C2 (n=7) to suppress cell body activity, did not affect cSCS-induced %□CBF (42.5±8.1% vs 36.8±7.1%, P>0.05□and %□CVR (−19.4±4.2% vs −15.2±5.6%, P>0.05). However, bilateral transection of the dorsal column at rostral C1 (n=8) abolished cSCS-induced changes in CBF and CVR. Also, rostral C1 transection (n=7) abolished cSCS-induced changes in CBF and CVR. Resinferatoxin (RTX), an ultra potent TRPV1 agonist, was used to inactivate TRPV1 containing nerve fibers / cell bodies. RTX (2 µg/ml□0.1ml) placed on the C1-C2 spinal cord (n=7) did not affect cSCS-induced %ΔCBF (60.2±8.1% vs 46.3±7.7%, P>0.05) and %ΔCVR (−25.5±3.5% vs −21.4±8.9%, P>0.05). However, intravenous RTX (2 µg/kg, n=9) decreased cSCS-induced %ΔCBF from 65.0±9.5% to 27.4±7.2% (P<0.05) and %ΔCVR from −28.0±7.6% to −14.8±4.2% (P<0.05). These results indicated that cSCS-increases in CBF and decreases in CVR occurred via rostral spinal dorsal column fibers and did not depend upon C1-C2 cell bodies. Also, our results suggested that cerebral but not spinal TRPV1 was involved in cSCS-induced cerebral vasodilation.
Keywords: Spinal cord stimulation, cerebral ischemia, vasodilation, transient receptor potential vanilloid type 1, resiniferatoxin
Introduction
Spinal cord stimulation (SCS) has been widely used as a clinically effective therapeutic modality to treat refractory neuropathic pain as well as ischemic pain resulting from peripheral vascular disease and angina pectoris (Cameron, 2004; Linderoth and Foreman, 1999; 2006). However, SCS has been used to a much less extent for treating cerebral vascular disturbances (Wu et al. 2007a). Hosobuchi (1985) was the first to report that cervical SCS (cSCS) produced an increase in cerebral blood flow (CBF) in humans. Subsequent clinical observations demonstrated that cSCS decreases cerebral vascular resistance and increases blood flow velocity, leading to an enhancement of the local-regional delivery of oxygen (Clavo et al., 2004; Mazzone et al. 1996; Meglio et al., 1991a, b). Therefore, the promising results of SCS-induced CBF augmentation have led some clinicians to use this procedure to treat various cerebral vascular disorders. These cerebral diseases and/or pathological conditions include cerebral ischemia (Broseta et al., 1994; De Andres et al., 2007; Hosobuchi, 1991), ischemic spastic hemiparesis (Visocchi et al., 1994), focal cerebral ischemia (Meglio et al., 1991a, b; Ebel et al., 2001; Sagher et al., 2003; Sagher and Huang 2006; Robaina et al., 2004), cerebral vasospasm (Gurelik et al., 2005; Karadag et al., 2005; Visocchi et al., 2001), stroke (Hosobuchi, 1991; Matsui and Hosobuchi, 1989; Visocchi et al., 1994, 2001), ischemic cerebral oedema (Gonzalez-Darder and Canadas-Rodriguez, 1991), postapoplectic spastic hemiplegia (Nakamura and Tsubokawa, 1985), prolonged coma (Fujii et al., 1998), persistent vegetative state (Funahashi et al. 1989; Kanno et al., 1987; Kuwata, 1993), as well as migraine and post-traumatic cervicogenic headache (Dario et al., 2005). However, the underlying mechanisms of blood flow improvement are not well understood (Wu et al. 2007a).
Animal models have been used to evaluate possible central and peripheral mechanisms of cSCS-induced increases in CBF. Blockade of autonomic ganglia with hexamethonium and blockade of α1-adrenergic receptors can suppress cSCS-induced increases in CBF (Patel et al., 2003; Sagher and Huang, 2000), but muscarinic receptor blockade with atropine had no effect (Garcia-March et al, 1989). Effects on brain vasomotor areas also are presumed to be of importance for increasing CBF (Patel et al., 2004; Sagher and Huang, 2000). In the case of SCS-induced hindlimb vasodilation, the response to SCS depends on TRPV1 containing peripheral fibers, as well as TRPV1 containing neurons in the spinal cord (Wu et al, 2006, 2007b), but it is unknown whether similar pathways are relevant to cSCS effects on CBF. Spinal cord transection in rats and dorsal column section in cats at the cervicomedullary junction abolish effects of cSCS on CBF (Isono et al., 1995; Patel et al. 2004). These effects may indicate that dorsal column fibers carry cSCS input to the brain; however, the upper cervical spinal cord also contains spinal neurons with projections to supraspinal structures, including various nuclei in caudal medulla, thalamus, hypothalamus, and periaqueductal gray in rats and cats (Malick et al., 2000; Mouton et al., 2005). It is unknown whether these spinal neurons play a role in changes of CBF by cSCS. Another important limitation of the SCS data discussed above is that cerebrovascular resistance (CVR) had not been calculated for each animal in previous studies. In some cases, a decrease in resistance might be inferred from mean changes in blood pressure versus mean changes in blood flow, but the occurrence of vasodilation is best verified by taking the mean of individual changes in vascular resistance.
To clarify and expand the results of previous studies, we addressed two issues with respect to upper cSCS-induced cerebral vasodilation in pentobarbital anesthetized rats. The first goal was to clarify the pathway from cervical spinal cord to brain through which cSCS produced cerebral vasodilation. Previous studies suggested that dorsal columns transmitted the information for vasodilation to the brain, but the effect could have been related to activation of cell bodies in the upper cervical spinal cord rather than axons. To differentiate between these mechanisms, effects on cSCS-induced cerebral vasodilation after C1 transections and after application of ibotenic acid to the C1-C2 spinal cord were compared. Ibotenic acid is excitotoxic to neuronal cell bodies but does not affect axons of passage (Ren et al. 1990). Our second goal was to assess the effects of TRPV1 containing neurons and nerve fibers during cSCS-induced cerebral vasodilation, since TRPV1-related mechanisms are crucial for SCS-induced peripheral vasodilation. We used resiniferatoxin (RTX), an ultrapotent TRPV1 agonist, to deactivate TRPV1 containing neurons / fibers involved in SCS (Wu et al. 2006; 2007). RTX was applied to the C1-C2 spinal cord or administered intravenously. All responses were examined with respect to changes in CBF and CVR for each tested animal. Because previous studies did not rigorously examine effects on resistance, we also verified that ganglionic blockade with hexamethonium suppressed upper cervical SCS-induced vasodilation. In addition, we considered whether the effects from the sympathetic chain could be excluded from the SCS response using C6-C7 spinal transection. Some of our results have been presented in abstract form (Yang et al., 2007a, b).
Materials and Methods
Animal preparation
Experiments were performed in 61 male Sprague-Dawley rats (310–430g Charles River, MA). The protocol for this study was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center and also followed the guidelines of animal experiments of the International Association for the Study of Pain. Anesthesia was initially induced by sodium pentobarbital (60 mg/kg i.p.) and was maintained throughout the experiment with constant intravenous infusion (15–25 mg/kg/h) through a catheter (PE-50) placed into the right jugular vein. Another catheter (PE-50) was inserted into the right carotid artery to monitor blood pressure (BP). A tracheotomy was performed for mechanical ventilation with a rodent ventilator (Model 683; Harvard Apparatus, Inc., S. Natick, MA) using a constant-volume pump (55–60 strokes/min, 3.0–5.0 ml stroke volume). Animals were paralyzed with pancuronium bromide (0.4 mg/kg, i.p.) and muscle relaxation was maintained with supplemental doses (0.2 mg/kg/h i.v.) during the experiment. Average blood pressure was kept between 80 and 120 mmHg, and pupils were constricted throughout experiments. Core body temperature was measured with a rectal probe and maintained between 36 and 38 °C at all times using a servo-controlled heating pad (Model 71A; Yellow Springs Instruments, Yellow Springs, OH). Animals were positioned in a stereotaxic frame and the vertebral column was stabilized with clamps. A laminectomy was performed to expose the dorsal surface of the cervical spinal cord segments (C1-C7). Room temperature was maintained between 22 and 24°C.
Spinal cord stimulation
A silver spring-loaded unipolar ball electrode with tip diameter of approximate 1 mm was placed on the left dorsal column 0.5 mm rostral to the C2 dorsal root entry zone to electrically activate C1-C2 spinal segments (Qin et al. 2007). The motor threshold (MT) stimulus intensity was determined in each animal at 50 Hz, 0.2 ms duration by slowly increasing the cSCS current from zero until a clear retraction of the left neck muscles was observed. Experimental cSCS (50 Hz; 0.2 ms, monophasic rectangular pulses), similar to clinical SCS, was performed for 1min at 30%, 60%, 90% of MT in random order of stimulus intensities (Tanaka et al. 2001; Wu et al., 2006). The lowest level of stimulation at 30% of MT is used because it was closest to the threshold of SCS that produced vasodilation. The level of stimulation at 60% of MT is also used because it approximates the parameters of clinical applications of SCS (Linderoth and Foreman 1999; 2006). The level of stimulation at 90% of MT intensity is used since it is close to, but below motor threshold. To obtain the stimulus- response relationships, different intensities of cSCS were applied at intervals >10 min and effects on CBF/CVR were assessed. In the experiments to examine the neural pathway and role of TRPV1, cSCS (90% MT) was reapplied after CBF/CVR recovered to control levels subsequent to spinal transection or chemical blockades (>20 min). Usually, cSCS was applied 5–8 times in each animal. In addition, to avoid potential interactions among drugs, testing trials for different chemical blockades were conducted in different groups of animals.
Measurement of CBF
A midline incision was made to expose the parietal region of the skull. A dental drill was used to produce a 3.0-mm-diameter hole that exposed the right and left parietal cortex at 4.0mm lateral and 3.0mm posterior to the bregma. These locations were held constantly to ensure reproducibility of recording CBF. The dura was exposed and kept intact. Under an operating microscope, a micromanipulator was used to position the 1-mm diameter laser Doppler probe (wavelength, 780 nm; probe 407, PeriFlux 5001; Perimed AB, Inc., Stockholm, Sweden) just above the dura over the exposed hemisphere. Care was taken to place the probe at a brain area with minimal vascularization. Both the laser probe and its holder were fixed to the skull by agar. Cortical CBF was assessed continually by using laser Doppler flowmetry (LDF) and arterial blood pressure was measured to calculate CVR. Responses to cSCS were determined as percent change from the baseline blood flow. Cerebral blood flow (CBF) and arterial blood pressure were recorded for 1 min of cSCS presented at different intensities in random order.
Spinal transections and pharmacological treatments
Spinal transections at C6-C7, dorsal column, and rostral C1 segment were carefully performed by using a sharp surgical knife. Hexamethonium (10 mg/kg), a ganglionic blocker, was intravenously administrated to interrupt sympathetic efferent activity (Patel et al. 2003; Tanaka et al. 2001). Chemical lesions with the soma-selective neurotoxin ibotenic acid were made at C1-C2 spinal segments to inactivate spinal neurons. Six to eight pieces of 2×2mm filter papers soaked with ibotenic acid (0.3mg/ml, 0.1ml) were placed on the C1-C2 spinal cord. To desensitize TRPV1 receptors, resiniferatoxin (RTX), an ultra potent analog of capsaicin and TRPV1 receptor agonist, was administered intravenously or on the C1-C2 surface of spinal cord (Wu et al. 2006; 2007b). Six to eight pieces of 2×2mm filter papers soaked with RTX (2 µg/ml, 0.1ml) were placed on the C1-C2 spinal cord. A stock solution of RTX (1 mg) was dissolved in 0.5 ml ethanol and 0.5 ml Tween 80. The bottle containing stock solution was wrapped in foil and stored in a −80° C freezer. On the day of an experiment, the stock solution of RTX was diluted in saline for intravenous injection (2 µg/kg). Agar (3–4% in saline) was used to make a well on the surface of spinal cord to prevent different drugs from diffusing to other spinal cord segments.
Statistical analysis
The unit of blood flow measurement was voltage. The change of CBF resulting from cSCS was calculated as the maximum voltage divided by the baseline voltage times 100 (%ΔCBF). Mean blood pressure was calculated by using the formula 2/3 × diastolic blood pressure+1/3×systolic blood pressure. When this value was divided by the voltage unit of flow, CVR in arbitrary units was obtained. The change in CVR resulting from cSCS was calculated as CVR at maximum flow divided by baseline CVR times 100 (%ΔCVR. This method may underestimate %ΔCVR if regional intracerebral venous resistance is raised sufficiently by cSCS to produce or increase a vascular waterfall effect. It is unclear whether such an effect occurs in this paradigm. The %ΔCBF and %ΔCVR were compared before and after various treatments. Data are presented as mean% ± SE. Differences in CBF and CVR between treatments were analyzed by one-way repeated measurement of variance ANOVA followed by Tukey's multiple comparison. Differences were considered statistically significant at P<0.05.
Results
Effects of cSCS on CBF
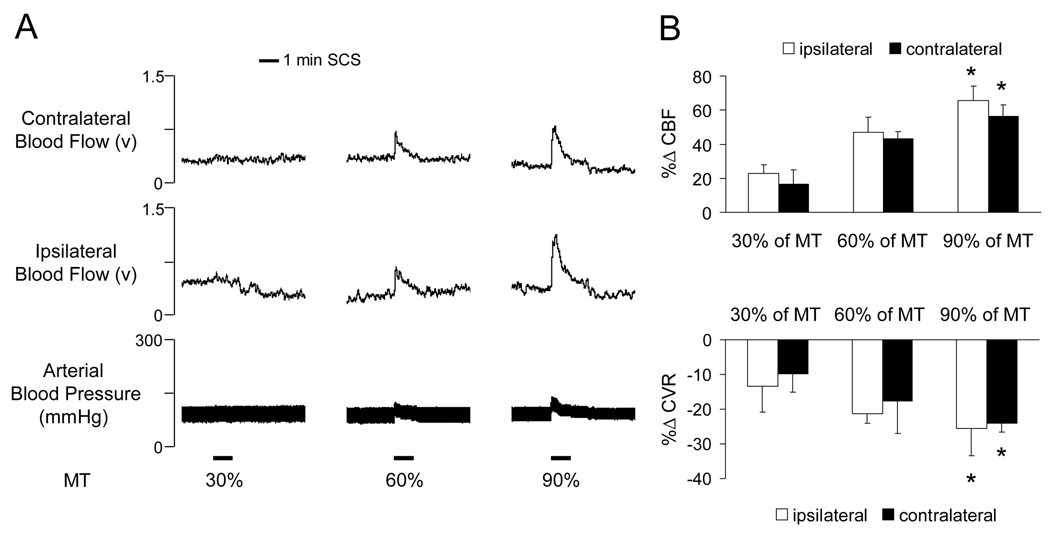
The average motor threshold (MT) in the first group of animals was 459±13µA (n=9). The cSCS at the different intensities (30%, 60%, 90% of MT) in random order increased CBF and decreased CVR from baseline (P<0.05). Changes of CBF and CVR with cSCS of 90% MT were greater than 30% of MT on both the ipsilateral and contralateral cortex (P<0.05). The maximal effect of cSCS on CBF and CVR occurred at 8.5±1.9s. Typical recordings of CBF in the ipsilateral and contralateral cortex and arterial blood pressure in response to different intensities of cSCS are shown in Fig. 1A. A summary for effects of cSCS on bilateral CBF and CVR are presented in Fig. 1B.
Fig. 1.
Effects of cSCS with different stimulus intensities on bilateral CBF and CVR. A: Typical recordings of bilateral CBF and arterial blood pressure in response to cSCS at different stimulus intensities. MT, motor threshold. B: Changes of bilateral cerebral blood flow (%ΔCBF, upper panel) and CVR (%ΔCVR, low panel) by cSCS at 30%, 60%, 90% of MT (n=9). * P <0.05 compared to response to cSCS at 30% of MT.
Neural pathway for cSCS-induced vasodilation
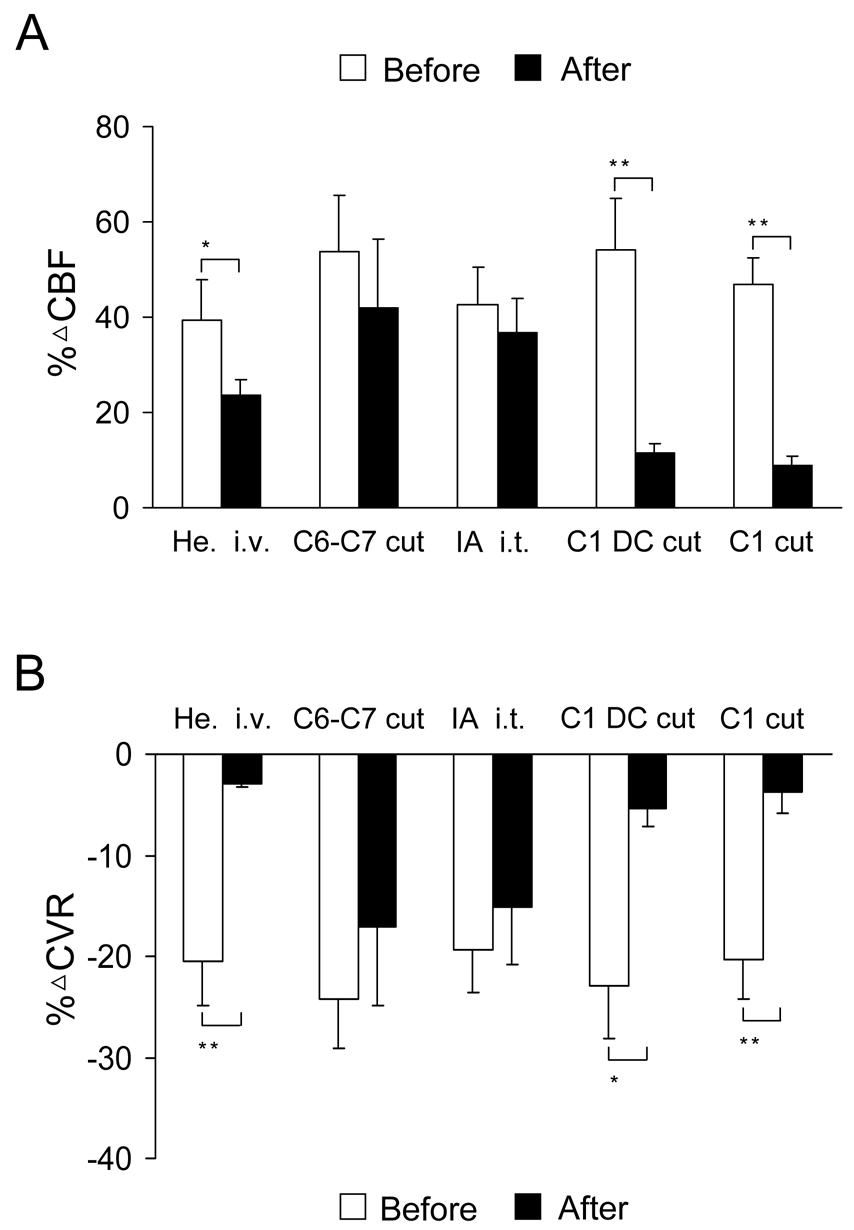
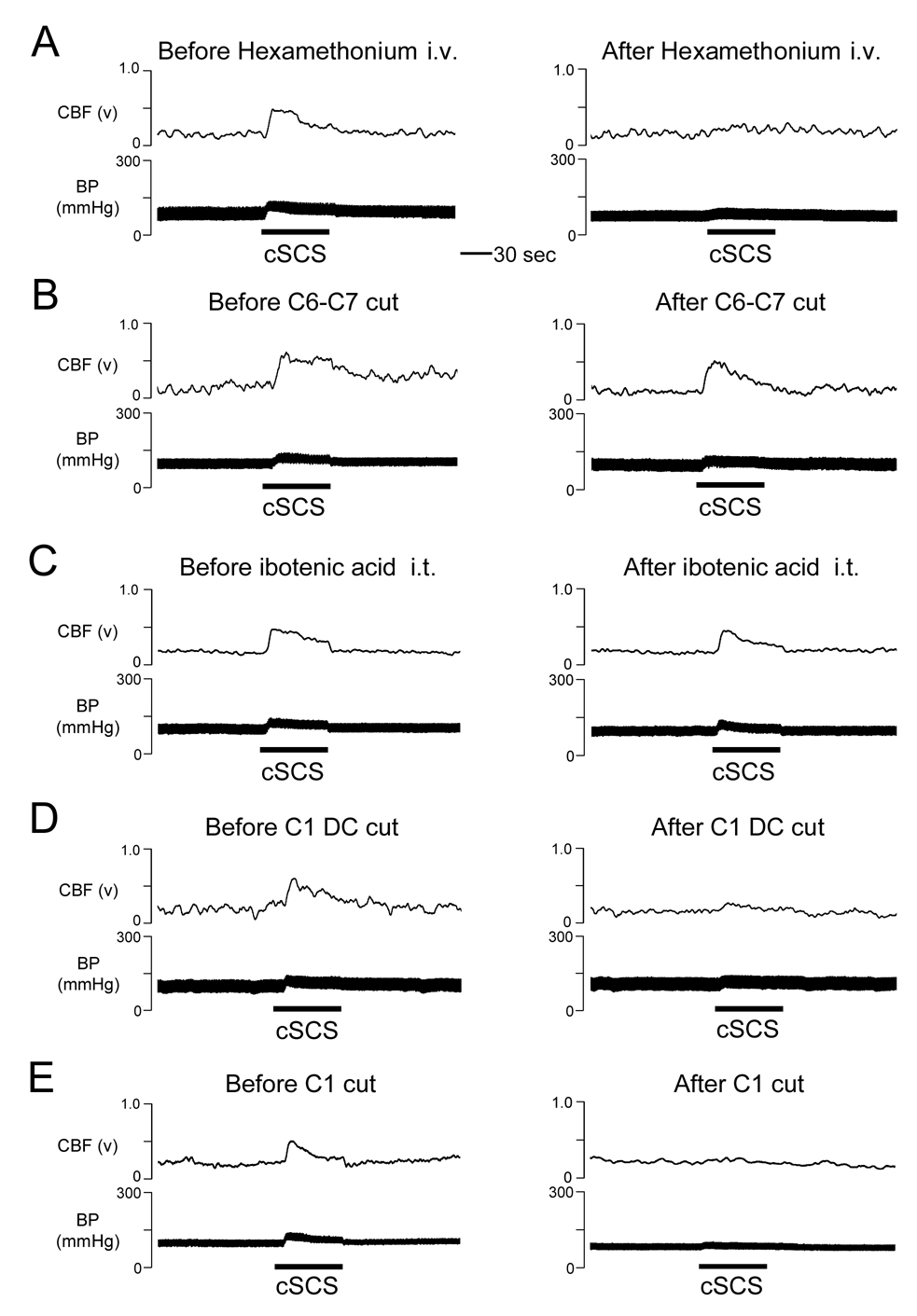
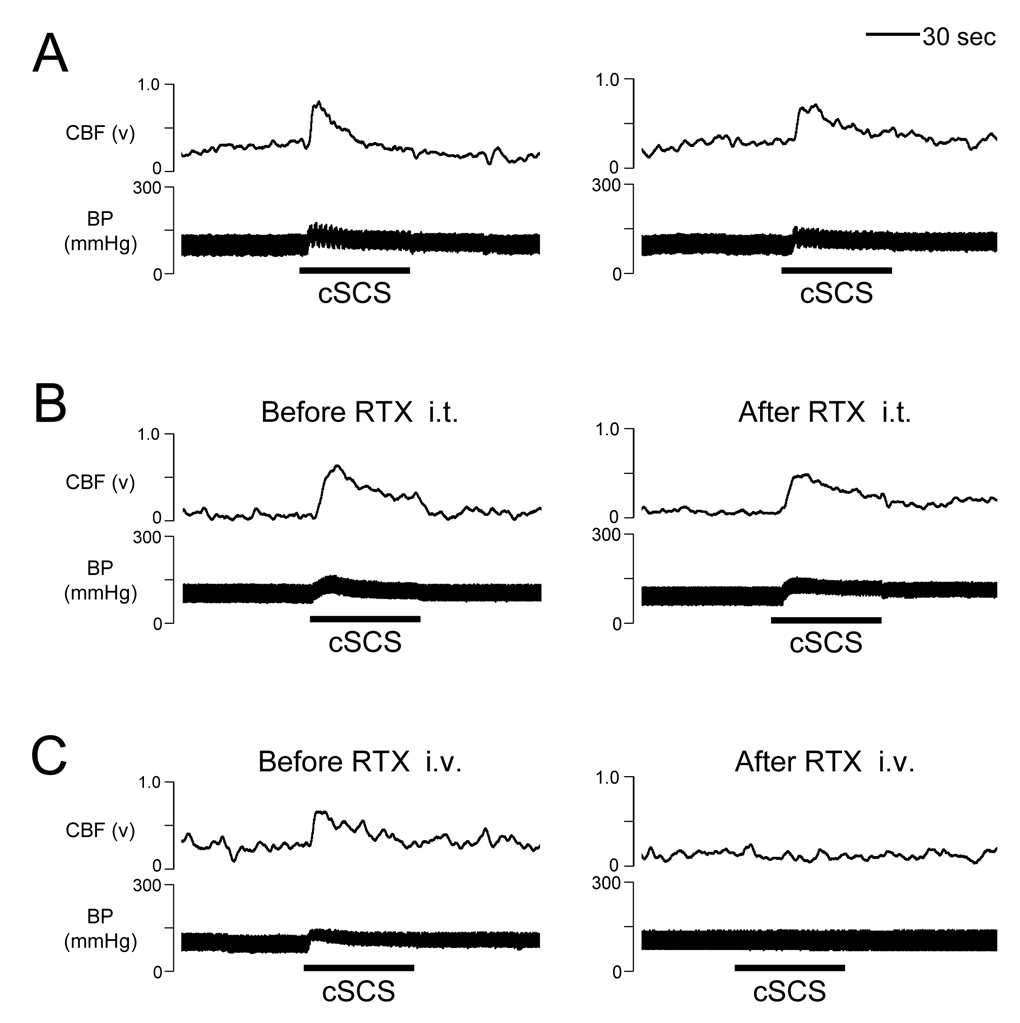
Spinal transections, intravenous hexamethonium, and C1-C2 ibotenic acid were used to determine the neural pathway for effect of cSCS-induced cerebral vasodilation. In the first group, intravenous hexamethonium significantly reduced effects of cSCS at 90% of MT (379±45µA, n=7) on %ΔCBF (39.5±8.3% vs 23.7±3.1%, P<0.05) and %ΔCVR (−20.5±4.3% vs −3.0±0.2%, P<0.01). Effects of intravenous hexamethonium on both CBF and CVR are summarized in Fig. 2 and an example of CBF augmented by cSCS but significantly attenuated by hexamethonium is shown in Fig. 3A. In the second group, spinal transection at C6-C7 segments did not affect %ΔCBF (53.8±11.7% vs 42.1±14.2%, P>0.05) and %ΔCVR (−24.3±4.8% vs −17.1±7.7%, P>0.05) to cSCS at 90% of MT (436±52µA, n=7). Statistical summary and example of effects of spinal transection at C6-C7 segments on CBF and CVR are shown in Fig. 2 and Fig. 3B. In the third group, ibotenic acid (0.3mg/ml, 0.1ml) placed on the dorsal surface of C1-C2 spinal segments did not affect %□CBF (42.5±8.1% vs 36.8±7.1%, P>0.05□and %□CVR (−19.4±4.2% vs −15.2±5.6%, P>0.05) to cSCS at 90% of MT (389±29µA, n=7). Fig. 2 and Fig. 3C show statistical summary and example of effects of ibotenic acid placed on C1-C2 spinal cord. In the fourth group, the average motor threshold was 370±17µA (n=8). Bilateral transection of the dorsal column at the rostral C1 segment abolished %ΔCBF from 54.1±10.8% to 11.6±1.8% (P<0.01) and %ΔCVR from −22.9±5.2% to −5.3±1.9% (P<0.05) with cSCS at 90% of MT. Effects of dorsal column transection at the rostral C1 segment on CBF and CVR are summarized in Fig. 2 and an example is shown in Fig. 3D. In the fifth group, the average motor threshold was 418±62µA (n=7). The rostral C1 transection abolished %ΔCBF (47.0±5.5% vs 8.8±2.0%, P<0.01) and %ΔCVR (−20.3±4.0% vs −3.8±2.1%, P<0.01) to cSCS at 90% of MT. Data are summarized Fig. 2 and Fig. 3E shows an example that C1 transection abolished the effects of cSCS on CBF and CVR.
Fig. 2.
Summary for effects of different treatments on cSCS-induced cerebral vasodilation. A: Responses of ipsilateral CBF to cSCS at 90% of MT before and after intravenous hexamethonium (He., n=7), spinal transection at C6-C7 segments (n=7), intrathecal ibotenic acid (IA, 0.3mg/ml, 0.1ml, 20min) on C1-C2 spinal segments (n=7), bilateral dorsal column (DC) transection at rostral C1 segment (n=8), and cutting at rostral C1 segment (n=7). B: Responses of ipsilateral CVR to cSCS at 90% of MT before and after various treatments. Values are expressed as the mean±SE. Statistical comparisons were made using two-way ANOVA. □P<0.05, □□ P<0.01, comparison before and after the treatments.
Fig. 3.
Examples of effects of cSCS at 90% of MT on CBF in different treatments. A: Responses of ipsilateral CBF to cSCS at 90% of MT before and after intravenous hexamethonium (10 mg/kg). B: spinal transection at C6-C7 segments. C: intrathecal ibotenic acid (0.3mg/ml, 0.1ml, 20min) on C1-C2 spinal segments. D: bilateral dorsal column (DC) transection at rostral C1 segment. E: spinal transection at rostral C1 segment.
The role of TRPV1
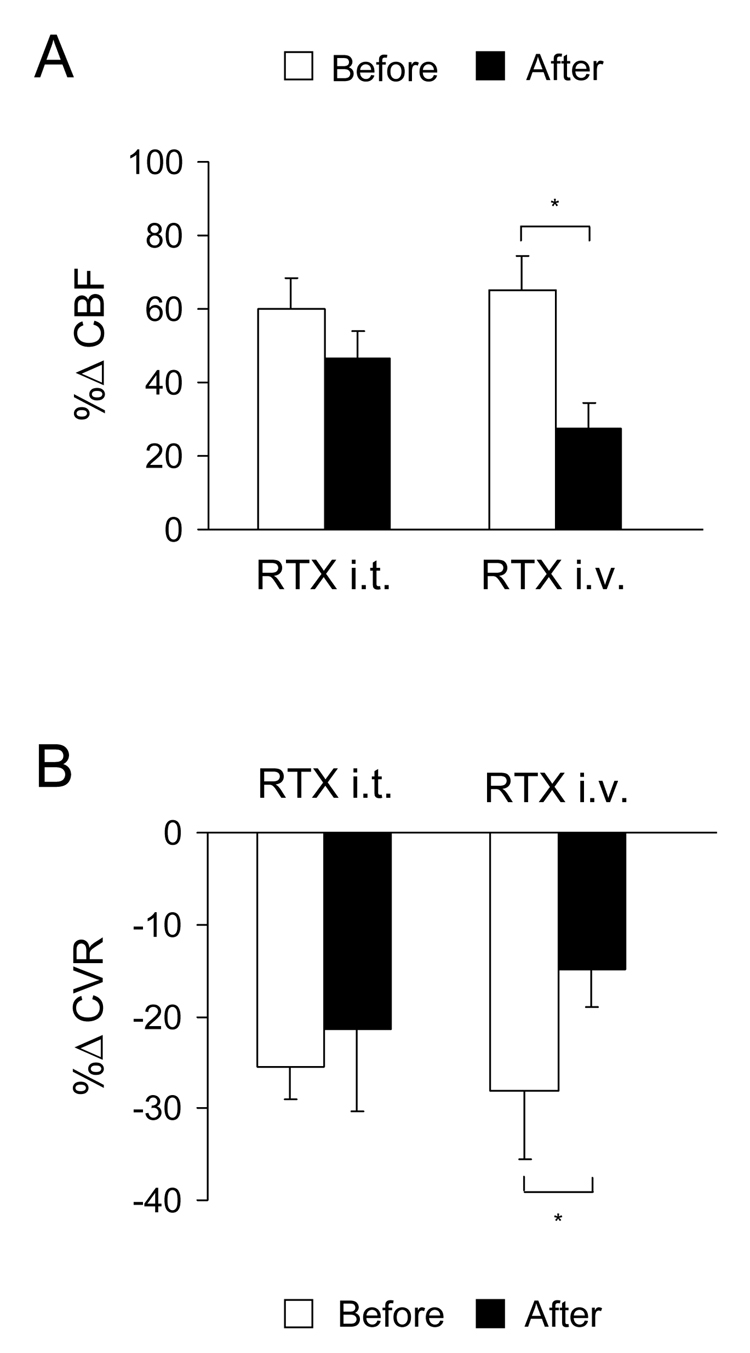
To determine if TRPV1 is involved in the effects of cSCS on ipsilateral CBF, an agonist of TRPV1 was administered intrathecally and intravenously. In the first group, application of RTX on the C1-C2 segments did not significantly affect the %ΔCBF (60.2±8.1% vs 46.3±7.7%, P>0.05) and %ΔCVR (−25.5±3.5% vs −21.4±8.9%, P>0.05) to cSCS at 90% of MT (472±45µA, n=7). Data are summarized Fig. 4 and a typical example of the effect of intrathecal RTX at C1-C2 segments on CBF is shown in Fig. 5B. In the second group, after desensitization of TRPV1 with intravenous RTX, cSCS-induced augmentation of %ΔCBF significantly decreased from 65.0±9.5% to 27.4±7.2% (P<0.05). It also reduced the %ΔCVR from −28.0±7.6% to −14.8±4.2% (P<0.05) with cSCS at 90% of MT (461±33µA, n=9). Nevertheless, cSCS-induced CBF and CVR values after RTX were changed in comparing to baseline levels at P<0.01 and P<0.05, respectively. Fig. 4 and Fig. 5C show a result of the analysis and an example of effect of intravenous RTX on CBF and CVR.
Fig. 4.
Summary for effects of cSCS at 90% of MT on CBF in different treatments. A: Responses of ipsilateral CBF before and after intrathecal administration of RTX on C1-C2 spinal segments (n=7), intravenous administration of RTX (n=9). B: Responses of ipsilateral CVR before and after intrathecal administration of RTX on C1-C2 spinal segments and intravenous administration of RTX. □P<0.05 in comparison before and after the treatments.
Fig. 5.
Examples of cSCS-induced cerebral vasodilation and the role of TRPV1. A: Responses of ipsilateral CBF to repeated cSCS at 90% of MT. B: Responses of ipsilateral CBF to cSCS at 90% of MT before and after intrathecal RTX at C1-C2 spinal segments. C: Responses of ipsilateral CBF to cSCS at 90% of MT before and after intravenous RTX.
Discussion
Effects of cSCS on CBF
The location of the stimulating electrode to apply cSCS often extends from C1 to C6, and the optimal placement is on the C1-C3 segments (Goksel et al., 2001; Patel et al.2003; Sagher et al., 2000). However, cSCS stimulation on the lower cervical and thoracic spinal cord does not significantly affect CBF (Hosobuchi, 1985; Isono et al., 1995; Sagher et al., 2000). Based on these observations, C1-C2 segments were selected as a place to set up cSCS in the present study. The stimulus parameters used for cSCS to augment CBF have been variously determined in previous studies. For example, cSCS with a frequency of 20 Hz gradually increased CBF up to 140% of the pre-SCS value in cats. This value remained high for 15 min after cSCS was terminated (Isono et al., 1995). In contrast, cSCS with 200–2,000 Hz does not increase CBF (Isono et al., 1995). In another study in cats, cSCS at a stimulus intensity of 4V (25 Hz and 0.1 ms) enhances CBF without producing any harmful effects, and this is sustained for 30 min after terminating cSCS (Inoue et al., 2000). In rabbits, cSCS (0.21 ms, 80 Hz, 2/3 MT, 20 min) increases CBF in 52.4% of animals, decreases CBF in 9.5% of animals, and did not affect the remaining animals (Visocchi et al., 1994). In rats, cSCS (0.25 ms, 50 Hz, 1.5 mA) increased CBF values by more than 80% over baseline (Zhong et al., 2004). A persistent increase in CBF can be produced when applying cSCS for up to 20 minutes. Tachyphylaxis to the effects of cSCS on CBF does not seem to limit its effectiveness in the experimental model (Zhong et al., 2004). Therefore, the adjustments in stimulation intensity, pulse width and frequency of cSCS are necessary to produce an increase in CBF. In the present study, cSCS (50 Hz, 0.2 ms) was applied low intensities (30, 60, 90% of MT) to the dorsal column of upper cervical (C1-C2) spinal segments and bilaterally increased CBF by 20–60%. The magnitude of the CBF and CVR response varied in a dose-dependent fashion with the changes in stimulation intensity of cSCS. These parameters of SCS were used since they are similar to clinical settings of SCS employed by physicians (Cameron 2004; Linderoth and Foreman 1999; 2006).
Pathways of cSCS effects
The neural pathways or mechanisms underlying cSCS-induced augmentation of CBF and reduction of CVR are not well understood. Some studies suggested the involvement of alterations in sympathetic tone in cSCS-induced cerebral vasodilation (Broseta et al., 1994; Isono et al., 1995; Visocchi et al., 1994). For example, intravenous administration of hexamethonium (a ganglionic blocker) and prazosin (a selective α-1 receptor blocker) prior to the initiation of cSCS in rats abolish the cSCS-induced cerebral vasodilation (Patel et al., 2003; Sagher et al., 2000). In contrast, idazoxan (a selective α-2 receptor blocker) and propranolol (a non-selective blocker) does not affect or partially attenuate the cSCS effect on CBF (Garcia-March et al., 1989; Patel et al., 2003). The present study in rats also showed that effects of cSCS on CBF and CVR were reduced by the intravenous injection of hexamethonium. It is important to note that in previous studies sympathetic tone was only assumed to play a role since CVR was not calculated for each animal (Patel et al. 2003). However, in the present study intravenous hexamethonium affected CVR as well as CBF. Therefore, this study confirms the idea that overall sympathetic tone is likely to play an important role in cSCS-induced cerebral vasodilation. This mechanism is mainly mediated by α-1 adrenergic receptors (Patel et al., 2003). Resection of superior cervical ganglion does not affect cerebral vasodilation produced by cSCS in rats (Patel et al., 2004). Furthermore, in the present study spinal transection at lower cervical spinal cord (C6-C7) did not affect the augmentation of CBF and reduction of CVR by cSCS. These results suggest that effects of cSCS on CBF and CVR do not likely depend on cervical and thoracic sympathetic outflow.
Since cSCS is directly applied to upper cervical spinal segments (C1-C2), the effects of cSCS might result from activation of passing fibers in spinal dorsal column with rostral projections to dorsal column nuclei in medulla. Another possibility is that cSCS activates spinal neurons with rostral projections to supraspinal sites (Malick et al., 2000; Mouton et al., 2005). In the present study, ibotenic acid applied to C1-C2 segment did not significantly reduce effects of cSCS on CBF and CVR, but complete bilateral dorsal column transection at the C1 segment or transection of the spinal cord at the spinal cervicomedullary junction attenuated the cSCS-induced CBF and CVR. These data indicated that ascending fibers in dorsal column rather than rostral spinal neuronal projections serve as a key pathway that might play an important role of cSCS-induced effects of CBF and CVR. It is consistent with the observations from previous studies in rats (Sagher and Huang, 2000) and cats (Isono et al., 1995) except the effects from cell bodies were not evaluated in these investigations. Apparently, low intensity cSCS performed in the present study might more likely activate the low-threshold large fibers in dorsal column (Linderoth and Foreman, 1999; 2006) so as to elicit rostral effects on supraspinal sites.
After reviewing the literature and the results of the present study, we propose that the central pathway involves ascending dorsal column fibers, preganglionic neurons in the rostral ventrolateral medulla and parasympathetic cerebrovascular innervation of sphenopalatine ganglion which is independent of the sympathetic chain. Some investigators have suggested that cSCS may initially activate vasomotor centers in the rostral region of ventrolateral medulla (Patel et al., 2004). In support of this, after injecting WGA-HRP into the dorsal column nuclei, anterograde and retrograde labeling neurons were found bilaterally within the rostral ventrolateral medulla (Kamiya et al. 1988). Preganglionic parasympathetic neurons in this region contain nitric oxide synthase and choline acetyltransferase, and send projections to the sphenopalatine ganglion in rabbits (Zhu et al. 1997) and rats (Suzuki et a. 1990). The sphenopalatine ganglion is the major source of postganglionic parasympathetic fibers that innervate the vascular beds of the cerebral hemispheres (Hara et al. 1993). Electrical stimulation of sphenopalatine ganglion or postganglionic fibers significantly increased CBF in the ipsilateral and contralateral parietal cortex independent of cerebral metabolism in rats (Seylaz et al. 1988; Suzuki et al. 1990), and cats (Goadsby 1990). Nitric oxide and vasoactive intestinal polypeptide often co-exist in these postganglionic fiber terminals perivascularly innervating the cerebral arteries in rats (Iadecola et al. 1993; Minami et al. 1994). Thus, it was reasonable to suggest that a potential pathway, i.e. dorsal column nuclei,rostral ventrolateral medulla, Sphenopalatine ganglion, and cortical vascular beds, could be activated by cSCS and produce an augmentation of CBF observed in the present study.
Role of TRPV1
Transient receptor potential vanilloid-1 (TRPV1), a ligand-gated nonselective cation channel, is activated by capsaicin, protons, and heat, as well as by some endogenous ligands (anandamide, ATP, lipoxygenase products, N-oleoyldopamine, etc.) (Steenland et al., 2006). This receptor is expressed in small-diameter, primary afferent neurons of the dorsal root, trigeminal, as well as in neurons of the nodose ganglia and spinal dorsal horn. It is considered to be an important integrator to detect various noxious stimuli and play a role in pain-related behaviors (Szallasi and Blumberg, 1999; Steenland et al., 2006). There is increasing evidence that TRPV1 expression not only occurs in spinal sensory neurons, but also in supraspinal structures including the cortex (Steenland et al., 2006). Therefore, wide distribution of TRPV1 expression in the brain is consistent with multiple functions within the central nervous system. There is TRPV1 expression in the cerebral microvasculature endothelial cells which may have a role in the control of the vasculature (Golech et al., 2004). It also has been reported that TRPV1 is found in the brain on astrocytes and pericytes, which are closely associated with vasculature (Toth et al., 2005). TRPV1 receptor is active in the brain microvasculature and exerts its permeability-increasing effect via substance P (Hu et al., 2005). In the present study, local administration of RTX on C1-C2 spinal segments did not interrupt the augmentation of CBF or the reductions of CVR by cSCS. However, intravenous administration of RTX significantly reduced the effect of cSCS on CBF and CVR. It is suggested that TRVP1 in the brain but not the spinal cord may be involved in cerebral vasodilation by cSCS. These intracerebral pathways might contain TRPV1 which could be involved in effects of cSCS on CBF observed in the present study. In addition, some vasodilators and neurotransmitters, such as CGRP, NO, SP, histamine, also possibly are involved in effects of cSCS on CBF (Garcia-March et al., 1989; Goksel et al., 2001).
Conclusions
The present study indicated that cSCS effects on CBF and CVR do not involve sympathetic outflow via the thoracic spinal cord but instead an ascending pathway in spinal dorsal column, which involved intracerebral rather than intraspinal TRPV1 was responsible for cSCS increases in CBF and decreases in CVR. These mechanisms provide the evidence to elucidate a novel target to improve therapeutic benefits of cSCS for patients suffering from cerebrovascular diseases.
Acknowledgments
The authors would like to thank Drs Bengt Linderoth (Department of Neurosurgery, Karolinska University Hospital, Stockholm, Sweden) and Miao Liu (Department of Surgery, Xi’an Jiaotong University First Hospital, Xi’an, P. R. China) for very helpful comments. The authors also thank Diana Holston for her excellent technical assistance. This study was supported by NIH grant (HL075524) and Shaanxi Science and Technology Foundation (2003K10-G80) in P. R. China.
A comprehensive list of abbreviations in the manuscript
- cSCS
Cervical spinal cord stimulation
- CBF
Cerebral blood flow
- CVR
Cerebral vascular resistance
- TRPV1
Transient receptor potential vanilloid type 1
- MT
Motor threshold
- RTX
Resiniferatoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Broseta J, Garcia-March G, Sanchez-Ledesma MJ, Goncalves J, Silva I, Barcia JA, Llacer JL, Barcia-Salorio JL. High-cervical spinal cord electrical stimulation in brain low perfusion syndromes: experimental basis and preliminary clinical report. Stereotact Funct Neurosurg. 1994;62:171–178. doi: 10.1159/000098614. [DOI] [PubMed] [Google Scholar]
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254–267. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- Clavo B, Robaina F, Catala L, Perez JL, Lloret M, Carames MA, Morera J, Lopez L, Suarez G, Macias D, Rivero J, Hernandez MA. Effect of cervical spinal cord stimulation on regional blood flow and oxygenation in advanced head and neck tumours. Ann Oncol. 2004;15:802–807. doi: 10.1093/annonc/mdh189. [DOI] [PubMed] [Google Scholar]
- Dario A, Scamoni C, Peron S, Tomei G. A case of post-traumatic cervicogenic headache treated by cervical cord stimulation. J Headache Pain. 2005;6:473. doi: 10.1007/s10194-005-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Andres J, Tatay J, Revert A, Valia JC, Villanueva V. The beneficial effect of spinal cord stimulation in a patient with severe cerebral ischemia and upper extremity ischemic pain. Pain Pract. 2007;7:135–142. doi: 10.1111/j.1533-2500.2007.00121.x. [DOI] [PubMed] [Google Scholar]
- Ebel H, Schomacker K, Balogh A, Volz M, Funke J, Schicha H, Klug N. High cervical spinal cord stimulation (CSCS) increases regional cerebral blood flow after induced subarachnoid haemorrhage in rats. Minim Invasive Neurosurg. 2001;44:167–171. doi: 10.1055/s-2001-18149. [DOI] [PubMed] [Google Scholar]
- Fujii M, Sadamitsu D, Maekawa T, Uesugi S, Ozaki S, Koizumi H, Uetsuka S, Sakamoto K, Yamashita T, Ito H. Spinal cord stimulation therapy at an early stage for unresponsive patients with hypoxic encephalopathy. No Shinkei Geka. 1998;26:315–321. [PubMed] [Google Scholar]
- Funahashi K, Komai N, Ogura M, Kuwata T, Nakai M, Tsuji N. Effects and indications of spinal cord stimulation on the vegetative syndrome. No Shinkei Geka. 1989;17:917–923. [PubMed] [Google Scholar]
- Garcia-March G, Sanchez-Ledesma MJ, Anaya J, Broseta J. Cerebral and carotid haemodynamic changes following cervical spinal cord stimulation. Acta Neurochir Suppl (Wien) 1989;46:102–104. doi: 10.1007/978-3-7091-9029-6_25. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Sphenopalatine ganglion stimulation increases regional cerebral blood flow independent of glucose utilization in the cat. Brain Res. 1990;506:145–148. doi: 10.1016/0006-8993(90)91211-x. [DOI] [PubMed] [Google Scholar]
- Goksel HM, karadag O, Turaclar U, Tas F, Oztoprak I. Nitric oxide synthase inhibition attenuates vasoactive response to spinal cord stimulation in an experimental cerebral vasospasm model. Acta Neurochir (Wien) 2001;143:383–391. doi: 10.1007/s007010170094. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Darder J, Canadas-Rodriguez D. Effects of cervical spinal cord stimulation in experimental ischaemic oedema. Neurol Res. 1991;13:229–232. doi: 10.1080/01616412.1991.11739997. [DOI] [PubMed] [Google Scholar]
- Gurelik M, Kayabas M, Karadag O, Goksel HM, Akyuz A, Topaktas S. Cervical spinal cord stimulation improves neurological dysfunction induced by cerebral vasospasm. Neuroscience. 2005;134:827–832. doi: 10.1016/j.neuroscience.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Hara H, Zhang QJ, Kuroyanagi T, Kobayashi S. Parasympathetic cerebrovascular innervation: an anterograde tracing from the sphenopalatine ganglion in the rat. Neurosurg. 1993;32:822–827. doi: 10.1227/00006123-199305000-00016. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y. Electrical stimulation of the cervical spinal cord increases cerebral blood flow in humans. Appl Neurophysiol. 1985;48:372–376. doi: 10.1159/000101161. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y. Treatment of cerebral ischemia with electrical stimulation of the cervical spinal cord. Pacing Clin Electrophysiol. 1991;14:122–126. doi: 10.1111/j.1540-8159.1991.tb04056.x. [DOI] [PubMed] [Google Scholar]
- Hu DE, Easton AS, Fraser PA. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br J Pharmacol. 2005;146:576–584. doi: 10.1038/sj.bjp.0706350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Beitz AJ, Renno W, Xu X, Mayer B, Zhang F. Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Res. 1993;606:148–155. doi: 10.1016/0006-8993(93)91583-e. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nakase H, Hirabayshi H, Hoshida T, Sakaki T. Effect of stimulation of the dorsal aspect of the cervical spinal cord on local cerebral blood flow and EEG in the cat. Neurol Res. 2000;22:386–392. doi: 10.1080/01616412.2000.11740688. [DOI] [PubMed] [Google Scholar]
- Isono M, Kaga A, Fujiki M, Mori T, Hori S. Effect of spinal cord stimulation on cerebral blood flow in cats. Stereotact Funct Neurosurg. 1995;64:40–46. doi: 10.1159/000098732. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: a morphological study in the cat. J Comp Neurol. 1988;15(2733):421–435. doi: 10.1002/cne.902730311. [DOI] [PubMed] [Google Scholar]
- Kanno T, Kamei Y, Yokoyama T, Jain VK. Neurostimulation for patients in vegetative status. Pacing Clin Electrophysiol. 1987;10:207–208. doi: 10.1111/j.1540-8159.1987.tb05949.x. [DOI] [PubMed] [Google Scholar]
- Karadag O, Eroglu E, Gurelik M, Goksel HM, Kilic E, Gulturk S. Cervical spinal cord stimulation increases cerebral cortical blood flow in an experimental cerebral vasospasm model. Acta Neurochir (Wien) 2005;147:79–84. doi: 10.1007/s00701-004-0410-5. [DOI] [PubMed] [Google Scholar]
- Kuwata T. Effects of the cervical spinal cord stimulation on persistent vegetative syndrome: experimental and clinical study. No Shinkei Geka. 1993;21:325–331. [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation. 1999;2:150–164. doi: 10.1046/j.1525-1403.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Foreman RD. Mechanisms of spinal cord stimulation in painful syndromes: role of animal models. Pain Med. 2006;7:S14–S26. [Google Scholar]
- Malick A, Strassman RM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol. 2000;84:2078–2112. doi: 10.1152/jn.2000.84.4.2078. [DOI] [PubMed] [Google Scholar]
- Matsui T, Hosobuchi Y. The effects of cervical spinal cord stimulation (cSCS) on experimental stroke. Pacing Clin Electrophysiol. 1989;12:726–732. doi: 10.1111/j.1540-8159.1989.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Rodriguez G, Arrigo A, Nobili F, Pisani R, Rosadini G. Cerebral haemodynamic changes induced by spinal cord stimulation in man. Ital J Neurol Sci. 1996;17:55–57. doi: 10.1007/BF01995709. [DOI] [PubMed] [Google Scholar]
- Meglio M, Cioni B, Visocchi M, Nobili F, Rodriguez G, Rosadini G, Chiappini F, Sandric S. Spinal cord stimulation and cerebral haemodynamics. Acta Neurochir (Wien) 1991a;111:43–48. doi: 10.1007/BF01402512. [DOI] [PubMed] [Google Scholar]
- Meglio M, Cioni B, Visocchi M. Cerebral hemodynamics during spinal cord stimulation. Pacing Clin Electrophysiol. 1991b;14:127–130. doi: 10.1111/j.1540-8159.1991.tb04057.x. [DOI] [PubMed] [Google Scholar]
- Minami Y, Kimura H, Aimi Y, Vincent SR. Projections of nitric oxide synthase-containing fibers from the sphenopalatine ganglion to cerebral arteries in the rat. Neurosci. 1994;60:745–759. doi: 10.1016/0306-4522(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Mouton LJ, Klop EM, Holstege G. C1-C3 spinal cord projections to periaqueductal gray and thalamus: a quantitative retrograde tracing study in cat. Brain Res. 2005;1043:87–94. doi: 10.1016/j.brainres.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tsubokawa T. Evaluation of spinal cord stimulation for postapoplectic spastic hemiplegia. Neurosurgery. 1985;17:253–259. doi: 10.1227/00006123-198508000-00001. [DOI] [PubMed] [Google Scholar]
- Patel S, Huang DL, Sagher O. Sympathetic mechanisms in cerebral blood flow alterations induced by spinal cord stimulation. J Neurosurg. 2003;99:754–761. doi: 10.3171/jns.2003.99.4.0754. [DOI] [PubMed] [Google Scholar]
- Patel S, Huang DL, Sagher O. Evidence for a central pathway in the cerebrovascular effects of spinal cord stimulation. Neurosurgery. 2004;55:201–206. doi: 10.1227/01.neu.0000126949.28912.71. [DOI] [PubMed] [Google Scholar]
- Qin C, Lehew RT, Khan KA, Wienecke GM, Foreman RD. Spinal cord stimulation modulates intraspinal colorectal visceroreceptive transmission in rats. Neurosci Res. 2007;58:58–66. doi: 10.1016/j.neures.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Randich A, Gebhart GF. Electrical stimulation of cervical vagal afferents. II. Central relays for behavioral antinociception and arterial blood pressure decreases. J Neurophysiol. 1990 Oct;64(4):1115–1124. doi: 10.1152/jn.1990.64.4.1115. [DOI] [PubMed] [Google Scholar]
- Robaina F, Clavo B, Catala F, Carames MA, Morera J. Blood flow increase by cervical spinal cord stimulation in middle cerebral and common carotid arteries. Neuromodulation. 2004;7:26–31. doi: 10.1111/j.1525-1403.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- Sagher O, Huang DL. Effects of cervical spinal cord stimulation on cerebral blood flow in the rat. J Neurosurg. 2000;93:71–76. doi: 10.3171/spi.2000.93.1.0071. [DOI] [PubMed] [Google Scholar]
- Sagher O, Huang DL, Keep RF. Spinal cord stimulation reducing infarct volume in a model of focal cerebral ischemia in rats. J Neurosurg. 2003;99:131–137. doi: 10.3171/jns.2003.99.1.0131. [DOI] [PubMed] [Google Scholar]
- Sagher O, Huang DL. Mechanisms of spinal cord stimulation in ischemia. Neurosurg Focus. 2006;21:E2. doi: 10.3171/foc.2006.21.6.5. [DOI] [PubMed] [Google Scholar]
- Seylaz J, Hara H, Pinard E, Mraovitch S, MacKenzie ET, Edvinsson L. Effect of stimulation of the sphenopalatine ganglion on cortical blood flow in the rat. J Cereb Blood Flow Metab. 1988;8:875–878. doi: 10.1038/jcbfm.1988.145. [DOI] [PubMed] [Google Scholar]
- Steenland HW, Ko SW, Wu LJ, Zhuo M. Hot receptors in the brain. Mol Pain. 2006;2:34. doi: 10.1186/1744-8069-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Hardebo JE, Skagerberg G, Owman C. Selective electrical stimulation of postganglionic cerebrovascular parasympathetic nerve fibers originating from the sphenopalatine ganglion enhances cortical blood flow in the rat. J Cereb Blood Flow Metab. 1990;10:383–391. doi: 10.1038/jcbfm.1990.68. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tanaka S, Barron KW, Chandler MJ, Linderoth B, Foreman RD. Low intensity spinal cord stimulation may induce cutaneous vasodilation via CGRP release. Brain Res. 2001;896:183–187. doi: 10.1016/s0006-8993(01)02144-8. [DOI] [PubMed] [Google Scholar]
- Toth A, Boczan J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Cioni B, Vergari S, Marano G, Pentimalli L, Meglio M. Spinal cord stimulation and cerebral blood flow: an experimental study. Stereotact Funct Neurosurg. 1994;62:186–190. doi: 10.1159/000098616. [DOI] [PubMed] [Google Scholar]
- Visocchi M, Argiolas L, Meglio M, Cioni B, Basso PD, Rollo M, Cabezas D. Spinal cord stimulation and early experimental cerebral spasm: the "functional monitoring" and the "preventing effect". Acta Neurochir (Wien) 2001;143:177–185. doi: 10.1007/s007010170126. [DOI] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Sensory fibers containing vanilloid receptor-1 (VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006;1107:177–184. doi: 10.1016/j.brainres.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2007a Dec 14; doi: 10.1016/j.autneu.2007.11.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Roles of peripheral terminals of transient receptor potential vanilloid-1 containing sensory fibers in spinal cord stimulation-induced peripheral vasodilation. Brain Res. 2007b;1156:80–92. doi: 10.1016/j.brainres.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Role of TRPV1 in augmentation of cerebral blood flow by cervical spinal cord stimulation in rats. FASEB J. 2007a;21:lb563. doi: 10.1016/j.neuroscience.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Farber JP, Wu M, Foreman RD, Qin C. Effects and pathways for upper cervical spinal cord stimulation on cerebral blood flow in rats. 2007 International Conference of Pain Research and Clinic Pain Control□Xi’an; P. R. China. 2007b. [Google Scholar]
- Zhong J, Huang DL, Sagher O. Parameters influencing augmentation of cerebral blood flow by cervical spinal cord stimulation. Acta Neurochir (Wien) 2004;146:1227–1234. doi: 10.1007/s00701-004-0364-7. [DOI] [PubMed] [Google Scholar]
- Zhu BS, Gibbins IL, Blessing WW. Preganglionic parasympathetic neurons projecting to the sphenopalatine ganglion contain nitric oxide synthase in the rabbit. Brain Res. 1997;769:168–172. doi: 10.1016/s0006-8993(97)00844-5. [DOI] [PubMed] [Google Scholar]