Abstract
An antibody-targeted radiation therapy (radioimmunotherapy, RIT) employs a bifunctional ligand that can effectively hold a cytotoxic metal with clinically acceptable complexation kinetics and stability while being attached to a tumor-specific antibody. Clinical exploration of the therapeutic potential of RIT has been challenged by the absence of adequate ligand, a critical component for enhancing the efficacy of the cancer therapy. To address this deficiency, the bifunctional ligand C-NETA in a unique structural class possessing both a macrocyclic cavity and a flexible acyclic moiety was designed. The practical, reproducible, and readily scalable synthetic route to C-NETA was developed, and its potential as the chelator of 212Bi, 213Bi, and 177Lu for RIT was evaluated in vitro and in vivo. C-NETA rapidly binds both Lu(III) and Bi(III), and the respective metal complexes remain extremely stable in serum for 14 days. 177Lu—C-NETA and 205/6Bi—C-NETA possess an excellent or acceptable in vivo biodistribution profile.
Introduction
Bifunctional ligands that possess both metal-binding moieties and a linker for conjugation to a targeting biomolecule such as monoclonal antibody (mAb)a have been employed for biomedical and pharmaceutical applications. In particular, development of adequate bifunctional ligands that can efficiently bind a therapeutic or diagnostic metal while being conjugated to a highly sensitive mAb without compromising its biological activity is a critical step for successful antibody-targeted cancer therapy and imaging. Radioimmunotherapy (RIT) has been recognized as a new promising therapeutic modality for treating numerous cancers by employing a tumor-targeting mAb for selective delivery of a cytotoxic radioactive metal while minimizing exposure of healthy cells.1–3 The proven efficacy4 of the first FDA-approved RIT drug, Ibritumomab, for treatment of B-cell non-Hodgkins lymphoma (NHL), has spurred numerous investigations of RIT modality on other cancer types including leukemia and cancers of the breast, colorectum, ovaries, and prostate.5 The RIT system generally consists of three components: a radionuclide, a mAb, and a bifunctional ligand.6 The modality requires the use of radioactive metals, which can be very toxic when deposited in vivo in normal tissue. Therefore, the success of clinical applications of RIT heavily depends on the performance of the bifunctional ligand that can rapidly form a stable complex, particularly with a relatively short-lived radioactive metal. Although RIT holds great promise for the treatment of many cancers, as evidenced by Ibritumomab therapy (overall response rate of ~80%),7 active clinical exploration of RIT using a variety of antibodies and cytotoxic radionuclides has been challenged by the absence of adequate bifunctional ligands that can bind the radionuclides with clinically acceptable kinetics and in vivo stability and thereby allow for practical and large-scale production of stable radioimmunoconjugates. Bone-marrow toxicity is reported to be a major side effect of Ibritumomab therapy, which has been proposed to be related to deposition of 90Y (t1/2 = 64.1 h, Emax = 2.3 MeV), the bone-seeking radionuclide prematurely leaking from the radiolabeled antibody conjugate.5,8
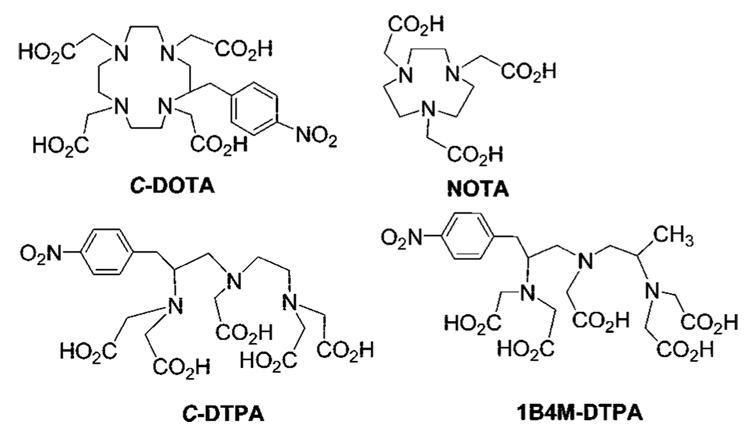
Research efforts have been directed toward the development of adequate bifunctional ligands for sequestering metallic radionuclides for RIT applications. The most frequently explored macrocyclic ligand for RIT, C-DOTA [2-(p-NO2-Bn)-1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid, Figure 1] forms a very stable complex with radionuclides.9,10 However, the extremely slow complex formation rate under mild conditions11–13 was found to be problematic in many RIT applications, particularly for short half-lived α-emitters such as 213Bi (t1/2 = 47 min).10 Acyclic C-DTPA [2-(p-NO2-Bn)-diethylenetriaminepentaacetic acid, Figure 1] rapidly forms a complex with radionuclides but is unstable, both in vitro and in vivo, resulting in toxic side effects.14 The acyclic ligand 1B4M-DTPA [2-(p-NO2-Bn)-6-methyl-DTPA, Figure 1]15 displays significantly improved complexation kinetics with several radionuclides as compared to C-DOTA. However, the corresponding complexes are still less stable both in vitro and in vivo compared to metal-DOTA complexes.16 Despite this shortcoming, 1B4M-DTPA is a component of the clinically approved Ibritumomab primarily due to practical and reproducible radiolabeling chemistry.17.
Figure 1.
Synthetic ligands currently in preclinical and clinical use.
In an effort to develop an improved bifunctional ligand for RIT, we have designed C-NETA ({4-[2-(bis(carboxymethyl)-amino)-3-(4-nitrophenyl)propyl]-7-carboxymethyl[1,4,7]triazonan-1-yl}acetic acid) in a unique structural class integrating both a macrocyclic and an acyclic moiety for metal binding. In this new bifunctional ligand, the macrocyclic component chosen is based upon 1,4,7-triazacyclononane-N,N′,N″-triacetic acid (NOTA, Figure 1), and the acyclic component is a pendant bis(carboxymethyl) amino donor group appended by an ethylene bridge to the macrocyclic ring. The cooperative and bimodal binding of the acyclic pendant donor groups coupled with the macrocyclic effect of the NOTA substructure is hypothesized to accelerate complexation with the metals while maintaining a high level of complex stability. The strategy is to use acyclic pendant donor groups to rapidly “capture” and initiate coordination to the metal, as in the case of DTPA. Thereafter, the macrocyclic component will envelop the cation trapped in the acyclic donor groups by wrapping around the metal ion to achieve maximum complex stability by saturating the metal coordination sphere, as in the case of DOTA. NOTA is known to be an inadequate chelate for lanthanides by virtue of its cavity size, an insufficient number of donors to saturate the coordination sphere, and an inability to assume an optimal geometry for the six donor groups toward the metal ion.18 The new bimodal ligand C-NETA is proposed to permit orientation for all donors to assume an optimal geometry with coordination sphere saturation, whereas cavity size becomes irrelevant with the metal sitting above the plane of the ring nitrogens.
Among the metallic radionuclides proposed for effective RIT, 212Bi, 213Bi, and 177Lu are of particular interest.19 The short-lived α-particle emitters, 213Bi and 212Bi (t1/2 = 60.6 m), were found to be very effective in inducing apoptosis even with a single particle traversal of the cell.20,21 The β−-emitting radionuclide, 177Lu (t1/2 = 6.7 days), possesses an imageable γ-emission (Emax) = 208 keV), which allows for imaging during cancer treatment. Its less energetic β− (Emax = 0.5 MeV) and thus shorter cell-killing range may be effective for treating smaller lesions while minimizing normal tissue damage.22.
We herein report the synthesis and evaluation of C-NETA as a potential bifunctional chelator of 212Bi, 213Bi, and 177Lu for cancer RIT. Complexation kinetics of C-NETA with Bi(III) and Lu(IIII) are determined using a UV spectroscopic Arsenazo III assay. In vitro and in vivo stability or dissociation of C-NETA labeled with 205/6Bi and 177Lu is evaluated in human serum and mice, respectively.
Results and Discussion
Synthesis of C-NETA and C-NETA Analogues
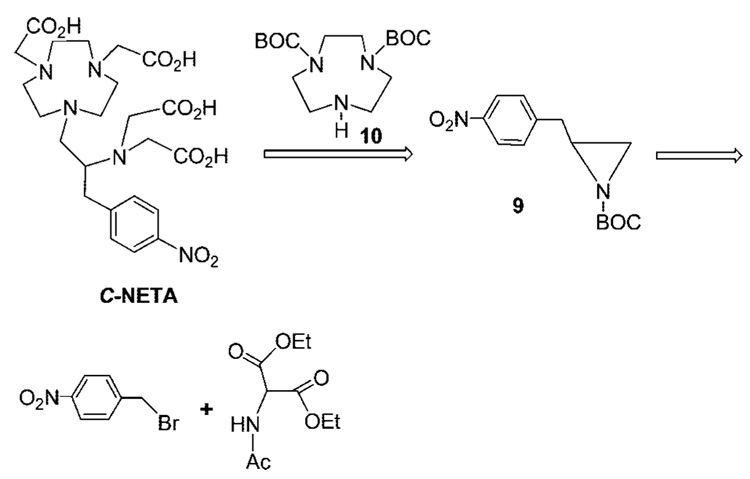
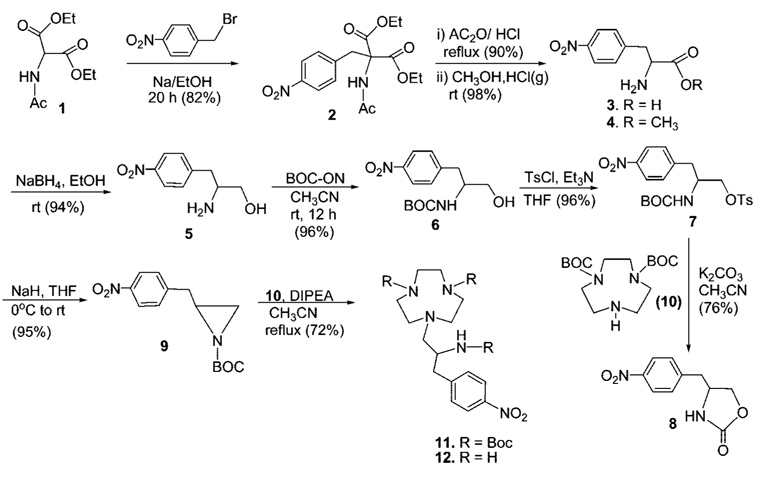
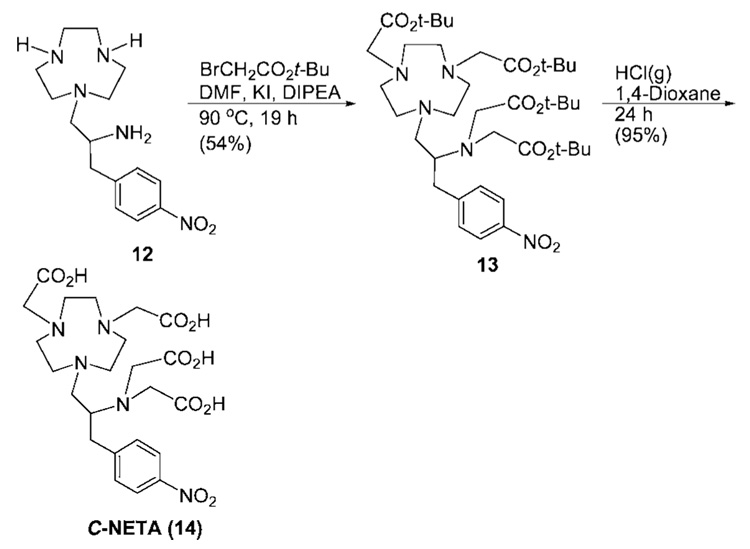
The synthetic strategy for the preparation of the bifunctional ligand C-NETA (Scheme 1) involves synthesis of 9 and 10 from readily available starting materials and their coupling reaction. The practical, reproducible, and readily scalable synthetic route and efficient purification of all precursor molecules for macrocyclic ligand backbone 12 was developed (Scheme 2). Racemic p-nitrophenylalanine 3 was prepared from the reaction of deprotonated diethyl acetamido malonic ester 1 and p-nitrobenzyl bromide and subsequent decarboxylation and removal of the acetyl protection group in 2. The simple two-step reaction route to the amino acid 3 does not involve column chromatographic purification and allows for the preparation of 3 in large quantities and high yield instead of employing the commercially available but relatively expensive amino acid 3. The amino acid 3 was converted to methyl ester 4, which was reduced to alcohol 5. Protection of the amino group in 5 with BOC-ON followed by reaction with TsCl provided precursor molecule 7 for synthesis of the backbone framework molecule 11 of C-NETA. N-BOC-protected 1,4,7-triazacyclononane (TACN) 10 was prepared from TACN according to a modification of an established method.23 Base-promoted coupling reaction of 7 with 10 in CH3CN was attempted using K2CO3. However, the reaction afforded oxazolidinone 8 from intramolecular cyclization of 7 instead of the desired macrocycle 11. Due to this unsuccessful intermolecular substitution, N-BOC-protected aziridine 9 was prepared by treatment of 7 with NaH and further reacted with 10 to provide N-BOC-protected macrocycle 11 in high yield (72%), and subsequent removal of BOC groups afforded tetraamine backbone 12. All precursor molecules 2–12 were prepared and purified in large quantities using recrystallization or after simple workup without extensive column chromatography. Compound 12 as its HCl salt was reacted with tert-butyl bromoacetate in DMF at 90 °C to provide tert-butyl C-NETA 13 in 54% yield (Scheme 3). Compound 13 was further treated with 4 M HCl/1,4-dioxane to provide the desired bifunctional ligand C-NETA (14) in the nitro form.
Scheme 1.
Retrosynthetic Route to C-NETA
Scheme 2.
Synthesis of Precursor Molecule 12 for C-NETA
Scheme 3.
Synthesis of C-NETA
AAIII Complexation Kinetics of NETA with Bi(III) and Lu(III)
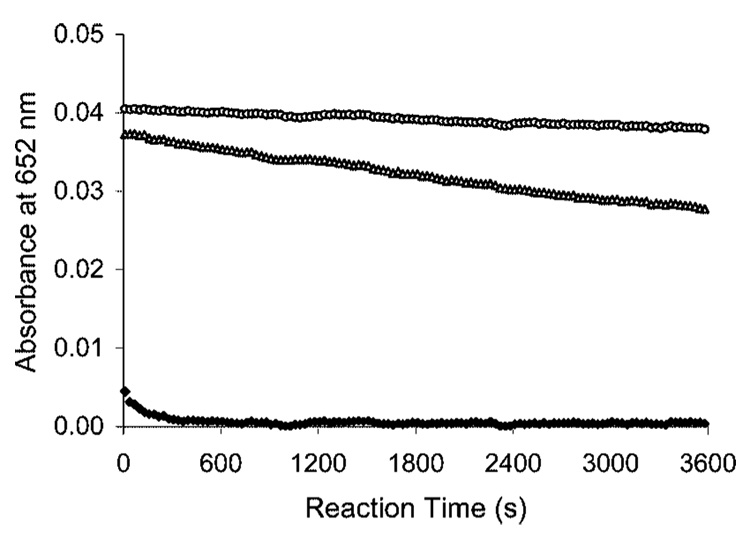
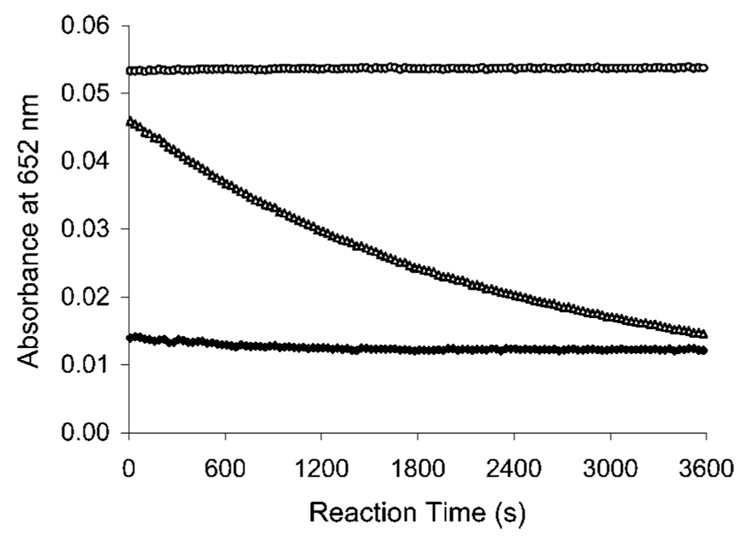
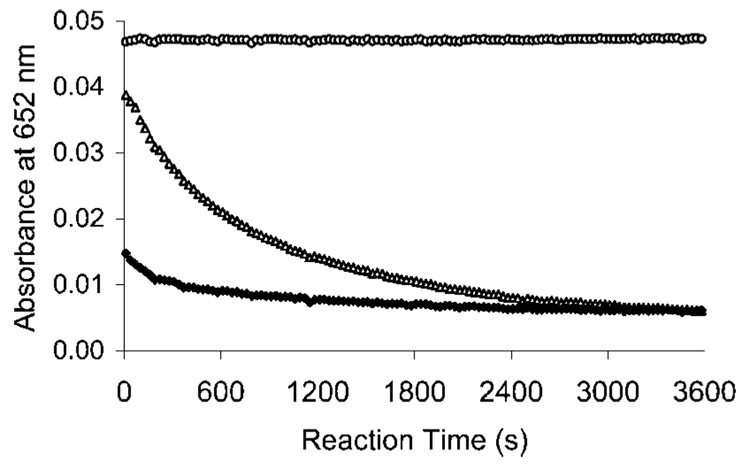
The complexation kinetics of new ligands with Bi(III) and Lu(III) was determined using a spectroscopic competing reaction with AAIII according to a modification of a previously reported procedure.12 AAIII is known to form a weak complex with many different metals, which produce a UV absorbance maximum at ~652 nm. However, uncomplexed AAIII absorbs little at this wavelength. When introduced to a solution containing the AAIII–metal complex, a chelate can compete with AAIII for the metal. If the chelate is more capable of binding the metal than AAIII, the metal will dissociate from the AAIII complex and form a complex with the chelate leading to the decrease in the absorbance at the wavelength. The absorbance (A652) for the AAIII–Bi(III) or AAIII–Lu(III) complex was measured in the absence and in the presence of the ligands over 1 h at room temperature (Figure 2–Figure 4). The complexation result of C-NETA was compared to that of C-DOTA, which is known to form an inert complex with metallic radionuclides, but with extremely slow kinetics. AAIII is known to form a relatively stable complex with Bi(III) producing strong UV absorbance at 652 nm in solution over the pH range of 1.5–4.0.24 Because of the lack of literature on AAIII–Bi(III) assay for complexation reaction kinetics, a series of experiments to measure the maximum absorbance and stability of the Bi(III)–AA(III)complex were performed using Bi(III)–AAIII solution in NH4OAc (0.15 M) in the pH range of 1.5–4.0 (Figure 2 and Figure 3). No kinetics experiments at a pH > 4.0 were performed, as Bi(III)–AAIII complex produced an unstable maximum absorbance. In NH4OAc buffer solution (pH 2, Figure 2), C-DOTA showed an extremely slow complexation with Bi(III), as expected.9 C-NETA displayed a greatly enhanced complexation kinetics compared to C-DOTA. Complexation of C-NETA with Bi(III) was essentially complete very shortly after the starting point of the measurement. At higher pH (0.15 M NH4OAc, pH 4), C-DOTA exhibited increased complexation kinetics, and C-NETA exhibited consistently rapid complexation kinetics (Figure 3). However, both ligands reached the reaction equilibrium, producing a saturation curve (A652 ~ 0.008). The complexation kinetics of Lu(III) was determined at pH 4.5, as hydrolysis occurs at a higher pH,12,25 and both ligands displayed no substantial complexation with Lu(III) at a pH <4.5. The absorbance (A652) was 0.053 for AAIII–Lu(III) in the absence of the ligand (Figure 4). C-NETA displayed substantial and rapid binding to Lu(III) but reached the reaction equilibrium at the starting point of measurement (A652 = 0.014). Slow complexation kinetics was observed with C-DOTA–Lu(III) complexes. The data in Figure 2–Figure 4 indicate that C-DOTA displayed sluggish complexation with both Lu(III) and Bi(III), whereas C-NETA formed a complex with Bi(III) and Lu(III) at a much faster rate than C-DOTA in all solutions studied. This result suggests that the pendant acyclic arm in C-NETA participates in binding of Bi(III) and Lu(III) and greatly contributes to achieving fast complexation kinetics, supporting the proposed bimodal binding nature of C-NETA.
Figure 2.
Plot of absorbance at 652 nm versus time of Bi(III)–AAIII (○), Bi(III)–C-DOTA (△), and Bi(III)–C-NETA (♦) at pH 2.0 (0.15 M NH4OAc) and 25 °C.
Figure 4.
Plot of absorbance at 652 nm versus time of Lu(III)–AAIII (○), Lu(III)–C-DOTA (△), Lu(III)–C-NETA (♦) at pH 4.5 (0.15 M NH4OAc) and 25 °C.
Figure 3.
Plot of absorbance at 652 nm versus time of Bi(III)–AAIII (○), Bi(III)–C-DOTA (△), Bi(III)–C-NETA (♦) at pH 4.0 (0.15 M NH4OAc) and 25 °C.
Radiolabeling
C-NETA was radiolabeled with 205/6Bi and 177Lu as described previously.26 The radiolabeling reactions were performed at both room temperature and an elevated temperature (80 °C). C-NETA was successfully labeled with both 177Lu and 205/6Bi both at room temperature and at 80 °C in 1 h in >90% yield. All of the formed complexes were purified via ion-exchange chromatography. C-NETA was labeled with 205/6Bi both at room temperature and at 80 °C with respective radiochemical yields of 95 and 94% as determined by radio-TLC. C-NETA was successfully labeled with 177Lu both at room temperature and at 80 °C in excellent radiochemical yield (94.3 and 94%, respectively) as determined by radio-TLC. The purified complexes were immediately prepared for serum stability or biodistribution work as outlined under Experimental Section.
In Vitro Stability of C-NETA Radiolabeled with 205/6Bi and 177Lu
205/6Bi–C-NETA and 177Lu–C-NETA were prepared at room temperature and purified via ion-exchange chromatography. In vitro serum stability of the radiolabeled complexes was performed to determine if C-NETA labeled with 205/6Bi and 177Lu remained stable without transchelation or loss of their respective radionuclide in human serum. This was assessed by measuring the transfer of radionuclide from the complex to serum proteins over 14 days. No measurable loss of radioactivity from 205/6Bi–C-NETA was recorded out to 14 days. C-NETA radiolabeled with 177Lu was stable in serum for up to 14 days without any measurable loss of radioactivity.
In Vivo Biodistribution of the Radiolabeled Complexes
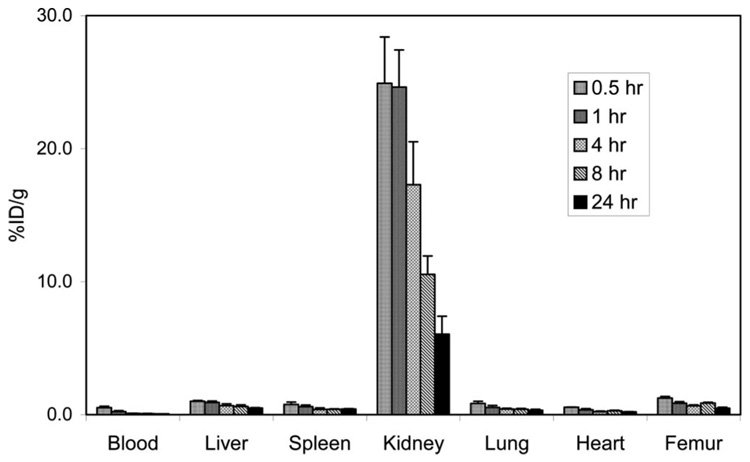
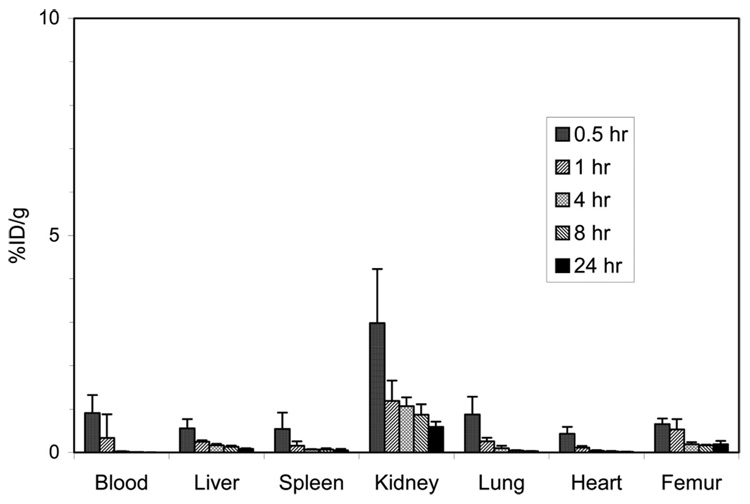
The radiolabeled complexes, 205/6Bi–C-NETA and 177Lu–C-NETA were prepared by labeling C-NETA with 205/6Bi or 177Lu at room temperature and purified via ion-exchange chromatography. The in vivo stability of the radiolabeled complexes was evaluated by performing biodistribution studies in normal athymic mice as described previously.26 Blood levels and organ uptake of the radiolabeled complexes in mice were measured at five time points, 0.5, 1, 4, 8, and 24 h postinjection of C-NETA radiolabeled with 205/6Bi or 177Lu. The results of the biodistribution studies for the radiolabeled complexes are shown in Figure 5 and Figure 6. The data in Figure 5illustrate that C-NETA radiolabeled with 205/6Bi displayed extremely low radioactivity levels in the blood and all of the organs except for kidney at all time points. 205/6Bi–C-NETA displayed relatively slow clearance from the kidney. At 24 h, kidney uptake of the complex was 6.06 ± 1.34% ID/g. The 177Lu-radiolabeled C-NETA complex was essentially inert in vivo and resulted in rapid blood clearance and very low organ uptake (Figure 6). At 24 h, uptake levels in the liver and bone were 0.56 ± 0.01 and 0.19 ± 0.08% ID/g, respectively. This low uptake is noteworthy in that unstable complexes radiolabeled with lanthanides were found to exhibit high liver/bone uptake.27 Higher uptake of 177Lu–C-NETA was observed in the kidney compared to other organs over all time points. Kidney uptake peaked at 2.98 ± 1.24% ID/g at 0.5 h and then decreased to 0.59 ± 0.12% ID/g at 24 h.
Figure 5.
Biodistribution of 205/6Bi–C-NETA in non-tumor-bearing athymic mice.
Figure 6.
Biodistribution of 177Lu–C-NETA in non-tumor-bearing athymic mice.
Conclusion
The structurally novel bifunctional ligand C-NETA was prepared and evaluated for its complex stability and kinetics with Bi(III) and Lu(III) to ascertain potential for use in cancer radioimmunotherapy. C-NETA binds either Lu(III) or Bi(III) with significantly enhanced complexation kinetics compared to the frequently explored ligand C-DOTA as evidenced in AAIII assay studies. C-NETA is efficiently radiolabeled with both 205/6Bi and 177Lu at mild conditions. C-NETA labeled with 205/6Bi and 177Lu at room temperature was stable in human serum without releasing any measurable radioactivity for 14 days, respectively. C-NETA radiolabeled with 177Lu displayed excellent in vivo stability as evidenced by rapid blood clearance and very low organ uptake. 205/6Bi–C-NETA possesses an acceptable biodistribution profile with rapid blood clearance and low radioactivity level at the organs other than the kidney.
In summary, the novel chelate C-NETA was designed on the basis of bimodal binding using both macrocyclic and acyclic moieties in the chelate. The complex stability and kinetics data suggest that C-NETA holds great promise for RIT of 205/6Bi and 177Lu. These findings open up active exploration of the new bifunctional ligand C-NETA and analogues for use in RIT. The relatively easy chemical modification of C-NETA due to the presence of both acyclic and macrocyclic moieties allows for the generation of a variety of novel NETA chelate templates that may be of value for biomedical therapeutic and diagnostic applications based on other biologically important metals such as Gd(III), Ga(III), Y(III), Pb(II), Ac(III), or In(III). The synthetic methodology employed for the preparation of C-NETA can be directly applied to construction of a wide range of new versatile bifunctional ligands by using modified macrocyclic or acyclic backbones.
Experimental Section
Instruments and Methods
1H, 13C, and APT NMR spectra were obtained using a Varian Gemini 300 or Bruker 300 instrument and chemical shifts are reported in parts per million on the δ scale relative to TMS, TSP, or solvent. Elemental microanalyses were performed by Galbraith Laboratories, Knoxville, TN. Fast atom bombardment (FAB) high-resolution mass spectra (HRMS) were obtained on an Extrel 4000 in the positive ion detection mode (NIDDK/NIH) or JEOL double sector JMS-AX505HA mass spectrometer (University of Notre Dame, South Bend, IN). The analytical HPLC was performed on an Agilent 1200 equipped with a dioarray detector (λ = 254 and 280 nm), themostat set at 35 °C, and a Zorbax Eclipse XDB-C18 column (4.6 × 150 mm, 80 Å). The mobile phase of a binary gradient (0–100% B/40 min; solvent A, 0.05 M AcOH/Et3N, pH 6.0; solvent B, CH3CN for method 1 or solvent A, 0.05 M AcOH/Et3N, pH 6.0; solvent B, CH3OH for method 2) at a flow rate of 1 mL/min was used. Semipreparative HPLC was performed on an Agilent 1200 equipped with a dioarray detector (λ = 254 and 280 nm), themostat set at 35 °C, and a Zorbax Eclipse XDB-C18 column (9.4 × 250 mm, 80 Å). The mobile phase of a binary gradient (0–100% B/40 min; solvent A, 0.05 M AcOH/Et3N, pH 6.0; solvent B, CH3CN) at a flow rate of 3 mL/min was used (method 3). All absorbance measurements for complexation formation kinetics were obtained on an Agilent 8453 diode array spectrophotometer equipped with a eight-cell transport system (designed for 1 cm cells). Size exclusion HPLC (SE-HPLC) chromatograms were obtained on a Laboratory Alliance isocratic system (model QGrad) with a Waters 717plus autosampler (Milford, MA), a Gilson 112 UV detector (Middleton, WI), and an in-line IN/US γ-Ram model 2 radiodetector (Tampa, FL), fitted with a TSK G3000PW column (Tosoh Biosep, Montgomeryville, PA).
Reagents
All reagents were purchased from Aldrich and used as received unless otherwise noted. Arsenazo III [AAIII, 2,2-(1,8-dihydroxy-3,6-disulfonaphthylene-2,7-bisazo)bis(benzenearsonic acid)] and copper, lutetium, and bismuth atomic absorption standard solutions were purchased and used as received. 177Lu in the chloride form was obtained from NEN Perkin-Elmer. 205/6Bi was produced using a CS30 cyclotron (PET Department, Clinical Center, NIH) and purified as described previously.28.
Caution:205/6Bi (t1/2 = 15.3/6.2 days) and 177Lu (t1/2 = 6.7 days) are α-, β-, or γ-emitting radionuclides. Appropriate shielding and handling protocols should be in place when using these isotopes.
Diethyl 2-(Acetylamino)-2-(4-nitrobenzyl)malonate (2)
To a three-neck, round-bottom flask fitted with a thermometer, a reflux condenser, and a dropping funnel was added anhydrous EtOH (150 mL). Na (1.71 g, 73.86 mmol) was portionwise added into EtOH, and the resulting mixture was stirred until all sodium disappeared. To the clear NaOEt solution was added a solution of diethyl acetamido malonate 1 (16.06 g, 73.86 mmol) in ethanol (200 mL) dropwise over 40 min. The reaction mixture was then heated at 50 °C for 1.5 h and refluxed for 10 min. The solution becomes cloudy and light brownish, indicating formation of deprotonated diethyl acetamido malonic ester. To the reaction mixture at reflux was added p-nitro benzyl bromide (15.99 g, 73.86 mmol) portionwise over 1 h. The resulting reaction mixture was maintained at reflux for 20 h. The reaction mixture was allowed to cool to room temperature, and the resulting precipitate was filtered while washing with EtOH (100 mL) and dried under vacuum. The filtrate was evaporated to ~50 mL and left in the freezer, and the resulting precipitate was collected. Pure 2 (20.82 g, 82%) was obtained as a white solid. An analytical sample was prepared by recrystallization using EtOH. 1H NMR (CDCl3) δ 1.28 (t, 6H), 2.02 (s, 3H), 3.75 (s, 2H), 4.27 (m, 4H), 6.26 (s, 1H), 7.18 (d, 2H), 8.11 (d, 2H); 13C NMR δ 13.89, 22.91, 37.48, 62.902, 66.74, 123.35, 130.61, 143.05, 147.17, 166.95, 169.28. Anal. Calcd for C19H26N2O7: C, 54.54; H, 5.72, N, 7.95. Found: C, 54.37; H, 6.05; N, 7.91.
2-Amino-3-(4-nitrophenyl)propionic Acid (3)
Diester 2 (10 g, 28.3 mmol) was dissolved in the mixture of CH3CO2H (85 mL) and concentrated HCl (30 mL), and the resulting solution was maintained under reflux for 24 h while the reaction progress was monitored using TLC. After the reaction was allowed come to room temperature, the white solid product precipitated was filtered with washing with isopropanol. The filtrate was evaporated to dryness, and the residue was treated with isopropanol to give a white product, which was recrystallized from isopropanol to afford 3 in a total yield of 6.44 g (90%). 1H NMR (D2O, pH 2) δ 3.22 (dd, 1H), 3.34 (dd, 1H), 4.23 (t, 1H), 7.45 (d, 2H), 8.16 (d, 2H); 13C NMR (D2O, pH 2) δ 37.69, 55.88, 126.55, 132.94, 144.49, 149.58, 173.15. The 1H and 13C NMR spectra of 3 are essentially identical to the data of the commercially available 3.
Methyl 2-Amino-3-(4-nitrophenyl)propionate (4)
A solution of 3 (12 g, 48.7 mmol) in MeOH (200 mL) at 0–5 °C was saturated with HCl (g) for 3 h, after which time the mixture was allowed to come to room temperature and then was stirred for 24 h. The precipitate was collected, and the filtrate was concentrated under vacuum to provide product 4 (11.7 g, 98%) as an acidic salt, which was used for the next step without further purification. 1H NMR (D2O) δ 3.33–3.43 (m, 2 H), 3.79 (s, 3 H), 4.42 (t, J = 6.0 Hz, 1 H), 7.48 (d, J = 8.2 MHz, 2 H), 8.21 (d, J = 8.2 MHz, 2 H); 13C NMR (D2O) δ 32.8 (t), 50.7 (d), 50.8 (q), 121.4 (d), 127.7 (d), 139.4 (s), 144.4 (s), 167.1 (s).
2-Amino-3-(4-nitrophenyl)propan-1-ol (5)
A slurry of 4 (11.2 g, 43.0 mmol) in dry CH3OH (5 mL) was treated with Et3N (6.45 mL). Anhydrous ether was added, and the solution was cooled at −10 °C for 1 h. The triethylamine hydrochloride was filtered off and the filtrate concentrated under vacuum to a light yellow oil. To a solution of the oil 4 in MeOH (100 mL) at 0 °C was added in portions NaBH4 (4.28 g, 107.1 mmol) over 30 min. The resulting mixture was allowed to warm to room temperature and stirred for 4 h. After evaporation of the solvent, the residue was treated with H2O (100 mL) and extracted with EtOAc (4 × 150 mL). The combined organic layer was dried, filtered, and concentrated under vacuum to provide white solid 5 (7.93 g, 94%) that was used for the next step without further purification. 1H NMR (CDCl3) δ 2.51 (dd, J = 8.1 MHz, 1 H), 2.76 (dd, J = 5.7 MHz, 1 H), 2.94–3.02 (m, 1 H), 3.24 (dd, J = 5.7 MHz, 1 H), 3.42 (dd, J = 4.6 MHz, 1 H), 3.90 (s, 3 H), 7.27 (d, J = 8.3 MHz, 2 H), 8.02 (d, J = 8.3 MHz, 2 H); 13C NMR (CDCl3) δ 39.95 (t), 54.2 (d), 65.8 (t), 123.1 (d), 130.4 (d), 145.9 (s), 148.5 (s).
tert-Butyl [2-Hydroxy-1-(4-nitrobenzyl)ethyl]carbamate (6)
To a solution of 5 (10 g, 51.0 mmol) in CH3CN (150 mL) at room temperature was added BOC-ON (25.1 g, 102 mmol) in CH3CN (200 mL). The resulting mixture was stirred for 4 h and evaporated. The residue was partitioned between ether (200 mL) and 10% NaOH solution (100 mL). The ether layer was separated and washed with water (10 mL) and 10% NaOH solution (10 mL) sequentially. The ether layer was dried, filtered, and concentrated under vacuum. The residue was washed with ether (20 mL) to provide 6 (14.5 g, 96%), which can be used for the next step without further purification. For preparation of an analytical sample, the residue could be purified via column chromatography on neutral alumina eluting with ether. Pure 6 was thereby obtained as a white solid. 1H NMR (CDCl3) δ 1.39 (s, 9 H), 2.98 (d, J = 6.0 MHz, 2 H), 3.57 (dd, J = 3.1 MHz, 1 H), 3.68 (dd, J = 3.1 MHz, 1 H), 3.90 (s, 1 H), 4.84 (d, J = 6.8 MHz, 1 H), 7.40 (d, J = 8.8 MHz, 2 H), 8.15 (d, J = 8.8 MHz, 2 H); 13C NMR (CDCl3) δ 28.06 (q), 37.22 (t), 53.0 (d), 63.2 (t), 79.7 (s), 123.4 (d), 130.1 (d), 146.3 (s), 146.5 (s), 155.9 (s). Anal. Calcd for C14H20N2O5: C, 56.75; H, 6.80. Found: C, 56.77; H, 7.03.
Toluene-4-sulfonic Acid 2-tert-Butoxycarbonylamino-3-(4-nitrophenyl)propyl Ester (7)
To a solution of 6 (7.15 g, 24.1 mmol) and Et3N (8.79 g, 86.9 mmol) in THF (50 mL) were added DMAP (50 mg) and TsCl (5.51 g, 28.9 mmol). The resulting mixture was stirred for 48 h at room temperature. Saturated NaHCO3 solution (50 mL) was added, and the reaction mixture was extracted with ether (3 × 200 mL). The combined ether layers were washed with saturated citric acid (100 mL), H2O (100 mL), 5% NaHCO3 (100 mL), and H2O (100 mL). The ether layer was dried, filtered, concentrated to ~150 mL, and left in the freezer, and the precipitate was filtered while washing with EtOH to provide 7 (10.4 g, 96%). For preparation of analytical sample, the residue could be purified via column chromatography on silica gel eluting with 30% EtOAc in hexanes. Pure 7 was thereby obtained as a white solid. 1H NMR (CDCl3) δ 1.38 (s, 9 H), 2.48 (s, 3 H), 2.85–3.04 (m, 2 H), 3.88 (d, J = 6.9 MHz, 1 H), 4.05 (d, J = 8.4 MHz, 2 H), 4.81 (d, J = 8.3 MHz, 1 H), 7.27 (d, J = 7.9 MHz, 2 H), 7.36 (d, J = 8.1 MHz, 2 H), 7.77 (d, J = 7.9 MHz, 2 H), 8.09 (d, J = 8.1 MHz, 2 H); 13C NMR (CDCl3) δ 21.6 (q), 28.1 (q), 37.1 (t), 50.5 (d), 70.1 (t), 80.0 (s), 123.6 (d), 127.9 (d), 130.0 (d, 2C), 132.1 (s), 144.8 (s), 145.4 (s), 146.7 (s), 154.9 (s). Anal. Calcd for C21H26N2O7S: C, 55.99; H, 5.82. Found: C, 55.81; H, 5.87.
4-(4-Nitrobenzyl)oxazolidin-2-one (8)
To a mixture of N-BOC-protected TACN 10 (278 mg, 0.84 mmol) and K2CO3 (116 mg, 4.2 mmol) in CH3CN (30 mL) was added 7 (378 mg, 0.84 mmol). The resulting mixture was heated to reflux and stirred for 5 days. The reaction mixture was cooled to room temperature and evaporated to dryness. The residue was purified via column chromatography on neutral alumina eluting with 2% MeOH in EtOAc to provide product 8 as a white solid (141 mg, 76%). 1H NMR (CDCl3) δ 2.96 (d, J = 6.4 MHz, 2 H), 4.08–4.18 (m, 2 H), 4.41–4.50 (m, 1 H), 7.36 (d, J = 9 MHz, 2 H), 8.18 (d, J = 9 MHz, 2 H); 13C NMR (CDCl3) δ 40.8 (t), 53.0 (d), 69.1 (t), 123.8 (d), 129.96 (d), 143.55 (s), 146.96 (s), 159.73 (s). HRMS (positive ion FAB) Calcd for C10H11N4O2 [M + H]+ m/z 223.0719. Found: [M + H]+ m/z 223.0713. Anal. Calcd for C10H10N2O4: C, 53.95; H, 4.56. Found: C, 54.05; H, 4.54.
tert-Butyl 2-(4-Nitrobenzyl)aziridine-1-carboxylate (9)
To a slurry of NaH (0.9 g, 44.6 mmol) in THF (150 mL) at 0 °C was added a solution of 7 (9.5 g, 44.6 mmol) in THF (150 mL) over 1 h. The resulting mixture was stirred for 2 h at 0 °C and continuously stirred for 6 h at room temperature while the progress of the reaction was monitored by TLC analysis. The reaction mixture was poured into cold saturated NH4Cl solution and extracted with EtOAc (2 × 100 mL). The combined EtOAc layer was washed with brine (100 mL), dried, filtered, and concentrated under vacuum. The residue solid was recrystallized to provide 9 (11.8 g, 95%). For preparation of analytical sample, the residue could be purified via column chromatography on silica gel eluting with 15% EtOAc in hexanes. Pure 9 was thereby obtained as a white solid. 1H NMR (CDCl3) δ 1.73 (s, 9 H), 2.02 (d, J = 3.37 MHz, 1 H), 2.35 (d, J = 5.1 MHz, 1 H), 2.58–2.66 (m, 1 H), 2.82 (dd, J = 4.0 MHz, 1 H), 2.97 (dd, J = 4.0 MHz, 1 H), 7.51 (d, J = 8.9 MHz, 2 H), 8.15 (d, J = 8.9 MHz, 2 H); 13C NMR (CDCl3) δ 27.7 (q), 31.1 (t), 37.2 (t), 38.1 (d), 81.3 (s), 123.5 (d), 129.6 (d), 145.8 (s), 146.7 (s), 162.0 (s). Anal. Calcd for C14H18N2O4: C, 60.42; H, 6.52. Found: C, 60.36; H, 6.70.
Di-tert-butyl [1,4,7]Triazanonane-1,4-dicarboxylate (10)
Compound 10 was prepared according to a modification of a synthetic procedure reported previously.23 To a solution of TACN (1 g, 7.7 mmol) in CHCl3 (25 mL) was added in portions BOC-ON (3.77 g, 15.3 mmol). The resulting mixture was stirred for 72 h and the solvent evaporated under vacuum. The residue was partitioned between 10% NaOH solution (10 mL) and diethyl ether (30 mL). The ether layer was separated and washed with 10% NaOH solution (10 mL) and water (10 mL) several times. The ether layer was dried (MgSO4), filtered, and concentrated under vacuum to provide pure 10 (2.33 g, 95%), which was directly used for the next step. 1H NMR (CDCl3) δ 1.48 (s, 18 H), 3.01–3.52 (m, 13 H); 13C NMR (CDCl3) δ 28.01 (q), 47.0 (t), 47.3 (t), 47.7 (t), 47.9 (t), 49.1 (t), 49.4 (t), 50.0 (t), 51.3 (t), 52.0 (t), 52.1 (t), 52.6 (t), 79.1 (s), 79.2 (s), 155.3 (s), 155.5 (s).
Di-tert-butyl 7-[2-tert-Butoxycarbonylamino-3-(4-nitrophenyl)-propyl][1,4,7]triazanonane-1,4-dicarboxylate (11)
To a solution of 10 (5.7 g, 17.3 mmol) in CH3CN (20 mL) were added 9 (4.58 g, 16.48 mmol) and diisopropylethylamine (2.34 g, 18.13 mmol). The resulting mixture was refluxed for 5 days, at which time the reaction mixture was cooled to room temperature and evaporated. The residue was purified via column chromatography on silica gel eluting with 25% EtOAc/hexane. Pure 11 (7.2 g, 72%) was thereby obtained as a light yellow solid. 1H NMR (CDCl3) 1.31–1.48 (m, 27 H), 2.51–2.64 (m, 6 H), 2.91–3.92 (m, 12 H), 7.34–7.37 (m, 2 H), 8.09–8.13 (m, 2 H); 13C NMR (CDCl3) δ 28.4 (q), 28.5 (2C, q), 39.2 (t), 39.5 (t), 40.2 (t), 47.8 (t), 48.8 (t), 49.6 (t), 50.2 (t), 50.5 (t), 50.6 (t), 51.3 (t), 53.0 (t), 53.3 (t), 53.4 (t), 54.0 (t), 54.1 (t), 55.3 (t), 60.2 (d), 60.5 (d), 79.3 (s), 79.4 (s), 79.7 (s), 79.8 (s), 79.9 (s), 123.35 (d), 123.41 (d), 123.5 (d), 130.1 (d), 146,1 (s), 146.5 (s), 146.8 (s), 147.0 (s), 155.4 (s), 155.5 (s), 155.8 (s), 156.0 (s). HRMS (positive ion FAB) Calcd for C30H49N5O8 [M + H]+ m/z 740.2635. Found: [M + H]+ m/z 740.2636. Anal. Calcd for C30H49N5O8: C, 59.29; H, 8.13. Found: C, 58.56; H, 8.31.
1-(4-Nitrobenzyl)-2-[1,4,7]triazanonan-1-ylethylamine (12)
11 (6.3 g, 10.4 mmol) in an ice bath was treated with 4 M HCl/dioxane (60 mL), gradually allowed to warm to ambient temperature, and stirred for 18 h, after which time ethyl ether (300 mL) was added into the reaction mixture with vigorous stirring. The resulting slurry was placed in the freezer for 2 h. The precipitate was collected and washed with ethyl ether, immediately dissolved in water, and lyophilized to provide pure 12 as a yellow solid (4.37 g, 93%). 1H NMR (D2O, pD 1) δ 2.36–2.79 (m, 10 H), 3.11–3.28 (m, 5 H), 3.40–3.61 (m, 5 H), 3.65–3.80 (m, 1 H), 7.16 (d, J = 8.6 Hz, 2 H), 7.82 (d, J = 8.6 Hz, 2 H); 13C NMR (D2O, pD 1) δ 36.4 (t), 41.3 (t), 43.3 (d), 48.2 (t), 49.3 (t), 58.0 (t), 123.7 (d), 130.1 (d), 142.6 (s), 146.3 (s). HRMS (positive ion FAB) Calcd for C15H25N5O2·4HCl [M + H]+ m/z 308.2087. Found: [M + H]+ m/z 308.2095. Anal. Calcd for C15H25N5O2(HCl)4(H2O)2: C, 39.79; H, 7.12. Found: C, 40.31; H, 7.09. Analytical HPLC (tR = 9.79 min, method 1).
tert-Butyl {4-[2-(Bis-(tert-butoxycarbonylmethyl)amino)-3-(4-nitrophenyl)propyl]-7-tert-butoxycarbonylmethyl[1,4,7]triazanonan-1-yl}acetate (13)
To a slurry of 12·HCl (454 mg, 1 mmol) in DMF (10 mL) at 0 °C were added diisopropylethylamine (1.72 g, 13.3 mmol) and KI (266 mg, 1.6 mmol). tert-Butyl bromoacetate (2.34 g, 4.4 mmol) was added dropwise over 20 min. The resulting mixture was stirred for 2 h at 0 °C and for 2 h at room temperature. The reaction mixture was heated to 90 °C and stirred for 24 h, after which time the reaction mixture was cooled to room temperature and then to 0 °C. HCl (6 M, 0.7 mL) and heptane (20 mL) were sequentially added to the solution. The resulting solution was vigorously stirred for 5 min, and the heptane layer was separated. The aqueous layer was extracted with heptane (2 × 20 mL) and treated with 10% Na2CO3 (10 mL). Additional heptane was added into the aqueous solution, the resulting mixture was stirred for 30 min, and the heptane layer was separated. The combined heptane layers were washed with water (20 mL), dried, filtered, and concentrated under vacuum. The residue was purified via column chromatography on silica gel (220–400 mesh) eluted with 4% CH3OH/CH2Cl2 starting from CH2Cl2 (gradual increase of 0.5% polarity) to provide pure 13 (412 mg, 54%). 1H NMR (CDCl3) δ 1.52 (s, 9 H), 1.55 (s, 18 H), 1.59 (s, 9 H), 2.01–2.05 (m, 1 H), 2.30–2.41 (m, 4 H), 2.60–3.15 (m, 12 H), 3.29 (s, 2 H), 3.37 (s, 2 H), 3.43 (s, 4 H), 7.34 (d, J = 7.72 Hz, 2 H), 8.14 (d, J = 7.72 Hz, 2 H); 13C NMR (CDCl3) δ 28.1 (q), 34.9 (t). 50.6 (t), 51.6 (t), 52.1 (t), 53.0 (t), 56.1 (t), 56.2 (t), 56.8 (t), 57.2 (t), 59.3 (d), 80.7 (s), 80.8 (s), 81.2 (s), 123.6 (d), 130.1 (d), 146.5 (s), 147.5 (d), 169.8 (s), 170.7 (s), 170.8 (s). HRMS (positive ion FAB) Calcd for C39H65N5O10 [M + H]+ m/z 764.9805. Found: [M + H]+ m/z 764.4810. Analytical HPLC (tR = 31.24 min, method 1). Isolation of 13 from the reaction mixture using semipreparative HPLC (method 3) was also successfully performed. The reaction mixture was dissolved in 1 mL of CH3OH, and the fraction at ~35 min was collected, evaporated, dissolved in CH2Cl2, and washed with H2O. The organic layer was dried, filtered, evaporated, and concentrated under vacuum to afford the desired 13.
{4-[2-(Bis(carboxymethyl)amino)-3-(4-nitrophenyl)propyl]-7-carboxymethyl[1,4,7]triazanonan-1-yl}acetic Acid (14)
Compound 13 (500 mg, 0.65 mmol) was treated with 4 M HCl in 1,4-dioxane (10 mL) at 0 °C. The resulting mixture was stirred at 0 °C for 30 min, gradually warmed to room temperature, and stirred for 18 h. Diethyl ether (100 mL) was added into the reaction mixture, which was placed in the freezer for 2 h. The precipitate was filtered, immediately dissolved in water (10 mL), and washed with diethyl ether (20 mL). The aqueous layer was lyophilized to provide pure 14 as a white creamy solid (423 mg, 95%). 1H NMR (D2O) δ 2.94–3.16 (m, 5 H), 3.23–3.56 (m, 12 H), 3.62–3.78 (m, 2 H), 3.84–4.30 (m, 10 H), 4.41–4.52 (m, 1 H), 7.46 (d, J = 7.9 Hz, 2 H), 8.14 (d, J = 7.9 Hz, 2 H); 13C NMR (D2O) δ 36.45 (t), 51.2 (t), 52.1 (t), 52.5 (t), 53.2 (t), 55.0 (t), 55.9 (t), 56.7 (t), 56.9 (t), 58.4 (t), 63.4 (d), 127.4 (d), 133.5 (d), 144.0 (s), 150.3 (s), 170.2 (s), 171.6 (s), 175.9 (d). HRMS (positive ion FAB) Calcd for C23H34N5O10 [M + H]+ m/z 541.2336. Found: [M + H]+ m/z 540.2311. Analytical HPLC (tR = 6.81 min, method 2).
General Procedure for Complexation Kinetics Using AAIII
Ultrapure ammonium acetate (Aldrich, 431311) and hydrochloric acid solution (J. T. Baker, JT6900-5) were purchased and used as received. All buffer solutions were prepared using deionized water (Milli-Q, 18Ω). Metal-free stock solutions of all NH4OAc buffers (0.15 M, pH 2 or 4) were prepared using Chelex-100 resin (100–200 mesh, Bio-Rad Laboratory, Hercules, CA). Chelex resin (5 g) was added into the buffer solution (500 mL), and the mixture was shaken for 1 h in a shaker (Eberbach), stored in the refrigerator overnight, and filtered through a Corning filter system (no. 430513, pore size = 0.2 µM). Fifteen micromolar AAIII solutions in 0.15 M NH4OAc (pH 4 or 2) were prepared by adding AAIII (5.82 mg) to the NH4OAc buffer (500 mL). A solution of Bi(III)-AAIII (7.5 µM) was freshly prepared by mixing AAIII solution (15 µM, 50 mL) in NH4OAc buffers (0.15 M, pH 4 or 2) with bismuth atomic absorption standard solution (78.8 µL, 995 µg/mL) and shaken for 2 h in a shaker (Eberbach). The multicell transporter of the UV – visible spectrometer was zeroed against a cell filled with 2 mL of AAIII solution. The solution in each sample cell was removed and filled with 2 mL of Bi(III)–AAIII solution, and the absorbance of the solution was measured for 1 h. Ten microliters of Bi(III)–AAIII solution was then removed from each sample cell, and each of the bifunctional ligands C-DOTA and C-NETA (10 µL, 10 mM) was added into their respective sample cells. Kinetics spectra were collected every 30 s at 652 nm for 1 h.
A solution of Lu(III)–AAIII (1.8 µM) was freshly prepared by mixing AAIII solution (5 µM, 50 mL) in NH4OAc buffers (0.15 M, pH 4.5) with lutetium atomic absorption standard solution (15.4 µL, 1014 µg/mL) and shaking for 2 h in the shaker. The resulting solution was stored in a brown bottle in the refrigerator to avoid degradation and disposed of after 24 h. The multicell transporter of the UV–visible spectrometer was zeroed against a cell filled with 2 mL of AAIII solution. The solution in each sample cell was removed and filled with 2 mL of Lu(III)–AAIII solution, and the absorbance of the solution was measured for 1 h. Ten microliters of Lu(III)–AAIII solution was then removed from each sample cell, and each of the bifunctional ligands C-DOTA and C-NETA (10 µL, 10 mM) was added into their respective sample cells; kinetics spectra were collected every 30 s at 652 nm for 1 h.
Radiolabeling of C-NETA
The labeling efficiency was determined by radio-TLC with silica gel plate as the stationary phase and 10% methanol/water as the mobile phase. The complexes formed were purified from free radioisotope, either 205/6Bi or 177Lu, by ion-exchange chromatography using a Chelex-100 column (1 mL volume bed, 100–200 mesh, Na+ form, Bio-Rad, Richmond, CA) eluted with PBS (pH 7.4). To a solution of 1.2 mg (1.75 µmol) of C-NETA in water (50 µL) was added 205/6Bi in HI (0.1M, 250 µL, 1.7 mCi). The pH of the mixture was adjusted to 5 with ammonium acetate buffer (5 M, 50 µL, pH 7). The reaction mixture was incubated at room temperature or 80 °C for 1 h. Labeling efficiencies of 95 and 94% were obtained at room temperature and 80 °C, respectively. The complex possesses an Rf value of 0.8.
To a solution of 1.2 mg (1.75 µmol) of C-NETA in water (40 µL) was added 177LuCl3 in 0.05 M HCl (2 µL, 0.5 mCi). The pH of the mixture was adjusted to 5 with 0.15 M ammonium acetate buffer (50 µL, pH 7). The total volume was brought up to 100 µL with water and the reaction mixture incubated at room temperature for 1 h. 177Lu–C-NETA was obtained with a labeling efficiency of 95.3%. The radiolabeling of C-NETA (1.75 µmol) with 177LuCl3 in 0.05 M HCl (0.5 mCi) was performed as described above at an elevated temperature (80 °C) overnight to provide the complex (Rf = 0.8) in 93.8% yield.
In Vitro Stability of the Radiolabeled Metal Complexes
The radiolabeled complexes were prepared by reacting C-NETA with 205/6Bi or 177Lu at room temperature. The stability of the purified radiolabeled complexes was evaluated in human serum (Gemini Bioproducts, Woodland, CA) for 14 days. The serum stability of the radiolabeled complexes was assessed by measuring the transfer of the radionuclide from each complex to serum proteins using SE-HPLC. Radiolabeled complexes were diluted to an appropriate volume that allowed for preparation of multiple samples containing 5–10 µCi and were filter-sterilized using a Millex-GV 0.22 µm filter. This stock solution was then mixed with 1400 µL of sterile normal human serum. Aliquots (200 µL) were drawn and separated into individual tubes for subsequent analysis using aseptic technique. The samples were incubated at 37 °C and, at designated intervals, subjected to analysis by SE-HPLC. Samples were loaded onto the HPLC and eluted with PBS, pH 7.4, isocratically at 1 mL/min. Radioactivity still associated with the chelate typically displayed a retention time of ~10 min at this flow rate. Radioactivity associated with a transfer to serum proteins generally appeared between ~4.5 and 8 min.
In Vivo Biodistribution Studies
The radiolabeled complexes were prepared by reacting C-NETA with 205/6Bi or 177Lu at room temperature. Female athymic mice were obtained from Charles River Laboratories (Wilmington, MA) at 4–6 weeks of age. The pH of the radiolabeled ligands was adjusted to ~7.0 with 0.5 M sodium bicarbonate (pH 10.5) and diluted in phosphate-buffered saline. The radiolabeled ligands (5–10 µCi for 205/6Bi and 177Lu) were administered to the mice in 200 µL of solution via tail vein injection. The mice (five per data point) were sacrificed by exsanguination at 0.5, 1, 4, 8, and 24 h. Blood and the major organs were harvested and wet-weighed, and the radioactivity was measured in a γ-scintillation counter (1480 WizardOne, Perkin-Elmer). The percent injected dose per gram (% ID/g) was determined for each tissue. The values presented are the mean and standard deviation for each tissue. All animal experiments were performed in compliance with current regulations and guidelines of the U.S. Department of Agriculture and approved by the NCI Animal Care and Use Committee.
Acknowledgment
We acknowledge financial support from the National Institutes of Health (K22CA102637 and R01CA112503-01A2 to H.-S.C.). This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Abbreviations: mAb, monoclonal antibody; RIT, radioimmunotherapy; NHL, non-Hodgkin’s lymphoma; C-NETA, {4-[2-(bis(carboxymethyl)amino)-3-(4-nitrophenyl)propyl]-7-carboxymethyl-[1,4,7]triazonan-1-yl}acetic acid; C-DTPA, 2-(p-NO2-Bn)-diethylenetriaminepentaacetic acid; C-DOTA, 2-(p- NO2-Bn)-1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid; NOTA, 1,4,7-triazacyclononane-N,N′,N″-triacetic acid; 1B4M-DTPA, 2-(p-NO2-Bn)-6-methyl-DTPA; BOC, tert-butoxycarbonyl; AAIII, Aresenazo III; % ID/g, percent injected dose per gram.
References
- 1.Knox SJ, Meredith RF. Clinical radioimmunotherapy. Semin. Radiat. Oncol. 2000;10:73–93. doi: 10.1016/s1053-4296(00)80045-4. [DOI] [PubMed] [Google Scholar]
- 2.McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, Wu K, Pellegrini V, Curcio MJ, Miederer M, Bander NH, Scheinberg DA. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava S, Dadachova E. Recent advances in radionuclide therapy. Semin. Nucl. Med. 2001;31:330–341. doi: 10.1053/snuc.2001.27043. [DOI] [PubMed] [Google Scholar]
- 4.Juweid M, DeNardo GL, Graham M, Vose J. Radioimmunotherapy: a novel treatment modality for B-cell non-Hodgkin’s lymphoma. Cancer Biother. Radiopharm. 2003;18:673–674. doi: 10.1089/108497803770418229. [DOI] [PubMed] [Google Scholar]
- 5.Jhanwar YS, Divgi CJ. Current status of therapy of solid tumors. Nucl. Med. 2005;46(S1):141S–150S. [PubMed] [Google Scholar]
- 6.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discovery. 2004;3:488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 7.Borghaei H, Schilder RJ. Safety and efficacy of radioimmunotherapy with yttrium 90 ibritumomab tiuxetan (Zevalin) Semin. Nucl. Med. 2004;34 Suppl. 1:4–9. doi: 10.1053/j.semnuclmed.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Parker D. Tumor targeting with radiolabeled macrocycle antibody conjugates. Chem. Soc. Rev. 1990;19:271–291. [Google Scholar]
- 9.Kumar K, Magerstadt M, Gansow OA. Lead(II) and bismuth(III) complexes of the polyazacycloalkane-N-acetic acids nota, dota, and teta. J. Chem. Soc., Chem. Commun. 1989:145–146. [Google Scholar]
- 10.Ruegg CL, Anderson-Berg WT, Brechbiel MW, Mirzadeh S, Gansow OA, Strand M. Improved in vivo stability and tumor targeting of bismuth-labeled antibody. Cancer Res. 1990;50:4221–4226. [PubMed] [Google Scholar]
- 11.Kumar K, Chang CA, Tweedle MF. Equilibrium and kinetic studies of lanthanide complexes of macrocyclic polyamino carboxylates. Inorg. Chem. 1993;32:587–593. [Google Scholar]
- 12.Kodama M, Koike T, Mahatma AB, Kimura E. Thermodynamic and kinetic studies of lanthanide complexes of 1,4,7,10,13-pentaazacyclopentadecane- N,N′,N″,N′″,N″″-pentaacetic acid and 1,4,7,10,13,16-hexaazacyclcootadecane-N,N′,N″,N′″,N″″-hexaacetic acid. Inorg. Chem. 1991;30:1270–1273. [Google Scholar]
- 13.Jang YH, Blanc M, Dasgupta S, Deire DA, Shively JE, Goddard WA., III Mechanism and energetics for complexation of 90Y with 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA), a model for cancer radioimmunotherapy. J. Am. Chem. Soc. 1999;121:6142–6151. [Google Scholar]
- 14.Harrison A, Walker CA, Parker D, Jankowski KJ, Cox JP. The in vivo release of 90-Y from cyclic and acyclic ligand–-antibody conjugates. Nucl. Med. Biol. 1991;18:469–476. doi: 10.1016/0883-2897(91)90107-v. [DOI] [PubMed] [Google Scholar]
- 15.Brechbiel MW, Gansow OA. Backbone-substituted DTPA ligands for 90Y radioimmunotherapy. Bioconjugate Chem. 1991:187–194. doi: 10.1021/bc00009a008. [DOI] [PubMed] [Google Scholar]
- 16.Camera L, Kinuya S, Garmestani K, Wu C, Brechbiel MW, Pai LH, McMurry TJ, Gansow OA, Pastan I, Paik CH, Carrasquillo JA. Evaluation of the serum stability and in vivo biodistribution of CHX-DTPA and other ligands for yttrium labeling of monoclonal antibodies. J. Nucl. Med. 1994;35:882–889. [PubMed] [Google Scholar]
- 17.Knox SJ, Goris ML, Trisler K, Negrin R, Davis T, Liles TM, Grillo-Lopez AJ, Chinn P, Varns C, Ning SC, Fowler S, Deb N, Becker M, Marquez C, Levy R. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin. Cancer Res. 1996;2:457–470. [PubMed] [Google Scholar]
- 18.Brucher E, Sherry AD. Kinetics of formation and dissociation of the 1,4,7-triazacyclononane-N,N′,N″-triacetate complexes of cerium(III), gadolinum(III), and erbium(III) ions. Inorg. Chem. 1990;29:1555–1559. [Google Scholar]
- 19.Srivastava SC. Criteria for the selection of radionuclides for targeting nuclear antigens for cancer radioimmunotherapy. Cancer Biother. Radiopharm. 1996;11:43–50. doi: 10.1089/cbr.1996.11.43. [DOI] [PubMed] [Google Scholar]
- 20.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, Ballangrud AM, Hamacher KA, Ma D, Humm JL, Brechbiel MW, Molinet R, Scheinberg DA. Targeted α particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 21.Hassfjell S, Brechbiel MW. The development of the α-particle emitting radionuclides 212Bi and 213Bi, and their decay chain related radionuclides for therapeutic applications. Chem. Rev. 2001;101:2019–2036. doi: 10.1021/cr000118y. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez RD, Partridge EE, Khazaeli MB, Plott G, Austin M, Kilgore L, Russell CD, Liu T, Grizzle WE, Schlom J, LoBuglio AF, Meredith RF. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol. Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs Z, Sherry AD. A general synthesis of mono- and disubstituted 1,4,7-triazacyclononanes. Tetrahedron Lett. 1995;36:9269–9271. [Google Scholar]
- 24.Savvin SB. Analytical use of arsenazo III, determination of thorium, zirconium, uranium and rare earth elements. Talanta. 1961:673–685. [Google Scholar]
- 25.Pippin CG, Parker TJ, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjugate Chem. 1992;3:342–345. doi: 10.1021/bc00016a014. [DOI] [PubMed] [Google Scholar]
- 26.Chong HS, Milenic DE, Garmestani K, Brady ED, Arora H, Pfiester C, Brechbiel MW. In vitro and in vivo evaluation of novel ligands for radioimmunotherapy. Nucl. Med. Biol. 2006;33:459–467. doi: 10.1016/j.nucmedbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Li WP, Ma DS, Higginbotham C, Hoffman T, Ketring AR, Cutler CS, Jurisson SS. Development of an in vitro model for assessing the in vivo stability of lanthanide chelates. Nucl. Med. Biol. 2001;28:145–154. doi: 10.1016/s0969-8051(00)00196-7. [DOI] [PubMed] [Google Scholar]
- 28.Garmestani K, Yao Z, Zhang M, Wong K, Park CW, Pastan I, Carrasquillo JA, Brechbiel MW. Synthesis and evaluation of a macrocyclic bifunctional chelating agent for use with bismuth radionuclides. Nucl. Med. Biol. 2001;28:409–418. doi: 10.1016/s0969-8051(00)00203-1. [DOI] [PubMed] [Google Scholar]