Abstract
Chronic tissue ischemia due to defective vascular perfusion is a hallmark feature of peripheral artery disease for which minimal therapeutic options exist. We have reported that sodium nitrite therapy exerts cytoprotective effects against acute ischemia/reperfusion injury in both heart and liver, consistent with the model of bioactive NO formation from nitrite during ischemic stress. Here, we test the hypothesis that chronic sodium nitrite therapy can selectively augment angiogenic activity and tissue perfusion in the murine hind-limb ischemia model. Various therapeutic doses (8.25–3,300 μg/kg) of sodium nitrite or PBS were administered. Sodium nitrite significantly restored ischemic hind-limb blood flow in a time-dependent manner, with low-dose sodium nitrite being most effective. Nitrite therapy significantly increased ischemic limb vascular density and stimulated endothelial cell proliferation. Remarkably, the effects of sodium nitrite therapy were evident within 3 days of the ischemic insult demonstrating the potency and efficacy of chronic sodium nitrite therapy. Sodium nitrite therapy also increased ischemic tissue nitrite and NO metabolites compared to nonischemic limbs. Use of the NO scavenger carboxy PTIO completely abolished sodium nitrite-dependent ischemic tissue blood flow and angiogenic activity consistent with nitrite reduction to NO being the proangiogenic mechanism. These data demonstrate that chronic sodium nitrite therapy is a recently discovered therapeutic treatment for peripheral artery disease and critical limb ischemia.
Keywords: wound healing, endothelial cell, nitric oxide, peripheral artery disease, tissue perfusion
Therapeutic angiogenesis remains an attractive treatment modality for peripheral vascular disease and chronic tissue ischemia. Numerous mediators, including growth factors, transcription factors, and signaling molecules, have been reported to augment chronic ischemia-induced angiogenesis in animal models (1). However, clinical trials of proangiogenic agents have revealed little to no practical utility in patients suffering from such disorders (2). Thus, there remains a clear need for better interventions with which to induce therapeutic angiogenesis.
The signaling molecule nitric oxide (NO) has been shown to be an important player in stimulating angiogenesis in a variety of settings (3, 4). NO is an important signaling intermediate governing VEGF-dependent angiogenesis (5–7). Multiple signaling pathways within endothelial cells may be affected in response to NO generation, including Erk1/2, PKC, tyrosine kinases, and transcription factors (4, 8, 9). Moreover, endothelial NO synthase (eNOS) has been shown to be a critical player in modulating postnatal angiogenesis activity, because genetic deficiency of this molecule diminishes ischemia-induced angiogenesis and pericyte recruitment (10, 11). Consistent with these observations, NO donors have been shown to have cardioprotective effects including augmentation of angiogenic responses in vitro and in vivo (9, 12–16). However, a major limitation with the use of NO donors is that these agents are nonselective and may induce undesired consequences, including cellular injury to healthy tissue and systemic alterations in tissue perfusion (17–19). Taken together, these observations indicate that NO donor therapy would be very beneficial for therapeutic angiogenesis, yet at present there are no effective means to selectively deliver NO to ischemic tissues to promote angiogenesis.
Recent discoveries from several laboratories have revealed that nitrite, a one-electron oxidation product of NO, can act as a selective NO donor because of reduction back to NO by several mechanisms, including, but not limited to, deoxyhemoglobin, deoxymyoglobin, xanthine oxidoreductase, acidic disproportionation, and mitochondrial complex IV (20–26). Common among these candidate nitrite-reducing transducers is that nitrite reduction will become more significant as the oxygen tension is lowered, a condition that exists in peripheral vascular disease (27, 28). Moreover, we and others have reported that sodium nitrite protects against acute ischemia/reperfusion injury and other disease states associated with acute tissue ischemia and NO deficiency (25, 29). These data led us to hypothesize that sodium nitrite therapy may also be beneficial during settings of chronic ischemia by augmenting ischemic tissue angiogenesis. We found that prolonged nitrite therapy robustly augmented ischemic tissue blood flow and angiogenesis, which did not occur in nonischemic tissues. These data provide compelling evidence that sodium nitrite therapy serves as a tissue-selective NO donor that could be of great clinical utility for peripheral vascular disease and critical tissue ischemic disorders.
Results
Chronic Sodium Nitrite Therapy Increases Ischemic Tissue Blood Flow.
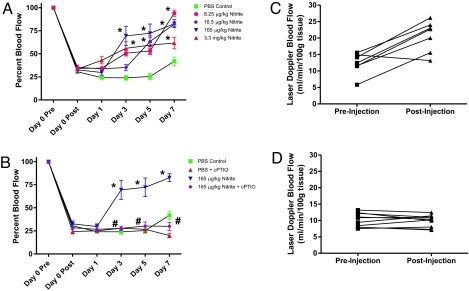
A recent study by Dejam et al. (30) has demonstrated that continuous infusion of sodium nitrite is safe and well tolerated in non-human primates. In our study, we examined a range of sodium nitrite doses from 8.25 to 3,300 μg/kg given via i.p. injection twice per day. Fig. 1A shows that nitrite doses of 8.25, 16.5, 165, and 3,300 μg/kg all increased the percentage blood flow in ischemic hind limbs by day 3 after ligation, which progressively increased by day 7. Interestingly, the 165-μg/kg sodium nitrite dose revealed optimal efficacy compared to higher and lower doses, which is consistent with maximum cardio and liver protection, as reported (29). Fig. 1B reports that the NO scavenger cPTIO (1 mg/kg daily) significantly attenuated the ability of sodium nitrite to restore ischemic limb blood flow. Fig. 1C demonstrates that injection of 165 μg/kg sodium nitrite enhances blood flow in hind limbs that were ischemic for 24 h compared to PBS injection (Fig. 1D). Importantly, neither PBS nor sodium nitrite altered nonischemic hind limb blood flow after injection [supporting information (SI) Fig. S1 A and B, respectively].
Fig. 1.
Chronic sodium nitrite therapy restores ischemic hind limb blood flow in an NO-dependent manner. A shows the effect of various doses of chronic sodium nitrite therapy on ischemic hind-limb blood flow over time compared to PBS control. B reports the effect of sodium nitrite therapy plus 1 mg/kg carboxy PTIO treatment on ischemic hind-limb blood flow. C shows the effect of 165 μg/kg sodium nitrite injection on ischemic limb blood flow. D illustrates the effect of PBS injection on ischemic limb blood flow. *, P < 0.01 vs. PBS control at each time point; #, P < 0.01 165 μg/kg sodium nitrite vs. 165 μg/kg sodium nitrite plus cPTIO at each time point. n = 15 mice per treatment group.
Chronic Sodium Nitrite Therapy Selectively Increases Ischemia-Induced Angiogenesis.
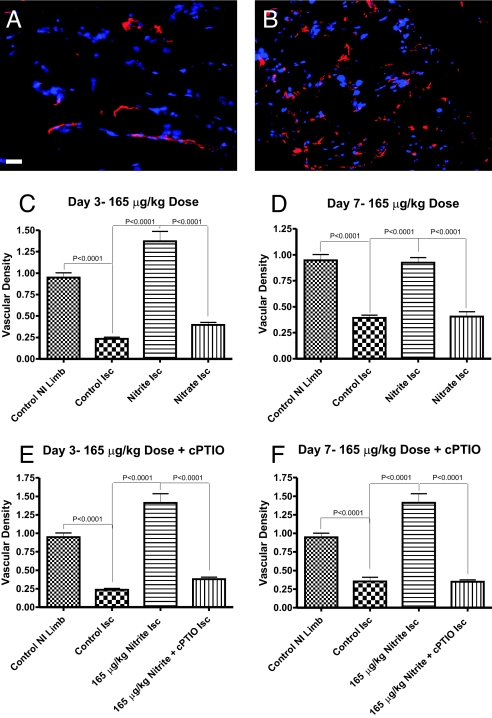
Successful reperfusion of ischemic tissues depends on stimulation of angiogenic activity necessary for restoration of tissue blood flow (27, 31). Fig. 2 A and B illustrate endothelial cell CD31 staining in red with DAPI nuclear counterstaining in blue of ischemic gastrocnemius muscle at day 7 from animals treated with PBS or sodium nitrite (165 μg/kg), respectively. Endothelial staining of CD31 was more abundant in ischemic muscle tissue in mice receiving sodium nitrite as compared to PBS or sodium nitrate controls. Quantitative analysis of vascular density revealed that sodium nitrite (165 μg/kg) therapy significantly increased vascular density at days 3 and 7 compared to control PBS treatment (Fig. 2 C and D). Interestingly, high-dose sodium nitrite (3,300 μg/kg) was less potent in augmenting ischemic tissue vascular density compared to low-dose (165 μg/kg) nitrite, consistent with observations of percentage blood flow changes in ischemic tissues (Fig. S2 A and B). Importantly, sodium nitrite did not significantly alter nonischemic tissue vascular density, highlighting the site-specific activity of chronic nitrite therapy (Fig. S2C). Consistent with changes in tissue blood flow, cPTIO cotreatment prevented nitrite augmentation of angiogenesis in the ischemic hind limbs at days 3 and 7 (Fig. 2 E and F).
Fig. 2.
Chronic sodium nitrite therapy increases ischemic tissue vascular density in an NO-dependent manner. A and B show representative images of CD31 (red) and DAPI nuclear (blue) staining from sodium nitrite and sodium nitrate ischemic gastrocnemius muscle tissue at day 7. C and D report the vascular density of ischemic gastrocnemius muscle tissue at days 3 and 7 for 165 μg/kg sodium nitrite and nitrate treatments, respectively. E and F demonstrate the vascular density of ischemic gastrocnemius muscle tissue at days 3 and 7 from 165 μg/kg sodium nitrite plus carboxy PTIO. (Scale bar, 150 μm.) n = 10 mice per treatment group.
Chronic Sodium Nitrite Therapy Increases Ischemic Endothelial Cell Proliferation.
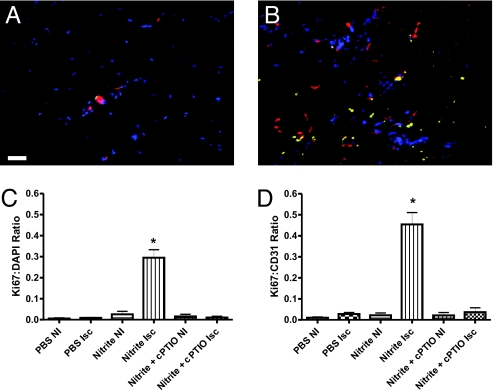
An obligatory requirement for increased angiogenic activity is endothelial cell proliferation. Fig. 3 A and B illustrate the amount of endothelial cell proliferation as determined by Ki67 staining (green) of ischemic tissues along with endothelial cell CD31 labeling (red) and DAPI nuclear staining (blue) from day 3 PBS or sodium nitrite (165 μg/kg) treatments, respectively. Fig. 3C reports the amount of Ki67 colocalization with DAPI nuclear counterstain at day 3, which revealed that sodium nitrite significantly increases Ki67 nuclear localization in ischemic but not nonischemic tissues that was blocked by cPTIO. Moreover, sodium nitrite significantly increased Ki67 to CD31 colocalization in ischemic tissues that was inhibited by cPTIO (Fig. 3D).
Fig. 3.
Chronic sodium nitrite therapy stimulates endothelial cell proliferation in an NO-dependent manner. A and B show representative images of Ki67 proliferation marker (green), CD31 (red), and DAPI nuclear (blue) staining from day 3 ischemic gastrocnemius muscle tissue from PBS and 165 μg/kg sodium nitrite-treated animals, respectively. C reports the amount of Ki67 colocalization with DAPI nuclear staining between PBS and sodium nitrite-treated tissues ± cPTIO. D shows the measurement of Ki67 colocalization with CD31 staining between PBS and sodium nitrite-treated tissues plus cPTIO. *, P < 0.001 sodium nitrite vs. PBS or nitrite plus PTIO. n = 10 mice per treatment group. (Scale bar, 150 μm.)
Blood and Tissue Nitrite Levels from Chronic Nitrite Therapy.
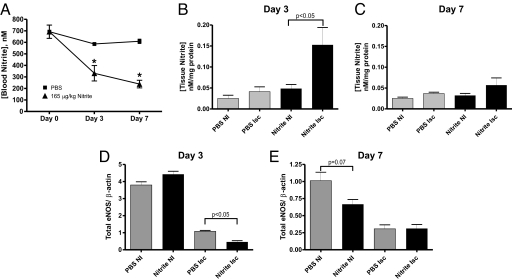
Recent reports demonstrate that administration of nitrite results in rapid accumulation of this anion in plasma and blood (32, 33). Fig. 4A reports the concentration of blood nitrite levels from 165 μg/kg sodium nitrite or PBS-treated animals over time. Interestingly, blood levels of nitrite were significantly decreased over time in the 165-μg/kg sodium nitrite therapy and minimally altered with PBS treatment. Fig. 4B reveals that nitrite therapy significantly increased ischemic but not nonischemic tissue nitrite accumulation at day 3. Conversely, Fig. 4C shows that day 7 tissue nitrite levels were not significantly different between sodium nitrite and PBS treatments. Together, these data demonstrate that chronic sodium nitrite therapy results in preferential early tissue accumulation of nitrite in ischemic versus nonischemic tissues.
Fig. 4.
Chronic sodium nitrite therapy alters blood and tissue nitrite levels. A reports blood nitrite levels at days 3 and 7 for PBS control and sodium nitrite (165 μg/kg). *, P < 0.01 treatments vs. PBS control blood nitrite levels. B and C show tissue nitrite levels at day 3 and 7 for PBS control and sodium nitrite (165 μg/kg). *, P < 0.01 ischemic vs. nonischemic tissue nitrite levels. n = 10 mice per treatment group. D and E report total eNOS protein expression normalized to β actin expression at day 3 and 7 for PBS and sodium nitrite treatments in ischemic and nonischemic hind limbs. n = 3 mice per experimental group.
Because of the interesting observation of decreased blood nitrite levels in response to chronic sodium nitrite therapy, we examined total eNOS protein levels in hind-limb muscle tissue from PBS or 165 μg/kg sodium nitrite therapy. Fig. 4D reveals that at day 3, chronic sodium nitrite therapy significantly decreases eNOS protein expression in ischemic hind-limb tissue without altering expression in nonischemic hind-limb tissue. Fig. 4E reports day 7 eNOS protein levels that show a clear trend for sodium nitrite inhibition of eNOS expression in nonischemic tissue with no effect on ischemic tissue. These data suggest that the decrease in blood nitrite levels could be due to decreased eNOS protein expression in response to chronic sodium nitrite therapy.
NO Tissue Metabolites and Tissue cGMP During Chronic Sodium Nitrite Therapy.
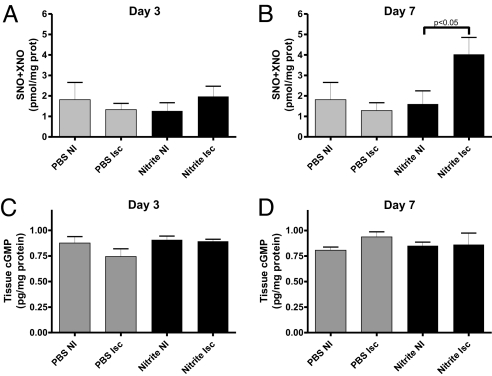
We have previously reported that nitrite therapy in the setting of acute ischemia/reperfusion injury results in increased NO metabolite production (29). Fig. 5 A and B illustrate tissue SNO+XNO levels for 165 μg/kg sodium nitrite or PBS at day 3 and 7. Neither sodium nitrite (165 μg/kg) nor PBS significantly altered tissue SNO+XNO levels at day 3. However, nitrite therapy significantly increased SNO+XNO levels in ischemic tissue at day 7 compared to PBS. These data reveal a delayed effect of nitrite therapy on tissue nitrosothiol, C-/N-nitroso compounds, and iron-nitrosyl proteins and demonstrate preferential production of NO containing intermediates in ischemic tissues.
Fig. 5.
Chronic sodium nitrite therapy effects on tissue NO metabolites and cGMP levels. A and B show the amount of SNO+XNO levels in nonischemic and ischemic tissues at day 3 and 7 for PBS control and 165 μg/kg sodium nitrite, respectively. C and D report the pg/mg total protein of cGMP in nonischemic and ischemic tissues at day 3 and 7 for PBS control and 165 μg/kg sodium nitrite, respectively. n = 20 mice per treatment group.
NO activates soluble guanylate cyclase, resulting in elevated cGMP levels that can augment ischemia-induced angiogenesis (31). Therefore, we examined whether chronic sodium nitrite therapy involved increased cGMP in augmenting ischemia-induced angiogenesis. Fig. 5 C and D report that 165 μg/kg sodium nitrite or PBS at day 3 or 7 did not significantly alter tissue cGMP levels.
Chronic Sodium Nitrite Therapy Augments Arteriogenesis and Acute Changes in Ischemic Tissue Blood Flow.
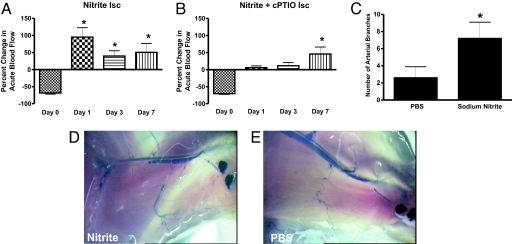
Stimulation of angiogenesis alone during chronic ischemia is insufficient for restoring tissue perfusion. Increased arteriogenesis through the recruitment and differentiation of smaller arterioles is important to supply newly formed microvasculature (34). Moreover, an acute increase in vascular shear stress due to increased blood flow is a critical mediator of arteriogenesis (35). We examined the effect of 165 μg/kg sodium nitrite on acute changes (30 sec) in ischemic and nonischemic tissue perfusion. Fig. 6A illustrates that 165 μg/kg sodium nitrite significantly enhances ischemic tissue blood flow by 92.3 ± 18% within 30 sec of administration at day 1 after ligation. The duration of increased blood flow after sodium nitrite injection persisted >10 min (data not shown). The ability of nitrite to induce large increases in acute blood flow was inversely proportional to the duration of tissue ischemia, because animals receiving chronic nitrite therapy showed a lesser, yet still significant, increase in acute blood flow change at days 3 and 7. This observation could be because significant angiogenic activity has occurred, thereby diminishing the degree of tissue ischemia. Moreover, Fig. 6B demonstrates that sodium nitrite-dependent changes in acute tissue blood flow involve NO, because cPTIO significantly blunted this response at early time points.
Fig. 6.
Chronic sodium nitrite therapy acutely increases ischemic tissue blood flow and stimulates arteriogenesis. A and B report 165 μg/kg sodium nitrite-induced acute changes in blood flow of chronically ischemic tissues at various time points with or without cPTIO, respectively. C reports the number of arterial branches between PBS and nitrite therapies. D and E illustrate vascular casting of the arterial vasculature in ischemic hind limbs of day 7 nitrite or PBS-treated mice, respectively. *, P < 0.01 vs. sodium nitrate. n = 10 mice per treatment group.
To further evaluate arteriogenesis activity, we performed hind-limb ligations distal to the profunda femoris and proximal to the knee to more easily distinguish changes in the arterial supply (36). Fig. 6C reports that sodium nitrite therapy significantly increased the number of arterial branch points compared to PBS treatment at day 7. Fig. 6D shows a representative example of an arterial cast from a sodium nitrite-treated ischemic limb at day 7, whereas Fig. 6E illustrates an arterial cast of a PBS-treated ischemic limb at day 7. Numerous collateral vessels can be observed throughout the tissue in response to nitrite therapy, indicating enhanced arteriogenesis. Conversely, PBS treatment did not enhance arterial perfusion, and minimal collateral vessels are observed. Together, these data demonstrate that chronic nitrite therapy augments arteriogenesis activity in ischemic tissue.
Discussion
Clinically useful strategies for enhancing therapeutic angiogenesis have largely been unrealized (2, 27). This is likely because stimulation of angiogenesis involves multiple complex events such as endothelial cell signal pathway activation, proliferation, directional migration, remodeling of extracellular matrix, and vessel maturation. As such, an ideal therapy for angiogenesis should augment as many of these events as possible. NO has been shown to positively regulate all of these endothelial cell responses, thereby identifying this molecule as a prime agent with which to enhance angiogenesis (1, 4). However, administration of NO can be problematic, with concerns over cellular toxicity, systemic pressor effects, ensuring tissue specificity, and delivery modalities being key conundrums (17–19). Recent studies have developed the concept that nitrite may act as a site-specific NO donor via mechanisms regulated by tissue hypoxia (25, 26, 29). Specifically, the general model proposed involves nitrite reduction by one electron to form NO, with this process occurring predominantly during hypoxia. The identity of the electron donor remains an active area of investigation, with xanthine oxidoreductase, oxygen-binding heme proteins (hemoglobin and myoglobin), mitochondrial heme proteins, and nitrite disproportionation at acidic pH all being possible candidates (25). We reasoned, therefore, that nitrite may represent a therapeutic intervention that stimulates ischemic angiogenesis with high efficacy and specificity by virtue of NO production localized primarily at the site of ischemia.
Our study demonstrates that continuous nitrite therapy is a very potent intervention for stimulating angiogenesis and reperfusion of chronically ischemic tissue. Previous studies have shown that restoration of tissue blood flow and angiogenic stimulation in the murine ischemic hind limb model typically require several weeks (10, 37, 38). We found that chronic nitrite therapy rapidly restored ischemic tissue blood flow and vascular density within 3–7 days. Moreover, we found that sodium nitrite therapy may acutely increase ischemic limb blood flow after injection indicative of a hypoxic vasodilator response, as suggested by Maher et al. (39) and Cosby et al. (40). However, direct intravital microscopy observation of ischemic arteriole dilation is necessary to firmly identify sodium nitrite as a hypoxic vasodilator. The profound effect of sodium nitrite on ischemic tissue reperfusion is likely because NO enhances many aspects of endothelial cell activity during angiogenesis, which was confirmed by the NO scavenger cPTIO, which blunted the effects of nitrite therapy. An alternative explanation could be that nitrite therapy increases recruitment of collateral circulation in the ischemic limb, thereby limiting tissue damage. However, the anatomical location and severity of the ligation minimize the number of collaterals available, and our endothelial cell proliferation data further indicate that nitrite therapy positively stimulates angiogenic activity.
Mechanisms of tissue uptake and distribution of nitrite remain poorly understood (32). However, we found distinct differences in blood and tissue nitrite levels in response to sodium nitrite therapy. A significant decrease in blood nitrite levels was observed over time, which could be related to changes in metabolic regulation and/or tissue uptake. This hypothesis is supported by the finding that tissue nitrite accumulation increased specifically in ischemic tissue. Interestingly, nitrite tissue uptake occurs early within 3 days, which was not sustained at 7 days even though blood nitrite levels continually decreased. Although this may be unexpected, this result could be due to differential nitrite uptake in other organs or increased excretion rates. Another possible explanation for decreased blood nitrite levels could be due to feedback inhibition of endogenous nitrite generators such as eNOS. Recent studies have shown that in vivo nitrite levels are predominantly governed by eNOS expression and that inhaled NO therapy can decrease tissue eNOS protein expression (41, 42). We discovered that chronic nitrite therapy significantly decreased total eNOS protein expression with an early influence on ischemic tissue and a later effect on nonischemic tissue. These data reinforce the notion of an endocrine function of nitrite in regulating tissue steady state levels of NO (43). Future studies will be necessary to understand the dynamic nature of sodium nitrite uptake and consumption during ischemia and its role in regulating tissue eNOS expression.
NO interacts with several intracellular targets to form various NO-containing species including S-nitrosothiols, C- or N-S-nitroso compounds, and nitrosylheme adducts. Moreover, these nitroso-products may serve as a biological reservoir for NO, which can be liberated under certain conditions (43, 44). We have reported that sodium nitrite therapy during acute ischemia/reperfusion injury increases tissue SNO and XNO formation (29). Interestingly, sodium nitrite therapy did not increase SNO+XNO formation at day 3 but did at day 7. These findings demonstrate that nitrite therapy is effective in generating a NO reservoir in tissues with greater metabolic demand and that this depot effect is temporally delayed compared to nitrite uptake. Last, the amount of nitrite required for therapeutic benefit and changes in tissue NO reservoir function is small, which is advantageous for dosing regimens and diminishing the potential for undesired side effects.
We have recently reported that increasing intracellular cGMP through the inhibition of phosphodiesterase 5 significantly enhances ischemia induced angiogenesis through a phosphoglycerate kinase pathway (31). However, we found that nitrite therapy did not sustain a significant increase in ischemic tissue cGMP levels. These data indicate that chronic sodium nitrite treatment modulates angiogenic activity through other signaling pathways, although we acknowledge that biological responses (i.e., angiogenic activity) may be more sensitive than experimental detection of very small changes in cGMP. It is important to reiterate that the molecular mechanism of augmenting ischemic angiogenesis, endothelial cell proliferation, and restoration of tissue blood flow requires NO production. NO has been shown to modulate several endothelial cell signaling pathways such as Erk1/2, PKC, and others (4, 8, 9). It is likely that chronic nitrite therapy involves one or more of these pathways, and future studies are necessary to precisely determine key signaling mechanisms.
Successful reperfusion of chronically ischemic tissue depends not only on stimulation of angiogenic activity but also on arteriogenesis (45). Stimulation of arteriogenesis through recruitment and differentiation of existing collateral vessels is essential in delivering blood flow to newly formed microvasculature. An acute increase in vascular shear stress due to increased blood flow is a key stimulant for arteriogenesis (35). Moreover, NO plays an important role in facilitating the process of arteriogenesis (46, 47). We found that chronic sodium nitrite therapy preferentially increased ischemic tissue blood flow after injection, which persisted for several minutes. This response was directly proportional to the duration of tissue ischemia and mediated by NO. These findings demonstrate that arteriogenesis is involved in sodium nitrite-mediated restoration of ischemic tissue perfusion.
In conclusion, we demonstrate that continuous sodium nitrite therapy is a highly potent and effective modality for selectively restoring perfusion to chronically ischemic tissues. This is because sodium nitrite stimulates multiple aspects of vascular remodeling such as angiogenesis and arteriogenesis in an NO-dependent manner. The well established safety profile of nitrite, along with its inexpensive nature, further emphasizes its therapeutic potential for disorders involving ischemia such as peripheral vascular disease and critical ischemic tissue disease. Future studies are required to evaluate and confirm the efficacy of nitrite therapy in other organs and disease states.
Materials and Methods
Animals and Reagents.
Male wild-type (C57BL/6J) mice weighing 20–25 gm and age 2–3 months were used. All experimental protocols were approved by the Louisiana State University Institutional Animal Care and Use Committee. Sodium nitrite, sodium nitrate, PBS, and all other chemicals were purchased from Sigma.
Hind-Limb Ischemia Model.
Hind-limb ischemia was induced in wild-type mice, as we have reported by ligating the left common femoral artery proximal to origin of profunda femoris artery (31).
Vascular Casting.
Hind-limb vascular casting of ischemic limbs was performed by using Microfil silicone injection MV120 as reported (38). Hind-limb muscle tissue was cleared in graded glycerol solutions of 40%, 60%, 80%, and 100% for 24 h each.
Laser Doppler Measurements of Tissue Blood Flow.
The Vasamedics Laserflo BPM2 deep-tissue-penetrating laser Doppler device was used to measure hind-limb blood flow, as we have reported (31). Daily blood flow measurements were taken before the initial nitrite or PBS injection of a 24-h period to obtain representative steady-state changes in perfusion.
Vascular Density Measurement.
Determination of the vascular density of muscle tissue was performed as we have reported (31). Briefly, ischemic (left) and nonischemic (right) tissues were stained with anti-CD31 (PECAM-1) and mounted by using Vectashield DAPI (4′,6-diamidine-2′-phenylindole dihydrochloride) nuclear counterstain. Vascular density was measured as the ratio between CD31 pixel density divided by DAPI pixel density.
Measurement of Nitrite, NO Metabolite, Tissue cGMP, and eNOS Protein Levels.
Nitrite and tissue NO metabolite levels were measured by using chemiluminescence techniques as we have reported (42). Gastrocnemius muscle tissue cGMP levels were determined by using the cGMP ELISA from Cayman Chemical according to the manufacturer's directions. Specimens were collected within 2 min after injection of either PBS or sodium nitrite. Total eNOS protein levels in hind-limb tissue were determined by Western blot analysis, as we have reported (48).
Statistical Analysis.
Blood flow, vascular density, endothelial cell proliferation, tissue nitrate and NO metabolites, cGMP, and eNOS expression data were analyzed by using Student's t test (unpaired) between sodium nitrite or sodium nitrate vs. PBS control groups with a minimum of P < 0.05 necessary for significance. Blood nitrite measurements and acute changes in blood flow from experimental groups were compared against PBS controls and day 0 time points, respectively, by using one-way ANOVA with Bonferroni's posttest with a minimum of P < 0.05 necessary for significance. Statistics were done with GraphPad Prism 4.0 software. The number of mice used per reported experiment is designated in the legends for Figs. 1–6.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health Grants HL80482 and DK43785 (to C.G.K.), AHA0655312B (to R.P.P.), and HL60849 (to D.J.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711480105/DCSupplemental.
References
- 1.Milkiewicz M, Ispanovic E, Doyle JL, Haas TL. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol. 2006;38:333–357. doi: 10.1016/j.biocel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Tirziu D, Simons M. Angiogenesis in the human heart: gene and cell therapy. Angiogenesis. 2005;8:241–251. doi: 10.1007/s10456-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 3.Cooke JP. NO and angiogenesis. Atherosclerosis. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 4.Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharmacol Des. 2003;9:521–530. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- 5.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morbidelli L, et al. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- 7.Ziche M, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murohara T, Asahara T. Nitric oxide and angiogenesis in cardiovascular disease. Antioxid Redox Signal. 2002;4:825–831. doi: 10.1089/152308602760598981. [DOI] [PubMed] [Google Scholar]
- 9.Jones MK, Tsugawa K, Tarnawski AS, Baatar D. Dual actions of nitric oxide on angiogenesis: possible roles of PKC, ERK, and AP-1. Biochem Biophys Res Commun. 2004;318:520–528. doi: 10.1016/j.bbrc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci USA. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumura D, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ignarro LJ, Napoli C, Loscalzo J. Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res. 2002;90:21–28. doi: 10.1161/hh0102.102330. [DOI] [PubMed] [Google Scholar]
- 13.Balasubramaniam V, Maxey AM, Fouty BW, Abman SH. Nitric oxide augments fetal pulmonary artery endothelial cell angiogenesis in vitro. Am J Physiol. 2006;290:L1111–L1116. doi: 10.1152/ajplung.00431.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ridnour LA, et al. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, et al. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- 16.Kevil CG, et al. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J Biol Chem. 2004;279:19230–19238. doi: 10.1074/jbc.M312025200. [DOI] [PubMed] [Google Scholar]
- 17.Loh E, Stamler JS, Hare JM, Loscalzo J, Colucci WS. Cardiovascular effects of inhaled nitric oxide in patients with left ventricular dysfunction. Circulation. 1994;90:2780–2785. doi: 10.1161/01.cir.90.6.2780. [DOI] [PubMed] [Google Scholar]
- 18.Kiss J, Kaapa P, Savunen T. Antioxidants combined with NO donor enhance systemic inflammation in acute lung injury in rats. Scand Cardiovasc J. 2007;41:186–191. doi: 10.1080/14017430601175459. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima A, Ueda K, Takaoka M, Yoshimi Y, Matsumura Y. Opposite effects of preischemic and postischemic treatments with nitric oxide donor on ischemia/reperfusion-induced renal injury. J Pharmacol Exp Ther. 2006;316:1038–1046. doi: 10.1124/jpet.105.092049. [DOI] [PubMed] [Google Scholar]
- 20.Bjorne HH, et al. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J Clin Invest. 2004;113:106–114. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford JH, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster KA. Therapeutic angiogenesis: a complex problem requiring a sophisticated approach. Cardiovasc Toxicol. 2003;3:283–298. doi: 10.1385/ct:3:3:283. [DOI] [PubMed] [Google Scholar]
- 28.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 29.Duranski MR, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dejam A, et al. Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 31.Senthilkumar A, et al. Sildenafil promotes ischemia-induced angiogenesis through a PKG-dependent pathway. Arterioscler Thromb Vasc Biol. 2007;27:1947–1954. doi: 10.1161/ATVBAHA.107.147421. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya K, et al. Nitrite is an alternative source of NO in vivo. Am J Physiol Heart Circ Physiol. 2005;288:H2163–2170. doi: 10.1152/ajpheart.00525.2004. [DOI] [PubMed] [Google Scholar]
- 33.Pannala AS, et al. The effect of dietary nitrate on salivary, plasma, and urinary nitrate metabolism in humans. Free Radic Biol Med. 2003;34:576–584. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 34.Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 36.Mees B, et al. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1926–1933. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- 37.Couffinhal T, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 38.He Y, et al. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–2355. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maher AR, et al. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 40.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 41.Kleinbongard P, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 42.Lang JD, Jr, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladwin MT, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol. 2006:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 44.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem. 2004;279:11065–11073. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 45.Wahlberg E. Angiogenesis and arteriogenesis in limb ischemia. J Vasc Surg. 2003;38:198–203. doi: 10.1016/s0741-5214(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 46.Prior BM, et al. Arteriogenesis: role of nitric oxide. Endothelium. 2003;10:207–216. doi: 10.1080/10623320390246388. [DOI] [PubMed] [Google Scholar]
- 47.Luque Contreras D, Vargas Robles H, Romo E, Rios A, Escalante B. The role of nitric oxide in the post-ischemic revascularization process. Pharmacol Ther. 2006;112:553–563. doi: 10.1016/j.pharmthera.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Langston W, Chidlow JH, Jr, Booth BA, Barlow SC, Lefer DJ, Patel RP, Kevil CG. Regulation of endothelial glutathione by ICAM-1 governs VEGF-A-mediated eNOS activity and angiogenesis. Free Radic Biol Med. 2007;42:720–729. doi: 10.1016/j.freeradbiomed.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.