Abstract
Alzheimer's disease (AD) is associated with progressive memory decline. Hippocampal place cells are a well understood candidate for the neural basis of one type of memory in rodents; these cells identify the animal's location in an environment and are crucial for spatial memory and navigation. We have recorded place cells in the Tg2576 mouse model of AD, and we report that aged (16 mo) but not young (3 mo) transgenic mice show degraded neuronal representations of the environment. The level of place cell degradation correlates with the animals' (poorer) spatial memory as tested in a forced-choice spatial alternation T-maze task and with hippocampal, but not neocortical, amyloid plaque burden. Place cell recording provides a sensitive assay for measuring the amount and rate of functional deterioration in animal models of dementia as well as providing a quantifiable physiological indication of the beneficial effects of potential therapies.
Keywords: Alzheimer's disease, hippocampus, spatial memory
It is now widely accepted that β-amyloid (Aβ) plays a central role in the pathogenesis of Alzheimer's disease (AD), and part of this disorder may consist of an Aβ-induced synaptic failure (1). A transgenic mouse model of AD (Tg2576) expresses a human amyloid precursor protein (APP) variant linked to AD, leading to an age-dependent deposition of amyloid plaques (2). Previous work has shown a spatial memory deficit in these animals believed to reflect pathological processes taking place in the hippocampus (3, 4). The exact neuropathology responsible for the cognitive deficits in AD is uncertain. Pathological entities specifically associated with cognitive impairments include amyloid plaques (5, 6), total Aβ (7, 8), soluble Aβ (9), and Aβ oligomeric assemblies (10–12).
Here, we directly examine the processing of spatial information in AD by recording the activity of hippocampal pyramidal cells in the Tg2576 model of AD. The major correlate of hippocampal pyramidal cells is the animal's location in a known environment (13). Different cells represent different parts of the environment, and their ensemble firing pattern is believed to provide a cognitive map that supports recognition of different environments and enables the animal to successfully navigate in them. Damage to the hippocampus causes a severe deficit in spatial memory (14). Here, we show that aged (16 mo) but not young (3 mo) Tg2576 mice have degraded hippocampal place cell representations of the environment and that these degraded representations correlate with spatial memory deficits in a forced-choice spatial alternation T-maze task and with amyloid plaque burden in the hippocampus.
Results
Place Cell Firing Is Degraded in Aged Tg2576 Mice.
Place cells recorded in aged Tg2576 mice show deficits in representing locations in a familiar environment (Fig. 1b). The deficits in the aged transgenic place fields are reflected in both a reduced spatial information content (Fig. 1c) and an increased place field size (Fig. 1d), whereas mean firing rates do not differ among the groups (effect of age, F1,17 = 0.79, P = 0.38; effect of genotype, F1,17 = 0.29, P = 0.60; age × genotype, F1,17 = 0.28, P = 0.60). The decrease in accuracy of spatial representation was not uniform across the aged Tg2576 mice (Fig. 1b). Some animals showed severely degraded place fields (mouse 4), whereas the place fields of others appeared normal (mouse 1). On average, place cells in the aged transgenic mice have lower spatial information content (age × genotype, F1,17 = 17.29, P = 0.001) and are larger (F1,17 = 15.05, P = 0.001) than in aged controls and young animals. A test of simple effects confirms that place cell quality does not differ across genotypes in young animals (spatial information: F1,17 = 0.46, P = 0.50; place field size: F1,17 = 0.33, P = 0.57) but does significantly differ in aged animals (spatial information: F1,17 = 8.46, P = 0.01; place field size: F1,17 = 12.22, P = 0.003).
Fig. 1.
Degraded place fields in aged Tg2576 mice. (a) Four representative place cells recorded from one young control mouse (Left) and one young Tg2576 mouse (Right). (b) Four representative place cells recorded from one aged control mouse (Left) and four different aged Tg2576 mice (Right four columns). Mouse 1 place cells show the highest mean spatial information across aged Tg2576 mice, mouse 4 the lowest, and mice 2 and 3 reflect average values. (c) Place cells in the aged transgenic mice have lower spatial information content than aged controls and young animals (effect of age, F1,17 = 17.29, P = 0.001; age × genotype, F1,17 = 4.89, P = 0.041). (d) Place fields are markedly larger in the aged transgenic animals (effect of age, F1,17 = 15.06, P = 0.001; age × genotype, F1,17 = 6.10, P = 0.024). Numbers to the upper left of rate maps in a and b indicate peak firing rates.
Spatial Memory Deficits Are Correlated with Disruption of Place Cell Firing.
To study the relationship between spatial representations and spatial performance in the Tg2576 mice, we trained them on a forced-choice T-maze alternation task. This task simultaneously tests both short- and long-term spatial memory: the quality of the animal's long-term representation of the environment and its short-term ability to remember the previous goal location during the period between the first and second runs of a trial. Each trial consists of two runs: on the first (sample) run, the animal is forced to go into one arm of the T, where it receives a reward; on the second (choice) run, both arms are available, and the animal must choose the previously nonvisited arm to receive a reward. Previous work has shown that these animals are deficient at this task (3, 4). Animals were given six trials per day for 12 d with a minimal delay of 15 s between the sample and choice runs.
All animals, with the exception of the aged transgenic group, steadily improved over the course of training (Fig. 2a). When a criterion of 80% correct or better on three consecutive training days was used, only 39% of the aged transgenic animals learned the task, as opposed to >69% of the other three groups (Fig. 2b). Post hoc analysis of performance on the final block showed that the aged transgenic group performed significantly worse than all of the other three groups (all Bonferroni-corrected P values ≤0.006), whereas none of these groups significantly differed from each other. Next, we increased the delay between the sample and choice runs to 2 and 6 min, in addition to the 15-s testing interval. All animals showed a reduction in performance as a function of the testing delay, and all groups were influenced to a similar degree by the introduction of delays (Fig. 2c). The aged transgenic mice performed at significantly lower levels than any other group (genotype × age, F1,38 = 5.08, P = 0.030), performing at chance at 2- and 6-min delays. Overall, these results are consistent with earlier studies on the same mouse model and confirm an age-dependent impairment of Tg2576 mice in spatial memory (3, 4).
Fig. 2.
Performance in the T-maze alternation task. (a) Acquisition of the T-maze alternation task (15-s-delay version) is selectively impaired in aged transgenic animals (training blocks × age × genotype: F3,174 = 3.23, P = 0.024). Each block consists of three training days (six trials per day). (b) A smaller percentage of aged transgenic mice reached the criterion of >80% correct over three consecutive days than the other three groups (χ2 (3) = 10.67, P = 0.014). (c) Overall performance on the T-maze task decreases with increasing delay (effect of delay, F2,76 = 9.70, P = 0.003). Aged mice perform significantly worse than young mice upon introduction of delays, but there is no additional deficit in the aged transgenic group (delay × genotype × age: F2,76 = 0.08, P = 0.782). Error bars show SEMs.
Performance on discrete trial spatial alternation depends highly on the hippocampus (15, 16): Does it correspond to the amount of spatial information carried by the place cell population? The variability of both place cell firing quality (Fig. 1b) and behavioral performance (Fig. 2) across the aged transgenic population allows us to answer this question. Using place field size and spatial information content as indices of the quality of place cell signaling in the aged (transgenic and control) animals, we found a good correlation with behavior averaged across all delays (Fig. 3). This pattern of correlations does not change if the performance of three aged transgenic animals performing at <50% correct is set to 50% (place field size: r = 0.76, P = 0.001; spatial information content: r = 0.76, P = 0.002).
Fig. 3.
Correlations between T-maze performance and place cell quality in aged mice. (a) Mean spatial information content is positively correlated with alternation rate in aged transgenic animals (red squares) and aged control animals (blue diamonds; r = 0.83, P < 0.001). (b) Mean place field size is inversely correlated with alternation rate (r = 0.83, P < 0.001). Alternation rates are means of three delays tested (15 s, 2 min, and 6 min).
Behavioral and Physiological Deficits Are Correlated with Amyloid Levels in Aged Tg2576 Mice.
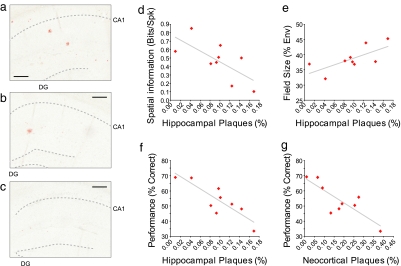
What are the molecular correlates of these physiological and behavioral deficits? To identify which amyloid species might be responsible for the behavioral and physiological alterations observed in the Tg2576 mice, we stained for dense core amyloid plaques in the hippocampus and overlying neocortex using Congo red, and we assessed the concentration of soluble and insoluble amyloid in the brain using a standard sandwich ELISA. There was a good correlation between congophilic (compact) amyloid plaque burden in the hippocampus (Fig. 4 a–c) and the spatial information content of hippocampal place cells (Fig. 4d) and their place field size (Fig. 4e). The plaque burden in the overlying neocortex did not correlate with hippocampal spatial representation quality (spatial information content, r = −0.61, P = 0.084; place field size, r = 0.62, P = 0.076), nor did the amount of soluble and total amyloid in the entire brain (Table 1). In addition, there was a strong correlation between each animal's overall performance on the spatial memory task and the burden of plaques in the hippocampus (Fig. 4f) and in the neocortex (Fig. 4g). Behavioral performance also correlated well with both soluble and total levels of Aβ42 (Table 1).
Fig. 4.
Percentage of hippocampal area occupied by amyloid plaques inversely correlates with place cell quality and behavioral performance. (a–c) Photomicrographs of 7-μm coronal sections through the dorsal hippocampus of three aged Tg2576 mice showing highest (a), mean (b), and lowest (c) hippocampal levels of congophilic plaque deposition. (d) Place cell spatial information is negatively correlated with the percentage area of the hippocampus covered by congophilic plaques of aged Tg2576 mice (r = −0.70, P = 0.036). (e) Place field size is positively correlated with hippocampal amyloid plaque deposition (r = 0.72, P = 0.029). (f and g) T-maze alternation rate is negatively correlated with the percentage area covered by congophilic plaques of the hippocampus (f) (r = −0.86, P = 0.003) and the overlying neocortex (g) (r = −0.83, P = 0.006). (Scale bars in a–c: 100 μm.)
Table 1.
Correlations of brain Aβ load with place cells' properties and behavior
| Spatial variables | Soluble Aβ1-40 | Soluble Aβ1-42 | Total Aβ1-40 | Total Aβ1-42 |
|---|---|---|---|---|
| Spatial information | r = −0.47, P = 0.20 | r = −0.37, P = 0.33 | r = −0.43, P = 0.25 | r = −0.42, P = 0.26 |
| Field size | r = 0.48, P = 0.19 | r = 0.39, P = 0.30 | r = 0.40, P = 0.28 | r = 0.38, P = 0.31 |
| Correct, % | r = −0.68, P = 0.04* | r = −0.70, P = 0.03* | r = −0.36, P = 0.34 | r = −0.70, P = 0.04* |
*, P value significant at the 0.05 level.
Discussion
The ability of hippocampal place cells to code for locations in an environment is impaired in an age-dependent manner in the Tg2576 animals. Place cells in older transgenic animals show lower spatial information content and increased place field size. The demonstration of a deficit at the level of place cell representations complements previous findings of a deficit in hippocampal LTP in these animals (3). At the behavioral level, aged transgenic animals were impaired in the acquisition of the T-maze spatial memory task. Aged Tg2576 animals took longer to reach criterion, and their performance at criterion was inferior to both young transgenic animals and older control animals. Although their poor performance even at the shortest delay prevented an adequate assessment of the effects of retention interval, previous work has shown no differential effect of delay in comparison with controls (4). This suggests that their deficit is primarily in the ability of the hippocampus to generate an adequate representation of the environment within which the task is set (4). This conclusion is strengthened by the strong correlation between the quality of place cell firing and performance levels in the T-maze alternation task. Previous work has shown that place field location can predict choice behavior, demonstrating that place cell activity is functionally related to spatial behavior (17, 18). In our study, place field degradation was accompanied by impaired spatial choice behavior, further supporting the view that place cells are part of a navigational system.
Our study also addresses the issue of which pathological changes underlie the behavioral and neurophysiological impairments in these animals. Both the quality of place cell firing and performance on the T-maze task were strongly correlated with the levels of congophilic plaque burden. In particular, although behavioral impairments correlated equally well with cortical and hippocampal plaque levels, place cells' firing quality correlated only with hippocampal pathology. There was also a weaker but near-significant trend toward correlation between place cells' quality and neocortical plaques. This trend could be interpreted to mean that the deficits in place cell physiology are mediated both by hippocampal and neocortical pathology, or it could simply reflect the fact that the area covered by plaques in these two brain structures is highly correlated. The fact that the strength of correlation between place cell physiology and hippocampal plaque burden is stronger supports the latter interpretation.
The amount of soluble and total amyloid did not correlate with place cell quality, probably because the assays were conducted on whole-brain tissue (not just hippocampal tissue). Total and soluble amyloid concentration scores showed, on the other hand, a robust correlation with behavioral performance (Table 1). These latter results, combined with the observation that behavioral performance correlated significantly with both the hippocampal and neocortical plaque burden, imply that both the hippocampus and the overlying neocortex are engaged by the T-maze alternation task in this aged population of Tg2576 mice.
Although two previous studies on AD animal models found correlations between amyloid plaque burden and cognitive decline (5, 6), most studies have failed to detect such a correlation (19–21). This is frequently interpreted as evidence that amyloid plaques represent end-stage remains or byproducts of the pathological processes in AD. Our study suggests that compact amyloid plaques are responsible for the physiological alterations we observed at the place cell level and that these, in turn, result in the spatial cognitive deficits observed in the Tg2576 mice. Accumulating evidence points to the in vivo toxicity of compact amyloid plaques. Dong et al. (22) observed a selective loss of dendritic synapses occurring in the proximity of amyloid plaques in the entorhinal cortex and the outer molecular layer of the dentate gyrus of aged Tg2576 mice (22). Indeed, amyloid plaques are selectively associated with dystrophic neurites in these mice (23), and such deposits have been associated with local structural disruption of synapses and breakage of neuronal branches (24). Another possibility is that plaque toxicity is mediated by elevated levels of soluble Aβ species in the vicinity of plaques. For instance, there is considerable evidence that soluble Aβ oligomers inhibit the maintenance of hippocampal LTP (10). Our results, however, cannot rule out the possibility that place cell degradation and behavioral deficits in the Tg2576 mice are caused by elevated soluble Aβ and are not directly related to plaque deposition or that plaques represent a surrogate marker for small assemblies of Aβ (25). The hypothesis that soluble Aβ species are contributing to the behavioral impairments observed in the Tg2576 mice is supported by the observation that soluble levels of Aβ42 (as well as total Aβ42) were correlated with aged Tg2576 animals' performance on the T-maze task.
A deficit in episodic memory is one of the hallmarks of human AD. Cognitive map theory (15) provides a basis for relating human episodic memory to spatial memory. The study of spatial working memory and place cells in rodent models of AD may provide a powerful tool for identifying the underlying pathology in AD and the respective contributions of hippocampal and neocortical pathology to the overall cognitive memory dysfunction. We are optimistic that, in combination, these paradigms may also provide a powerful testing platform for therapeutic interventions.
Methods
Animals.
Male Tg2576 mice overexpressing human APP harboring the Swedish double mutation (K670N, M671L) (2), in a hybrid strain of C57BL/6J with SJL (for details on breeding procedures, see ref. 3) were obtained from Merck Sharp & Dohme. For all experiments, transgenic animals were compared with littermate controls to ensure that age and background strain were comparable. Subjects were maintained on a 12-h light/dark schedule (lights off at 1500 hours) and were maintained at 90% of free feeding weight. All experiments were carried out blind to genotype and in accordance with the U.K. Animals (Scientific Procedures) Act 1986.
Behavioral Testing.
A total of 62 animals were trained on the T-maze forced-alternation task. Animals were assigned to four groups on the basis of age and genotype: young littermate controls (n = 15; mean age, 2.5 mo; range, 1.9–3.2 mo), young transgenic (n = 16; mean age, 2.7 mo; range, 1.9–3.2 mo), aged littermate controls (n = 13; mean age, 15.4 mo; range, 13.4–16.4 mo), and aged transgenic (n = 18; mean age, 15.4 mo; range, 13.5–16.8 mo).
Behavioral Apparatus.
The T-maze was constructed of three arms, each of which was 7 cm wide. The start arm was 45 cm long, and the goal arms were each 35 cm long. A square start box (side 30 × 30 cm) was positioned next to the start arm and separated from it by a vertically sliding door. The T-maze was elevated 30 cm from the floor. The T-maze was placed in the center of a black-curtained, circular testing arena 1.7 m in diameter. Four external cue cards differing in brightness, shape, and size suspended inside the curtains provided directional constancy. Mice ran for a condensed milk reward.
T-Maze Alternation Acquisition.
All mice received 5 d of habituation to the apparatus and the reward (5 min of exposure to the maze each day, with reward placed at both ends of the T-maze). Mice were then trained for 12 d, six trials per day. On the first (sample) run of each trial, both goal arms were baited, but the mouse was forced to choose one of the goal arms (the other being blocked by a removable door). After entering the preselected goal arm, the mouse was allowed 20 s to consume the reward and then was placed back in the start box. On the second (choice) run, which during the acquisition phase of training followed 15 s after the sample run, both goal arms were open, and the mouse was rewarded for choosing the previously unvisited arm. The location of the sample arm (left or right) was varied pseudorandomly across trials so that mice received equal numbers of left and right presentations, but no more than two consecutive trials had the same sample location. The mice were run in squads of six to eight (including both transgenic and littermate control animals) to minimize variation in intertrial interval. Intertrial interval was ≈12 min for all animals throughout the 12 d of training.
T-Maze Alternation Delay Probes.
A subset (n = 42) of mice (n = 9 young control, n = 6 young transgenic, n = 13 aged control, n = 14 aged transgenic) were run on a modified T-maze alternation protocol, which included: (i) the introduction of a presample phase (lasting 6 min and preceding the sample phase of each trial), during which place cell recording took place in those animals that had been implanted with microelectrodes and (ii) the introduction of delays between the sample and choice runs of the task. Each mouse was trained for a total of five consecutive days. At the beginning of each day, the mouse received four 15-s-delay trials, followed by six trials of increased delay. During training days 1 and 2, the delay between the sample and choice runs was 2 min, whereas for days 3, 4, and 5, the delay was 6 min.
In Vivo Electrophysiology.
A subset of animals trained in the T-maze alternation task underwent implantation of microelectrodes for chronic hippocampal recording (young littermate controls, n = 4; young transgenic mice, n = 3; aged littermate controls, n = 5; aged transgenic mice, n = 9).
Surgery and Electrodes.
Under deep isofluorane anesthesia, mice were chronically implanted with microdrives loaded with four tetrodes. Tetrodes were constructed from HM-L-coated 90% platinum/10% iridium 17-μm diameter wire (California Fine Wire). Details of the surgical procedure were similar to those in Cacucci et al. (26). Mice were allowed a 1-wk postoperative recovery, after which microelectrodes were advanced ventrally by 30 μm/day. When hippocampal pyramidal cells were found, recording sessions began. After completion of the experiments, the mice were killed with an overdose of sodium pentobarbital (50 mg of Lethobarb; Merial Animal Health U.K.). The hemibrain in which the electrodes were implanted was immersion fixed in 4% paraformaldehyde with 0.1 M PBS (pH 7.6) and embedded in paraffin. The hemibrain was then sliced coronally, and 7-μm-thick sections were obtained and stained with cresyl violet for electrode localization.
Place Cell Recording.
Place cell recording took place during a maximum of eight recording days for each mouse. Place cells were recorded during the presample phase of each testing trial (see behavioral procedures for details of behavioral protocol) while animals foraged for chocolate flakes in the start box. Each recording trial lasted 6 min.
Quantitative Analysis of Place Fields.
Isolation of single units from multiunit data was performed manually, blind to genotype, on the basis of peak-to-trough amplitude, by using custom software (TINT; Neil Burgess, University College London). Cells were included in the analysis if they had: (i) spike width >300 μs and (ii) peak firing rate >1 Hz. Spike width was measured as the time interval between the peak and trough of the 1-ms-long waveform. Spike amplitude was measured as the voltage difference between the peak and trough of the waveform. Position data were sorted into 1.5 × 1.5-cm bins. For a given cell, firing rates in each bin were calculated by dividing the total number of action potentials during occupancy of the bin by the total duration of occupancy. The firing rate in each bin was smoothed by using a boxcar average over the surrounding 5 × 5 bins. The five colors of firing-rate maps were autoscaled to represent 20% of the peak rate—red (top 20%) to dark blue (bottom 20%). Unvisited bins are shown in white. Place field size represents the percentage of sampled environment in which the cell's firing rate was greater than the overall mean firing rate during that trial. Spatial information is a measure of the extent to which the cell's firing can be used to predict the position of the animal (27).
Measuring Amyloid Plaque Deposition.
Hemibrains where electrodes had been implanted were fixed in 4% paraformaldehyde with 0.1 M PBS (pH 7.6) and embedded in paraffin. Coronal brain sections of 7 μm thickness were cut on a microtome and mounted on gelatin-coated slides. Plaques were identified with Congo red staining. For each hemibrain, amyloid plaque quantification was assessed by using a total of 10 coronal sections through the hippocampus (sections were spaced 280 μm apart). Brain sections were routinely deparaffinized, incubated in alkaline sodium chloride solution (2.5 mM NaOH in 80% ethanol) for 20 min and then stained with alkaline Congo red solution (0.2% in 80% ethanol saturated with sodium chloride; Sigma), washed three times in absolute ethanol, and coverslipped with DPX mounting medium. Congo red-positive Aβ plaques were visualized with a Leica DMLB microscope (with a total ×100 magnification factor), and images were captured by using a DC300F Leica camera. Quantitative image analysis was performed by using Image J as the image-analysis system. The software uses red, green, and blue levels to segment objects in the image field. Segmentation thresholds were established by using a series of standard slides that have extremes of intensity and remained constant throughout the analysis. Hippocampal and cortical measurements were performed by manually circumscribing the areas of interest. Hippocampal measurements refer to an area encompassing all hippocampal fields (CA1, CA3, and dentate gyrus), whereas cortical measurements refer to the neocortical areas overlying the hippocampus.
Aβ Concentration Assessment.
Hemibrains contralateral to electrode implantation were immediately frozen on dry ice and stored at −80°C until use.
Soluble Aβ Concentration.
The frozen hemibrains were homogenized in 10 volumes (wt/vol) of 0.2% diethanolamine containing 50 mM NaCl (pH 10), and protease inhibitors (Complete; Roche Diagnostics) and then centrifuged at 355,000 × g at 4°C for 30 min (Optima MAX ultracentrifuge; Beckman Coulter). The resulting supernatant was retained as the soluble fraction and neutralized by the addition of 10% 0.5 M Tris·HCl (pH 6.8). Samples were frozen at −80°C awaiting analysis by immunoassay.
Total Aβ Concentration.
The protocol used for extraction of total amyloid from the brain sections is based on the GnHCl extraction method described previously (based on ref. 28). The hemibrains were homogenized in 10 volumes of 5 M GnHCl, 50 mM Hepes (pH 7.3), 5 mM EDTA plus 1× EDTA-free protease inhibitor mixture (Complete). After mixing at room temperature for 3 h, the homogenate was diluted 10-fold into ice-cold 25 mM Hepes (pH 7.3), 1 mM EDTA, 0.1% BSA plus 1× protease inhibitor mixture, and centrifuged at 16,000 × g for 20 min at 4°C. Aliquots of supernatant were stored at −80°C to prevent degradation.
Immunoassay Analysis.
The biotinylated antibody 4G8 was used in combination with the monoclonal antibodies G2-10 or G2-11 for detection of Aβ40 or Aβ42, respectively. These species reflect subpopulations of peptides with heterogeneous N termini encompassing at least the 4G8 epitope at residues 17–24. Analysis of the samples was performed by using the SECTOR Imager 6000 (Meso Scale Discovery), as described previously (29).
Acknowledgments.
We thank David Becker for assistance with microscopy, Thomas Rosahl (MSD, Harlow, U.K.) for the provision of mice, Mark Jay for running the Aβ concentration assays, Gabriel Landini for Image J segmentation algorithms, and Neil Burgess, Colin Lever, and Alastair McClelland for useful discussions. This research has been supported by the Biotechnology and Biological Sciences Research Council, Merck Sharp & Dohme, and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
References
- 1.Selkoe D. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao K, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 3.Chapman PF, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P, Hale G, Good M. Intramaze and extramaze cue processing in adult APPSWE Tg2576 transgenic mice. Behav Neurosci. 2004;118:1184–1195. doi: 10.1037/0735-7044.118.6.1184. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, et al. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MN, et al. Correlation between cognitive deficits and Aβ deposits in transgenic APP+PS1 mice. Neurobiol Aging. 2001;22:377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 7.Naslund J, et al. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. J Am Med Assoc. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- 8.Kotilinek LA, et al. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean CA, et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DM, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 11.Lesne S, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 12.Malm T, et al. β-Amyloid infusion results in delayed and age-dependent learning deficits without role of inflammation or β-amyloid deposits. Proc Natl Acad Sci USA. 2006;103:8852–8857. doi: 10.1073/pnas.0602896103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 14.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 15.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford Univ Press; 1978. [Google Scholar]
- 16.Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 17.O'Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- 18.Lenck-Santini PP, Save E, Poucet B. Evidence for a relationship between place-cell spatial firing and spatial memory performance. Hippocampus. 2001;11:377–390. doi: 10.1002/hipo.1052. [DOI] [PubMed] [Google Scholar]
- 19.Terry RD, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 20.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E, Bohl J, Bratzke H. Evolution of Alzheimer's disease related cortical lesions. J Neural Transm Suppl. 1998;54:97–106. doi: 10.1007/978-3-7091-7508-8_9. [DOI] [PubMed] [Google Scholar]
- 22.Dong H, Martin MV, Chambers S, Csernansky JG. Spatial relationship between synapse loss and β-amyloid deposition in Tg2576 mice. J Comp Neurol. 2007;500:311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noda-Saita K, et al. Exclusive association and simultaneous appearance of congophilic plaques and AT8-positive dystrophic neurites in Tg2576 mice suggest a mechanism of senile plaque formation and progression of neuritic dystrophy in Alzheimer's disease. Acta Neuropathol (Berlin) 2004;108:435–442. doi: 10.1007/s00401-004-0907-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 25.Westerman MA, et al. The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cacucci F, Wills TJ, Lever C, Giese K, O'Keefe J. Experience-dependent increase in CA1 place cell spatial information, but not spatial reproducibility, is dependent on the autophosphorylation of αCaMKII. J Neurosci. 2007;27:7854–7859. doi: 10.1523/JNEUROSCI.1704-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. Adv Neural Inf Process Syst. 1993;5:1030–1037. [Google Scholar]
- 28.Johnson-Wood K, et al. Amyloid precursor protein processing and A β42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Best JD, et al. Quantitative measurement of changes in amyloid-β(40) in the rat brain and cerebrospinal fluid following treatment with the γ-secretase inhibitor LY-411575 [N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-ox o-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-l-alaninamide] J Pharmacol Exp Ther. 2005;313:902–908. doi: 10.1124/jpet.104.081174. [DOI] [PubMed] [Google Scholar]