Abstract
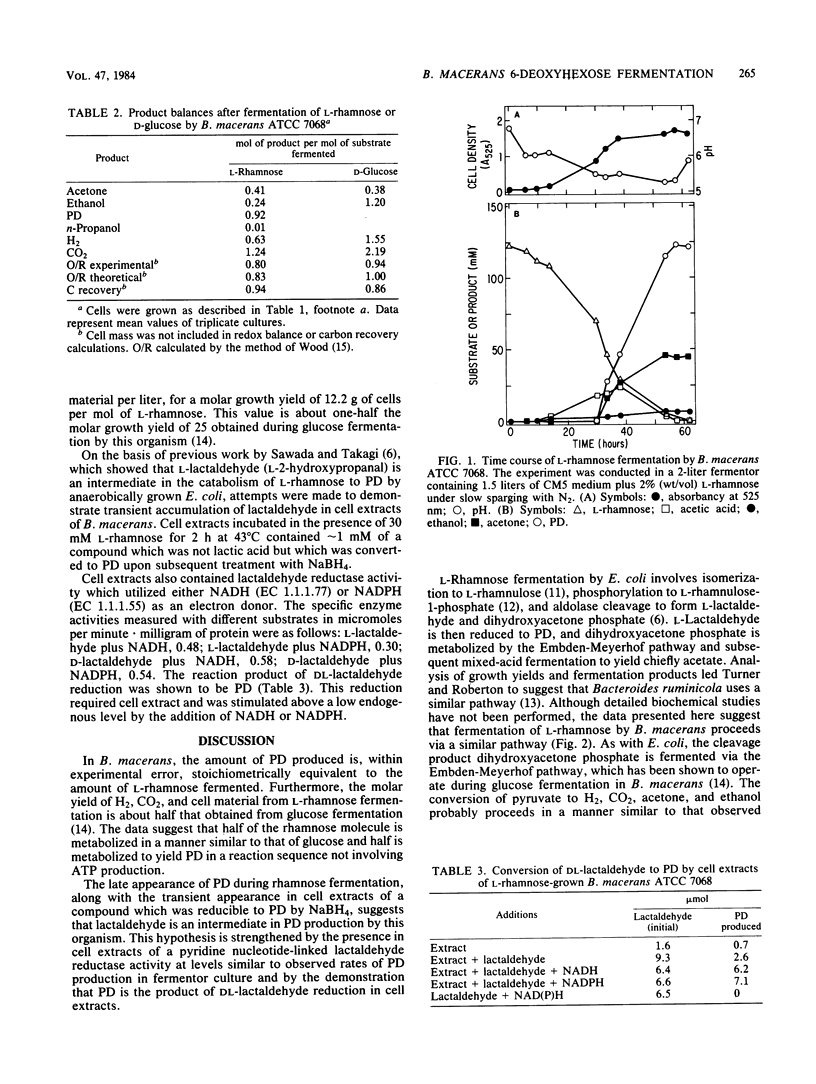
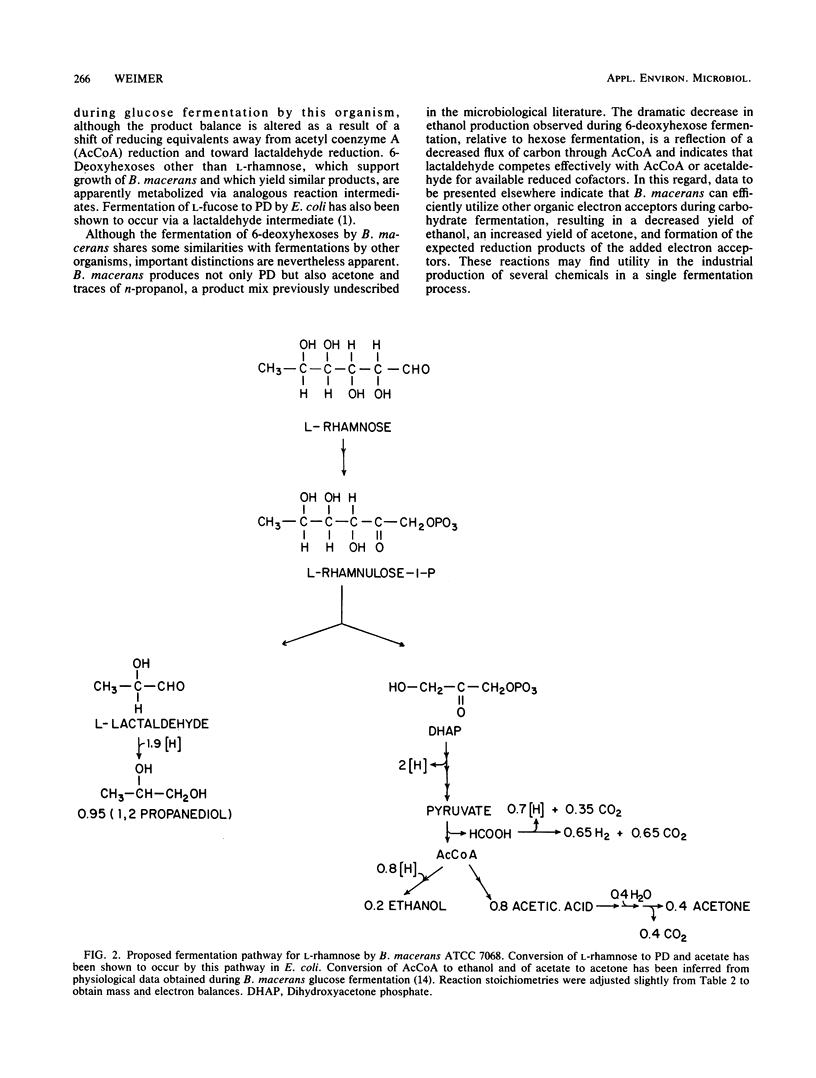
Under anaerobic conditions Bacillus macerans ATCC 7068 fermented 6-deoxyhexoses (l-rhamnose, l-fucose, and d-fucose) to a mixture of 1,2-propanediol (PD), acetone, H2, CO2, and ethanol. The final PD concentration was proportional to the amount of l-rhamnose fermented (∼0.9 mol of PD per mol of rhamnose). PD was not produced from hexoses (e.g., d-glucose or l-mannose), despite active fermentation of these substrates. Relative to the fermentation of d-glucose, the fermentation of l-rhamnose was accompanied by a twofold reduction in yield of H2, CO2, and cell mass. Exposure of cell extracts to l-rhamnose resulted in the transient appearance of an aldehyde intermediate. Cell extracts contained a pyridine nucleotide-linked lactaldehyde reductase activity which converted synthetic d- or l-lactaldehyde to PD. The data suggest an Embden-Meyerhof pathway for 6-deoxyhexose catabolism, with the formation of lactaldehyde by a conventional aldolase cleavage reaction and subsequent reduction to PD.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cocks G. T., Aguilar T., Lin E. C. Evolution of L-1, 2-propanediol catabolism in Escherichia coli by recruitment of enzymes for L-fucose and L-lactate metabolism. J Bacteriol. 1974 Apr;118(1):83–88. doi: 10.1128/jb.118.1.83-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUFF E., RUDNEY H. The enzymatic oxidation of 1,2-propanediol phosphate to acetol phosphate. J Biol Chem. 1959 May;234(5):1060–1064. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWADA H., TAKAGI Y. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. 3. L-RHAMULOSE-PHOSPHATE ALDOLASE. Biochim Biophys Acta. 1964 Oct 23;92:26–32. doi: 10.1016/0926-6569(64)90265-2. [DOI] [PubMed] [Google Scholar]
- TAKAGI Y., SAWADA H. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. I. L-RHAMNOSE ISOMERASE. Biochim Biophys Acta. 1964 Oct 23;92:10–17. doi: 10.1016/0926-6569(64)90263-9. [DOI] [PubMed] [Google Scholar]
- TAKAGI Y., SAWADA H. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. II. L-RHAMNULOSE KINASE. Biochim Biophys Acta. 1964 Oct 23;92:18–25. doi: 10.1016/0926-6569(64)90264-0. [DOI] [PubMed] [Google Scholar]
- Turner K. W., Roberton A. M. Xylose, arabinose, and rhamnose fermentation by Bacteroides ruminicola. Appl Environ Microbiol. 1979 Jul;38(1):7–12. doi: 10.1128/aem.38.1.7-12.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



