Abstract
Recent proposals have conceptualized piriform cortex as an association cortex, capable of integrating incoming olfactory information with descending input from higher order associative regions such as orbitofrontal cortex (OFC). If true, encoding in piriform cortex should reflect associative features prominent in these areas during associative learning involving olfactory cues. To test this hypothesis, we recorded from neurons in OFC and anatomically related parts of the anterior piriform cortex (APC) in rats, learning and reversing novel odor discriminations. Findings in OFC were similar to what we have reported previously, with nearly all the cue-selective neurons exhibiting substantial plasticity during learning and reversal. Also, many of the cue-selective neurons were originally responsive in anticipation of the outcomes early in learning, thereby providing a single-unit representation of the cue-outcome associations. Some of these features were also evident in firing activity in APC, including some plasticity across learning and reversal. However, APC neurons failed to reverse cue selectivity when the associated outcome was changed, and the cue-selective population did not include neurons that were active prior to outcome delivery. Thus, although representations in APC are substantially more associative than expected in a purely sensory region, they do appear to be somewhat more constrained by the sensory features of the odor cues than representations in downstream areas of OFC.
Keywords: olfactory, orbitofrontal, piriform, rat, reversal
Introduction
Piriform cortex, the largest of the olfactory cortical areas, is part of a parallel, distributed, and bidirectional system involved in processing olfactory information (Haberly 2001). On one hand, piriform cortex, and particularly the anterior piriform cortex (APC), has strong reciprocal connections with olfactory bulb and has traditionally been thought of as primary olfactory cortex. In fact, it is the largest recipient of afferent fibers from the bulb. Afferents from both mitral and tufted cells send projections to neurons in APC and convey information regarding olfactory information in the animal's environment. Based on this anatomy, the APC may function in a manner analogous to that of other primary sensory areas that extract highly specific stimulus features.
On the other hand, piriform cortex also receives substantial descending input from downstream brain regions, including many neocortical and subcortical areas that are involved in processing multi-modal sensory input and in representing associative rather than sensory information. For example, APC receives input from the orbitofrontal cortex (OFC) (Johnson and others 2000). Damage to OFC impairs the acquisition and retention of odor discriminations (Eichenbaum and others 1980, 1983), and neurons in these areas in rats appear to encode the associative significance of cues, even odor cues, rather than their sensory features (Schoenbaum and Eichenbaum 1995; Schoenbaum and others 1998, 1999). Similarly, studies in monkeys demonstrate the importance of associative significance to encoding of olfactory cues in OFC (Tremblay and Schultz 1999, 2000a, 2000b; Rolls 2000; Wallis and Miller 2003; Roesch and Olson 2004).
Based on these connections, it has been proposed that piriform cortex may function not as a primary sensory region but as an olfactory association cortex, integrating incoming olfactory information with descending input from higher order associative regions such as OFC. If this is true, one might expect encoding in piriform cortex to reflect associative features prominent in these afferent areas during associative learning involving olfactory cues. Single-unit studies provide some support for this hypothesis. For example, in rats trained to perform an 8-odor discrimination task, neuronal activity in APC and OFC is very similar, with firing in both areas reflecting both the identity and the valence of the odors (Schoenbaum and Eichenbaum 1995a, 1995b). However, these rats were heavily overtrained on the odor set used, and there was no attempt to manipulate the cue-outcome associations. Moreover, learning and reversal studies have since provided a much more detailed account of encoding in OFC and related limbic areas (Schoenbaum and others 1998, 1999; Setlow and others 2003). These reports have demonstrated that representations in these areas are uniformly associative. If piriform cortex is integrating this information with incoming olfactory input, one might expect the encoding in this area to represent some of these associative features. To test this hypothesis, we recorded from neurons in OFC and anatomically related parts of the APC in rats, learning and reversing novel 2-odor discrimination problems.
Materials and Methods
This research was conducted at the University of Maryland School of Medicine in accordance with university and the National Institutes of Health guidelines. Note that prior to the start of this experiment, these rats received saline injections, intraperitoneally, for 14 days, as part of another experiment that examined the effects of drug exposure on encoding in OFC. The OFC recordings reported here served as control data for that study.
Surgical Procedures
Eight adult male Long-Evans rats served as subjects (Charles River Laboratories, Wilmington, MA). Procedures for implanting electrodes were identical to those used previously (Schoenbaum and Eichenbaum 1995; Schoenbaum and others 1999, 2003). A drivable electrode bundle was chronically implanted in the left hemisphere at 3.0 mm anterior to bregma, 3.2 mm laterally, and 4.0 mm ventral to the surface of the brain. This electrode bundle was composed of 10 25-μm diameter FeNiCr wires (Stablohm 675, California Fine Wire, Grover Beach, CA) in a 27-gauge thin wall cannula (Small Parts, Miami Lakes, FL). Immediately prior to implantation, these wires were freshly cut with surgical scissors to extend ∼1 mm beyond the cannula and electroplated with platinum (H2PtCl6, Aldrich, Milwaukee, WI) to an impedance of ∼300 kOhms. During recording, the electrode bundle was advanced in 40-μm increments to acquire activity from new neurons for the following day. In a given session, neural activity was acquired from neurons in either OFC or APC.
Histology
Following testing, rats were given an overdose of pentobarbital and prepared for perfusion. Immediately prior to perfusion, the final electrode position was marked by passage of a 15-μA current through each microwire for ∼10 s to create a small iron deposit. The rats were then perfused intracardially with 0.9% saline followed by 4% formaldehyde followed by 100 mL of 3% potassium ferrocyanide in perfusate to visualize the iron deposit. Brains were removed from the skulls and stored in a 30% sucrose/4% formaldehyde/3% potassium ferrocyanide solution for several days until sectioning. The brains were sectioned on a freezing microtome and coronal sections (40 μm) collected through APC. Sections were mounted on glass slides, stained with thionin, and coverslipped with Permount. Lesion and electrode placements were verified under a light microscope and drawn onto plates adapted from the atlases of Paxinos and Watson (1997) and Swanson (1992).
Behavioral Methods
The apparatus and methods are described in more detail elsewhere (Schoenbaum 2001). Briefly, odor discrimination training was conducted in aluminum chambers ∼18″ on each side with sloping walls narrowing to an area of 12″ × 12″ at the bottom. An odor port and fluid well were located on a panel, which was located in the right wall of each chamber below 2 panel lights. Discrimination problems were composed of odor compounds obtained from International Flavors and Fragrances (New York, NY; e.g., isopropyl hydratropic ald para, verbena oliffac, petinerol, ald C-8 orange fraction florex, cedryl acet trubek, vanoris, geranyl formate, auralva, camekol DH, dimeth phen eth carb acetate, hexenol B gamma extra, celeriax, cyclemone A, and phenoxanol; contact G.S. for full list). Discrimination problems were constructed from dissimilar odors, according to a subjective classification scheme (fruity, spicy, herbal, etc) developed by Staubli and others (1987), and the odor discrimination sequence was arranged such that similar compounds were counterbalanced by valence and did not repeat across days. Task control and data acquisition were implemented by a custom program written in C++ and running in DOS on a Pentium II PC. During training, rats were maintained on water restriction. After each session, the rats were given ad lib access to water for 10-30 min depending on the fluid intake of each rat during the session.
The events in a trial are depicted in Figure 1. Trials were signaled by illumination of the panel lights inside the box. When these lights were on, nose poke into the odor port resulted in delivery of the preselected odor cue to a small hemicylinder located behind this opening. The rat terminated odor sampling by leaving the odor port and then had 3 s to make a go response at the fluid well located below the port. If a response was made after sampling a positive odor, then a 0.05-ml bolus of an appetitive 10% sucrose solution was delivered to the well after a variable delay (500-1500 ms). If the same response was made after sampling a negative odor, then a 0.05-ml bolus of an aversive 0.02-M quinine solution was delivered after a similar delay. If the rat did not respond within 3 s, the trial was counted as a no-go. A behavioral criterion was defined as 18 correct responses in a moving block of 20 trials.
Figure 1.
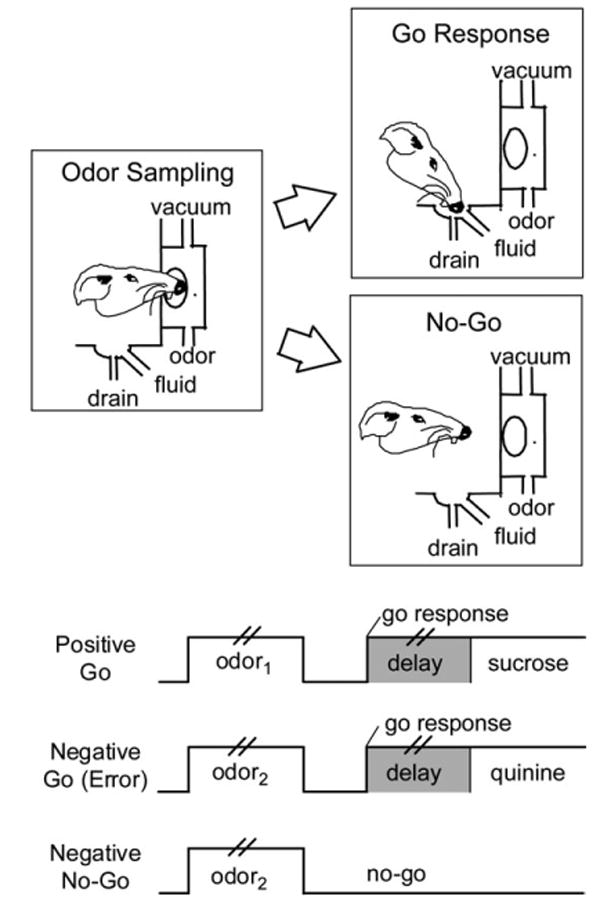
Schematic illustrating behaviors in the task. Pairs of vertical lines during odor presentation and the delay between a go response and fluid delivery denote the variable duration of these events; odor sampling typically lasted 250-750 ms, and the delay was programmed to vary from 500 to 1500 ms.
The rats received training on several problems prior to surgery, and then neural data were collected as the rats acquired novel discriminations in sessions after surgery. In these sessions, the rats were trained until they met the behavioral criterion (∼50 trials on average) and for an additional 60-100 trials after this criterion was achieved. In some sessions, the discrimination problem was also reversed, and neural data were obtained as the rats acquired the reversal problem.
Data Acquisition and Analysis
Experimental recording sessions after surgery were conducted in 2 aluminum chambers identical in all respects to the set of chambers used for training prior to surgery. These chambers included a behavioral computer running the same program used in presurgical training; this computer was interfaced with the recording systems to signal events. For each recording session, the rat was placed in the training chamber, and the electrode wires were screened for neural activity while the rat explored the open chamber. If no activity was detected, the rat was removed, and the electrode assembly was advanced 40 or 80 μm. Otherwise, active wires were selected for recording, and a training session was begun.
Neural activity was recorded using 2 identical Plexon Multichannel Acquisition Processor systems (Dallas, TX). Signals from the wires were amplified 20× by an operational amplifier headstage (Plexon Inc, HST/8o50-G20-GR), located on the electrode array. Immediately outside the recording chamber, the signals were passed through a differential preamplifier (Plexon Inc, PBX2/16sp-r-G50/16fp-G50). From there, the single-unit signals were amplified 50×, filtered at 150-9000 Hz, and then sent to the Multichannel Acquisition Processor box, where they were further filtered at 250-8000 Hz, digitized at 40 kHz, and amplified at 1-32×. Waveforms (>2.5:1 signal-to-noise) were extracted from active channels and recorded to disk by an associated workstation along with time stamps from the behavior computer indicating when significant events occurred (odor onset, responding, fluid delivery, etc).
Units were sorted off-line using software from Plexon Inc. For this analysis, files were first imported into Offline Sorter where waveforms on each channel were sorted using a template matching algorithm. These waveforms were compared with notes regarding the waveforms made during the session, and the interspike interval histograms were inspected to ensure that spike events were separated by >1 ms. Typically, 1-3 waveforms could be isolated on an active channel.
Sorted files were then processed in Neuroexplorer to extract these unit time stamps and relevant event markers. These data were subsequently analyzed using statistical routines in Matlab (Natick, MA) to examine activity during odor sampling (from 50 ms after odor onset to 50 ms after odor offset), during the variable delay after a response at the fluid well (from 50 ms before the response until fluid delivery), and after fluid delivery (first 500 ms). Firing activity (spikes/second) in each time window was compared on positive and negative trials during pre- and postcriterion trial blocks using analysis of variance (P < 0.05), and neurons with a significant difference in activity were categorized as “selective” in that time window and phase.
A Pearson chi-square test (P < 0.05) was used to compare the proportions of neurons with different firing properties and to ask whether particular firing patterns (e.g., neurons that fired before sucrose delivery that became selective for the positive odor after learning) were observed at a greater frequency than expected by chance in the population of neurons. For these comparisons, chance was calculated based on the probabilities of neurons in the population exhibiting each type of response. This expected occurrence was compared with the actual proportion observed in our experimental groups.
Results
Neural activity was recorded from drivable arrays of microelectrodes. By moving the electrodes in 40- to 80-μm increments overnight, we were able to acquire neural activity from a new location in OFC or APC each day. Neural activity was recorded as rats learned and reversed a series of 2-odor go, no-go discrimination problems. In each problem, one “positive” odor signaled the availability of an appetitive sucrose solution, and the other “negative” odor signaled the availability of an aversive quinine solution. When presented with a novel odor pair, the rats initially responded at the fluid well on every trial but subsequently learned to respond only after sampling the positive odor. Rats acquired the odor problem when they met a behavioral criterion of 18 correct go, no-go responses in the last 20 trials. Data were acquired during this learning phase (precriterion) and after learning (postcriterion). In some sessions, we also recorded neural activity as the rats acquired a reversal of the discrimination problem (reversal).
OFC and APC Respond Similarly to Odor Cues after Learning
We acquired data from 538 OFC neurons in 44 sessions and from 215 APC neurons in 36 sessions. Figure 2 shows the locations in OFC and APC from which recordings were obtained. Performance did not differ between these 2 groups of sessions. When recordings were made in OFC, the rats met a criterion of 18/20 correct on the initial discrimination problem in 50 trials on average and on the reversal in 72 trials. When recordings were made in APC, the rats acquired the initial problem in 56 trials and the reversals in 76 trials.
Figure 2.

Location of recording sites in OFC and APC. Vertical bars on the drawing indicate the center of the electrode track in each rat; boxes indicate approximate extent of recording sessions vertically during transition through each area and give an estimate of lateral (and AP) spread of the wires (∼1 mm).
As expected from previous studies, neurons in both OFC and APC fired differentially during odor sampling after rats had achieved the behavioral criterion for learning. During this postcriterion phase, comparison of neural activity in trials involving different odors revealed that 165 (31%) of the 538 neurons recorded in OFC and 53 (25%) of the 215 neurons recorded in APC fired selectively during evaluation of the odor cues. These results did not differ significantly between areas (chi-squared test, P > 0.05). Of the 165 OFC neurons selective during odor presentation, 84 neurons fired more strongly to the odor cue predicting sucrose, whereas 81 fired more strongly to the odor cue predicting quinine. Likewise, of the 53 odor-selective neurons, 34 and 19 neurons fired significantly more strongly to odor cues predicting sucrose and quinine, respectively. The difference in frequencies was only significant in APC (chi-squared test, P = 0.041); however, these results did not differ significantly between areas (chi-squared test, P = 0.127). Thus, examination in postcriterion shed little light on the functional differences of odor selectivity between OFC and APC.
Cue-Selective Firing in APC Is Less Associative than in OFC
To dissociate sensory encoding of the olfactory cues from more associative representations, we examined activity in the 2 areas during learning and after reversal. The different patterns we analyzed for are illustrated in Figure 3. For example, a purely sensory neuron should exhibit the same odor selectivity through a session, including before learning and even after reversal (Fig. 3A). A purely associative neuron—for example, a neuron that encodes only what the odor means—should develop a cue-selective response with learning and reverse it during reversal learning (Fig. 3B). A neuron that encodes some combination of sensory and associative information—for example, a neuron that represents the conjunction of a particular odor and a particular associative significance—might develop a selective response before reversal and then lose it after reversal or vice versa (Fig. 3C). In the analyses that follow, we analyzed neural activity in OFC and APC for firing that approximated these simple patterns and then compared the proportions of these correlates across the 2 areas.
Figure 3.

Predicted cue-selective patterns across learning and reversal. (A) Example of putative sensory encoding. (B) Example of putative associative encoding. (C) Two examples of putative conjunctive encoding of sensory + associative information.
Orbitofrontal Cortex
Examination of the population response of the cue-selective neurons in OFC, shown in Figure 4A, indicated that cue-selective activity in OFC was almost uniformly associative, consistent with the pattern illustrated in Figure 3C. Cue selectivity developed with learning in this population and declined when the odor-outcome associations changed with reversal. Analysis of cue selectivity in the single units, presented in Figure 5, supported this conclusion. The vast majority of the neurons that were cue selective in the postcriterion phase (142, 86%) were “slowly selective” in that they only developed cue selectivity in the postcriterion trial block. This pattern, illustrated by the examples in Figure 6A, is consistent with encoding the associative significance of the cues (Fig. 3B or 3C). Only 23 (14%) neurons exhibited odor selectivity during precriterion trials, consistent with the encoding of sensory information, and analysis of activity after reversal revealed that even most of these neurons were “rapidly selective” rather than sensory—only 5 maintained selectivity for the same odor cue after reversal as required by our criteria for sensory encoding (Fig. 3A). Indeed, the majority of the cue-selective neurons exhibited marked plasticity in their response across reversal; this included 104 neurons (61%) that became nonselective among the odor cues after reversal and 40 neurons (25%) that reversed firing selectivity entirely (Fig. 5). In addition, cue selectivity emerged in 84 OFC neurons that had been nonselective prereversal. These patterns, illustrated by the examples in Figure 6A, are consistent with associative encoding (Fig. 3B,C).
Figure 4.

Effect of learning and reversal training on cue-selective firing in OFC and APC. Population histogram shows the average response of neurons that were selective in the postcriterion phase in (A) OFC and (B) APC. Activity is shown separately for trials involving each odor cue during the precriterion, postcriterion, and reversal trial blocks. Black bars show the response to the preferred odor cue in the postcriterion phase. White bars show the response to the nonpreferred odor. Activity is synchronized to odor onset, displayed in 100 ms bins in spikes/second, and box overlays indicate the approximate duration of odor sampling, which was terminated by the rat.
Figure 5.
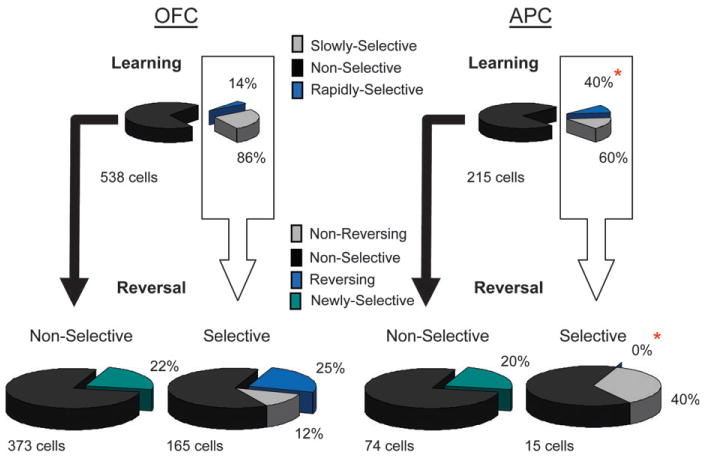
Effect of learning and reversal training on cue-selective firing in OFC and APC. The upper pie charts show the proportions of all neurons recorded in each area that developed cue-selective firing during odor discrimination learning. Rapidly selective neurons (blue) developed an odor preference in the precriterion phase, which persisted postcriterion; slowly selective neurons (gray) developed an odor preference only in the postcriterion phase. APC had more rapidly selective neurons than OFC (*). The lower pie charts show the odor preferences in selective and nonselective neurons during reversal learning. Nonreversing neurons (gray) maintained the same odor preference during reversal learning; reversing neurons (blue) became selective for the other odor cue during reversal learning. APC had more nonreversing neurons and fewer reversing neurons than OFC (*).
Figure 6.
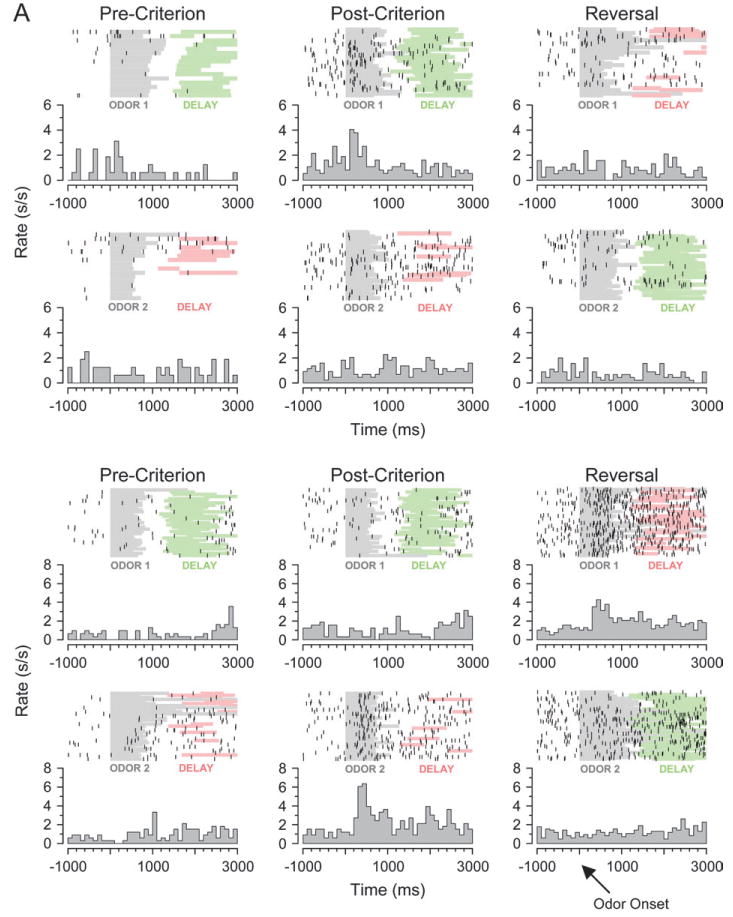

Neural activity during cue sampling, learning, and reversal training. (A) Top panel: OFC neuron with a selective response to the positive odor cue in the postcriterion trials (middle column). This selective response is not present precriterion (left column) or after reversal (right column). Bottom panel: OFC neuron with a selective response to the negative odor cue in the postcriterion trials (middle column). This selective response is not present precriterion (left column), and it switches to the other odor cue after reversal (right column). (B) Top panel, left: APC neuron with a selective response to the positive odor cue in the postcriterion trials (right column). Top panel, right: APC neuron with a selective response to the negative odor cue in the postcriterion trials (right column). In both of these neurons, the response is not significantly different among the odor cues precriterion (left column). Bottom panel: APC neuron with a selective response to the positive odor cue in the postcriterion trials (middle column). The same selective response is present precriterion (left column) and after reversal (right column). Raster displays show neural activity on individual trials, and each histogram shows average activity in spikes/second in 100 ms bins. The timing of odor sampling and the delay after responding are shown by shading on the rasters (odor, gray; delay before sucrose, green; delay before quinine, red).
Piriform Cortex
A similar analysis of the effect of learning and reversal on cue-selective firing in APC revealed substantially more sensory encoding than was observed in OFC. This was evident in the population response for cue-selective neurons in APC, illustrated in Figure 4B. Unlike their counterparts in OFC, these neurons were selective for the same odor cue before and after learning and also after reversal, consistent with the sensory encoding pattern illustrated in Figure 3A. Analysis of cue selectivity in the single units revealed more associative encoding but still a higher degree of sensory representation. This was evident during learning in the relative proportions of “rapidly” and “slowly” selective neurons. Of the 53 APC neurons that were cue selective in the postcriterion trial block, 32 (60%) were slowly selective. This correlate, illustrated by examples in the upper panel of Figure 6B, indicates that there was substantial associative encoding in APC. However, APC also had a large population of “rapidly selective” neurons (21, 40%), illustrated by the example in the lower panel of Figure 6B, which had a preference for the same odor cue in the pre- and postcriterion phases. This population was larger in APC than in OFC (chi-squared test, P < 0.001), suggesting a stronger influence of sensory features on activity in APC.
The importance of both sensory and associative encoding in APC was more clearly evident in the effect of reversal learning on cue-selective firing, illustrated in Figure 5. Eighty-nine APC neurons were recorded in sessions in which the rats acquired a reversal of the discrimination problem, including 15 cue-selective neurons from the population described above. The encoding characteristics of these neurons during learning were similar to those of the full population described above. Consistent with plasticity observed in APC during learning, the majority of these neurons (60%) lost odor preference during reversal learning. In addition, 15 nonselective neurons became cue selective after reversal. These patterns are consistent with associative encoding (Fig. 3B,C).
However, reversal training revealed greater sensory encoding in APC compared with OFC. For example, as illustrated in Figure 5, many cue-selective neurons in APC (6/15, 40%) maintained the same odor preference across reversal. This proportion was significantly larger than the proportion of such correlates in OFC (chi-squared test, P < 0.05). These neurons typically maintained the same odor preference across the entire session, as illustrated by the population response in Figure 4B and by the example in the lower panel of Figure 6B. This population amounted to 4/30 cue-selective neurons in APC but only 5/165 in OFC. Although these numbers are small, the differences are highly significant (chi-squared test, P < 0.001). Conversely, not a single cue-selective neuron in APC reversed firing selectivity when the associated outcomes were switched during reversal learning, which represented a significant difference from OFC where many neurons reversed (chi-squared test, P < 0.0001).
In summary, activity in APC was more associative than one would expect for a sensory region but did represent substantially more sensory information than did activity in OFC. For example, of the 249 OFC neurons with selective responses before or after reversal, only 5 met our criteria for pure sensory encoding, shown in Figure 3A, whereas 40 met our criterion for pure associative encoding, shown in Figure 3B. These proportions were reversed in APC, where 4/30 neurons met the criteria for sensory encoding, whereas none met the criteria for associative encoding. The relative import of the sensory features of the odor cue was also evident in the conjunctive encoding (Fig. 3C) observed in the 2 regions. In OFC, 188/249 cue-selective neurons exhibited one of these patterns, whereas in APC, all 26 neurons that were not purely sensory did so. These findings are perhaps not surprising; however, they provide the first direct comparison of sensory and associative encoding between these 2 areas.
Cue-Selective Activity in APC Fails to Activate Outcome Representations
We also examined whether neurons in OFC and APC fired during the outcome-related periods of the trial early in learning and whether those populations would become incorporated into the cue-selective population after learning, thereby potentially activating information about the outcome during odor sampling.
Orbitofrontal Cortex
As previously reported, many OFC neurons fired differently on positive and negative precriterion trials during a delay after responding but prior to outcome delivery. In this study, this population amounted to 100 neurons or 19% of the cells recorded in OFC. Notably, many (31/100, 31%) of these neurons were active during sampling of the corresponding odor cues after learning in the postcriterion trials. This population included 28 neurons with outcome-expectant activity during precriterion trials that only developed selective firing to the corresponding cue during the postcriterion trials. This percentage was significantly above that expected by chance (chi-squared test, P < 0.0001).
Piriform Cortex
Many APC neurons also fired during the delay period preceding either sucrose or quinine delivery in the precriterion trial block. This population consisted of 27 neurons or 13% of the APC neurons recorded in this study. These neurons differed from their counterparts in OFC in that very few (2/27, 7%) became active during sampling of the corresponding odor cue after learning. This proportion is no more than one would expect by chance alone (chi-squared text, P > 0.05) and is significantly smaller than that observed in OFC (chi-squared test, P < 0.0001). Thus, odor-selective populations in APC did not include neurons that were active to the outcomes.
Discussion
Although piriform cortex receives primary olfactory sensory input, recent proposals have conceptualized this area as an association cortex, capable of integrating incoming olfactory information with descending input from higher order associative regions such as OFC (Haberly 2001). To test this hypothesis, we recorded from single neurons in OFC and APC in rats learning and reversing novel 2-odor discrimination problems. In APC, cue- and outcome-selective neurons were observed in proportions similar to those observed in OFC. APC neurons also exhibited a significant plasticity across learning and reversal, in that selective firing in many neurons only developed after learning and often disappeared after reversal of the cue-outcome associations. However, unlike OFC, cue selectivity in APC failed to reverse when the associated outcome changed, and many cue-selective APC neurons did maintain the same odor preference across the entire session. In addition, the cue-selective population in APC did not include neurons that were active prior to outcome delivery, which comprise a substantial part of the cue-selective population in OFC. Thus, representations in APC are substantially more associative than expected in a purely sensory region but appear to be more constrained by the sensory features of odor cues than are representations in OFC.
Neuronal Activity in APC Represents Associative Information
APC is part of a parallel, distributed, and bidirectional system involved in processing olfactory information (Haberly 2001). These projections include strong reciprocal connections with olfactory bulb and other primary olfactory areas. This circuitry may be used to identify and discriminate among odors in the environment. In fact, APC neurons are capable of making very fine discriminations among odors. In anesthetized preparations, it has been shown that APC neurons can discriminate among alkanes differing only in chain length (Wilson 2000a, 2000b), like mitral/tufted cells in the olfactory bulb (Mori and others 1999). These results suggest that neuronal firing in APC represents olfactory information. Our data support this idea, in that many APC neurons fired based on the identity of odors prior to correct behavioral performance and continued to fire to the identity of the odor after cue-outcome contingences were reversed. Indeed, we agree with these previous studies that neuronal activity in APC is rooted in the sensory process of olfaction. These encoding characteristics are fundamentally different from those we have observed in downstream associative areas in this task (Schoenbaum and others 1998, 1999, 2003b; Setlow and others 2003; Saddoris and others 2005).
However, APC also receives input from an extensive network of brain regions that are involved in processing multi-modal sensory input and in representing associative rather than sensory information. Based on these connections, subpopulations of neurons in APC may integrate incoming olfactory information with descending input from higher order associative regions, such as OFC, and thus be directly involved in associative and behavior-level processes. The APC is well suited to serve such a role because long associational axons are directed both rostrally and caudally throughout APC, resulting in a spatially distributed recurrent connectivity that is likely to support autoassociative processes (Haberly and Price 1978; Luskin and Price 1983a, 1983b; Datiche and Cattarelli 1996; Datiche and others 1996; Haberly 2001). Furthermore, a large number of APC neurons project back to the olfactory bulb, suggesting that APC influences sensory information processing (Haberly 2001). Notably, these are heaviest in the anterior part of pirifrom cortex from which we recorded (de Olmos and others 1978; Haberly and Price 1978; Shipley and Adamek 1984). As a result of these connections, APC is in an excellent position to modulate incoming sensory information based on the past experience or expectations of the animal.
Consistent with this idea, we found that many APC neurons fired differentially during odor sampling but only before or after the reversal. Thus, APC neurons responded to particular odors, but only when they had become associated with a particular outcome in our task. Further, we found that some APC neurons developed selective firing in anticipation of the outcomes. These findings, which resemble neural activity we have observed in OFC and basolateral amygdala (ABL) in this task (Schoenbaum and others 1998, 1999, 2003b; Setlow and others 2003; Saddoris and others 2005), could be explained if input from these areas were able to influence responses to sensory attributes of the olfactory cues.
The Role of APC within an Associative Learning Circuit
The APC receives sensory input regarding olfactory cues, but it also has strong reciprocal connections with brain regions concerned with associative learning and behavior. For example, the OFC receives input from APC and projects back to layer III of APC (Johnson and others 2000; Illig 2005), where the input converges with autoassociative (Haberly 1998) and amygdaloid projections (Majak and others 2004). It has been suggested that, through these connections, OFC might actively modulate afferent input to APC so that cells fire in certain contexts but not others or that OFC input might even initiate activity in APC in the absence of any odor stimulus allowing for recall of odors and odor-related associations (Haberly 2001). This could be mediated by OFC projections that synapse proximal to the afferent input (layer Ib) on the apical dendritic tree of pyramidal cells in APC (Haberly 1998, 2001). According to this account, activity in APC is heavily influenced by input from associative areas and works together with them to promote appropriate responses to olfactory cues.
Yet lesion studies suggest that OFC and APC have unique functions within this circuit. Damage to OFC can impair the acquisition and retention of odor discriminations without affecting odor detection (Eichenbaum and others 1980, 1983), whereas lesions of APC impair odor discrimination ability but can also impair odor detection (Slotnick and Schoonover 1992). According to these reports, OFC plays a unique role within a closely interconnected system of structures that mediate odor-guided learning and behavior, whereas APC may be more involved in representation of olfactory information.
Our findings are consistent with both of these accounts, inasmuch as we found both sensory and associative representations of the odor cues in APC. However, viewing our results along these lines would miss an important point of the study, which is that it is to some extent nonsensical to ask whether APC is important for encoding odors' sensory features or their significance; instead, our data as well as findings from other laboratories (Kay and Laurent 1999) show that from the time sensory information becomes nerve impulses, these 2 variables are confounded. In other words, sensory information is represented in the central nervous system because it is significant. Indeed, it is axiomatic that the central nervous system is optimized to shape incoming sensory information, at the earliest possible stage, to extract the most relevant features and minimize the irrelevant or unimportant features as noise. Relevant and irrelevant are clearly defined based on the evolutionary history of the organism, which has shaped the functionality of the structures involved in perception; however, studies such as ours show that the personal history of the animal also has a powerful effect on processing of sensory information, even at very early stages.
Consistent with this view, our results suggest that APC functions as a way station by which olfactory information might be distributed to higher order cognitive centers, while at the same time allowing those brain regions to influence that sensory processing to reflect the past experience and expectations of the animal, both directly in APC and indirectly via projections back to olfactory bulb. Thus, we found subpopulations of APC neurons that appeared to be primarily responding to the sensory features of the odor cues, subpopulations that seemed to reflect the learned significance of the cues, and a third subpopulation that fired selectively in expectation of differently preferred outcomes. Among these populations, the sensory encoding was relatively unique to APC; we have not observed sensory encoding in OFC, ABL, or ventral striatum in any substantial number of neurons (Schoenbaum and others 1998, 1999, 2003b; Setlow and others 2003; Saddoris and others 2005). As a result, we would speculate that this population reflects the strong influence of sensory input from olfactory bulb on a subset of neurons in APC.
The associative and outcome-expectant encoding, however, has been a prominent feature of neural activity in downstream associative regions in our studies (Schoenbaum and others 1998, 1999, 2003b; Setlow and others 2003; Saddoris and others 2005) and those of a number of other laboratories (Sanghera and others 1979; Thorpe and others 1983; Quirk and others 1995; Tremblay and others 1998, 1999; Lipton and others 1999; Ramus and Eichenbaum 2000; Roesch and Olson 2004). Much of this information could reach APC through input from OFC. We would speculate that this is particularly true for the neural activity observed during the delay, in anticipation of the different outcomes. We and others have identified such outcome-expectant activity as a prominent feature of neural activity in OFC. It appears early in learning, to both differently valenced and even differently preferred outcomes of the same valence, and it appears to develop progressively earlier as the animal learns to expect a particular outcome with greater certainty. Moreover, although such activity is evident in other brain regions, notably amygdala, striatum, and other parts of prefrontal cortex (Schoenbaum and others 1998; Cromwell and Schultz 2003; Roesch and Olson 2003; Setlow and others 2003; Wallis and Miller 2003), we have found that damage to OFC disrupts these correlates in other structures (Saddoris and others 2005). Further, OFC is particularly critical to behaviors that depend on such outcome expectancies (Schoenbaum and Roesch 2005).
By contrast, although associative encoding in APC may also reflect input from OFC, we would suggest that these features ultimately result from processing in ABL. Although neurons in both OFC and ABL develop associative encoding in this task (Schoenbaum and others 1998, 1999; Saddoris and others 2005), and both areas are responsive to the value or valence of olfactory cues in humans (Gottfried and others 2002, 2003; Anderson and others 2003), ABL neurons acquire associative encoding earlier than those in OFC and appear to pass this information to OFC during learning (Schoenbaum and others 1999, 2003). Indeed, neural correlates in OFC in ABL-lesioned rats are much more sensory driven, much like activity in APC in the present study.
Although APC receives little direct input from this part of the amygdala (Krettek and Price 1977; Luskin and Price 1983a, 1983b; Majak and others 2004; Santiago and Shammah-Lagnado 2004), this information could influence processing in APC indirectly, both through connections with OFC and also via projections to posterior piriform cortex (Haberly and Price 1978; Luskin and Price 1983a, 1983b; McIntyre and others 1996; Burwell and Amaral 1998; Johnson and others 2000; Haberly 2001). A circuit from ABL to APC via posterior piriform cortex would provide an alternate account of how odor identification and appropriate responding might occur, even in the absence of OFC (Schoenbaum and others 2002, 2003; Brown and McAlonan 2003). Further, behavioral studies that combine specific lesions of these areas with single-unit recording could differentiate between these possibilities.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (DA015718, GS), the National Institute of Neurological Disorders and Stroke (T32-NS07375, MR), and the National Institute on Deafness and Other Communication Disorders (T32-DC00054, TS). We would like to thank Drs Adam Puche and Don Katz for their insightful comments on this manuscript and Dr Stephen Warrenburg at International Flavors and Fragrances for his assistance.
Footnotes
Conflict of Interest: None declared.
References
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:106–108. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Brown VJ, McAlonan K. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–130. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- Datiche F, Cattarelli M. Reciprocal and topographic connections between the piriform and prefrontal cortices in the rat: a tracing study using the B subunit of the cholera toxin. Brain Res Bull. 1996;41(6):391–398. doi: 10.1016/s0361-9230(96)00082-2. [DOI] [PubMed] [Google Scholar]
- Datiche F, Litaudon P, Cattarelli M. Intrinsic association fiber system of the piriform cortex: a quantitative study based on a cholera toxin B subunit tracing in the rat. J Comp Neurol. 1996;376(2):265–277. doi: 10.1002/(SICI)1096-9861(19961209)376:2<265::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- de Olmos J, Hardy H, Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978;181(2):213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79(2):434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Shedlack KJ, Eckmann KW. Thalamocortical mechanisms in odor-guided behavior. I. Effects of lesions of the mediodorsal thalamic nucleus and frontal cortex on olfactory discrimination in the rat. Brain Behav Evol. 1980;17(4):255–275. doi: 10.1159/000121803. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:10829–10837. doi: 10.1523/JNEUROSCI.22-24-10829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Olfactory cortex. In: Sheperd GM, editor. The synaptic organization of the brain. New York: Oxford University Press; 1998. [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26(5):551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. J Comp Neurol. 1978;178(4):711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Illig KR. Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Comp Neurol. 2005;488:224–231. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20(18):6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. J Comp Neurol. 1977;172(4):723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- Lipton P, Alvarez AP, Eichenbaum H. Crossmodal associative memory representations in rodent orbitofrontal cortex. Neuron. 1999;22:349–359. doi: 10.1016/s0896-6273(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The laminar distribution of intracortical fibers originating in the olfactory cortex of the rat. J Comp Neurol. 1983a;216(3):292–302. doi: 10.1002/cne.902160306. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983b;216(3):264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Majak K, Ronkko S, Kemppainen S, Pitkanen A. Projections from the amygdaloid complex to the piriform cortex: a PHA-L study in the rat. J Comp Neurol. 2004;476(4):414–428. doi: 10.1002/cne.20233. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, Staines WA. Efferent projections of the anterior perirhinal cortex in the rat. J Comp Neurol. 1996;369(2):302–318. doi: 10.1002/(SICI)1096-9861(19960527)369:2<302::AID-CNE10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286(5440):711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Repa JC, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Ramus SJ, Eichenbaum H. Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. J Neurosci. 2000;20:8199–8208. doi: 10.1523/JNEUROSCI.20-21-08199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10(3):284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Sanghera G, Rolls ET, Roper-Hall A. Visual responses of neurons in the dorsolateral amygdala of the alert monkey. Exp Neurol. 1979;63:610–626. doi: 10.1016/0014-4886(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Santiago AC, Shammah-Lagnado SJ. Efferent connections of the nucleus of the lateral olfactory tract in the rat. J Comp Neurol. 2004;471(3):314–332. doi: 10.1002/cne.20028. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G. Olfactory learning and the neurophysiological study of rat prefrontal function. In: Nicolelis MAL, Simon SA, editors. CRC series: methods in chemosensory research. Boca Raton, FL: CRC Press; 2001. pp. 371–427. [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19(5):1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995a;74(2):733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995b;74(2):751–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003b;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Setlow B, Schoenbaum G, Gallagher M. Neural encoding in ventral striatum during olfactory discrimination learning. Neuron. 2003;38(4):625–636. doi: 10.1016/s0896-6273(03)00264-2. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Adamek GD. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull. 1984;12(6):669–688. doi: 10.1016/0361-9230(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Schoonover FW. Olfactory pathways and the sense of smell. Neurosci Biobehav Rev. 1992;16(4):453–472. doi: 10.1016/s0149-7634(05)80187-3. [DOI] [PubMed] [Google Scholar]
- Staubli U, Fraser D, Faraday R, Lynch G. Olfaction and the “data” memory system in rats. Behav Neurosci. 1987;101:757–765. doi: 10.1037//0735-7044.101.6.757. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. New York: Elsevier; 1992. [Google Scholar]
- Thorpe SJ, Rolls ET, Maddison S. The orbitofrontal cortex: neuronal activity in the behaving monkey. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. J Neurophysiol. 1998;80:964–977. doi: 10.1152/jn.1998.80.2.964. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate orbitofrontal cortex. J Neurophysiol. 2000a;83(4):1877–1885. doi: 10.1152/jn.2000.83.4.1877. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. J Neurophysiol. 2000b;83(4):1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18(7):2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol. 2000a;84(6):3036–3042. doi: 10.1152/jn.2000.84.6.3036. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Odor specificity of habituation in the rat anterior piriform cortex. J Neurophysiol. 2000b;83(1):139–145. doi: 10.1152/jn.2000.83.1.139. [DOI] [PubMed] [Google Scholar]


