Abstract
The inhalation of airborne pollutants, such as asbestos or silica, is linked to inflammation of the lung, fibrosis, and lung cancer. How the presence of pathogenic dust is recognized and how chronic inflammatory diseases are triggered are poorly understood. Here, we show that asbestos and silica are sensed by the Nalp3 inflammasome, whose subsequent activation leads to interleukin 1β secretion. Inflammasome activation is triggered by reactive oxygen species, which are generated by a NADPH oxidase upon particle phagocytosis (NADPH is the reduced form of nicotinamide adenine dinucleotide phosphate). In a model of asbestos inhalation, Nalp3−/− mice showed diminished recruitment of inflammatory cells to the lungs, paralleled by lower cytokine production. Our findings implicate the Nalp3 inflammasome in particulate matter–related pulmonary diseases and support its role as a major proinflammatory “danger” receptor.
Inhalation of asbestos or silica in occupational exposures can result in pulmonary fibrosis (asbestosis, silicosis), and lung cancer, especially in smokers (1). Asbestos fibres have been associated with development of malignant mesotheliomas after environmental exposures (2). The mechanisms of injury to cells of the lung and pleura and disease development by these pathogenic particulates are unclear (3, 4). Inflammation is a hallmark of exposure to asbestos or silica and is observed both in animal models and in the lungs of patients with asbestos-related lung disease (1, 3). Bronchial epithelial cells and alveolar macrophages are in prolonged contact with the inhaled particulates when clearing them from the lung, and can initiate and sustain inflammatory responses. Likewise inflammatory responses are observed after exposure to other particulates such as found in diesel exhaust (5).
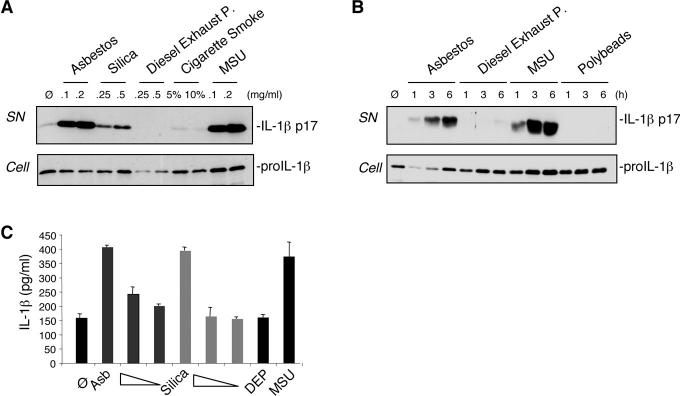
The reported proinflammatory activities of asbestos and silica prompted us to investigate the potential of these particulates in activating the secretion of the proinflammatory cytokine IL-1β in human macrophages (6). We indeed observed that the macrophage-like cell line THP1 secreted mature IL-1β upon stimulation with asbestos or silica (Fig. 1A). In contrast, the addition of diesel exhaust particles (DEP) or cigarette smoke extract (CSE) did not result in the production of detectable IL-1β when measured after 6 h of exposure (Fig. 1A). The secretion of mature IL-1β induced by asbestos was comparable to amounts observed with monosodium urate crystals (MSU), a known potent activator of IL-1β processing (7) (Fig. 1, A and B). Inert particles, such as polystyrene beads, did not activate IL-1β maturation (Fig. 1B). Primary human monocyte-derived macrophages also produced mature IL-1β after stimulation with asbestos or silica (Fig. 1C), while DEP exposure again did not result in IL-1β secretion.
Fig 1.
Asbestos and silica activate IL-1β secretion in human macrophages. (A) THP1 cells were stimulated for 6 h with the indicated amounts (mg/ml media) of asbestos, silica, diesel exhaust particles (DEP), cigarette smoke extract (CSE, in % solution) and MSU. (B) THP1 cells were stimulated with 0.2 mg/ml asbestos, 0.5 mg/ml DEP and 0.2 mg/ml MSU for the indicated times. Media supernatants (SN) were analysed for the presence of mature IL-1β, and cell extracts (Cell) for the presence of proIL-1β by Western blotting. (C) LPS-primed primary human monocyte-derived macrophages (M-CSF) were stimulated for 6 h with asbestos (0.2, 0.1, 0.05 mg/ml), silica (0.5, 0.2, 0.1 mg/ml), DEP (0.2 mg/ml) and MSU (0.2 mg/ml) and analysed by ELISA for IL-1β released into the media. Values are means ± S.E.M.
Mature IL-1β is produced by cleavage of the inactive proIL-1β precursor by caspase-1, which is activated within a large multi-protein complex, termed the inflammasome (8). The Nalp3 inflammasome, composed of the Nod-like receptor (NLR) protein Nalp3 (Cryopyrin/NLRP3), Cardinal, the adaptor ASC and caspase-1, is implicated in the production of mature IL-1β in response to a variety of signals. For example, the presence of bacteria is recognized through binding of the pathogen-associated molecular pattern (PAMP) muramyldipeptide (MDP) or via the action of bacterial toxins (9, 10). In addition to PAMPs, Nalp3 has the extraordinary capacity of sensing endogenous stress-associated danger signals (DAMPs) such as ATP (9) or MSU (7). Since both MSU and asbestos are crystalline structures, we wondered whether production of IL-1β by asbestos would also occur through the Nalp3 inflammasome. To clarify this, we determined whether the particulates activate caspase-1 in THP1 cells and indeed found processing of caspase-1 into the p10 fragment (fig. S1). We then measured IL-1β maturation in cells in which the different inflammasome components were downregulated. Highly reduced secretion of mature IL-1β in Nalp3-, ASC- and caspase-1-knocked-down cells was observed, similar to patterns observed with MSU or the bacterial toxin nigericin (Fig. 2A). The residual IL-1β secretion observed in these cells is most likely due to incomplete shutdown of the corresponding proteins (fig. S2). In contrast, Toll-like receptors (TLRs) or IL-1 receptors do not seem to be essential for asbestos signalling, since the knock-down or knock-out of MyD88, an intracellular adaptor protein mediating TLR and IL-1 receptor signalling, had no or little effects on IL-1β secretion and caspase-1 activation (Fig. 2A and fig. S3). To further analyse the requirement for the Nalp3 inflammasome in particulate-induced IL-1β processing, we made use of primary murine bone-marrow-derived macrophages primed with LPS to induce proIL-1β synthesis and then stimulated with asbestos, silica or MSU (Fig. 2B). Caspase-1 was no longer cleaved and secreted in the presence of the pan-caspase inhibitor zVAD or in macrophages from Nalp3 and ASC deficient mice and as a consequence, mature IL-1β processing and secretion was abolished (Fig. 2B). By contrast, IL-1β production was independent of the Ipaf inflammasome, which has been shown to activate caspase-1 and produce IL-1β in response to flagellin from Salmonella typhimurium and Legionella pneumophila (fig. S4) (11, 12).
Fig 2.
Asbestos-induced IL-1β secretion is Nalp3 inflammasome-dependent. (A) THP1 cells stably expressing shRNA against the indicated target genes were stimulated for 6 h with 0.2 mg/ml asbestos, 0.2 mg/ml MSU and 3.4 μM nigericin. Media supernatants (SN) and cell extracts (Cell) were analysed by Western blotting as indicated in the text. (B) LPS-primed, murine bone-marrow-derived macrophages (BMDM) from wild-type (WT), Nalp3- or ASC-deficient mice were stimulated for 6 h with the indicated amounts (mg/ml) of asbestos, silica and MSU in the presence of 20 μM zVAD where indicated and analysed for IL-1β secretion and activation of caspase-1 by Western blotting.
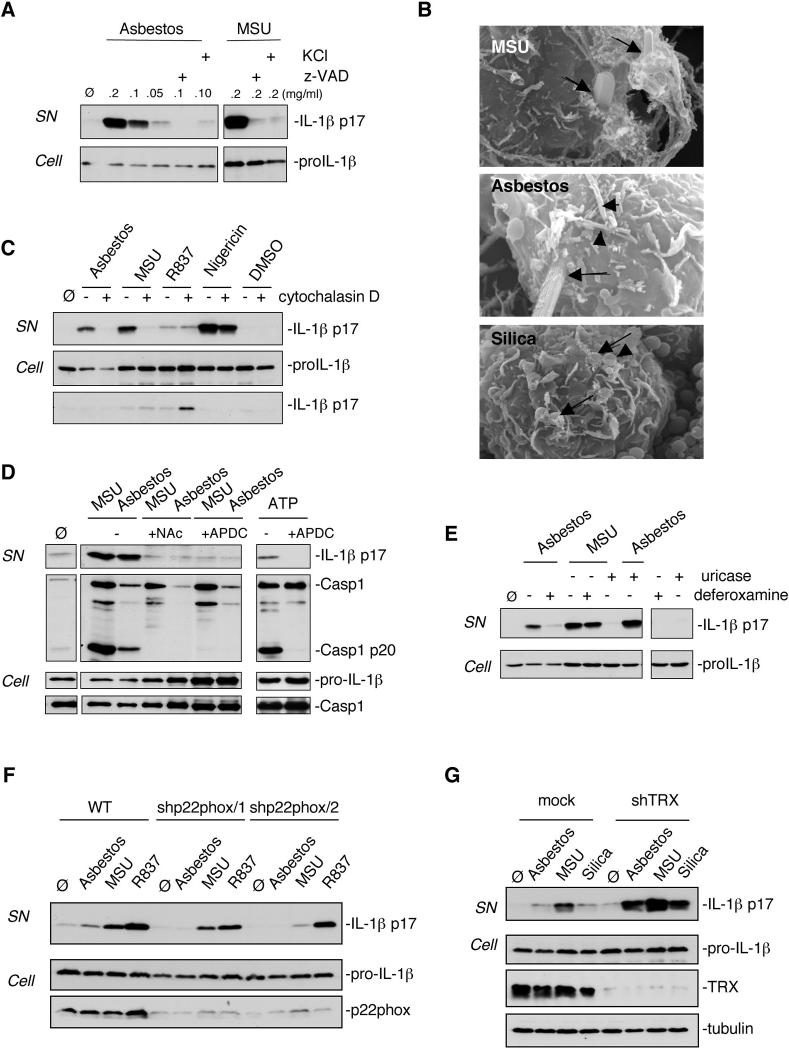
The mechanism of Nalp3 inflammasome activation by PAMPs or DAMPs is still poorly understood. There are at least two possible hypotheses: 1) the activating molecules could directly interact with the LRR domain of Nalp3 after entering the cell, or 2) they could modify one or more membrane-associated proteins, which then trigger a signalling cascade leading to Nalp3 activation. In favour of the latter assumption is the recent finding that potassium (K+) efflux, lowering intracellular K+ levels, is a requirement for Nalp3 inflammasome activation triggered by all known activators including MSU (13–15). We inhibited K+ efflux by adding high concentrations (130 mM) of KCl to the culture media of THP1 cells and found that asbestos-induced IL-1β production was blocked (Fig. 3A).
Fig 3.
Nalp3 inflammasome activation upon asbestos stimulation is dependent on endocytosis and ROS production. (A) THP1 cells were stimulated with the indicated amounts of asbestos or MSU in the presence of 20 μM caspase inhibitor zVAD or 130 mM extracellular KCl. (B) THP1 cells were treated for 6 h with 0.2 mg/ml asbestos, 0.5 mg/ml silica and 0.2 mg/ml MSU. Particles/fibers entering cells are marked with arrows and fibers/particles on cell surface with arrowheads. (C) THP1 cells were stimulated for 6 h with 0.2 mg/ml asbestos, 0.2 mg/ml MSU, 10 μg/ml R837 or 3.4 μM nigericin in the absence or presence of 0.2 μM cytochalasin D. (D) Murine peritoneal macrophages were stimulated with asbestos, MSU or ATP in the presence of N-acetyl-L-cysteine (NAC) (25 mM) or APDC (50 μM). (E) THP1 cells were stimulated with asbestos (0.1 mg/ml) or MSU (0.1 mg/ml) in the presence or absence of deferoxamine mesylate (2 mM) or uricase (0.1 U/ml) respectively. (F) THP1 control cells (mock) or knock-down for the NADPH oxidase subunit p22phox cells were stimulated for 6 h with 0.1 mg/ml asbestos, 0.1 mg/ml MSU or 0.01 mg/ml R837. (G) THP1 control cells (mock) or knock-down for thioredoxin (TRX) cells were stimulated for 6 h with 0.1 mg/ml asbestos, 0.1 mg/ml MSU or 0.5 mg/ml silica. Media supernatants (SN) or cell extracts (Cell) were analysed by Western blotting as indicated in the text.
In order to delineate the asbestos-induced signalling pathway leading to inflammasome activation in more detail, we started with the question of whether asbestos fibres needed to be endocytosed. Phagocytic cells can endocytose small particles, whereas bigger crystals or fibres are subject to so-called “frustrated” phagocytosis (16) and remain trapped at the surface (Fig. 3B). To test the importance of the endocytic process, we treated THP1 cells with cytochalasin D, which disrupts actin filaments. Cytochalasin D inhibited not only secretion of mature IL-1β by asbestos (Fig. 3C), but also by the particulate MSU, while for non-crystalline Nalp3 activators such as R837 and nigericin, IL-1β secretion was not affected (Fig. 3C). Since the mature form of IL-1β did not accumulate inside the cell (Fig. 3C), we conclude that the actin cytoskeleton is necessary for the attempt to phagocytose, and not to drive mature IL-1β secretion. The same effect was achieved by treating the cells with the inhibitor of actin polymerisation, latrunculin A (fig. S5).
Asbestos fibres have been shown to participate in redox reactions to generate reactive oxygen species (ROS) that are widely linked to signalling pathways causing inflammation and carcinogenesis [reviewed in (17)]. The generation of ROS correlates with toxicity and pathogenicity of different types of asbestos. Recent data also suggest a role for ROS in activating caspase-1 upon ATP treatment (18) and in Nalp3 inflammasome activation by MSU and R837 (13). It is therefore possible that asbestos particles, through activation of a NADPH oxidase triggered by “frustrated” phagocytosis, generate ROS, which in turn contribute to inflammasome activation. In order to test this hypothesis, we investigated whether asbestos led to ROS generation under our experimental conditions, which was the case (fig. S6). We then treated THP1 cells with the ROS inhibitors N-acetyl-L-cysteine (NAC) or (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC) to determine whether the redox status of the cell affected inflammasome activation. IL-1β production was indeed impaired in response to asbestos, MSU and ATP when using these inhibitors (Fig. 3D), suggesting that ROS production was a necessary step in inflammasome activation. Crocidolite asbestos fibres appear to be particularly potent ROS producers due to the presence of iron on the fibre surface. The iron is thought to play an important role in the generation of free radicals (3). We observed that iron chelation of asbestos fibres by deferoxamine strongly reduced IL-1β maturation, whereas it had no effect in response to MSU crystals (Fig. 3E). On the other hand, uricase treatment blocked the activity of MSU but not of asbestos.
Our model predicts that inflammasome-activating ROS are generated by a NADPH oxidase known to be assembled and activated upon phaygocytosis of microbes. In support of this, ROS production was inhibited by the NADPH oxidase inhibitors diphenylene iodonium (DPI) and apocynin, but not with rotenone (an inhibitor of mitochondrial complex I) or TTFA (an inhibitor of mitochondrial complex II) (fig. S7). To further corroborate NADPH oxidase as a source of ROS production, we knocked down the common NADPH oxidase subunit p22phox and found highly diminished IL-1β secretion (Fig. 3F). If ROS play a central role in NALP3 inflammasome activation, ROS detoxifying proteins such as thioredoxin (TRX) should play a regulating role in inflammasome activation. Indeed, when asbestos and MSU were added to macrophages in which TRX was downregulated by shRNA, increased IL-1β secretion was observed (Fig. 3G).
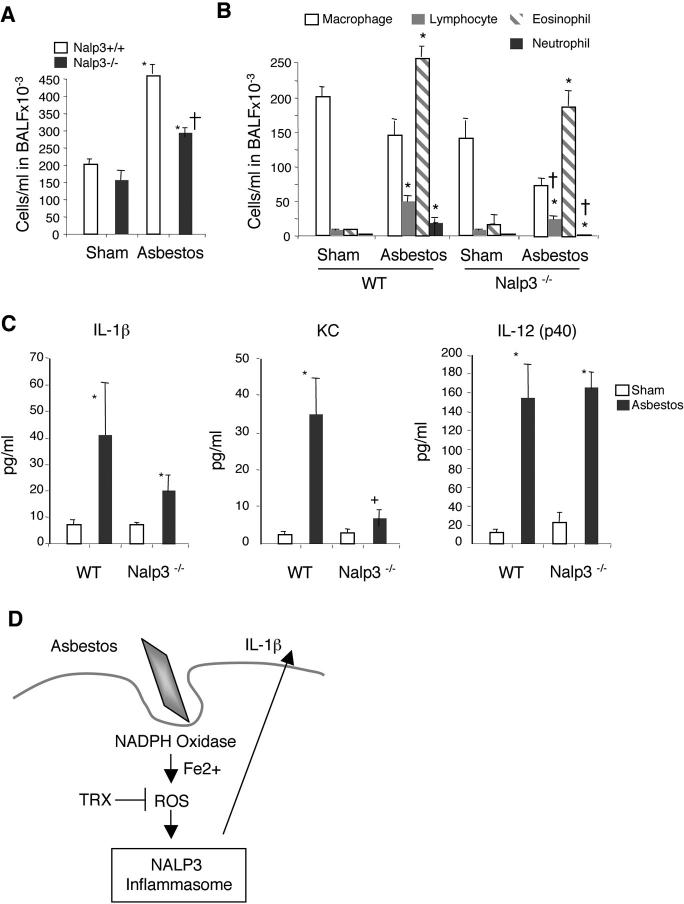
Models of asbestos inhalation have revealed that the airway epithelium and alveolar macrophages play important roles in cell proliferation, inflammation and fibrogenesis triggered by the asbestos fibres (reviewed in (1)). To investigate the in vivo significance of the Nalp3 inflammasome in asbestos-induced inflammation, Nalp3−/− and Nalp3+/+ littermate mice were exposed for 9 days to chrysotile asbestos and markers of injury, inflammation and cytokine production were analysed on day 10. As previously shown (19, 20), asbestos-exposed mice exhibited increased total cell numbers in bronchoalveolar lavage fluid (BALF) compared to air-exposed mice. However, significantly fewer cells were recruited to the lungs of Nalp3−/− mice after exposure to asbestos (Fig. 4A). Notably, lymphocyte, eosinophil and neutrophil infiltration was reduced in Nalp3−/− asbestos-exposed mice (Fig. 4B). Several cytokines were induced by asbestos, such as IL-1β, KC, and IL-12(p40) (Fig. 4C). KC, a potent neutrophil chemoattractant, and IL-1β were decreased in Nalp3−/− mice, whereas IL-12(p40) production was independent of Nalp3. Although mucin production, which parallels inflammatory changes in lungs and may occur to facilitate clearance of asbestos fibers (21), was similarly increased by asbestos in Nalp3−/− and Nalp3+/+ lungs, significant increases in the number of mucin-producing distal bronchioles, sites of impact of chrysotile asbestos fibers after inhalation, occurred in Nalp3−/− mice (table S1).
Fig 4.
In vivo inhalation of asbestos results in reduced pulmonary inflammation in Nalp3-deficient mice. (A) Total and (B) differential cell counts in bronchoalveolar lavage fluid (BALF) in 9 day sham- and asbestos-exposed groups. (C) Cytokine/chemokine levels were measured in BALF of 9 day asbestos-exposed mice. Values are means ± S.E.M. *Significantly different from sham of same genotype. †Significantly different from +/+ Asb. (D) A proposed model for asbestos induced inflammasome activation. See text for details.
The Nalp3 inflammasome is implicated in the pathological increase of IL-1β production in autoinflammatory syndromes, such as Muckle-Wells syndrome (22), as well as inflammatory processes, such as gout and pseudogout (7). Our findings support the implication of the Nalp3 inflammasome in pulmonary inflammatory diseases that are linked to pathogenic air pollutants and can ultimately lead to lung cancer and fibrosis. Asbestos, MSU and probably other particles activate the NALP3 inflammasome in a similar way, requiring actin-mediated cellular uptake in contrast to small, non-particulate molecules such as R837, ATP or nigericin. How the phagocytosed fibres, particles, and MSU crystals are sensed by the Nalp3 inflammasome is not completely clear. It seems unlikely, however, that each of the particles is “specifically” recognized by Nalp3. Rather, our data support a model in which ROS generated by a NADPH oxidase are implicated in Nalp3 inflammasome activation (Fig. 4D). ROS constitute one of the most ancient danger signals and are generated in large amounts by NADPH oxidase upon microbe (and fine particle) phagocytosis.
An important role for IL-1β has been proposed in the pathogenesis of asbestos-induced mesothelioma by regulating human mesothelial cell proliferation (23) and IL-1β-driven inflammation is well known to promote the development and invasiveness of several tumour types in vivo (24). Moreover, in a mouse model of bleomycin- or silica-induced pulmonary fibrosis, treatment with IL-1 receptor antagonist (IL-1ra) reduces the proportion of damaged lung (25). Silicosis and asbestosis continue to be a common cause of chronic lung disease, despite evidence that they can be prevented by environmental dust control.
Since Anakinra (IL-1ra) is efficiently used in the clinical treatment of autoinflammatory syndromes (26) as well as gout patients (27), the present study suggests a potential use of Anakinra in order to slow down progression of asbestosis, silicosis and possibly other inflammatory lung diseases.
Acknowledgments
Supporting Online Material www.sciencemag.org/cgi/content/full/1156995/DC1 Materials and Methods Figs. S1 to S8 Table S1 References
References and Notes
- 1.Mossman BT, Churg A. Am J Respir Crit Care Med. 1998;157:1666. doi: 10.1164/ajrccm.157.5.9707141. [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Nino ME, et al. J Cell Biochem. 2006;98:723. doi: 10.1002/jcb.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamp DW, Weitzman SA. Thorax. 1999;54:638. doi: 10.1136/thx.54.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mossman BT, Bignon J, Corn M, Seaton A, Gee JB. Science. 1990;247:294. doi: 10.1126/science.2153315. [DOI] [PubMed] [Google Scholar]
- 5.Rimal B, Greenberg AK, Rom WN. Curr Opin Pulm Med. 2005;11:169. doi: 10.1097/01.mcp.0000152998.11335.24. [DOI] [PubMed] [Google Scholar]
- 6.Materials and methods are available as supporting material on Science Online.
- 7.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Nature. 2006;440:237. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Burns K, Tschopp J. Mol Cell. 2002;10:417. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 9.Mariathasan S, et al. Nature. 2006 [Google Scholar]
- 10.Martinon F, Agostini L, Meylan E, Tschopp J. Curr Biol. 2004;14:1929. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Miao EA, et al. Nat Immunol. 2006;7:569. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 12.Amer A, et al. J Biol Chem. 2006;281:35217. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 13.Petrilli V, et al. Cell Death Differ. 2007;14:1583. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 14.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. J Biol Chem. 2007 doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes-Alnemri T, et al. Cell Death Differ. 2007;14:1590. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen K, Mossman BT. Cancer Res. 1987;47:1681. [PubMed] [Google Scholar]
- 17.Shukla A, et al. Free Radic Biol Med. 2003;34:1117. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 18.Cruz CM, et al. J Biol Chem. 2007;282:2871. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haegens A, et al. J Immunol. 2007;178:1800. doi: 10.4049/jimmunol.178.3.1800. [DOI] [PubMed] [Google Scholar]
- 20.Robledo RF, et al. Am J Pathol. 2000;156:1307. doi: 10.1016/S0002-9440(10)65001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabo-Attwood T, et al. Am J Pathol. 2005;167:1243. doi: 10.1016/S0002-9440(10)61212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agostini L, et al. Immunity. 2004;20:319. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, et al. Int J Oncol. 2004;25:173. [PubMed] [Google Scholar]
- 24.Krelin Y, et al. Cancer Res. 2007;67:1062. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 25.Piguet PF, Vesin C, Grau GE, Thompson RC. Cytokine. 1993;5:57. doi: 10.1016/1043-4666(93)90024-y. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins PN, Lachmann HJ, McDermott MF. N Engl J Med. 2003;348:2583. doi: 10.1056/NEJM200306193482523. [DOI] [PubMed] [Google Scholar]
- 27.So A, De Smedt T, Revaz S, Tschopp J. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.