Abstract
Isoform A of phosphatidylinositol 3-kinase enhancer (PIKE-A) is a newly identified prooncogenic factor that has been implicated in cancer cell growth. How PIKE-A activity is regulated in response to growth signal is poorly understood. Here, we demonstrate that cyclin dependent kinase 5 (Cdk5), a protein known to function mainly in postmitotic neurons, directly phosphorylates PIKE-A at Ser-279 in its GTPase domain in glioblastoma cells. This phosphorylation event stimulates PIKE-A GTPase activity and the activity of its downstream effector Akt. Growth signal activates Cdk5 and results in a Cdk5-dependent accumulation of phosphorylated PIKE-A and activation of Akt in the nucleus. Furthermore, PIKE-A phosphorylation and Cdk5 are increased in human glioblastoma specimens. Phosphorylation of PIKE-A by Cdk5 mediates growth factor-induced migration and invasion of human glioblastoma cells. Together, these findings identify PIKE as the first Cdk5 target in cancer cells, revealing a previously undescribed regulatory mechanism that mediates growth signal-induced activation of PIKE-A/Akt and tumor invasion.
PIKEs [phosphatidylinositol 3 (PI3)-kinase enhancer] are a group of recently identified GTP-binding proteins shown to play an important role in regulating the PI3K-Akt signaling pathway (1, 2). Three forms of PIKEs (S, L, and A) are generated from a single gene by means of alternative splicing and differential transcriptional initiation. The newly identified PIKE-A differs from PIKE-S and -L in important ways (3, 4). Unlike PIKE-S and L, PIKE-A is not brain specific, but rather is expressed ubiquitously. Instead of binding to PI3K like PIKE-S and L, PIKE-A exerts its effects by directly regulating Akt, the key downstream effector of the PI3K/PTEN pathway frequently dysregulated in cancer (5, 6). PIKE-A, whose gene is amplified in neuroblastoma cells (7), has been shown to regulate the invasive behavior of several cancer cell lines and promote cellular transformation (8). However, the precise molecular mechanisms by which PIKE-A is regulated remain largely unknown.
Cyclin dependent kinase 5 (Cdk5) is a member of the Cdk family of serine/threonine kinases (9) and is activated by its regulators p35 or p39. Cdk5 does not regulate the cell cycle. Instead, its expression and activity are highest in postmitotic neurons, where Cdk5 controls many processes including neuronal migration. Cdk5 activity has been detected in oligodendrocytes (10, 11). However, its role in astrocytes is not clear. Cdk5 has been implicated in the regulation of the motility of prostate cancer cells, control of apoptosis in leukemia cells and astrocytoma, and modulation of proliferation of medullary thyroid carcinoma cells (12–16). Cdk5 has been detected in human glioblastomas (17), but how Cdk5 affects glioblastoma cell growth remains unknown.
Here, we investigated the role of Cdk5 in glioblastoma cells. We showed that, in response to growth signal, Cdk5 phosphorylates PIKE-A and stimulates its GTPase activity, which activates nuclear Akt and promotes glioblastoma cell migration and invasion.
Results
Phosphorylation of PIKE-A by Cdk5.
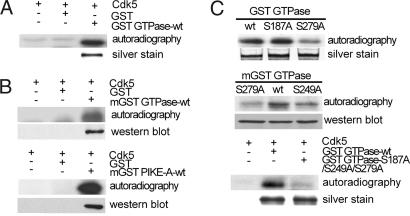
To test whether Cdk5 directly phosphorylates PIKE-A, purified GST PIKE-A GTPase domain was incubated with purified active Cdk5 kinase complex in an in vitro kinase assay (18). Coincubation with active Cdk5 resulted in phosphorylation of the GST GTPase domain (Fig. 1A Upper). To confirm this result, we overexpressed the GST PIKE-A GTPase (mGST GTPase) or GST full-length PIKE-A (mGST PIKE-A) in HEK293 cells, pulled the protein down with glutathione beads [an approach used successfully in other studies (7)], and performed the in vitro Cdk5 kinase assay. Cdk5 phosphorylated both the GTPase domain and full-length PIKE-A (Fig. 1B). Next, we identified the site of phosphorylation in PIKE-A. Site-directed mutagenesis was performed to change the putative Cdk5 recognition sites to alanine. These mutants were then tested as described above. Mutation of S187 in the GTPase domain to Ala had no effect on phosphorylation by Cdk5 (Fig. 1C Top), whereas mutation at either S279 or S249 reduced the level of phosphorylation (Fig. 1C Middle). Mutating all three sites to alanine led to a near complete loss of phosphorylation (Fig. 1C Bottom), indicating that S279 and S249 are the major sites phosphorylated by Cdk5 under our experimental condition.
Fig. 1.
Phosphorylation of PIKE-A by Cdk5 in vitro. (A) Phosphorylation of bacteria-expressed GTPase domain of PIKE-A by Cdk5 in vitro. GST alone was used as negative control. Lower is silver staining of purified GST GTPase. (B) Phosphorylation of mammalian-expressed GST GTPase domain (mGST GTPase WT) and full-length PIKE-A (mGST PIKE-A-WT) by Cdk5 in vitro. (Upper) Kinase results. (Lower) The membrane was reprobed with anti-GST antibody. (C) Identification of Cdk5 phosphorylation sites in GTPase domain. Various GST fusion proteins purified from bacteria were assayed for phosphorylation by Cdk5 as described in A (Top and Bottom). Mammalian-expressed fusion proteins immunoprecipitated from cells were assayed for phosphorylation by Cdk5 (Middle).
Cdk5 Regulates PIKE-A GTPase Activity and Its Binding to Akt.
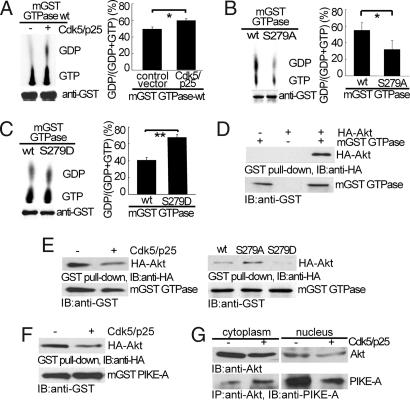
PIKE-A GTPase activity is linked directly to its ability to regulate downstream effectors. To determine how phosphorylation by Cdk5 may affect PIKE-A GTPase activity, we overexpressed the mGST GTPase in HEK293, isolated it, and incubated with [α-32P]GTP in a GTPase assay. The GST control alone showed no GTPase activity (data not shown). Coexpression of Cdk5/p25 significantly increased the conversion of GTP to GDP by mGST-GTPase (Fig. 2A). To show that this activity is an direct effect of Cdk5, we immunoprecipitated the GTPase domain, phosphorylated it in vitro using purified Cdk5 complex, and then determined GTPase activity. In vitro phosphorylation by Cdk5 directly stimulated the GTPase activity [supporting information (SI) Fig. S1]. To determine the sites involved, we tested the mutants for their GTPase activity using a similar assay. Compared with WT GTPase, mutation at S249 did not significantly affect GTPase activity (Fig. S2) but S279A converted few amount GTP to GDP (Fig. 2B). Moreover, mutation of S279 to the charged amino acid residue aspartic acid (S279D) enhanced the conversion of GTP to GDP (Fig. 2C). Taken together, these findings indicate that phosphorylation at S279 by Cdk5 stimulates PIKE-A GTPase activity.
Fig. 2.
Phosphorylation by Cdk5 on PIKE-A GTPase activity and binding of PIKE-A and Akt. (A) Stimulation of PIKE-A GTPase activity by Cdk5. The hydrolysis of GTP to GDP was separated on TLC, measured by autoradiography (Upper Left) and quantified (Right). (Lower Left) Loading of mGST GTPase. (B) Reduction of GTPase activity in S279A mutant. GTPase WT and S279A mutant overexpressed in HEK293 cells were compared for their GTPase activity as described in A. (C) Increase in GTPase activity in S279D mutant. All data above were calculated as means (± SD) of three independent experiments (*, P < 0.05 and **, P < 0.01, Student's t test). (D) Interaction of PIKE GTPase domain and Akt. mGST GTPase and HA-Akt expressed in HEK293 was pulled down with glutathione sepharose 4B beads, and coprecipitated proteins were analyzed by anti-HA antibody. (E) Decreased binding of GTPase domain to Akt. The experiments were carried out as in D under the indicated conditions. (Left) Inhibition of mGST GTPase binding to Akt by Cdk5. (Right) The interaction between Akt and mGST GTPase mutants. (F) Decreased binding of Akt binding to full-length PIKE-A. Binding of Akt and full-length PIKE-A was carried out as in E. (G) Decreased binding of endogenous PIKE-A to Akt in the nucleus. After transfection, Akt was immunoprecipitated from LN-Z308 glioblastoma cells, and coprecipitated PIKE-A was detected by anti-PIKE-A antibody.
Because PIKE-A binds to Akt directly, we tested the effects of phosphorylation at S279 on PIKE-A-Akt interaction. HA-Akt and mGST GTPase were coexpressed in HEK293 cells. mGST GTPase was precipitated from lysates, and the precipitates were blotted for HA-Akt by using anti-HA antibody. This study showed that the WT mGST GTPase readily interacts with Akt (Fig. 2D Upper). Coexpression of Cdk5/p25 clearly reduced the interaction between mGST GTPase and Akt (Fig. 2E Left). Compared with WT mGST GTPase, mGST GTPase S279A showed increased binding to Akt whereas mutant S279D had reduced binding to Akt (Fig. 2E Right). Binding of full-length PIKE-A (mGST PIKE-A) and Akt was inhibited by coexpression of Cdk5/p25 (Fig. 2F). Similarly, overexpression of Cdk5 in LN-Z308 glioblastoma cells significantly reduced the binding between endogenous Akt and PIKE-A in the nucleus (Fig. 2G). These findings indicate that S279 phosphorylation weakens PIKE-A's interaction with Akt.
The effect of Phosphorylation of PIKE-A S279 on Akt Activity.
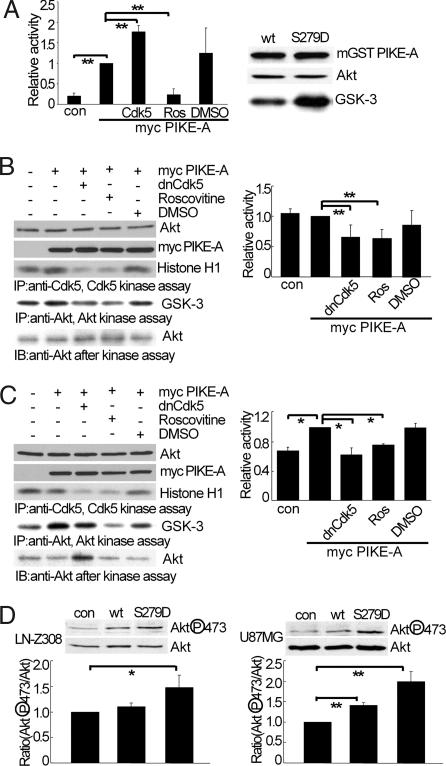
Because PIKE-A is known to enhance Akt activity, we overexpressed PIKE-A in HEK293 cells, immunoprecipitated endogenous Akt, and assayed for kinase activity by measuring phosphorylation of a GSK3β based peptide (cross-tide) in a liquid scintillation based assay. Overexpression of PIKE-A enhanced Akt activity over control (Fig. 3A Left). Coexpression of Cdk5/p25 led to an even greater increase in Akt activity. Treating cells with a selective Cdk5 inhibitor roscovitine attenuated PIKE-A-induced activation of Akt whereas vehicle control had no significant effect. We then compared the ability of WT PIKE-A and PIKE-A S279D mutant to stimulate Akt by measuring phosphorylation of Akt substrate GSK-3 fusion protein. Compared with WT PIKE-A, PIKE-A S279D mutant significantly increased the activity of endogenous Akt (Fig. 3A Right), suggesting that phosphorylation of PIKE-A at S279 is sufficient to enhance Akt activity.
Fig. 3.
Phosphorylation by Cdk5 on PIKE-A-stimulated Akt activity. (A) Stimulation of Akt activity by PIKE-A upon Cdk5-induced phosphorylation. HEK293 was transfected with myc-tagged PIKE-A, cotransfected with Cdk5 or treated with 10 μM Ros (roscovitine). Akt activity was determined by kinase assay using substrate cross tide and analyzed by liquid scintillation counter (Left. con, control). (Right) Wild-type PIKE-A or S279D mutant expressed in HEK293 was immunoprecipitated and assayed for Akt kinase activity by using purified GSK3β as a substrate. (B) The effect of inhibiting Cdk5 on Akt activity in LN-Z308 glioblastoma cells. LN-Z308 cells were transfected and then treated as indicated. (Left) Akt activity was done as in A. (Right) The relative ratio of Akt activity adjusted for the amount precipitated. (C) The effect of inhibiting Cdk5 on Akt activity in U87MG glioblastoma cells. The experiments were carried out as described in B. (D) The effects of S279D mutation on PIKE-A stimulated Akt phosphorylation. After expression of indicated PIKE-A, endogenous Akt phosphorylation was determined. All of the data above were calculated as means (± SD) of three independent experiments (*, P < 0.05 and **, P < 0.01, Student's t test).
The PIKE-A gene is amplified in the highly invasive glioblastoma LN-Z308 cells but not in the less invasive U87MG (19). Blocking endogenous PIKE-A in LN-Z308 and U87MG using siRNA confirmed that PIKE-A is required for Akt activity in these cells (Fig. S3). We then performed experiments similar to Fig. 3A in LN-Z308 cells. Transient overexpression of PIKE-A did not significantly increase Akt activity because of the high level of endogenous PIKE-A already present in these cells. Interestingly, overexpression of a dominant negative Cdk5 (dnCdk5) or treating cells with roscovitine greatly reduced Akt kinase activity (Fig. 3B). In contrast with LN-Z308 cells, overexpression of PIKE-A in U87MG cells resulted in a significant increase in Akt activity (Fig. 3C). This increase was significantly attenuated by blocking endogenous Cdk5 with dnCdk5 or roscovitine. These data suggest that Cdk5 is required for PIKE-A-mediated activation of Akt in glioblastoma cells. To strengthen this conclusion, we examined the effect of PIKE-A mutation in glioblastoma cells. Overexpression of WT PIKE-A in LN-Z308 cells had an insignificant effect on Akt phosphorylation at S473 (Fig. 3D Left) whereas overexpression of PIKE-A S279D greatly increased S473 phosphorylation. In contrast, overexpression of PIKE-A WT and S279D in U87MG increased S473 phosphorylation (Fig. 3D Right). Consistently, overexpression of PIKE-A S279A reduced the activity of endogenous Akt in HEK293 cells whereas the S249A mutant had little effect (Fig. S4). Together, these findings indicate that phosphorylation at S279 by Cdk5 is necessary and sufficient for PIKE-A-mediated activation of Akt.
Regulation of Nuclear Translocation of PIKE-A by Phosphorylation at S279.
Previous studies show that PIKE-A is localized both in the cytoplasm and nucleus. We studied whether phosphorylation of PIKE-A by Cdk5 alters its subcellular localization. To facilitate this study, we generated a phospho antibody and tested it using PIKE-A either unphosphorylated or phosphorylated in vitro or in cells. This phospho antibody specifically recognizes either purified or endogenous PIKE-A in LN-Z308 cells when phosphorylated at S279 (Fig. S5).
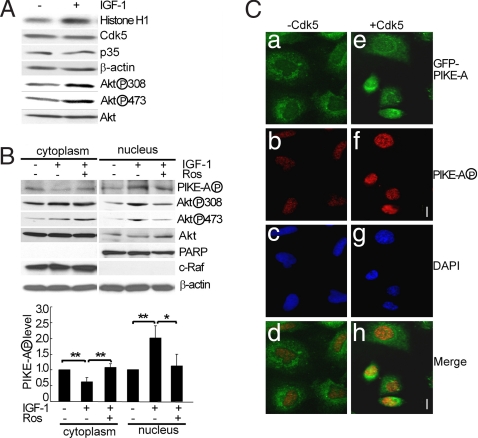
With this new phospho specific PIKE-A antibody, we tested the effects of phosphorylation of PIKE-A on its subcellular distribution in glioblastoma cells in response to IGF-1. We first determined whether IGF-1, a potent activator of PIKE-A and Akt in glioblastoma cells, activates Cdk5. Cdk5 was immunoprecipitated from lysates of LN-Z308 cells treated with IGF-1 and assayed for kinase activity with a widely used substrate Histone 1 (H1) (19). IGF-1 treatment increased Cdk5 activity without significantly altering the levels of Cdk5 or its activator p35 (Fig. 4A. Similar results were obtained by using EGF and U87MG cells. Data not shown), which paralleled an increase in Akt phosphorylation at T308 and S473. We then tested the effect of inhibiting Cdk5 on the phosphorylation of endogenous PIKE-A and its subcellular localization. LN-Z308 cells were stimulated with IGF-1 in the presence or absence of roscovitine. IGF-1 treatment reduced phospho PIKE-A levels in the cytoplasmic fraction but increased phospho PIKE-A signal in the nucleus (Fig. 4B. The same membranes were then reprobed with the nuclear marker PARP and with the cytoplasmic marker c-Raf, showing minimum cross contamination between these fractions. The right graph shows quantification of phospho PIKE-A). The increase in nuclear phospho PIKE-A correlated well with increased Akt phosphorylation. Furthermore, inhibition of Cdk5 with roscovitine reduced the phosphorylation levels for both PIKE-A and Akt in the nucleus. In contrast, roscovitine attenuated the IGF-1-induced change in PIKE-A signal, but did not reduce Akt phosphorylation in the cytoplasm. Similarly, overexpression of dnCdk5 also reduced IGF-1-induced phosphorylation of PIKE-A and Akt in the nucleus (Fig. S6). To corroborate these findings, we determined the subcellular localization of GFP-PIKE-A in LN-Z308 cells after transfection by immunohistochemistry using phospho PIKE-A antibody. Without coexpression of Cdk5/p25, the phospho GFP-PIKE-A signal was low and present largely in the cytoplasm (Fig. 4 Ca–Cd). Coexpression of Cdk5/p25 significantly increased the phospho PIKE-A signal (Fig. 4 Ce–Ch). Furthermore, the phospho PIKE-A signal was present predominantly in the nucleus, suggesting that phosphorylation by Cdk5 leads to preferential accumulation of phospho PIKE-A in the nucleus.
Fig. 4.
Stimulation of Cdk5 activity and PIKE-A phosphorylation by IGF-1. (A) Activation of Cdk5 by IGF-1. Cdk5 kinase activity and levels of other markers were determined in LN-Z308 cells treated with 100 ng/ml of IGF-1 for 10 min. (B) IGF-1-induced phosphorylation of Akt and PIKE-A in the nucleus. LN-Z308 cells were starved overnight and stimulated with IGF-1 as described above (roscovitine was added 30 min before IGF-1 where indicated). Cytoplasmic and nuclear fractions (Sigma EZ nuclei isolation kit) were assayed for various phospho signals and proteins (C-Raf-1, cytoplasmic marker; PARP, nuclear marker). Graph is quantification of three experiments. (C) Immunocytochemistry study of IGF-1-induced phosphorylation of PIKE-A in the nucleus. LN-Z308 cells transfected with GFP-PIKE-A construct with or without constructs for Cdk5/p25 were fixed and stained with the purified phospho PIKE-A antibody. Nuclei were stained with DAPI. (Scale bar: 10 μm).
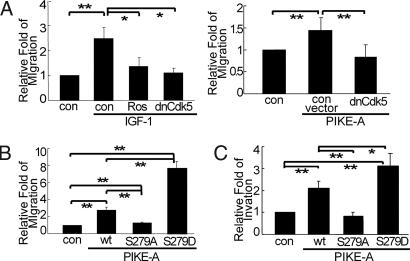
Cdk5-Mediated Phosphorylation of PIKE-A Induces Glioblastoma Cell Migration and Invasion.
Previous studies showed that PIKE-A is required for IGF-1-mediated invasion of glioblastoma cells (8, 20). We investigated whether phosphorylation by Cdk5 modulates PIKE-A-mediated glioblastoma cell migration and invasion. For migration studies, LN-Z308 cells were transfected with a construct encoding GFP alone or in combination with other constructs, plated in a transwell chamber, starved overnight, treated with IGF-1, and scored in a blind manner for the number of GFP positive cells that migrated to the bottom well. Compared with unstimulated controls, IGF-1 greatly increased the number of migratory cells (Fig. 5A Left). Importantly, this increased glioblastoma cell migration was significantly attenuated by overexpression of dnCdk5 or roscovitine. To corroborate this finding, we tested the effect of dnCdk5 on PIKE-A-mediated migration in U87MG cells. Overexpression of PIKE-A in U87MG stimulated cell migration (Fig. 5A Right). Coexpression of dnCdk5 effectively reduced the number of PIKE-A-induced migratory U87MG cells. These results demonstrate that both IGF-1- and PIKE-A-induced glioblastoma cell migration require Cdk5.
Fig. 5.
Phosphorylation by Cdk5 on PIKE-A-mediated glioblastoma cell migration and invasion. (A) The effect of blocking Cdk5 on glioblastoma cell migration. After transfection and starvation, U87MG cells (Right) were scored for migration in a blind manner whereas LN-Z308 (Left) cells were first stimulated with IGF-1 before the scoring. (B) The effect of PIKE-A phosphorylation status on U87MG cell migration. Equal numbers of GFP-PIKE WT and RFP-S279A or RFP-S279D positively transfected cells were mixed, plated into transwell chambers, and scored for the migration of GFP and RFP positive cells as described in Materials and Methods. (C) The effect of PIKE-A phosphorylation status on U87MG cell invasion. Experiment was performed as described in B except for that the cells were placed in Matrigel-coated chamber. All of the data above were calculated as means (± SD.) of three independent experiments (*, P < 0.05 and **, P < 0.01, Student's t test).
To link phosphorylation of PIKE-A at S279 with migration, we examined the effects of PIKE-A mutation in the migration assay. To facilitate quantification, U87MG cells grown in separated dishes were transfected with a vector encoding GFP-WT PIKE-A, RFP-PIKE-A S279A or RFP-PIKE-A S279D. The transfection efficiency was assessed by fluorescence microscope, and the efficiency of transfection was found to be the same for all constructs (data not shown). Equal numbers of GFP and RFP positive cells from different dishes were mixed, plated in a transwell chamber, and scored for the number of GFP and RFP positive cells that migrated into the bottom wells. This method allowed us to compare directly in a single assay the migration rate of cells carrying WT vs. mutated PIKE-A. Our control studies indicated that GFP or RFP alone did not alter the rate of migration (data not shown). Compared with the GFP control, GFP-PIKE-A WT expressing cells showed an enhanced ability to migrate (Fig. 5B). Compared with cells expressing GFP-PIKE-A WT, cells carrying RFP-PIKE-A S279A were far less likely to migrate whereas cells expressing RFP-PIKE-A S279D migrated at a much higher rate. Using the same approach, we tested the effect of PIKE-A mutation on U87MG invasion. Overexpression of GFP-PIKE-A WT stimulated U87MG invasion (Fig. 5C). The RFP-PIKE-A S279A mutant lost its ability to promote cell invasion. In contrast, the RFP-PIKE-A S279D mutant showed an enhanced capacity to induce cell invasion. Because the GFP-PIKE-A S249D mutant promoted invasion to the same degree as PIKE-A WT (Fig. S7), these data demonstrate clearly that Cdk5-dependent phosphorylation of PIKE-A at S279 is critical in regulating glioblastoma cell migration and invasion.
Increased Phosphorylation of PIKE-A at S279 in Human Glioblastoma Specimens.
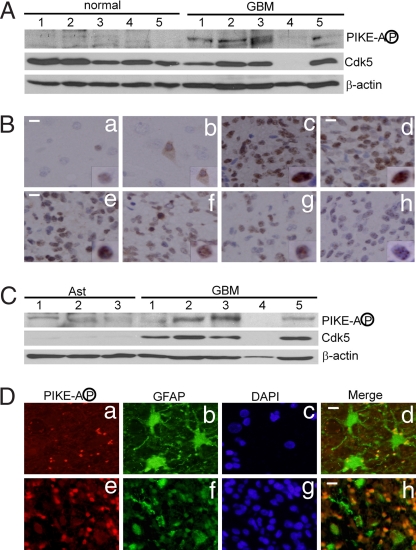
To complement these findings (Fig. 5), we investigated the expression of Cdk5 and the level of S279 phosphorylation in human glioblastoma multiforme (GBM) specimens. Western blot analysis showed that phospho PIKE-A signal is significantly higher in GBM specimens than in normal brain tissue (Fig. 6A). Four of the five glioblastoma specimens had high levels of phospho PIKE-A, whereas this signal was undetectable under our experimental conditions in the normal brain controls. The high levels of phospho PIKE-A were confirmed by immunohistochemistry studies using a glioblastoma tissue array (58 cases and 6 controls). Intense phospho PIKE-A signal in glioblastoma resided predominantly in the nucleus. Representative photomicrographs are shown in Fig. 6B. Immunostaining of normal brain tissue revealed positive staining in neurons (Fig. 6 Ba and Bb). Consistent with the Western blotting data, glioblastoma tissue showed high levels of phospho PIKE-A compared with controls although the levels and patterns of expression varied considerably among individual cases and between regions (Fig. 6 Bc–Bh).
Fig. 6.
Phosphorylation of PIKE-A at S279 in human GBM tumors. (A) Levels of PIKE-A phosphorylation and Cdk5 in GBM tumors and normal human brain tissue. (B) Immunohistochemistry study of human GBM specimens. GBM tissue arrays (58 pathologically confirmed GBM cases and 6 control cases) were stained with purified anti-phospho PIKE-A antibody (Ba and Bb, normal brain tissue controls; Bc–Bh, GBM tumors). (Scale bar: 20 μm.) (C) Levels of PIKE-A phosphorylation and Cdk5 in human GBM tumors and three independent samples of normal human astrocytes (Ast). (D) Immunofluorescence studies of phospho PIKE-A in GBM tumors. GBM tissue arrays were stained with purified anti-phospho PIKE-A, anti-GFAP, and DAPI (Da–Dd, morphologically mature astrocytes in normal area of brain; De–Dh, GBM tumor cells. (Scale bar: 10 μm.)
In contrast with phospho PIKE-A, the level of Cdk5 in glioblastoma is somewhat lower than that in normal brain controls (Fig. 6A). Because glioblastomas are astroglial tumor and normal astrocytes express lower levels of Cdk5 than normal neurons, we determined the levels of Cdk5 in GBM and normal human astrocytes. Compared with signals in glioblastomas, both cdk5 and phospho PIKE-A levels were very low in three independent samples of normal human astrocytes (Fig. 6C). To strengthen this finding, we performed double immunofluorescence on human glioblastoma specimens (Fig. 6 Da–Dh). Mature reactive astrocytes adjacent to the tumor expressed high levels of GFAP but were largely negative for phospho PIKE-A (Fig. 6 Da–Dd). In contrast, the malignant glioblastoma cells generally showed low levels of GFAP but high levels of phospho PIKE-A (Fig. 6 Be and Bf).
Discussion
The diffuse invasiveness of glioblastomas is one of their most striking and deadly features. Some but not all of the molecular players involved in glioblastoma invasion have been identified (6). Included among them is PIKE-A. However, the mechanisms that control PIKE-A are poorly understood. Our present study identifies a critical mechanism that regulates PIKE-A in response to IGF-1, a growth signal that is often altered in glioblastomas, and promotes glioblastoma cell migration and invasion (21). Our findings indicate that Cdk5-mediated regulation of the PIKE-A-Akt pathway plays an important role in the invasive growth of glioblastoma. Because blocking Cdk5 reduces PIKE-A or IGF-1 induced activation of Akt and glioblastoma cell invasion, targeting Cdk5 may offer a therapeutic strategy to inhibit glioblastoma growth and invasiveness. Given the wide tissue distribution of Cdk5 and PIKE-A, Cdk5-mediated phosphorylation of PIKE-A could also be involved in regulating the growth and invasiveness of other tumor types. In this regard, recent studies have revealed the roles of Cdk5 in nonneuronal cells (22) including several cancer models (12–16, 23). It would be interesting to test Cdk5-PIKE-A in these other models.
The presence of Cdk5 in glioblastoma samples has been reported (17). However, it was not clear from the previous study whether Cdk5 was aberrantly expressed in GBM specimens. Our studies showed that the level of Cdk5 in glioblastomas is a little lower than that in normal human brain tissues but significantly higher than that in normal human astrocytes. This result is consistent with the fact that Cdk5 level is higher in neurons than in normal astrocytes, indicating a substantial increase in Cdk5 in glioblastoma cells. Our immunohistochemistry studies of glioblastoma specimens revealed a complex expression pattern for phospho PIKE-A. Although phospho PIKE-A signal is generally high in glioblastomas, both the level and pattern of expression vary among individual cases and different regions of the same samples. The phospho PIKE-A signal is present primarily in the nucleus, although low levels of cytoplasmic staining can also be detected in some samples. This result is consistent with our cellular findings (see below) and with the previously reported role of PI3K/PIKE-A in the nucleus (24). The significance of these variations is unclear at the present time and requires further study.
Our current data reveal a previously uncharacterized mode of regulation of PIKE-A. In response to IGF-1 stimulation, the level of phospho PIKE-A decreases in the cytoplasm and increases in the nucleus. Overexpression of Cdk5 leads to a significant increase in phospho PIKE-A signal concentrated mainly in the nucleus. Coupled with the finding that roscovitine and dnCdk5 clearly reduce Akt phosphorylation in the nucleus but not in the cytoplasm, our results suggest a model in which Cdk5-mediated phosphorylation of PIKE-A functions primarily or even specifically within the nucleus to regulate nuclear Akt. Because Cdk5 level is higher in the cytoplasm than in the nucleus, our study raises the question of whether IGF-1-induced activation of Cdk5 is also compartmentalized.
The precise mechanisms by which PIKE-A regulates Akt activity remain unknown. Previous studies have suggested that the GTPase domain of PIKE-A directly interact with Akt. This interaction is in part regulated by GTPase activity and stimulated by GTP (20). However, analysis of various PIKE-A mutants, some of which are derived from patients, provides a far more complex picture (25) without a clear and consistent correlation between GTPase activity and binding to Akt. Our data showed that phosphorylation of PIKE-A by Cdk5 stimulates PIKE-A GTPase activity, reduces binding between PIKE-A and Akt, and activates Akt kinase. Clearly, the precise mechanism involved in these interactions needs further study. It is possible that the binding between PIKE-A and Akt reflects only part of a dynamic process by which PIKE-A affects Akt, and it may depend on the input signals under stimulated vs. basal conditions.
The PI3K/Akt pathway performs many important functions, including survival (7). It has been noted that invading or migrating glioblastoma cells are more resistant to apoptosis because of increased PI3K/Akt signaling (26, 27), suggesting that glioblastoma cell invasion and survival may be linked. Because Cdk5 has been implicated in cell survival in the brain and many other organ systems (19, 23, 28, 29), it is possible that Cdk5-mediated modulation of PIKE-A/Akt may regulate a number of different functions including survival in glioblastoma as well as other cell types.
Materials and Methods
Cells and Reagents.
HEK293, human glioblastoma LN-Z308, and U87MG cells were maintained in Dulbecco's modified Eagle's medium with 10% FBS. Antibodies to Cdk5 and p35 were from Santa Cruz Biotechnology. Polyclonal rabbit antibody to phospho Ser-279 of PIKE was raised against a peptide corresponding to a sequence containing the phospho Ser-279 motif in PIKE-A. Roscovitine, cross tide, and IGF-1 were purchased from Sigma–Aldrich. GST-GSK3 fusion protein was from Cell Signaling. Recombinant Cdk5/p25 complex and Histone H1 were from Millipore.
Cdk5 and Akt Kinase Assay.
Purified recombinant GST-GTPase or mGST-PIKE-A isolated from mammalian cells by glutathione-sepharose 4B beads were incubated with purified Cdk5/p25 complex in a kinase buffer containing [γ-32P]ATP (18). To determine Cdk5 activity, Cdk5 from lysates (100 μg) was immunoprecipitated with anti-Cdk5 antibody, washed, and incubated in the kinase buffer containing 1 μg of histone H1 and [γ-32P]ATP. Phosphorylation of substrates after SDS/PAGE was determined by autoradiography. Akt kinase assay was carried out as described by Ahn et al. (20).
GTPase Activity Assay.
The GTPase assay was carried out as described by Hu et al. (25). Isolated mGST-GTPase of PIKE-A was incubated with 0.1 μM [α-32P]GTP. The eluted GTP and GDP were separated by TLC on polyethyleneimine-cellulose plates.
Cell Migration and Invasion Assay.
Cell migration and invasion assay was carried out as described by Ahn et al. (20) with some modifications. Cells (1 × 105) were added to each chamber (without Matrigel coating) and incubated with media containing 100 ng/ml IGF-1 without serum for 6 h in an incubator. The membrane of the chambers was rinsed three times on both sides with DME medium. Cells on the lower surface of the scrubbed membranes were fixed, and random fields were counted under the fluorescence microscope. The assay was performed in duplicate. For the invasion test, cells were placed in a Matrigel-coated chamber. The chambers were incubated for 16 h in media.
Supplementary Material
Acknowledgments.
We thank Deborah Cooper at the Center for Neurodegenerative Disease at Emory University for her help with the immunohistochemical studies and Brian Ciliax for critique of the manuscript. This work was supported by National Institutes of Health Grants R01 NS048254 and AG023695, and by The Robert W. Woodruff Health Sciences Center Fund (Z.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712306105/DCSupplemental.
References
- 1.Ye K, Hurt K-J, Wu F-Y, Fang M, Luo H-R, et al. PIKE. A nuclear GTPase that enhances PI3 kinase activity and is regulated by protein 4.1N. Cell. 2000;103:919–930. doi: 10.1016/s0092-8674(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 2.Ye K, Snyder S-H. PIKE GTPase: A novel mediator of phosphoinositide signaling. J Cell Sci. 2004;117:155–161. doi: 10.1242/jcs.00924. [DOI] [PubMed] [Google Scholar]
- 3.Nagase T, Seki N, Ishikawa K, Tanaka A, Nomura N. Prediction of the coding sequences of unidentified human genes. V. The coding sequences of 40 new genes (KIAA0161-KIAA0200) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1996;3:17–24. doi: 10.1093/dnares/3.1.17. [DOI] [PubMed] [Google Scholar]
- 4.Xia C, et al. GGAPs, a new family of bifunctional GTP-binding and GTPase-activating proteins. Mol Cell Biol. 2003;23:2476–2488. doi: 10.1128/MCB.23.7.2476-2488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser M-M, et al. PTEN loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–7779. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 6.Newton H-B. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis. Expert Rev Anticancer Ther. 2004;4:105–128. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Ahn J-Y, Hu Y, Kroll T-G, Allard P, Ye K. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA. 2004;101:6993–6998. doi: 10.1073/pnas.0400921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Hu Y, Hao C, Rempel S-A, Ye K. PIKE-A is a proto-oncogene promoting cell growth, transformation and invasion. Oncogene. 2007;26:4918–4927. doi: 10.1038/sj.onc.1210290. [DOI] [PubMed] [Google Scholar]
- 9.Cruz J-C, Tsai L-H. A Jekyll and Hyde kinase: Roles for Cdk5 in brain development and disease. Curr Opin Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Tang X-M, Strocchi P, Cambi F. Changes in the activity of cdk2 and cdk5 accompany differentiation of rat primary oligodendrocytes. J Cell Biochem. 1998;68:128–137. doi: 10.1002/(sici)1097-4644(19980101)68:1<128::aid-jcb13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto Y, et al. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci. 2007;24:4355–4366. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- 12.Gao C, et al. Cdk5 mediates changes in morphology and promotes apoptosis of astrocytoma cells in response to heat shock. J Cell Sci. 2001;114:1145–1153. doi: 10.1242/jcs.114.6.1145. [DOI] [PubMed] [Google Scholar]
- 13.Sandal T, Stapnes C, Kleivdal H, Hedin L, Doskeland S-O. A novel, extraneuronal role for cyclin-dependent protein kinase 5 (CDK5): Modulation of cAMP-induced apoptosis in rat leukemia cells. J Biol Chem. 2002;277:20783–20793. doi: 10.1074/jbc.M112248200. [DOI] [PubMed] [Google Scholar]
- 14.Strock C-J, et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66:7509–7515. doi: 10.1158/0008-5472.CAN-05-3048. [DOI] [PubMed] [Google Scholar]
- 15.Goodyear S, Sharma M-C. Roscovitine regulates invasive breast cancer cell (MDA-MB231) proliferation and survival through cell cycle regulatory protein cdk5. Exp Mol Pathol. 2007;82:25–32. doi: 10.1016/j.yexmp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Chen M-C, Chiu C-Y, Song Y-M, Lin S-Y. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J Biol Chem. 2007;282:2776–2784. doi: 10.1074/jbc.M607234200. [DOI] [PubMed] [Google Scholar]
- 17.Catania A, et al. Expression and localization of cyclin-dependent kinase 5 in apoptotic human glioma cells. Neuro Oncol. 2001;3:89–98. doi: 10.1093/neuonc/3.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, et al. Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2005;25:4823–4834. doi: 10.1523/JNEUROSCI.1331-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong X, et al. Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron. 2003;38:33–46. doi: 10.1016/s0896-6273(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 20.Ahn J-Y, et al. PIKE (phosphatidylinositol 3-kinase enhancer)-A GTPase stimulates Akt activity and mediates cellular invasion. J Biol Chem. 2004;279:16441–16451. doi: 10.1074/jbc.M312175200. [DOI] [PubMed] [Google Scholar]
- 21.Trojan J, Cloix J-F, Ardourel M-Y, Chatel M, Anthony D-D. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience. 2007;145:795–811. doi: 10.1016/j.neuroscience.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Rosales J-L, Lee K-Y. Extraneuronal roles of cyclin-dependent kinase 5. BioEssays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 23.Smith P-D, et al. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci. 2006;26:440–447. doi: 10.1523/JNEUROSCI.2875-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn J-Y, Rong R, Liu X, Ye K. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J. 2004;23:3995–4006. doi: 10.1038/sj.emboj.7600392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Liu Z, Ye K. Phosphoinositol lipids bind to phosphatidylinositol 3 (PI3)-kinase enhancer GTPase and mediate its stimulatory effect on PI3-kinase and Akt signalings. Proc Natl Acad Sci USA. 2005;102:16853–16858. doi: 10.1073/pnas.0507365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joy A-M, et al. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 27.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, et al. Neuronal cyclin-dependent kinase 5 activity is critical for survival. J Neurosci. 2001;21:550–558. doi: 10.1523/JNEUROSCI.21-02-00550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung Z-H, Ip N-Y. Cdk5: mediator of neuronal death and survival. Neurosci Lett. 2004;361:47–51. doi: 10.1016/j.neulet.2003.12.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.