Abstract
Luteolin, a flavonoid found in high concentrations in celery and green pepper, has been shown to reduce production of proinflammatory mediators in LPS-stimulated macrophages, fibroblasts, and intestinal epithelial cells. Because excessive production of proinflammatory cytokines by activated brain microglia can cause behavioral pathology and neurodegeneration, we sought to determine whether luteolin also regulates microglial cell production of a prototypic inflammatory cytokine, IL-6. Pretreatment of primary murine microlgia and BV-2 microglial cells with luteolin inhibited LPS-stimulated IL-6 production at both the mRNA and protein levels. To determine how luteolin inhibited IL-6 production in microglia, EMSAs were performed to establish the effects of luteolin on LPS-induced binding of transcription factors to the NF-κB and activator protein-1 (AP-1) sites on the IL-6 promoter. Whereas luteolin had no effect on the LPS-induced increase in NF-κB DNA binding activity, it markedly reduced AP-1 transcription factor binding activity. Consistent with this finding, luteolin did not inhibit LPS-induced degradation of IκB-α but inhibited JNK phosphorylation. To determine whether luteolin might have similar effects in vivo, mice were provided drinking water supplemented with luteolin for 21 days and then they were injected i.p. with LPS. Luteolin consumption reduced LPS-induced IL-6 in plasma 4 h after injection. Furthermore, luteolin decreased the induction of IL-6 mRNA by LPS in hippocampus but not in the cortex or cerebellum. Taken together, these data suggest luteolin inhibits LPS-induced IL-6 production in the brain by inhibiting the JNK signaling pathway and activation of AP-1 in microglia. Thus, luteolin may be useful for mitigating neuroinflammation.
Keywords: cytokines, flavonoids, MAPK, brain, neuroinflammation
Lipopolysaccharide (LPS) is an outer membrane component of Gram-negative bacteria that is recognized by the immune system as a pathogen-associated molecular pattern. It binds the Toll-like receptor 4 (TLR4)/CD14 complex on the surface of mammalian cells (1) and activates cell signaling pathways to induce expression of inflammatory genes including IL-1β, IL-6, and TNF-α (2). Peripheral injection of LPS in rodents stimulates cells of the innate immune system and increases the production of inflammatory cytokines. Cytokines produced in the periphery convey a message to the CNS and induce microglial cells to generate a similar cytokine response within the brain. In this way, microglial cells participate in the formation of the sickness behavior syndrome, which when moderately expressed, is adaptive (3). In some instances, however, peripheral infection results in an exaggerated brain cytokine response and behavioral pathology. For example, old mice that expressed high levels of inflammatory cytokines in the brain after peripheral injection of LPS remained anorectic longer (4), exhibited depression-like behavior longer (5), and experienced deficits in hippocampal-dependent working memory not seen in young adult cohorts (6). Peripheral infection also exacerbated neurodegeneration in animal models of multiple sclerosis, Alzheimer's disease, and prion disease by inducing an exaggerated inflammatory cytokine response in the brain (7). Therefore, inhibiting inflammatory cytokine production by activated microglia may be useful for preventing neurobehavioral deficits and neurodegeneration.
Flavonoids, plant polyphenolic compounds abundant in fruits and vegetables, exhibit a wide variety of biological effects, including antioxidant free-radical scavenging and antiinflammatory properties (8). The flavonoid luteolin (3′,4′,5,7-tetrahydroxyflavone), abundant in celery, green pepper, parsley, perilla leaf, and chamomile tea (9), is of particular interest for modulating immune reactions as several studies comparing the antiinflammatory properties of luteolin with other flavonoids such as quercetin, genistein, or hesperetin in peripheral macrophages found luteolin to be most potent (10, 11). Luteolin has been shown to inhibit LPS-induced NF-κB transcriptional activity in intestinal epithelial cells, mouse bone-marrow derived dendritic cells (12), murine macrophage cells (10), and rat fibroblasts (13). In another study (10), luteolin inhibited LPS-stimulated TNF-α and IL-6 in a murine macrophage cell line. These studies suggest luteolin modulates cell signaling pathways activated by LPS and subsequent production of inflammatory cytokines.
Although the effects of flavonoids on the NF-κB inflammatory pathway have received considerable attention, expression of the inflammatory cytokine IL-6 is mediated by an activator protein-1 (AP-1) regulatory element in addition to NF-κB (14). It is not known whether luteolin inhibits AP-1 activitiy in microglia. AP-1 is regulated by c-Jun and the MAPK, JNK, which is stimulated by LPS binding to the TLR4/CD14 complex. Activated JNK phosphorylates the NH2-terminal activation domain of c-Jun, resulting in DNA binding and AP-1 activation. The IL-6 gene promoter contains functional AP-1 consensus sequences (14), and only recently have proinflammatory actions in microglia been shown to be mediated by JNK (15). JNK is now viewed as a pharmacological target for inhibiting brain inflammation and protecting neurons (16, 17).
We examined the effects of luteolin on LPS-induced production of IL-6 by microglial cells in vitro and found evidence suggesting luteolin inhibits the JNK and AP-1 signaling pathway and production of IL-6. Furthermore, in mice provided drinking water supplemented with luteolin, IL-6 was reduced in peripheral blood and discrete brain areas after peripheral injection of LPS. Thus, luteolin is a promising agent for preventing and treating neuroinflammation mediated by the JNK and AP-1 signaling pathway, and it may be useful for mitigating a dysregulated linkage between the immune system and brain.
Results
Effect of Luteolin on LPS-Induced IL-6 Production in Microglial Cells.
Primary murine microglia and BV-2 cells were pretreated with luteolin (0, 10, 25, and 50 μM) and stimulated with 10 ng/ml LPS for a 24-h incubation period. IL-6 concentration in supernatant of microglial cells stimulated with LPS increased to 1,100 pg/ml. Pretreatment of primary microglia with 10 and 25 μM luteolin reduced LPS-induced IL-6 production by 40% and 90%, respectively (Fig. 1A). When luteolin was increased to 50 μM, IL-6 secretion by LPS-stimulated microglia was completely blocked. In a separate, but similar, study, the effect of luteolin on IL-6 secretion by LPS-stimulated BV-2 cells was assessed (Fig. 1B). IL-6 concentration in supernatant of BV-2 cells stimulated with LPS increased to 2,800 pg/ml. Pretreatment of BV-2 cells with 10 and 25 μM reduced LPS-stimulated IL-6 by 40% and 80%, respectively. The highest concentration of luteolin suppressed IL-6 secretion completely. Neither LPS nor luteolin affected cell survival or proliferation (data not shown).
Fig. 1.
Suppression of LPS-induced IL-6 secretion in primary microglia and BV-2 cells. Primary microglia (A) and BV-2 cells (B) were pretreated with luteolin for 1 h and stimulated with LPS for a 24 h incubation period. IL-6 secretion was measured by ELISA. Concentration of IL-6 in supernatants from primary microglia and BV-2 cells stimulated with LPS was 1,100 and 2,800 pg/ml, respectively. Data are presented as percentage of IL-6 release compared with IL-6 level of LPS alone treatment (100%). Bars represent the mean ± SEM from three independent experiments. Means with different letters are different (P < 0.05).
To determine whether the decrease in LPS-induced IL-6 protein measured in supernatants was associated with a decrease in steady-state levels of IL-6 mRNA, primary microglia and BV-2 cells were incubated 1 h with the indicated concentrations of luteolin and then incubated for 8 h with 10 ng/ml LPS (Fig. 2). As anticipated, LPS induced a marked increase in IL-6 mRNA in primary microglia (1,200-fold increase) and BV-2 cells (3,000-fold increase). Pretreatment with luteolin reduced steady-state levels of IL-6 mRNA in a dose-dependent manner in both primary microglia (Fig. 2A) and BV-2 cells (Fig. 2B). Taken together, these data indicate that luteolin is a potent inhibitor of LPS-induced IL-6 production in both primary microglia and BV-2 cells.
Fig. 2.
Suppression of LPS-induced IL-6 mRNA expression in primary microglia and BV-2 cells. Primary microglia (A) and BV-2 cells (B) were pretreated with luteolin for 1 h and stimulated with LPS for an 8-h incubation period. Expression of IL-6 mRNA was measured by RT-PCR. In primary microglia the threshold cycle of GAPDH and IL-6 was 32 and 19, respectively. In BV-2 cells the threshold cycle of GAPDH and IL-6 was 27 and 13, respectively. Data are presented as percentage of IL-6 mRNA expression compared with IL-6 mRNA expression level of LPS-alone treatment (100%). Bars represent the mean ± SEM from three independent experiments. Means with different letters are different (P < 0.05).
Effect of Luteolin on NF-κB and AP-1 DNA Binding Activity in Microglia.
To determine the effects of luteolin on transcription factor signaling pathways that might mediate LPS-induced IL-6 production, EMSA was performed. Because BV-2 cells and primary microglia responded similarly to LPS and luteolin, and the number of microglia that can be cultured from neonatal mice is limited, BV-2 cells were used. Cells were pretreated with 0 and 50 μM luteolin for 1 h after which they were stimulated with LPS (10 ng/ml) for a 1-h incubation period. The 1-h treatment of LPS was determined to be optimal in a preliminary study (data not shown). Nuclear protein was extracted and subjected to EMSA using oligonucleotide probes corresponding to the consensus binding sites for NF-κB or AP-1. Representative gels are shown in Fig. 3 A and B, and the average DNA binding as determined by densitometric scanning of gels from three separate studies is shown in Fig. 3 C and D. NF-κB and AP-1 binding activity was induced by LPS treatment (Fig. 3 A and B, lanes 1 and 5). The binding specificity was verified by incubating nuclear extracts from LPS-stimulated BV-2 cells with excess unlabeled specific competitor oligonucleotide probe (Fig. 3 A and B, lane 2) or unlabeled nonspecific ologonucleotide probe (Fig. 3 A and B, lane 3). Whereas treatment with the NF-κB inhibitor pyrrolidinedithiocarbamate (PDTC) completely blocked LPS-induced NF-κB DNA binding (Fig. 3A, lane 8), luteolin did not affect this response to LPS (Fig. 3A, lane 7). However, the increased AP-1 binding activity induced by LPS was attenuated by pretreatment with luteolin (Fig. 3B, lane 7).
Fig. 3.
Luteolin inhibits LPS-induced AP-1 activation. BV-2 cells were pretreated with luteolin for 1 h and stimulated with LPS for a 1 h incubation period. (A and B) Nuclei were extracted for NF-κB (A) and AP-1 (B) EMSA. PDTC was used as a NF-κB inhibitor. (C and D) Transcription factor binding activity for NF-κB (C) and AP-1 (D) was determined by densitometric scanning. Bars represent the mean ± SEM from three independent experiments. Means with different letters are different (P < 0.05).
Effects of Luteolin on IκB-α Degradation and Phosphorylation of JNK.
To determine whether luteolin modulates IκB-α and MAPK, which are upstream molecules in the NF-κB and AP-1 signaling pathways, respectively, BV-2 cells were pretreated with 0, 25, or 50 μM luteolin for 1 h and stimulated with LPS for a 30-min incubation period. The 30-min treatment of LPS was determined to be optimal in a preliminary study that examined IκB-α degradation and MAPK phosphorylation at 5, 15, 30, 45, and 60 min after LPS treatment (data not shown). Treatment with LPS resulted in degradation of IκB-α, a necessary prelude to NF-κB activation (Fig. 4A). As anticipated, prior treatment with PDTC inhibited IκB-α degradation. However, luteolin did not inhibit LPS-induced IκB-α degradation, which is consistent with the failure of luteolin to inhibit NF-κB DNA binding activity (Fig. 3A). As anticipated, stimulation with LPS resulted in the phosphorylation of Erk, p38, and JNK (Fig. 4 B–D). However, pretreatment of BV-2 cells with 25 and 50 μM luteolin inhibited only JNK phosphorylation (Fig. 4D).
Fig. 4.
Luteolin inhibits LPS-induced phosphorylation of JNK. BV-2 cells were pretreated with luteolin for 1 h and stimulated with LPS for a 30-min incubation period. Cells were collected, and cell lysates were separated by 12% SDS/PAGE, transferred to membranes, and blotted with specific antibodies to IκB-α (A), Erk and phosphorylated Erk (B), p38 and phosphorylated p38 (C), and JNK and phosphorylated JNK (D). β-Actin was blotted as an internal control. Bars represent the mean ± SEM from three independent experiments. Means with different letters are different (P < 0.05).
Effect of Luteolin on IL-6 Production in the Periphery and Brain After Injection of LPS.
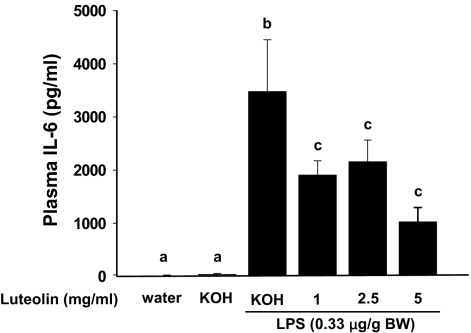
Peripheral administration of LPS stimulates peripheral macrophages to produce IL-6 and other inflammatory cytokines. The peripheral cytokines convey a message to the brain and stimulate microglia to produce a similar set of cytokines. Therefore, we investigated whether intake of luteolin would attenuate IL-6 production both in the periphery and brain of mice injected with LPS. Mice were provided 0, 1, 2.5, and 5 mg/ml luteolin in drinking water for 21 days and then injected i.p. with LPS [0.33 μg/g body weight (BW)]. Based on average daily intake, mice given water with 0, 1, 2.5, and 5 mg/ml luteolin ingested 0, 4.7, 10.6, and 19.5 mg of luteolin per day, respectively. As anticipated, LPS induced IL-6 in both the periphery and brain (Figs. 5 and 6). Mice given luteolin, however, had lower plasma levels of IL-6 protein irrespective of the supplementation level (Fig. 5). The highest supplemental level of luteolin reduced LPS-induced IL-6 mRNA in the hippocampus, but IL-6 expression in other brain areas was not affected (Fig. 6).
Fig. 5.
Luteolin reduces LPS-stimulated IL-6 production in plasma. Mice fed luteolin (0, 1, 2.5, or 5 mg/ml) for 21 days were injected with LPS (0.33 μg/g BW). At 4 h postinjection, mice were killed, and plasma was collected for IL-6 measurement. Bars represent the mean ± SEM (n = 9). Means with different letters are different (P < 0.05).
Fig. 6.
Luteolin reduces LPS-stimulated IL-6 production in hippocampus. Mice fed luteolin (0, 1, 2.5, or 5 mg/ml) for 21 days were injected with LPS (0.33 μg/g BW). Four hours after LPS injection, cortex (Left), cerebellum (Center), and hippocampal (Right) tissue were collected, and IL-6 mRNA was measured by RT-PCR. Bars represent the mean ± SEM (n = 9). Means with different letters are different (P < 0.05).
Discussion
In this study, we showed that the polyphenolic plant-derived flavonoid, luteolin, inhibited binding to the AP-1 site on the IL-6 promoter and subsequent production of IL-6 by microglial cells stimulated with LPS. Luteolin reduced AP-1 transcription factor DNA binding and inhibited phosphorylation of the MAPK, JNK. The inhibitory properties of luteolin on kinase activity were discrete, as several steps in the NF-κB activation pathway were uninterrupted. Additionally, the antiinflammatory properties of luteolin were evident in vivo, as LPS-induced IL-6 production in both the periphery and hippocampus was reduced in mice provided luteolin-supplemented drinking water. These results suggest that luteolin inhibits the JNK signaling pathway and activation of AP-1 in microglia. Thus, luteolin is a promising agent for preventing and treating neuroinflammation mediated by the JNK and AP-1 signaling pathway and may be useful for mitigating a dysregulated linkage between the immune system and brain.
There are three isoforms of JNK, which are also referred to as stress-activated kinases (18). Whereas JNK1 and JNK2 are ubiquitously expressed, JNK3 is restricted to the brain, heart, and testis. In neurons, activated JNK targets substrates that induce apoptotic responses (19), and as a result, therapeutic strategies aimed at inhibiting JNK have emerged as a means to reduce cell death resulting from cerebral ischemia or diseases such as Parkinson's and Alzheimer's (17, 20). In brain, however, JNK activity is not restricted to neurons and apoptotic pathways, because all three isoforms of JNK are present in microglia where they mediate proinflammatory actions (15). In primary microglia cultures from neonatal rat brain, inhibition of JNK with SP600125 reduced LPS-induced metabolic activity and the induction of several genes regulated by AP-1, including IL-6 (15). Thus, our finding in the present study that luteolin inhibited LPS-induced JNK phosphorylation, AP-1 DNA binding activity, and IL-6 expression and release confirms the proinflammatory nature of the JNK pathway in microglial cells. It demonstrates, further, an additional antiinflammatory property of luteolin.
It is important to note that we do not contend that all of the antiinflammatory effects of luteolin are mediated via JNK and AP-1. The JNK inhibitor, SP600125, only partially reduced (30–40%) LPS-induced IL-6 secretion in rat microglial cells (15). Thus, if luteolin acted only via the JNK pathway we would not have seen the nearly complete inhibition of IL-6 production (Fig. 1). It is more likely that luteolin inhibited both AP-1 and NF-κB transcriptional activity. A previous study in rat fibroblasts found that luteolin inhibited LPS-induced NF-κB transcriptional activity without preventing degradation of IκB-α and translocation of NF-κB from the cell cytoplasm to the nucleus (13). In another study, luteolin partially inhibited TNF-α-induced NF-κB DNA binding activity but completely blocked NF-κB transcriptional activity in mouse intestinal epithelial cells (21). In our study, luteolin also failed to inhibit IκB-α degradation and NF-κB DNA binding. Although we did not measure transcriptional activity, based on these earlier reports and our own findings showing NF-κB is involved in IL-6 expression in microglia (22–24), we suspect the nearly complete blockade of LPS-induced IL-6 production by luteolin was the net result of its effects on the JNK/AP-1 signaling pathway and NF-κB transcriptional activity, which act independently or coordinately to regulate expression of target genes (25). Interaction between components of NF-κB (p65) and AP-1 (c-Jun/c-Fos) presents a possible mechanism for coordinate control of these factors (26). It has been reported that expression of dominant negative c-jun results in AP-1 and NF-κB down-regulation (27). In any case, our data are intriguing because they suggest that luteolin inhibits LPS-induced JNK phosphorylation in microglia, thus demonstrating the functional diversity of luteolin in inhibiting inflammatory pathways.
An important objective of our study was to determine whether the effects of luteolin on IL-6 production in vitro translated to the in vivo setting. We were encouraged by previous studies suggesting an inverse relationship between dietary flavonoid concentration and degree of inflammation associated with pathologic conditions. For example, Commenges et al. (28) examined records from 1,367 subjects 65 years of age or older and concluded that the intake of flavonoids is inversely related to dementia. Flavonoids also reduce the incidence of cerebrovascular disease in humans (29), are protective in cardiovascular disease and cancer (30), reduce clinical symptoms in a rodent model of multiple sclerosis (31), and inhibit inflammation in an animal model of arthritis (32).
In our mouse model, provision of luteolin for 21 days attenuated LPS-induced expression of IL-6 in the periphery (as assessed by measuring plasma IL-6 concentration) and in the hippocampus (as assessed by measuring IL-6 mRNA). These results are noteworthy because IL-6 in the periphery plays a role in the pathogenesis of immunologically mediated fatigue and loss of strength (33, 34). Furthermore, the hippocampus is densely populated with microglia, and inflammatory cytokines are well known to inhibit working memory (35) and memory consolidation (36) in hippocampal-dependent tasks. Although flavonoids can penetrate the blood–brain barrier (37), it is unclear whether luteolin entered the brain to directly inhibit IL-6 expression or whether it reduced hippocampal IL-6 indirectly by reducing peripheral responses to LPS. Regardless, we provide evidence to suggest luteolin may be useful for mitigating neuroinflammation.
Materials and Methods
Reagents.
LPS from Escherichia coli (serotype 0127:B8) was obtained from Sigma. Luteolin was obtained from Calbiochem and Synorex. The antibodies and standards for the IL-6 ELISA were purchased from PharMingen. The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay was purchased from Promega. DMEM and HBSS were purchased from Bio-Whittaker. FBS was purchased from HyClone. Penicillin and streptomycin were purchased from Invitrogen.
Animals.
Neonatal (<3 days old) and adult (3–6 months) male BALB/c mice were obtained from our breeding colony kept in barrier-reared conditions in a specific pathogen free facility at the University of Illinois. Mice were housed in polypropylene cages and maintained at 21°C under a reverse-phase 12-h light-dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
Primary Microglial Cell Culture.
Primary microglial cell cultures were established from neonatal mice, as described (38). In brief, whole brains were aseptically removed and mechanically dissociated after a 15-min trypsinization (0.25% trypsin). Brain tissue was passed through a 100-mm nylon mesh, washed twice in HBSS, and plated on poly-l-lysine-coated 162-cm2 culture flasks in growth medium (DMEM supplemented with 20% FBS, 3.7 g/liter sodium bicarbonate, 200 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone, 50 μg/ml gentamicin). Mixed glia cultures were maintained at 37°C, 95% humidity, and 5% CO2. Growth medium was replenished every other day until confluency. Mixed glia cultures were shaken for 3 h at 37°C, and nonadherent microglia were collected, counted by trypan blue staining, and plated at a density of 1 × 105 cells/ml on poly-l-lysine-coated 24-well plates. Resultant cultures contained >95% microglia. After 48 h, microglia were washed twice with DMEM and supplemented with warm growth medium containing experimental treatments. Cell viability was measured by the MTS cell proliferation assay according to the manufacturer's instructions.
BV-2 Microglial Cell Culture.
The immortalized murine microglia cell line, BV-2 (a gift from Linda Van Eldik, Northwestern University, Evanston, IL), has been used as a model to investigate the neuroimmune system (39, 40). BV-2 cells were maintained in 150-cm2 tissue culture flasks (BD Falcon) in DMEM supplemented with 10% FBS and 100 units/ml penicillin/streptomycin at 37°C in a humidified incubator under 5% CO2. Confluent cultures were passed by trypsinization. Cells were centrifuged (5 min at 4°C, 250 × g), and culture medium was removed. In all experiments, cells were resuspended in DMEM supplemented with 10% FBS and seeded in six-well plates (BD Falcon) before being subjected to treatments. Cell viability was measured by the MTS cell proliferation assay according to the manufacturer's instructions.
IL-6 Production.
Primary microglia and BV-2 cells were pretreated with vehicle (0.05% DMSO, vol/vol) or luteolin (0–50 μM) for 1 h and incubated with 10 ng/ml LPS for 8 h to determine IL-6 mRNA expression or 24 h to determine supernatant IL-6 concentration. Cytokine gene expression was determined at 8 h because a preliminary study that examined IL-6 mRNA at various times after introduction of LPS (0, 2, 4, 8, 16, and 24 h) revealed maximal induction of IL-6 mRNA at this time point. IL-6 protein in supernatant was determined 24 h after LPS treatment as we have reported (23). Luteolin was introduced at the 0- to 50-μM range because the physiologically relevant concentration of flavonoids is reportedly 10 μM (41, 42), and one study showed that plasma luteolin increased up to 14 μM after 50 μmol/kg of luteolin administration (9).
Total RNA was isolated from primary microglia and BV-2 cells by using the Tri Reagent protocol (Sigma). RNA samples were subjected to a DNase I digestion procedure and then reverse-transcribed to cDNA by using a RT Retroscript kit (Ambion). Quantitative real-time PCR was performed by using the Applied Biosystems Assay-on Demand Gene Expression protocol as described (4). In brief, cDNA was amplified by PCR where a target cDNA (IL-6; Mm00446190_m1) and a reference cDNA (glucose-3 phosphate dehydrogenase; Mm99999915_g1) were amplified simultaneously by using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCRs were performed at the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (PerkinElmer). Data were analyzed by using the comparative threshold cycle (Ct) method, and results are expressed as fold difference.
IL-6 protein in cell culture supernatants was measured with an in-house customized ELISA that we have described in detail (43). The assay was sensitive to 10 pg/ml IL-6, and the interassay and intraassay variation was <10%.
NF-κB and AP-1 Binding to the IL-6 Promoter.
BV-2 cells were pretreated with vehicle (0.05% DMSO, vol/vol) or luteolin (0–50 μM) for 1 h and stimulated with 10 ng/ml LPS for a 1-h incubation period. Transcription factor DNA-binding activity was determined at 1 h, because a preliminary study that examined NF-κB at various times after introduction of LPS (0, 0.5, 1, and 2 h) revealed maximal activity at this time point. Nuclear proteins were extracted from BV-2 cells by using procedures similar to what we described (23). In short, ≈1 × 107 microglia were collected with a cell scraper, centrifuged at 500 × g, and washed three times in HBSS. The remaining steps of the nuclear extraction procedure were performed at 4°C. Cells were resuspended for 5 min in hypotonic buffer containing 10 mM Hepes, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.5 mM PMSF, and 10 μg/ml leupeptin at pH 7.9. After a 5-min spin at 2,060 × g, cells were twice treated for 10 min with hypotonic buffer plus 0.5% Nonidet P-40. Cells were then centrifuged at 2,900 × g, and the liquid (cytosolic) layer was removed and frozen at −80°C. The remaining pellet was resuspended in hypotonic buffer, and nuclei were examined and counted with trypan blue staining. After two washes with hypotonic buffer, the pellet was subjected to a high-salt extraction buffer containing 20 mM Hepes, 50 mM KCl, 420 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.5 mM PMSF, 10 μg/liter leupeptin, 25% glycerol, and 0.2 mM EDTA for 30 min. Nuclei were then centrifuged twice for 5 min each at 11,600 × g to pellet cell debris, and the remaining clear layer (nuclear extract) was collected, assayed for protein (Bio-Rad), diluted to a concentration of 1 μg/μl protein, and frozen at −80°C until EMSA was performed.
EMSA was performed as described (22, 23); purified synthetic oligonucleotide probes corresponding to NF-κB and AP-1 binding sequences of the IL-6 promoter were purchased from Qiagen. The sequences for NF-κB and AP-1 were 5′-atgtgggattttcccatgag-3′ and 5′-ctcaagtgctgagtcacttt-3′, respectively. The oligonucleotides were end-labeled with [γ-32P] ATP by using T4 polynucleotide kinase (Promega) and purified with a nucleotide removal kit (Qiagen). Resulting radiolabeled single-stranded probes were annealed according to standard methods. Nuclear extracts (20 μg) were incubated with or without competitor DNA for 10 min at room temperature in a buffer containing 10 mM Tris·HCl (pH 7.5), 2 μg poly(di–dc), 10 mM MgCl2 100, mM KCl, 1 mM EDTA, 20% glycerol, and 1 mM DTT. Either radiolabeled AP-1 or NF-κB probe (100 fmol, 1–2 × 105 cpm/pmol) was added to the samples for 30 min at room temperature. Reactions were terminated by addition of 10× EMSA loading buffer, and resulting protein–DNA complexes were resolved by electrophoresis on a 4% polyacrylamide gel in a Tris–glycine-EDTA running buffer (pH 8.5). Gels were dried and exposed to autoradiography overnight at −80°C. Band densities were quantitated by densitometric analysis using ImageJ software.
Immunoblotting for IκB-α and MAPK.
BV-2 cells were pretreated with luteolin (0–50 μM) and stimulated with 10 ng/ml of LPS for a 30-min incubation period. MAPK phosphorylation was determined at 30 min because a preliminary study that examined phosporylation at various times after introduction of LPS (0, 5, 10, 15, 20, 30, 45, and 60 min) revealed maximal phosphorylation at this time point. Cells were washed with cold HBSS and lysed with a lysis buffer [150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 0.25% sodium depxycholate, 1% Nonidet P-40, 1 mM NaF and 1 mM NaVO3], including protease inhibitors (1 mM PMSF, 1 μg/ml leupeptin, pepstatin, aprotinine and 1 mM EDTA). Protein content was determined with a BCA protein assay kit (Bio-Rad). Cell lysates were denatured with SDS/PAGE sample buffer at 92°C and frozen at −20°C. Protein samples were separated by 12% SDS/PAGE and electrophoretically transferred to nitrocellulose membranes (Osmonics). The membranes were subsequently blocked in 5% nonfat milk/TPBS (10 mM Tris·HCl, 150 mM NaCl, 0.05% Tween 20). Anti-IκB-α (Santa Cruz), anti-Erk, anti-phosphorylated Erk, anti-p38, antiphosphorylated p38, anti-JNK, and antiphosphorylated JNK antibodies (Cell Signaling) were used at a dilution of 1:1,000 in 1% BSA/TPBS. β-Actin (Sigma) was used as an internal control. The immunoreactive bands were visualized by using secondary antibodies conjugated to HRP (Santa Cruz) and a Lumi-Glo chemiluminescent detection kit (Amersham). Protein bands were quantitated by densitometric analysis using ImageJ software.
Animal Studies.
Luteolin was dissolved in 50 mM KOH and added to drinking water to achieve final concentrations of 0, 1, 2.5, and 5 mg/ml. Aspartame (1.25 mg/ml) and 0.2% (wt/vol) white chocolate flavor were added to mask any taste difference and improve palatability. All drinking solutions were standardized to pH 9.5 by addition of HCl. Mice had free access to the drinking solutions for 21 days. The duration and level of luteolin water supplementation was chosen based on several previous studies (31, 44). Fresh drinking solution was provided every other day, and average daily intake and body weight were monitored. Mice drank ≈4 ml per day, and luteolin treatment did not alter daily intake of water. Drinking water was subjected to Folin–Ciocalteu assay to verify that luteolin did not degrade (data not shown). At the end of the 21-day water supplementation, mice were injected i.p. with saline or Escherichia coli LPS (0.33 μg/g BW). Mice were killed by CO2 asphyxiation 4 h after injection, and plasma was collected, stored (−80°C), and later assayed for IL-6 as described for cell culture supernatants. Brain tissue was dissected, and cortex, cerebellum and hippocampus were placed in RNAlater and stored (−80°C). The time for collecting samples after LPS injection was determined by previous studies (45, 46). As described for BV-2 cells, RNA was later isolated from brain tissue, and IL-6 mRNA was measured by quantitative real-time PCR.
Statistical Analysis.
All data were analyzed by using Statistical Analysis System (SAS) General Linear Model procedures. Data were subjected to one-way ANOVA, and differences between treatment means were determined by t tests using the least-significant difference procedure of SAS. All data are expressed as the treatment means ± SEM.
Acknowledgments.
We thank Dr. Linda Van Eldik for providing the BV-2 cell line and Synorex Co. for providing dietary luteolin for feeding. This research was supported by National Institutes of Health Grants AG16710, AG023580, and MH069148 (to R.W.J.) and AG029573 (to K.W.K.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Laflamme N, Rivest S. Toll-like receptor 4: The missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 2.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godbout JP, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 5.Godbout JP, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301649. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham J, et al. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 8.Rice-Evans C. In: Flavonoids in Health and Diseases. Rice-Evans C, Packer L, editors. Boca Raton, FL: CRC; 1998. pp. 329–395. [Google Scholar]
- 9.Shimoi K, et al. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998;438:220–224. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 10.Xagorari A, et al. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296:181–187. [PubMed] [Google Scholar]
- 11.Comalada M, et al. Inhibition of proinflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem Pharmacol. 2006;72:1010–1021. doi: 10.1016/j.bcp.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-κB signaling and gene expression by blocking IκB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology. 2005;115:375–387. doi: 10.1111/j.1365-2567.2005.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SH, et al. Luteolin inhibits the nuclear factor-κB transcriptional activity in Rat-1 fibroblasts. Biochem Pharmacol. 2003;66:955–963. doi: 10.1016/s0006-2952(03)00465-9. [DOI] [PubMed] [Google Scholar]
- 14.Dendorfer U, Oettgen P, Libermann TA. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol. 1994;14:4443–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waetzig V, et al. c-Jun N-terminal kinases (JNKs) mediate proinflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- 16.Borsello T, Forloni G. JNK signaling: A possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 17.Waetzig VHT. Neurodegenerative and physiological actions of c-Jun N-terminal kinases in the mammalian brain. Neurosci Lett. 2004;361:64–67. doi: 10.1016/j.neulet.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 18.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: Regulation, function, and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putcha GV, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 20.Repici MBT. JNK pathway as therapeutic target to prevent degeneration in the central nervous system. Adv Exp Med Biol. 2006;588:145–155. doi: 10.1007/978-0-387-34817-9_13. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz PA, Haller D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-κB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J Nutr. 2006;136:664–671. doi: 10.1093/jn/136.3.664. [DOI] [PubMed] [Google Scholar]
- 22.Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor κB. J Neuroimmunol. 2001;117:87–96. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]
- 23.Heyen JR, Ye S, Finck BN, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-κB. Brain Res Mol Brain Res. 2000;77:138–147. doi: 10.1016/s0169-328x(00)00042-5. [DOI] [PubMed] [Google Scholar]
- 24.Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- 25.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 26.Stein B, et al. Cross-coupling of the NF-κB p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li JJ, Cao Y, Young MR, Colburn NH. Induced expression of dominant-negative c-jun down-regulates NF-κB and AP-1 target genes and suppresses tumor phenotype in human keratinocytes. Mol Carcinog. 2000;29:159–169. doi: 10.1002/1098-2744(200011)29:3<159::aid-mc5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Commenges D, et al. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- 29.Knekt P, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 30.Ross JA, Kasum CM. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 31.Hendriks JJ, et al. Flavonoids influence monocytic GTPase activity and are protective in experimental allergic encephalitis. J Exp Med. 2004;200:1667–1672. doi: 10.1084/jem.20040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotelli AE, Guardia T, Juarez AO, de la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003;48:601–606. doi: 10.1016/s1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]
- 33.Sheng WS, Hu S, Lamkin A, Peterson PK, Chao CC. Susceptibility to immunologically mediated fatigue in C57BL/6 versus BALB/c mice. Clin Immunol Immunopathol. 1996;81:161–167. doi: 10.1006/clin.1996.0172. [DOI] [PubMed] [Google Scholar]
- 34.Visser M, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 35.Sparkman NL, et al. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrientos RM, et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radical Biol Med. 2004;36:592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Yao J, Johnson RW. Induction of interleukin-1 β-converting enzyme (ICE) in murine microglia by lipopolysaccharide. Brain Res Mol Brain Res. 1997;51:170–178. doi: 10.1016/s0169-328x(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 39.Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: Down-regulation of inducible nitric oxide synthase by 15-deoxy-delta12,14-prostaglandin J2. Proc Natl Acad Sci USA. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrova TV, Akama KT, Van Eldik LJ. Selective modulation of BV-2 microglial activation by prostaglandin E(2). Differential effects on endotoxin-stimulated cytokine induction. J Biol Chem. 1999;274:28823–28827. doi: 10.1074/jbc.274.40.28823. [DOI] [PubMed] [Google Scholar]
- 41.Kanazawa K, Uehara M, Yanagitani H, Hashimoto T. Bioavailable flavonoids to suppress the formation of 8-OHdG in HepG2 cells. Arch Biochem Biophys. 2006;455:197–203. doi: 10.1016/j.abb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Lee-Hilz YY, Ter Borg S, van Berkel WJ, Rietjens IM, Aarts JM. Shifted concentration dependency of EpRE- and XRE-mediated gene expression points at monofunctional EpRE-mediated induction by flavonoids at physiologically relevant concentrations. Toxicol In Vitro. 2008 doi: 10.1016/j.tiv.2008.01.008. in press. [DOI] [PubMed] [Google Scholar]
- 43.Godbout JP, Berg BM, Kelley KW, Johnson RW. α-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. J Neuroimmunol. 2004;149:101–109. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 44.van Praag H, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godbout JP, Berg BM, Krzyszton C, Johnson RW. α-tocopherol attenuates NF-κB activation and proinflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169:97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, et al. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]