Abstract
Plant steroid hormones, brassinosteroids (BRs), are of great importance for plant growth and development. BRs signal through a cell surface receptor kinase, BRI1, and a GSK3-like kinase, BIN2, to regulate the BES1/BZR1 family of transcription factors, which directly bind to target gene promoters to activate or repress gene expression and mediate BR responses. To understand how BES1 regulates target gene expression, we identified two BES1-interacting proteins, ELF6 (early flowering 6) and its homolog REF6 (relative of early flowering 6), both of which are Jumonji N/C (JmjN/C) domain-containing proteins and were previously found to regulate flowering time. The interactions between BES1 and ELF6/REF6 were confirmed by GST pull-down and BiFC (bimolecular fluorescence complementation) experiments. Mutations in ELF6 or REF6 genes in Arabidopsis lead to BR-related phenotypes, including impaired cell elongation and reduced expression of BR target genes. Chromatin immunoprecipitation (ChIP) experiments indicated that histone 3 lysine 9 (H3K9) methylation status was changed in elf6 and ref6 mutants, consistent with recent findings that many Jmj proteins are histone demethylases. Our results demonstrate that BES1 recruits other transcriptional regulators such as ELF6 and REF6 to regulate target gene expression and coordinate BR responses with other developmental processes such as control of flowering time. Jmj domain-containing histone demethylases are involved in gene expression in many developmental processes and diseases, but how these proteins affect specific pathways is not well understood. Thus, our study establishes an important mechanism by which Jmj domain proteins modulate specific gene expression by interacting with pathway-specific transcription factors such as BES1.
Brassinosteroids (BRs) are plant steroid hormones with structural similarities to their animal counterparts. They are widely distributed in the plant kingdom and are active at very low concentrations. BRs are involved in multiple plant growth and development processes, such as cell elongation, vascular development, senescence, photomorphogenesis, flowering time control, and stress responses (1–3). BR-deficient or -insensitive mutants usually display dwarfism due to a defect in cell elongation; but these mutants also have delayed senescence, late flowering, and reduced fertility.
Molecular genetic studies in the past decade have dramatically increased our understanding of the BR signaling pathway in the reference plant, Arabidopsis thaliana (4–6). Unlike animal steroid hormones, which mostly bind to nuclear receptors, BRs are perceived by a membrane-bound receptor, BRI1 (7–10). BRI1's activity is regulated by BR binding, which relieves repression by its C-terminal tail and a negative regulator BKI1, while increasing its affinity for BAK1, its coreceptor (11–13).
The ultimate target of BR signaling is the dephosphorylation of a family of plant-specific transcription factors, defined by their founding members, BES1 and BZR1 (14, 15). In the absence of BRs, a GSK3-like kinase, BIN2, phosphorylates BES1, BZR1, and their homologs to negatively regulate their function (14, 16–18). BIN2 phosphorylation likely inhibits BES1/BZR1 function through several mechanisms, such as targeted protein degradation, reduced DNA binding, and retention of the protein in the cytoplasm through interaction with 14–3-3 proteins (18–21). In the presence of BRs, BIN2 is inhibited through an unknown mechanism, leading to the accumulation of dephosphorylated BES1 in the nucleus, a process likely facilitated by the BSU1 phosphatase (14, 22). Nuclear accumulated BES1 can then regulate target gene expression (14, 16).
The BES1/BZR1 proteins are previously uncharacterized, although they contain some known motifs. The N terminus of BES1 contains a bipartite nuclear localization signal (NLS) and perhaps an atypical basic helix–loop–helix (bHLH) motif, which confers upon BES1 the ability to bind to E-box (CANNTG) elements to activate gene expression (16). BZR1 was found to bind to BRRE (CGTGT/CG) to repress gene expression (23). The central domain of BES1 has multiple BIN2 phosphorylation sites and a PEST domain. A single proline-to-leucine substitution in the PEST domain leads to the accumulation of both phosphophorylated and dephosphosphorylated BES1 protein and, therefore, constitutive BR responses (14). BES1 interacts with bHLH protein BIM1 to synergistically bind DNA and regulate gene expression (16).
Despite these significant advances, little is known about the mechanisms by which BES1 regulates BR target gene expression. To address this question, we performed a yeast genetic screen and identified two JmjN/C domain-containing proteins, ELF6 (early flowering 6) and its close homolog REF6 (relative of early flowering 6), as putative BES1 partners. ELF6 and REF6 were initially identified in a genetic screen for mutants with altered flowering phenotypes (24). Genetic analysis has shown that ELF6 and REF6 have opposite effects in the regulation of flowering time. Whereas ELF6 is a repressor in the photoperiodic flowering pathway and its loss-of-function mutation causes early flowering, loss-of-function mutation of REF6 leads to increased expression of flowering repressor FLC (flowering locus C) and hence late flowering (24).
Jmj domains are present in many transcriptional regulators implicated in chromatin modifications. Jmj has recently been found as a signature motif of a group of histone demethylases (25). Jmj family members have been shown to demethylate different methyl-lysines (26–33) or arginines (34) on histone tails. They are implicated in diverse biological processes, such as macrophage-mediated inflammation response (30), posterior development (26), X-linked mental retardation (28), prostate cancer (35), rRNA expression (29), and androgen nuclear receptor-mediated gene expression (31).
In this paper, we report that ELF6 and REF6 interact with BES1 in yeast and in Arabidopsis. The interaction is highly specific and depends on a small domain in the N terminus of BES1 and the C-terminal regions of ELF6 or REF6. Mutation of either ELF6 or REF6 in Arabidopsis causes BR- responsive phenotypes, apparently due to altered BES1 target gene expression. We also provide some evidence that ELF6 and REF6 likely modify BR target gene expression by affecting histone modifications, such as H3K9 methylation, in chromatin associated with the target gene promoters.
Results
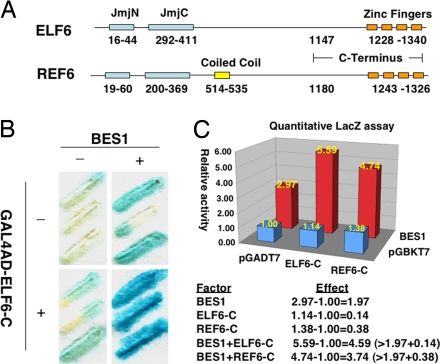
ELF6 and REL6 Interact with BES1 in a Yeast One-Hybrid System.
We used a yeast one-hybrid system to identify BES1 partners. To avoid high background activation caused by BES1, we used a BES1 binding site (E2 box: CACTTG) that binds BES1 with moderate affinity (data not shown) to construct the reporters. From ≈5 million colonies screened, several clones were able to activate reporter gene expression. One of the clones carried a cDNA fragment encoding the C-terminal part of ELF6 (ELF6-C, Fig. 1A). To confirm the interaction between ELF6-C and BES1, we transformed GAL4AD-ELF6-C (or control vector) into a yeast reporter strain without or with BES1 and determined the LacZ activity by qualitative or quantitative assays (Figs. 1 B and C). Whereas ELF6-C alone had little effect on reporter gene expression, BES1 by itself can activate reporter gene expression. More important, coexpression of BES1 and ELF6-C led to synergistic activation of the reporter gene, suggesting that BES1 interacts with ELF6-C. Predicted full-length ELF6 has 1,340 amino acids and is composed of three recognizable functional domains: JmjN, JmjC, and four tandem C2H2-type zinc fingers (Fig. 1A). A close homolog of ELF6, REF6, has the same architecture, except for a predicted coiled-coil region (Fig. 1A). Because of the high homology between ELF6 and REF6, especially at their C termini, we tested whether REF6 also interacted with BES1. Like ELF6-C, REF6-C fused with GAL4AD could also synergistically activate the lacZ reporter with BES1 (Fig. 1C). Taken together, these results suggest that BES1 interacts with both ELF6 and REF6 in the yeast system.
Fig. 1.
ELF6 and REF6 interact with BES1 at the C termini in yeast. (A) Schematic representation of the ELF6 and REF6 proteins. Functional domains were predicted by using SMART (http://smart.embl-heidelberg.de/). (B and C) β-Galactosidase activities were measured with X-gal in a filter-lift assay (B) or with ONPG in a liquid-culture assay (C). ELF6-C/REF6-C (in fusion with GAL4AD) and BES1 can synergistically activate lacZ reporter gene expression. Enzyme activities are normalized against control containing both pGADT7 and pGBKT7, and gene activation effect calculations are shown in C (Bottom).
ELF6 and REF6 Interact with BES1 Through Specific Domains.
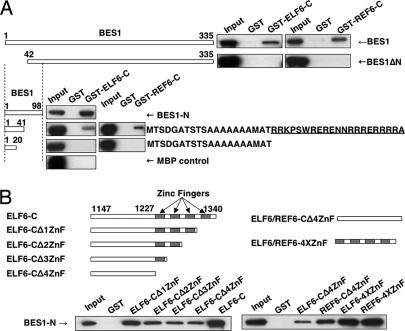
To confirm the direct interactions between BES1 and ELF6/REF6, we fused full-length BES1 with MBP and ELF6-C and REF6-C with GST and performed a GST pull-down experiment. GST–ELF6-C or GST–REF6-C, but not GST alone, was able to pull down full-length BES1 (Fig. 2A), indicating that ELF6 and REF6 directly interact with BES1 in vitro.
Fig. 2.
Mapping the interaction regions on BES1 and ELF6/REF6 by GST pull-down assays. (A) Full length and truncated forms of BES1 protein were used to test their interaction with GST-tagged ELF6-C/REF6-C proteins. (B) ELF6-C/REF6-C proteins with serial deletions of zinc finger motifs and MBP-BES1-N were used for GST pull-down assay. All BES1 fragments were fused with MBP and detected with anti-MBP antibody in Western blots.
To determine which domain of BES1 was required for the interaction with ELF6, we mapped the binding site of ELF6 on BES1 using a series of truncated BES1 and GST–ELF6-C and GST–REF6-C proteins. Deletion of the N-terminal 41 amino acid of BES1 (BES1ΔN) disrupted the interaction between BES1 and ELF6-C or REF6-C (Fig. 2A). The fragment containing the first 41 residues of BES1 was sufficient for the interaction, although the binding was much weaker (BES1 1–41). When we further deleted amino acid 21–41, the interaction was abolished (BES1 1–20). Thus, the first 41 amino acids of BES1 were necessary and sufficient for the BES1-EFL6/REF6 interaction, and residues 21 to 41 are important for this interaction. Interestingly, these residues overlap with the basic region of the bHLH domain of BES1 (underlined in Fig. 2A).
We then took a similar approach to map the BES1 interaction regions in ELF6-C and REF6-C using a fragment of BES1 containing the first 98 amino acids (BES1-N). While deletion of individual zinc finger weakened the interaction (Fig. 2B Left), zinc fingers in both ELF6 and REF6 can bind BES1 (Fig. 2B Right). These results demonstrated that both the zinc fingers and the fragment immediately upstream of zinc fingers of ELF6 and REF6 contributed to the interaction with BES1.
ELF6 Interacts with BES1 in Arabidopsis.
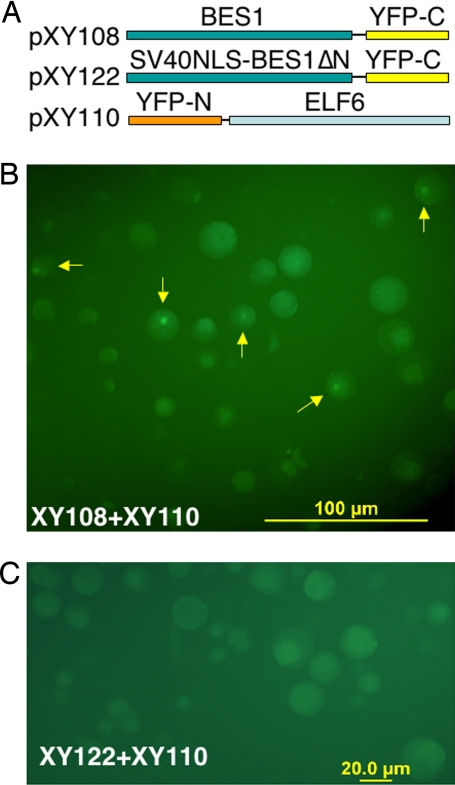
To test whether full-length ELF6 interacts with BES1 in vivo, we constructed plasmids for BiFC. In the assay, we fused EYFP C terminus downstream of BES1 (pXY108) and EYFP N terminus upstream of ELF6 (pXY110) (Fig. 3A). When both constructs were introduced to Arabidopsis protoplasts, strong fluorescence was observed in the nucleus (Fig. 3B), indicating an interaction of BES1 and ELF6. Among the cells observed, ≈10% cells showed positive signals. To determine whether the observed fluorescence was truly due to an ELF6-BES1 interaction, we deleted the ELF6/REF6-interacting domain in BES1 and tested the interaction again. Because the deletion also disrupts the BES1 nuclear localization signal, the NLS from SV40 was fused to the BES1 fragment (BES1ΔN, amino acid 42–335, which does not interact with ELF6 as shown in Fig. 2A). The fusion protein indeed is localized in the nucleus when tagged with full-length EYFP (data not shown). The NLS-BES1ΔN-EYFP-C (pXY122) is cotransfected into protoplasts with pXY110, and no positive signal was observed (Fig. 3C). We conclude that BES1 interacts with ELF6 in Arabidopsis cells.
Fig. 3.
ELF6 interacts with BES1 in Arabidopsis mesophyll protoplasts, as shown by BiFC. (A) Schematic representations of constructs used in this experiment. (B) The interaction of full-length BES1 and ELF6 leads to reconstruction of EYFP. Arrows indicate cells with EYFP signals. (C) No EYFP signal was detected when pXY122 and pXY110 was used in this assay.
ELF6/REF6 Knockout Mutants Display BR-Response Phenotypes.
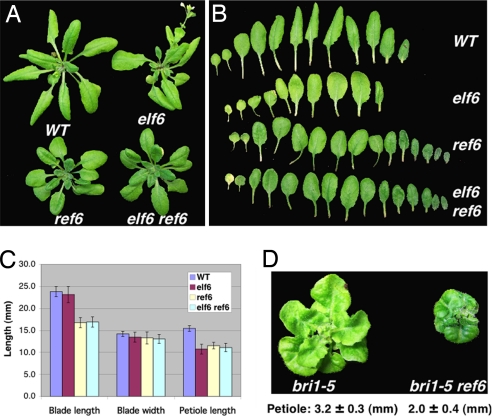
To determine the biological functions of ELF6 and REF6 in BR responses, we obtained T-DNA insertion alleles of both ELF6 and REF6 and named them as elf6 and ref6, respectively. Light grown seedlings of elf6 or ref6 did not show obvious phenotypes; however, adult elf6 plants were early flowering and ref6 plants flowered late, as previously reported (24). In addition, adult plants of both mutants have reduced cell elongation phenotypes, characterized by shorter leaf petioles compared to a wild-type control (about 30% reduction) (Fig. 4). Interestingly, ref6, but not elf6, also produces shorter leaf blades, suggesting that these two genes probably function in a tissue-specific manner. Both mutants have similar leaf widths as the wild type. To determine the genetic relationship between ELF6 and REF6, we also made double mutant elf6 ref6, which has essentially the same phenotype as ref6 (Fig. 4), suggesting that ELF6 and REF6 function in a common pathway, at least with respect to the cell elongation process.
Fig. 4.
ELF6 and REF6 knockout mutants show BR-related phenotype. (A) Wild-type, elf6, ref6, and elf6 ref6 double mutants were grown in soil until the WT plants began bolting. (B) Leaf morphology of the mutants compared to WT. (C) Compare blade length, blade width and petiole length of the sixth leaves. Error bar shows standard deviation (SD); n = 10. (D) Three-week-old plants of bri1-5 and bri1-5 ref6 double mutant.
These elf6 and ref6 phenotypes are reminiscent of BR-deficient or -insensitive mutants. To further confirm that the observed cell elongation phenotype is related to BR responses, we made a ref6 and bri1-5 (a weak allele of bri1) double mutant. As shown in Fig. 4D, ref6 mutation could enhance bri1-5's phenotype, displaying even shorter hypocotyls as well as darker green and more curled leaves. The result is consistent with our hypothesis that REF6 modulates BR response.
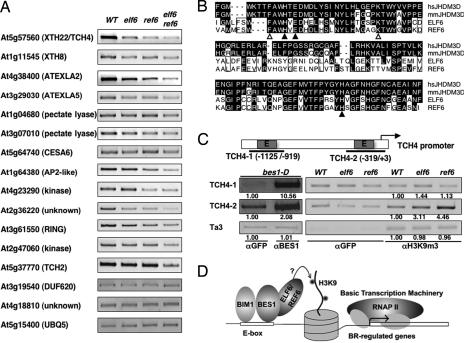
Because ref6 displayed a stronger cell elongation phenotype, we determined the gene expression profile of ref6 adult plants using Affymetrix Arabidopsis Genomic Arrays (ATH1). Among the 342 genes previously shown to be induced by BRs (36), we found that 45 of them were reduced in ref6 by about 30–80% [supporting information (SI) Table S1]. A semiquantitative RT-PCR was carried out with BR-induced genes in ref6, elf6, and elf6 ref6 double mutant to confirm the results from the microarray experiment (Fig. 5A). Most genes we tested (9 of 11) were indeed reduced in ref6, elf6 ref6, and to a lesser extent in elf6, which is consistent with the severity of the cell elongation phenotypes. Quantitative RT-PCR studies indicated that the BL induction of TCH4 and ATEXLA2 genes is attenuated in elf6 ref6 double mutant (Fig. S1). Taking together, these results suggest that ELF6 and REF6 are required for BR-induced gene expression.
Fig. 5.
ELF6 and REF6 modulate the expression of some BR-regulated genes, likely by affecting the histone modifications. (A) Many BR-regulated genes were down-regulated in elf6, ref6, and elf6 ref6 double mutants, compared to WT. The genes tested were from BR-induced genes that are reduced in ref6 (Table S1) with three controls. (B) The JmjC domains of ELF6 and REF6 are highly homologous to those from the JHDM3D family from mouse (mm) and human (hs), which demethylate H3K9me3. Key residues required for cofactor binding were conserved [filled triangles, residues for Fe(II) binding and, open triangles, residues for α-ketoglutarate binding]. (C) ChIP assays with bes1-D, WT, elf6, or ref6 with indicated antibodies. The ChIP products were analyzed by PCR with primers from TCH4 gene promoter. αGFP was used as antibody control, and Ta3 served as input control. The numbers under the gel panels indicate relative enrichment of PCR products compared to αGFP control. (D) A model for ELF6 and REF6 function in BES1-mediated gene expression.
BES1 Recruits ELF6 and REF6 to Regulate BR Target Gene Expression Through Histone Modifications.
Given the facts that ELF6 and REF6 can interact with BES1 and that the expression levels of some BR-regulated genes decrease in elf6 and ref6 mutants, we hypothesized that ELF6 and REF6 were recruited by BES1to modulate the expression of some BR target genes. As an example, we used the TCH4 gene, which was known to be up-regulated during BR-regulated cell elongation (37) and was also consistently reduced in both elf6 and ref6. By ChIP with anti-BES1 antibody, we found that BES1 bound to two E-box-containing fragments in the TCH4 promoter, TCH4–1 (−1125/−919) and TCH4–2 (−319/+3) (Fig. 5C). As a negative control, there was no enrichment of Ta3, a retrotransposable element in Arabidopsis. These results indicate that TCH4 is a direct target of BES1.
Based on the conserved residues required for binding cofactors, ELF6 and REF6 seem most closely related to the JHDM3 subfamily (Fig. 5B). The JHDM3 family members have been shown to remove methyl groups from trimethyl histone 3 lysine 9 (H3K9me3) (33). If ELF6 and REF6 have same enzyme activity, we should expect elevated H3K9me3 in loss-of-function mutants at the promoter regions of ELF6/REF6-affected genes. ChIP assays were then performed with anti-H3K9m3 antibody. As shown in Fig. 5C (middle gel), H3K9me3 level is significantly elevated at the TCH4–2 site in both elf6 and ref6 mutants. Interestingly, at the TCH4–1 site (which is further upstream from the TCH4 gene core promoter), there is no enrichment of H3K9me3 level compared with antibody control (Fig. 5C, top gel). Although the Ta3 locus appears to have H3K9me3 modification, there is no change in H3K9me3 level between wild-type and elf6 and ref6 (Fig. 5C, bottom gel). Taking together, our ChIP studies indicate that ELF6 and REF6 are recruited to BR target gene promoters by BES1 and likely modulate gene expression through histone modifications (Fig. 5D).
Discussion
BRs control many diverse processes throughout plant growth and development by regulating the expression of hundreds of genes (36). Recent studies suggest that BR signaling regulates the protein level, cellular distribution, and/or DNA-binding activity of BES1/BZR1 family transcriptional factors. BES1 and BZR1 have been found to bind to E-box and BRRE elements to activate and repress target gene expression, respectively (16, 23). However, the intrinsic DNA binding activity cannot account for all of the regulatory properties of BES1, because BES1 regulates only a small portion of the genes with predicted E-box elements in their promoters. The ubiquitous expression of BES1 (14) also suggests that additional factors are required to modulate BES1 function in different tissues and developmental stages. In this study, we provide evidence that BES1 recruits two JmjN/C domain-containing proteins, ELF6 and REF6, to modulate BR-regulated gene expression. Our results therefore establish one mechanism by which BES1 activity is fine-tuned to regulate downstream target gene expression.
ELF6 and REF6 can function as activators of BR-regulated genes because many BR-induced genes are down-regulated in re6 and elf6 knockout mutants (Fig. 5 and Table S1). Many of the BR-regulated genes reduced in ref6 and/or elf6 encode xyloglucan endotransglucosylases/hydrolases (XTHs), expansins, and pectate lyases, cell wall-modifying enzymes implicated in cell elongation. Reduced accumulation of these RNAs could explain the reduced stem elongation phenotype of elf6 and ref6.
How do ELF6 and REF6 influence BR-induced gene expression? Although the ELF6/REF6 interaction site is located within the DNA binding domain of BES1, expression of ELF6 and REL6 in yeast did not compromise BES1-mediated activation (Fig. 1B and data not shown), indicating that the binding of ELF6 and REL6 does not affect BES1 DNA binding ability. One obvious scenario is that ELF6 and REF6 exert their functions through their JmjC domain, which has been shown to serve as the active site for a number of histone demethylases (38). ELF6 and REF6 are most closely related to the mammalian JHDM3 subfamily, members of which can demethylate trimethyl H3K9 (H3K9me3) (33). Our ChIP data showed that H3K9me3 level is elevated in the immediate promoter region of TCH4 in elf6 and ref6 mutants, but not at 1kb upstream of the transcriptional start site (Fig. 5C), which is consistent with the observation that H3K9me3 was most concentrated in the promoter region adjacent to the transcription start site (39). Thus, our in vivo data are consistent with the idea that ELF6 and REF6 are conserved histone demethylases that target H3K9me3.
Despite the in vivo observation, no in vitro histone demethylase activity was detected with either full-length or JmjC-containing fragments of ELF6 and REF6 in a well established assay using various substrates (K. Gardner, X. Yu, Y. Yin, and Y. Zhang, unpublished results). One possibility is that other proteins may be required for ELF6 and REF6 enzyme activity, which is different from the case in animal systems. Similarly, Arabidopsis lysine-specific demethylase 1 (LSD1) was found to repress flowering repressors FLC and FWA by reducing H3K4 methylation levels in vivo (40), but by itself did not show in vitro demethylase activity, whereas its human homolog did. It was proposed that a complex containing Arabidopsis LSD1 and other factors may be required for the demethylase activity (40). Alternatively, ELF6/REF6 may affect chromatin modification status by other unknown mechanisms, as in the case of the yeast JmjC-containing protein Epe1 (41).
Although both ELF6 and REF6 mainly promote BR-induced gene expression, they appear to be able to repress gene expression in other pathways. For example, REF6 was reported to function as a transcriptional repressor of FLC (24). The effects of ELF6 and REF6 on gene expression can be well explained by the H3K9me3 mark. In Arabidopsis, H3K9me3 is distributed mostly within the euchromatin regions (42, 43). At the gene level, H3K9me3 shows a 5′ concentrated pattern, covering the immediate promoter region and the whole gene afterward (39). It is well established that the effect of a histone modification mark on transcription depends on both the distribution of the mark in a gene and the nearby companion mark(s). Because H3K9me3 keeps chromatin in a closed and inactive form, its existence in a promoter may result in gene repression by limiting the accessibility of the general transcription machinery to DNA, whereas its existence in the coding region can actually promote gene expression by reducing cryptic transcription initiation and facilitating transcription elongation process (39).
Our results also expand the previously known function of ELF6 and REF6 in flowering time control to hormone-regulated gene expression. Microarray data show that about 9% of all Arabidopsis genes are reduced in ref6 (although not all of them are direct target of REF6, data not shown), indicating that REF6 and ELF6 might serve as coordinators for multiple developmental programs in plants. Our results suggest that these genes may play important roles in coordinating the BR signaling pathway and flowering pathway. BR-insensitive or deficient mutants were recently reported to enhance the phenotype of a late-flowering mutant (ld), which is at least in part due to higher expression of FLC in bri1 ld and cpd ld double mutants compared to ld single mutant (44). Although the mechanism is not known, BRs may affect flowering time control through the interaction between BES1 and ELF6/REF6, which may have important implications in coordinating growth and reproductive processes. This hypothesis is supported by the fact that the cell elongation phenotypes of elf6 and ref6 are most apparent in adult tissues when flowering occurs (Fig. 4). In contrast to the fact that both ELF6 and REF6 interact with BES1 to positively regulate BR target gene expression and promote cell elongation, they might be recruited by different transcription factors and/or target distinct genes in the flowering pathway, and thus exert opposite effects on flowering time control. Identification of ELF6 and REF6 partners in the flowering pathway should help address the question.
In summary, our study establishes a new mechanism by which BES1 regulates BR target gene expression. Our results also reveal a mechanism by which JmjN/C family transcriptional regulators achieve a specific function through interactions with pathway-specific transcription factors such as BES1. Further functional studies of the BES1 and ELF6/REF6 interaction should provide significant insights into the mechanisms by which BES1 regulates gene expression and controls BR responses during growth and development in changing environmental conditions.
Materials and Methods
Plant Materials and Growth Conditions.
A. thaliana ecotype Columbia (Col-0) was the wild type. T-DNA insertion mutants, elf6 and ref6, were obtained from ABRC (Arabidopsis Biological Resource Center) and corresponded to lines SALK_074694 and SALK_001018, respectively. elf6 has a T-DNA insertion at amino acid 169, and ref6 at amino acid 1082, which were previously designated as elf6–3 and ref6–1 (24). Plants were grown in MS plates or soil under long day (16-h light/8-h dark) conditions at 22°C.
Plasmid Construction.
The ELF6 coding region was amplified from Col-0 cDNA and incorporated into the pETMALc-H vector (Merck). BES1 and the C-terminal domains of ELF6 and REF6 were cloned into pGBKT7 and pGADT7 (Clontech), respectively, for expression in yeast. BES1 and ELF6/REF6 as well as deletion mutants were cloned into pMAL-p2x (NEB) and pET42a(+) (Novagen), respectively, for recombinant protein production.
For BiFC assay (45), the N (amino acids 1–174) or C (amino acids 175–239) terminus of EYFP was amplified from pEYFP-N1 (Clontech). The N-terminal parts of the EYFP and ELF6 coding region were cloned into pCHF3 (14) to yield pXY110. The C terminus of EYFP and then the full-length or N-terminal-truncated form of BES1 with SV40 nuclear localization signal (NLS) (46) were cloned into pCHF3 to create pXY108 and pXY122, respectively. The omega translational enhancer is placed in the plant expression vectors before the fusion genes. The primer sequences used for cloning, genotyping, and PCR are listed in Table S2.
Yeast Screen and lacZ Assays.
The Clontech yeast one-hybrid system was used. Four tandem copies of E2 sites (CACTTG) were cloned into the yeast minimal promoters directing lacZ (β-galactosidase) and His reporter genes, which are integrated into yeast strain YM4271 that has a his−trp−leu− genotype. Yeast transformed with both BES1 (with TRP marker) and an Arabidopsis cDNA library (with LEU marker) (47) was first screened in media lacking His, Trp, and Leu. Positive clones were then assayed for LacZ activity by using X-gal (5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside) or ONPG (ortho-nitrophenyl-b-d-galactopyranoside).
GST Pull-Down Assay.
BES1 and BES1 fragments fused with maltose binding protein (MBP) were purified with amylose resin (NEB). ELF6 and REF6 fragments fused with GST were purified with glutathione beads (Sigma). GST pull-down assays were performed as described (14).
Biomolecular Fluorescence Complementation (BiFC).
Arabidopsis mesophyll protoplasts were prepared and transformed by PEG-mediated transfection (48). After culturing for 2 days, cells were observed under an OLYMPUS IX71 fluorescence microscope with an EYFP filter.
Gene Expression Analysis.
Total RNA was extracted from adult plants with TRIzol reagent (Invitrogen) and then purified with RNeasy Mini kit (Qiagen). Microarray experiments were performed with GeneChip Facility (http://www.biotech.iastate.edu/facilities/genechip/Genechip.htm). Data were analyzed by R with the affy package (http://bioconductor.org/packages/2.1/bioc/html/affy.html). Genes that are reduced by at least 33% in both biological replicates were considered to be significant. For RT-PCR, 2.5 μg total RNA was reverse-transcribed to cDNA by SuperScript II Reverse transcriptase (Invitrogen). Equal amount of cDNA was used for PCR with 25 to 31 cycles, which were in the linear range of amplification (data not shown). RT-PCRs were repeated twice, and typical results are presented.
ChIP.
ChIP was performed as described (49). Antibodies against BES1 or trimethylated H3K9 (Abcam) were used to precipitate chromatin, and GFP antibody (Molecular Probes) was used as a negative control. The ChIP assays were repeated two to three times, and the typical results are presented.
Supplementary Material
Acknowledgments.
We thank Dan Voytas for comments on the manuscript. We are grateful to Kathryn Gardner and Yi Zhang (University of North Carolina, Chapel Hill, NC) for help with the in vitro demethylase assays and Yi Hou for help with the BiFC experiment. The research was supported by a faculty startup fund from Iowa State University, National Science Foundation Grant IOS0546503 (to Y.Y.) and by the U.S. Department of Agriculture and the National Science Foundation (J.C.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 7345.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802254105/DCSupplemental.
References
- 1.Krishna P. Brassinosteroid-mediated stress responses. J Plant Growth Regul. 2003;22:289–297. doi: 10.1007/s00344-003-0058-z. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Chory J. Brassinosteroid actions in plants. J Exp Bot. 1999;50:332–340. [Google Scholar]
- 3.Clouse SD. Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 1996;10:1–8. doi: 10.1046/j.1365-313x.1996.10010001.x. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Jin H. Regulation of brassinosteroid signaling. Trends Plants Sci. 2007;12:37–41. doi: 10.1016/j.tplants.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Belkhadir Y, Chory J. Brassinosteroid signaling: A paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- 6.Clouse SD. Brassinosteroid signal transduction: Clarifying the pathway from ligand perception to gene expression. Mol Cell. 2002;10:973–982. doi: 10.1016/s1097-2765(02)00744-x. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita T, et al. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. doi: 10.1038/nature03227. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 9.He Z, et al. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science. 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, et al. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell. 2005;8:855–65. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, et al. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis brassinosteroid-insensitive1 receptor kinase. Plant Cell. 2005;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin Y, et al. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZY, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell. 2002;2:505–513. doi: 10.1016/s1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 16.Yin Y, et al. A new class of transcription factors mediate brassinosteroid-regulated gene expression in Arabidopsis. Cell. 2005;120:249–259. doi: 10.1016/j.cell.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Nam KH. Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science. 2002;295:1299–1301. doi: 10.1126/science.1065769. [DOI] [PubMed] [Google Scholar]
- 18.He JX, Gendron JM, Yang Y, Li J, Wang ZY. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu H, et al. Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell. 2007;19:2749–2762. doi: 10.1105/tpc.107.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gampala SS, et al. An essential role for 14–3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev Cell. 2007;13:177–189. doi: 10.1016/j.devcel.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vert G, Chory J. Downstream nuclear events in brassinosteroid signalling. Nature. 2006;441:96–100. doi: 10.1038/nature04681. [DOI] [PubMed] [Google Scholar]
- 22.Mora-Garcia S, et al. Nuclear protein phosphotases with Kelch-repeat domains modulate the response to bassinosteroids in Arabidopsis. 18: 448–460. Genes Dev. 2004;18:448–460. doi: 10.1101/gad.1174204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He JX, et al. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noh B, et al. Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell. 2004;16:2601–213. doi: 10.1105/tpc.104.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–18. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- 26.Lan F, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature. 2007;449:689–94. doi: 10.1038/nature06192. [DOI] [PubMed] [Google Scholar]
- 27.Klose RJ, et al. The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Iwase S, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Frescas D, et al. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309–313. doi: 10.1038/nature06255. [DOI] [PubMed] [Google Scholar]
- 30.De Santa F, et al. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Yamane K, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Tsukada Y, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 33.Klose RJ, et al. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- 34.Chang B, et al. JMJD6 is a histone arginine demethylase. Science. 2007;318:444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 35.Xiang Y, et al. JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci USA. 2007;104:19266–19231. doi: 10.1073/pnas.0700735104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemhauser JL, Mockler TC, Chory J. Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol. 2004;2:E258. doi: 10.1371/journal.pbio.0020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu W, et al. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 39.Pfluger J, Wagner D. Histone modifications and dynamic regulation of genome accessibility in plants. Curr Opin Plant Biol. 2007;10:1–8. doi: 10.1016/j.pbi.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang D, Yang W, He Y, Amasino RM. Arabidopsis relatives of the human lysine-specific Demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell. 2007;19:2975–2987. doi: 10.1105/tpc.107.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zofall M, Grewal SI. Swi6/HP1 recruits a JmjC domain protein to facilitate transcription of heterochromatic repeats. Mol Cell. 2006;22:681–92. doi: 10.1016/j.molcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Turck F, et al. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer A, Hofmann I, Naumann K, Reuter G. Heterochromatin proteins and the control of heterochromatic gene silencing in Arabidopsis. J Plant Physiol. 2006;163:358–368. doi: 10.1016/j.jplph.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Domagalska MA, et al. Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering. Development. 2007;134:2841–2850. doi: 10.1242/dev.02866. [DOI] [PubMed] [Google Scholar]
- 45.Citovsky V, et al. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol. 2006;362:1120–1131. doi: 10.1016/j.jmb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 46.van der Krol AR, Chua NH. The basic domain of plant B-ZIP proteins facilitates import of a reporter protein into plant nuclei. Plant Cell. 1991;3:667–675. doi: 10.1105/tpc.3.7.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Harter K, Theologis A. Protein-protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho YH, Yoo SD, Sheen J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell. 2006;127:579–589. doi: 10.1016/j.cell.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Johnson L, Cao X, Jacobsen S. Interplay between two epigenetic marks: DNA methylation and histone H3 lysine 9 methylation. Curr Biol. 2002;12:1360–1367. doi: 10.1016/s0960-9822(02)00976-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.