Abstract
Retinoic acid (RA) displays potent anticarcinogenic activities that are mediated by the nuclear retinoic acid receptors (RARs). However, use of RA in oncology is limited by RA resistance acquired during carcinogenesis. Moreover, in some cancers, RA facilitates rather than inhibits growth. A clue to this paradoxical behavior was recently suggested by the findings that RA also activates PPARβ/δ, a receptor involved in mitogenic and anti-apoptotic activities. The observations that partitioning of RA between its two receptors is regulated by two intracellular lipid-binding proteins—CRABP-II, which targets RA to RAR, and FABP5, which delivers it to PPARβ/δ—further suggest that RA resistance may stem from the deregulation of the binding proteins, resulting in activation of PPARβ/δ rather than RAR. Here, we show that, in the RA-resistant mouse model of breast cancer MMTV-neu, RA indeed activates the nonclassical RA receptor PPARβ/δ. This behavior was traced to an aberrantly high intratumor FABP5/CRABP-II ratio. Decreasing this ratio in mammary tissue diverted RA from PPARβ/δ to RAR and suppressed tumor growth. The data demonstrate the existence of a mechanism that underlies RA resistance in tumors, indicate that CRABP-II functions as a tumor suppressor, and suggest that the inhibition of FABP5 may comprise a therapeutic strategy for overcoming RA resistance in some tumors.
Keywords: cancer, lipid-binding proteins, nuclear receptors, CRABP, FABP
All-trans retinoic acid (RA) is a potent anticarcinogen and is currently used or is being tested for therapy of several types of human cancer. Most notably, RA is a powerful agent in the treatment of promyelocytic leukemia (1). The anticarcinogenic activities of this hormone are mediated by the ligand-activated transcription factors termed retinoic acid receptors (RARα, RARβ, and RARγ). The first step in the activation of RAR entails the delivery of RA from the cytosol to the receptor in the nucleus, a step mediated by cellular retinoic acid-binding protein II (CRABP-II) (2–5). After binding of RA, RAR undergoes a large conformational change, leading to the dissociation of corepressors and the recruitment of coactivators, which in turn loosen chromatin structure and bridge to the general transcription machinery to enhance transcriptional rates.
Activated RAR regulates the expression of multiple target genes, including genes involved in differentiation (6, 7), cell-cycle control (8), and apoptosis (9, 10), and it thus often inhibits cell growth. However, despite promising preclinical and clinical results, use of retinoids in cancer therapy remains limited. Such therapy is hampered by both the pronounced toxicity of RA and the development of RA resistance during carcinogenesis (11). It has been demonstrated that RA resistance may stem from the deregulation of various aspects of RA signaling, e.g., defects in RA synthesis (12), down-regulation of CRABP-II (13), loss of expression of RAR (14), and impaired ligand-induced corepressor/coactivator exchange (15).
Notably, some carcinomas not only fail to become growth-inhibited upon treatment with RA, but instead respond to RA treatment with enhanced proliferation (16, 17). In addition, the β-Carotene and Retinol Efficacy Trial, a lung cancer chemoprevention trial, was terminated 21 months ahead of schedule because the treatment increased lung cancer incidence (18). Hence, under some conditions, retinoids appear to be procarcinogenic, a characteristic that is unlikely to be mediated by RAR. A clue to a possible basis for this activity was recently provided by the observations that RA also serves as a ligand for PPARβ/δ (17, 19), a nuclear receptor that targets genes that support cell proliferation and survival (20, 21). It was further shown that, while RA is delivered to RAR by CRABP-II, it is shuttled to PPARβ/δ by another intracellular lipid-binding protein, namely FABP5 (17, 22). These observations raise the possibility that the RA resistance of some tumors may result from the targeting of RA to PPARβ/δ, rather than to RAR, and that this behavior may stem from deregulation of expression of the two RA-binding proteins, CRABP-II and FABP5.
To examine this possibility, we used the FVB/N-Tg(MMTVneu)202Mul/J (MMTV-neu) transgenic mouse model of breast cancer, which has been reported to be profoundly RA-resistant (17, 23). In this model, the EGF receptor Neu/Erb-B2/Her2 is specifically overexpressed in mammary epithelium, resulting in the spontaneous development of mammary tumors (24). Amplification of this gene has been observed in a significant proportion of human breast cancers (25, 26) and is correlated with poor outcome in human patients (27). We thus generated MMTV-neu mice models with varying mammary FABP5/CRABP-II ratios and investigated the transcriptional activities of RA and the consequences of these activities for tumor development in these mice.
Results
Generation of Transgenic Mice with Varying Mammary FABP5/CRABP-II Ratios.
Two MMTV-neu mouse models were generated. One of these models consisted of MMTV-neu mice in which the expression of CRABP-II is disrupted, leading to a very high FABP5/CRABP-II ratio. This model was established by crossing MMTV-neu mice with CRABP-II-null mice (28), resulting in MMTV-neu+/−/CRABP-II−/− mice (termed here MCRABP-II−/−). A second model entailed MMTV-neu mice that specifically overexpress CRABP-II in mammary tissue and thus display a low FABP5/CRABP-II ratio. These mice were generated by using a transgenic construct consisting of the mammary epithelium-specific promoter/enhancer MMTV-LTR, a synthetic human CRABP-II cDNA, and a human β-globin polyadenylation signal. Transgenic founders were identified by PCR, and the mammary-specific expression of the transgene was verified by immunoblotting and by real-time quantitative PCR (Q-PCR) analyses of various tissues [supporting information (SI) Fig. S1]. These mice were crossed with MMTV-neu mice to generate MMTV-neu+/−/MMTV-CRABP-II animals (termed MTgCRABP-II).
Examination of the expression levels of the two RA-binding proteins, CRABP-II and FABP5, and the two RA receptors, RAR and PPARβ/δ, revealed that, as previously noted, carcinogenesis in MMTV-neu mice is accompanied by the down-regulation of CRABP-II and the up-regulation of FABP5 (Fig. 1a) (17). As expected, CRABP-II was not detectable in tumors of the MCRABP-II−/− mice and was markedly elevated in tumors of mice that transgenically overexpress the protein in mammary tissue (Fig. 1a). Immunoblots using pan-RAR antibodies revealed that this receptor is down-regulated upon tumor development (Fig. 1b). Expression levels of mRNA for all RAR isotypes were lower in tumors compared with normal mammary tissue, with RARβ displaying the largest response (Fig. 1c). Importantly, similar expression levels of RAR mRNAs were observed in all mice models, indicating that they can be directly compared. RAR expression could not be restored by RA treatment of the mice. Expression of both FABP5 and PPARβ/δ were similar in all three mouse models (Fig. 1 a and d and Fig. S2). Hence, tumors that develop in the three MMTV-neu mouse models express similar levels of RARs and PPARβ/δ, but display large differences in their FABP5/CRABP-II ratio.
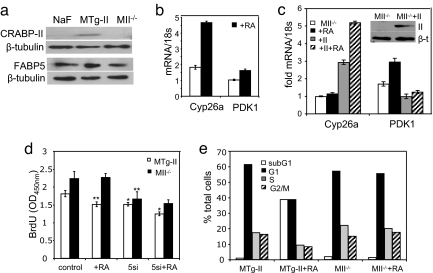
Fig. 1.
RA receptors and binding proteins in tumors in the mouse models. (a) Immunoblots of CRABP-II and FABP5 in normal mammary tissue (n) and in tumors of MMTV-neu (Mneu), MTgCRABP-II (MTg-II), and MCRABP-II−/− (MII−/−) mice. (b) Immunoblots of total RAR in normal mammary tissue (n) and in mammary tumor in MMTV-neu mice. (c) Q-PCR analyses of mRNA for RARα, RARβ, and RARγ in denoted tumors and in tumors of MMTV-neu mice treated with RA as of age 140 days (+RA). (d) PPARβ/δ mRNA in tumors in the mouse models and in MMTV-neu mice treated with RA. Data (mean ± SEM, n = 3) are normalized to 18-s mRNA.
In MMTV-neu Mouse Tumors, RA Signals Through PPARβ/δ and Is Diverted to RAR Upon Increasing the CRABP-II/FABP5 Ratio.
MMTV-neu mice were systemically treated with RA, tumors were allowed to develop, and the intratumor expression of mRNA for known target genes for RAR and PPARβ/δ were assessed. RA treatment markedly up-regulated the expression of the PPARβ/δ target genes fasting-induced adipose factor (FIAF) (29), adipose differentiation-related protein (ADRP) (30), and PDK1 (31), a kinase that activates the Akt1 survival pathway (Fig. 2a). In contrast, RA had little effect on the direct RAR targets caspase 9 and the cell cycle control gene BTG2 or the indirect RAR target cyclin D1 (Fig. 2b). Hence, in MMTV-neu tumors, RA does not activate RAR, but instead induces transcriptional activation by PPARβ/δ.
Fig. 2.
Decreasing the FABP5/CRABP-II ratio in MMTV-neu mice diverts RA from PPARβ/δ to RAR. (a and b) mRNA of target genes for PPARβ/δ (a) and RAR (b) in tumors in MMTV-neu mice and in mice treated with RA as of age 140 days. *, P < 0.01 versus untreated mice. (c and d) Expression of target genes for PPARβ/δ (c) and RAR (d) in tumors in the denoted mouse models. (Upper) mRNA levels measured by Q-PCR. *, P < 0.01 versus MMTV-neu controls. Data (mean ± SEM, n = 3) were normalized to 18-s mRNA. (Lower) Western blots of PDK1 (c) and caspase 9 (d).
Expression levels of RAR and PPARβ/δ target genes were then measured in tumors that arose in the different mouse models. The level of mRNA for three PPARβ/δ target genes was significantly reduced in the presence of a low FABP5/CRABP-II ratio and up-regulated in the absence of CRABP-II (Fig. 2c Upper). Western blot analyses of one of these genes, PDK1, confirmed that modulation of protein expression corresponded to changes in mRNA (Fig. 2c Lower and Fig. S3). In contrast, the RAR targets caspase 9, BTG2, and Cyp26a were markedly up-regulated upon overexpression of CRABP-II and down-regulated in mice lacking this binding protein (Fig. 2d). Correspondingly, the expression of the BTG2 downstream effector cyclin D1 (8, 32) decreased upon overexpression of CRABP-II and was elevated at a high FABP5/CRABP-II ratio (Fig. 2d). Interestingly, Apaf1, the major protein in the apoptosome, is not subject to regulation by RAR, but it has been reported that its expression is induced in mammary carcinoma cells overexpressing CRABP-II (9). The observations that Apaf1 also is markedly up-regulated upon overexpression of CRABP-II in vivo (Fig. 2d) suggests that, in addition to its cooperation with RAR, this binding protein may contribute to apoptotic responses through another as yet unknown mechanism. Taken together, these data show that a low FABP5/CRABP-II ratio depresses the expression of PPARβ/δ target genes, including antiapoptotic genes, and leads to gene expression commensurate with cell-cycle arrest and apoptosis
Varying the CRABP-II/FABP5 Ratio Alters RA Activities in Cell Lines Generated from Tumors of the Mouse Models.
Cell lines were generated from tumors that developed in the different MMTV-neu models (see Experimental Procedures). In accordance with the expression profile in vivo, NaF cells, generated from MMTV-neu tumors (33), expressed a high FABP5/CRABP-II ratio. Binding protein profiles of cells generated from tumors that overexpress or lack CRABP-II also corresponded to the intratumor profiles (Fig. 3a). Also similarly to the in vivo behavior, cells overexpressing CRABP-II showed a higher expression of the RAR target gene Cyp26a (34) and a decreased expression of the PPARβ/δ target PDK1 (compare Fig. 3 b and c). RA treatment of the cells up-regulated the expression of Cyp26a in cells with a high CRABP-II level, but induced PDK1 in CRABP-II−/− cells (Fig. 3 b and c). Additional cell lines, independently generated from tumors of MTgCRABP-II and MCRABP-II−/− mice, displayed characteristics similar to those reflected by data shown in Fig. 1 a–c (see also Fig. S4). To verify that these responses indeed originated from changes in the ratio of the binding protein, MII−/− cells were transfected with an expression vector for CRABP-II and the expression levels of the target genes examined. The data (Fig. 3c) revealed that the ectopic expression of CRABP-II in these cells rescued the ability of RA to induce the expression of the RAR target Cyp26a and dampens RA-induced induction of the PPARβ/δ target PDK1.
Fig. 3.
Decreasing the FABP5/CRABP-II ratio diverts RA from PPARβ/δ to RAR and inhibits proliferation in cell lines derived from MMTV-neu mice tumors. (a) Immunoblots of CRABP-II and FABP5 in cell lines derived from tumors that arise in MMTV-neu (NaF), MCRABP-II (MTg-II), and MCRABP-II−/− (MII−/−) mice. (b) mRNA for Cyp26a and PDK-1 in MTg-II cells. Cells were treated with 0.1 μM RA for 4 or vehicle. (c) mRNA for Cyp26a and PDK-1 in MIIminus]/− cells and in MII−/− cells ectopically overexpressing CRABP-II (+II). Cells were treated with RA or vehicle. (Inset) Western blot demonstrating ectopic overexpression of CRABP-II in transfected cells. (d) BrdU incorporation assays in denoted cell lines and in cell lines transfected with FABP5 siRNA (5si). Cells were treated with 1 μM RA for 24 h or vehicle. *, P = 0.001; **, P = 0.02 versus corresponding untreated controls. (e) Cell-cycle distribution. Denoted cells were treated with vehicle or 1 μM RA for 5 days, fixed in ethanol, stained with propidium iodide, and analyzed by FACS.
The ability of RA to inhibit the growth of the cell lines was assessed by measuring BrdU incorporation and by cell-cycle analyses. MTg-CRABP-II cells displayed a lower proliferation index and were more sensitive to RA-induced growth inhibition, compared with cells lacking CRABP-II (Fig. 3d). Furthermore, reducing FABP5 levels by transfection of FABP5siRNA (Fig. S5) inhibited the growth of both MTg-CRABP-II and MCRABP-II−/− cells (Fig. 3d), demonstrating that observed effects stemmed from alterations of the FABP5/CRABP-II ratio and not merely from manipulating CRABP-II expression. Treatment with RA further inhibited the growth of CRABP-II overexpressing cells, but did not affect the growth of cells lacking CRABP-II (Fig. 3d). Correspondingly, FACS analyses showed that RA treatment of MTg-CRABP-II cells evoked an apoptotic response, reflected by a marked increase in the subG1 population (Fig. 3e). In contrast, RA had little effect on cell-cycle distribution or apoptosis in CRABP-II-null cells (Fig. 3e).
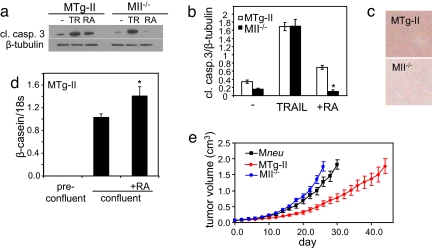
To further examine the ability of RA to induce apoptosis in the two lines, cells were treated with either RA or the apoptosis inducer TRAIL, and apoptosis was assessed by monitoring caspase 3 cleavage (Fig. 4 a and b). In cells expressing a high CRABP-II/FABP5 ratio, both TRAIL and RA induced apoptosis. Strikingly, cells lacking CRABP-II displayed a markedly lower level of cleaved caspase 3, and RA, unlike TRAIL, decreased this level even further. Hence, RA induced apoptosis in the presence of a high CRABP-II/FABP5 ratio, but became an anti-apoptotic agent in cells in which this ratio is reversed. Finally, the effects of changing the binding proteins' ratio on the differentiation status of the cells was examined. Oil-red-O staining showed that confluent MTg-CRABP-II cells, but not MCRABP-II−/− cells, displayed marked lipid accumulation, a hallmark of differentiated mammary epithelial cells (Fig. 4c). Correspondingly, the expression of the mammary differentiation marker β-casein was highly induced in postconfluent MTg-CRABP-II cells, and its expression was further up-regulated upon treatment with RA (Fig. 4d). In contrast, β-casein mRNA was undetectable in McRABP-II−/− cells either in the absence or presence of RA.
Fig. 4.
Decreasing the FABP5/CRABP-II ratio triggers apoptosis and differentiation in cell lines derived from MMTV-neu mice tumors and suppresses tumor growth in vivo. (a) Denoted cell lines were treated with 1 μM RA for 12 h or 10 ng/ml TRAIL (TR) for 12 h. Cells were lysed and the level of cleaved caspase 3 (cl. casp. 3) was assessed by immunoblots. (b) Quantitation of data in a. Values are mean ± SEM (n = 3). *, P = 0.05 versus untreated II−/− cells. (c) Denoted cells were stained with Oil-red-O 2 days postconfluence. (d) β-casein mRNA in MTg-CRABP-II cells preconfluence and 1.5 days postconfluence. Cells were treated with 1 μM RA throughout the experiment. *, P = 0.04 versus nontreated confluent MTg-II cells. (e) Tumor growth in MMTV-neu (Mneu), MTg-CRABP-II (MTg-II), and M-CRABP-II−/− (M-II−/−) mice. Tumor sizes were measured with a start point (day 0) of tumor volume = 0.065 cm3.
Decreasing the FABP5/CRABP-II Ratio Suppresses Tumor Development in MMTVneu Mice.
The rate of tumor growth in MCRABP-II−/− mice was significantly facilitated, whereas mammary overexpression of CRABP-II markedly retarded tumor growth, compared with control MMTV-neu mice (Fig. 4e). Mice with an elevated CRABP-II/FABP5 ratio also were burdened by fewer tumors. Notably, although tumors developed in 100% of mice in other groups, 4 of 13 MTg-CRABP-II mice remained tumor-free throughout the 650 days of the experiment (Fig. S6 and Table S1).
Mammary neoplasms in the three cohorts of mice were adenocarcinomas, but some differences were observed. In agreement with previous studies of the MMTV-neu model (35, 36), adenocarcinomas in control mice were composed of solid sheets of neoplastic epithelial cells with little or no glandular differentiation (Fig. 5a). The neoplastic population consisted of uniform, densely packed, relatively small cells with eosinophilic cytoplasm, rounded to elongated nuclei, and diffuse chromatin pattern. Mitotic figures were often observed. Similarly, adenocarcinomas in the MCRABP-II−/− mice displayed solid/nodular growth with densely packed cells containing pale eosinophilic cytoplasm and scanty connective tissue (Fig. 5c). In 8 of 12 of these tumors, there were areas of cells with nuclei of intermediate size and an open chromatin pattern. Numerous mitoses and few apoptotic cells were seen. In contrast, in MTg-CRABP-II mice, 16 of 18 tumors contained areas of adenocarcinomas with larger cells organized in nests or bundles separated by thin bands of connective tissue and blood vessels (Fig. 5b). The nuclei of these cells were larger, with more open chromatin pattern with prominent nucleoli and clumped heterochromatin. Such areas varied from 10–30% to >90% of neoplasms. Single mitotic figures as well as apoptotic cells were seen.
Fig. 5.
Decreasing the FABP5/CRABP-II ratio decreases proliferation and induces differentiation and apoptosis in tumors in vivo. (a–c) H&E staining. (a) Mammary carcinoma in an MMTV-neu mouse. Dense, relatively small tumor cells with round dark nuclei. Mitotic figures (arrows) and few apoptotic cells (arrowhead) are seen. (b) Carcinoma cells in an MTg-CRABP-II mouse are larger, arranged in bundles separated by thin connective tissue bands, and display vesicular nuclei and prominent nucleoli and heterochromatin. Apoptotic cells (arrowhead) are seen. (c) In an MCRABP-II−/− mouse, solid growth of cells with dense chromatin and frequent mitotic figures (arrows). (d–f) Immunohistochemical detection of β-casein (brown, arrow). (g–i) Immunostaining for the proliferation marker Ki67 (brown nuclei) (see Fig. S7). (j–l) Detection of apoptotic cells by immunostaining for cleaved-caspase 3 (brown nuclei). (Scale bars: 45 μm.)
Consistent with the behavior of the cultured cells, neoplastic cells of MTg-CRABP-II mice, but not those of MMTV-neu and MCRABP-II−/− models, expressed the differentiation marker β-casein (Fig. 5 d–f). Immunostaining for the proliferation marker Ki67 (Fig. 5 g–i) showed a significant decrease in proliferation of MTg-CRABP-II neoplasm and a slight increase in proliferation in the MCRABP−/− tumor. However, the fraction of apoptotic cells, evaluated by immunostaining for cleaved caspase 3, was markedly higher in mammary tumors of mice overexpressing CRABP-II, compared with CRABP-II-null mice or MMTV-neu controls (Fig. 5 j–l and Fig. S7).
Discussion
General use of RA in oncology is hampered by toxicity and the development of RA resistance in tumors. Moreover, in some cases, RA treatment not only fails to inhibit carcinoma cell growth, but, paradoxically, facilitates tumor development. Although it is well established that inhibition of cancer cell growth by RA is mediated by RAR, the mechanisms through which this agent enhances tumor development has remained an enigma. The recent reports that, in addition to activating RAR, RA also serves as a ligand for PPARβ/δ, a receptor that targets genes involved in mitogenic responses and in survival pathways (20, 21), suggests such a mechanism. The observations that the partitioning of RA between RAR and PPARβ/δ is controlled by CRABP-II and FABP5 further suggest that these binding proteins may be involved in regulating proliferative versus growth-inhibitory activities of the hormone.
Thus, we set out to explore whether the facilitated development of mammary tumors in MMTV-neu mice in response to RA may originate from an aberrantly high FABP5/CRABP-II ratio that, in turn, may divert RA from RAR to PPARβ/δ. The data show that, in mammary tumors of female MMTV-neu mice, RA up-regulates the expression of PPARβ/δ target genes, including the survival factor PDK1, but does not induce the expression of cell-cycle control and proapoptotic RAR targets. Increasing the already high FABP5/CRABP-II ratio further elevated PPARβ/δ targets and down-regulated genes regulated by RAR. However, a decreased FABP5/CRABP-II ratio in tumors directed RA to RAR, up-regulated the apoptosis factor caspase 9 and the cell-cycle control protein BTG2, and down-regulated cyclin D1. Correspondingly, neoplastic cells in MCRABP-II−/− and MMTV-neu mice displayed numerous mitotic figures and a high proliferation rate, whereas those of MTg-CRABP-II tumors exhibited reduced proliferation and a high apoptotic rate. Slower growth of neoplasms that overexpress CRABP-II also was accompanied by changes in morphology, growth pattern, and β-casein expression, indicating a more differentiated state. Thus, lowering the FABP5/CRABP-II ratio inhibited tumor growth by attenuating proliferation, enhancing apoptosis, and inducing differentiation.
Remarkably, the FABP5/PPARβ/δ pathway was found to facilitate, and CRABP-II/RAR signaling suppressed tumor growth at the level of RA endogenously present in the mouse. Thus, CRABP-II appears to function as a tumor suppressor, and FABP5 may comprise a therapeutic target whose inhibition may bypass both the RA resistance and RA toxicity encountered in current therapies. Although no antagonists for this protein currently exist, a recent report demonstrated the development an inhibitor for the homologous protein FABP4. In the case of FABP4, such an inhibitor was found to be effective against atherosclerosis and type 2 diabetes in mice (37). Compounds that target intracellular lipid-binding proteins may thus comprise a class of therapeutic agents. Development of such compounds could be assisted by the recent delineation of the structural features that underlie the ability of specific ligands to activate intracellular lipid-binding proteins (5, 38, 39).
Experimental Procedures
Transgenic Mice.
MMTV-CRABP-II mice were generated by using a transgenic construct consisting of the mammary epithelium-specific promoter/enhancer MMTV-LTR, a synthetic human CRABP-II cDNA, and a human β-globin polyadenylation signal (see SI Experimental Procedures).
Mouse Breeding.
The MCRABP-II−/− model was established by crossing FVB/N-Tg(MMTVneu)202Mul/J mice with C57BL/6 CRABP-II−/− mice. F1 mice were bred with CRABP-II−/− males to yield the MCRABP-II−/− cohort and control MMTV-neu littermates. Rate of mammary carcinogenesis on the F1 FVB/NXC57BL/6 background was similar to that on FVB/N background. MTgCRABP-II mice were generated by crossing MMTV-CRABP-II with MMTV-neu mice.
Genotyping.
DNA was isolated as described previously (41). MTg-II and MCRABP-II−/− mice were identified by PCR using apporpriate primers (see SI Experimental Procedures) (42).
Biochemical Procedures.
Cell and tissue extractions, immunoblotting, and Q-PCR analyses were performed as described previously (see SI Experimental Procedures) (17).
Carcinogenesis Studies.
Experiments were performed on multiparous females bred thrice. RA was administered by 90-day release 15-mg pellets (Innovative Research of America) implanted s.c. Mammary tumor development was monitored by palpation three times per week, and tumor sizes were measured with calipers and recorded without investigator's knowledge of study groups.
Immunohistochemical Analyses.
Tissues samples were collected and processed as described in SI Experimental Procedures. Apoptosis and cell proliferation were examined by immunohistochemical analyses using antibodies against cleaved caspase-3 (see SI Experimental Procedures). Apoptotic and proliferation indices were determined essentially as described previously (see SI Experimental Procedures) (44).
Cell Lines.
Tumors were collected after reaching a volume of 0.524 cm3, and cell lines were generated as described previously (see SI Experimental Procedures) (33).
Proliferation and Cell-Cycle Distribuition Assays.
For BrdU assays, cells were grown overnight in DMEM supplemented with 2% charcoal-treated FBS, treated with 1 μM RA for 24 h, and analyzed by using a BrdU cell proliferation assay (Calbiochem). For FACS, cells were treated with RA or vehicle in DMEM containing 1% FBS and incubated with 30 μg/ml 5′-Bromo-2′-deoxyuridine for 20 min at 37°C. Cells were processed as described previously (see SI Experimental Procedures) (8) and analyzed by FACS.
Statistical analyses were carried out by using Prism 3.02 (Graphpad) software. Survival fractions were calculated by using the Kaplan–Meier method and compared by log-rank Mantel–Haenszel tests. Means were compared by estimation of two-tailed P with unpaired t tests.
Supplementary Material
Acknowledgments.
We thank Pierre Chambon [Institute of Genetics and Molecular and Cellular Biology (IGBMC), Illkirch, France] for providing the CRABP-II−/− mice, Philip Leder (Harvard Medical School, Boston, MA) for the NaF cells, and Cecile Rochette-Egly (IGBMC, Illkirch, France) for CRABP-II antibodies. This work was supported by National Institutes of Health Grants R01 CA107013 (to N.N.), R01 CA96823 (to A.Y.N.), and 5T32CA009682 (to T.T.S.) and National Center for Research Resources/National Institutes of Health Midcareer Award K26 RR017595 (to A.Y.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709981105/DCSupplemental.
References
- 1.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 2.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 3.Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Mol Cell Biol. 2002;22:2632–2641. doi: 10.1128/MCB.22.8.2632-2641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budhu A, Gillilan R, Noy N. Localization of the RAR interaction domain of cellular retinoic acid binding protein-II. J Mol Biol. 2001;305:939–949. doi: 10.1006/jmbi.2000.4340. [DOI] [PubMed] [Google Scholar]
- 5.Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol Cell. 2005;18:343–353. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Park DJ, et al. CCAAT/enhancer binding protein epsilon is a potential retinoid target gene in acute promyelocytic leukemia treatment. J Clin Invest. 1999;103:1399–1408. doi: 10.1172/JCI2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochette-Egly C, Chambon P. F9 embryocarcinoma cells: A cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol Histopathol. 2001;16:909–922. doi: 10.14670/HH-16.909. [DOI] [PubMed] [Google Scholar]
- 8.Donato LJ, Suh JH, Noy N. Suppression of mammary carcinoma cell growth by retinoic acid: The cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res. 2007;67:609–615. doi: 10.1158/0008-5472.CAN-06-0989. [DOI] [PubMed] [Google Scholar]
- 9.Donato LJ, Noy N. Suppression of mammary carcinoma growth by retinoic acid: Proapoptotic genes are targets for retinoic acid receptor and cellular retinoic acid-binding protein II signaling. Cancer Res. 2005;65:8193–8199. doi: 10.1158/0008-5472.CAN-05-1177. [DOI] [PubMed] [Google Scholar]
- 10.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 11.Garattini E, Gianni M, Terao M. Retinoids as differentiating agents in oncology: A network of interactions with intracellular pathways as the basis for rational therapeutic combinations. Curr Pharm Des. 2007;13:1375–1400. doi: 10.2174/138161207780618786. [DOI] [PubMed] [Google Scholar]
- 12.Arapshian A, Kuppumbatti YS, Mira-y-Lopez R. Methylation of conserved CpG sites neighboring the beta retinoic acid response element may mediate retinoic acid receptor beta gene silencing in MCF-7 breast cancer cells. Oncogene. 2000;19:4066–4070. doi: 10.1038/sj.onc.1203734. [DOI] [PubMed] [Google Scholar]
- 13.Jing Y, Waxman S, Mira-y-Lopez R. The cellular retinoic acid binding protein II is a positive regulator of retinoic acid signaling in breast cancer cells. Cancer Res. 1997;57:1668–1672. [PubMed] [Google Scholar]
- 14.Xu XC. Tumor-suppressive activity of retinoic acid receptor-beta in cancer. Cancer Lett. 2007;253:14–24. doi: 10.1016/j.canlet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slack JL. The biology and treatment of acute progranulocytic leukemia. Curr Opin Oncol. 1999;11:9–13. doi: 10.1097/00001622-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Verma AK, Conrad EA, Boutwell RK. Differential effects of retinoic acid and 7,8-benzoflavone on the induction of mouse skin tumors by the complete carcinogenesis process and by the initiation-promotion regimen. Cancer Res. 1982;42:3519–3525. [PubMed] [Google Scholar]
- 17.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omenn GS, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 19.Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor beta/delta. J Biol Chem. 2003;278:41589–41592. doi: 10.1074/jbc.C300368200. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, et al. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci USA. 2006;103:19069–19074. doi: 10.1073/pnas.0607948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan NS, Michalik L, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor-beta as a target for wound healing drugs. Expert Opin Ther Targets. 2004;8:39–48. doi: 10.1517/14728222.8.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Tan NS, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tari AM, Lim SJ, Hung MC, Esteva FJ, Lopez-Berestein G. Her2/neu induces all-trans retinoic acid (ATRA) resistance in breast cancer cells. Oncogene. 2002;21:5224–5232. doi: 10.1038/sj.onc.1205660. [DOI] [PubMed] [Google Scholar]
- 24.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985;229:974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 26.Yokota J, et al. Amplification of c-erbB-2 oncogene in human adenocarcinomas in vivo. Lancet. 1986;1:765–767. doi: 10.1016/s0140-6736(86)91782-4. [DOI] [PubMed] [Google Scholar]
- 27.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 28.Lampron C, et al. Mice deficient in cellular retinoic acid binding protein II (CRABPII) or in both CRABPI and CRABPII are essentially normal. Development. 1995;121:539–548. doi: 10.1242/dev.121.2.539. [DOI] [PubMed] [Google Scholar]
- 29.Kersten S, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 30.Schmuth M, et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 31.Tan NS, et al. Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guardavaccaro D, et al. Arrest of G(1)-S progression by the p53-inducible gene PC3 is Rb dependent and relies on the inhibition of cyclin D1 transcription. Mol Cell Biol. 2000;20:1797–1815. doi: 10.1128/mcb.20.5.1797-1815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elson A, Leder P. Protein-tyrosine phosphatase epsilon. An isoform specifically expressed in mouse mammary tumors initiated by v-Ha-ras OR neu. J Biol Chem. 1995;270:26116–26122. doi: 10.1074/jbc.270.44.26116. [DOI] [PubMed] [Google Scholar]
- 34.White JA, et al. Identification of the human cytochrome P450, P450RAI-2, which is predominantly expressed in the adult cerebellum and is responsible for all-trans-retinoic acid metabolism. Proc Natl Acad Sci USA. 2000;97:6403–6408. doi: 10.1073/pnas.120161397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardiff RD, Sinn E, Muller W, Leder P. Transgenic oncogene mice. Tumor phenotype predicts genotype. Am J Pathol. 1991;139:495–501. [PMC free article] [PubMed] [Google Scholar]
- 36.Cardiff RD, et al. The mammary pathology of genetically engineered mice: The consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 37.Furuhashi M, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry. 2007;46:6744–6752. doi: 10.1021/bi700047a. [DOI] [PubMed] [Google Scholar]
- 39.Gillilan RE, Ayers SD, Noy N. Structural basis for activation of fatty acid-binding protein 4. J Mol Biol. 2007;372:1246–1260. doi: 10.1016/j.jmb.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaub MP, et al. Nuclear detection of cellular retinoic acid binding proteins I and II with new antibodies. J Histochem Cytochem. 1998;46:1103–1111. doi: 10.1177/002215549804601002. [DOI] [PubMed] [Google Scholar]
- 41.Nikitin A, Rajewsky MF, Pozharisski KM. Development of malignant fibrous histiocytoma induced by 7,12-dimethylbenzanthracene in the rat: Characterization of early atypical cells. Virchows Arch. 1993;64:151–159. doi: 10.1007/BF02915108. [DOI] [PubMed] [Google Scholar]
- 42.Manor D, et al. N Mammary carcinoma suppression by cellular retinoic acid binding protein-II. Cancer Res. 2003;63:4426–4433. [PubMed] [Google Scholar]
- 43.Nikitin AY, et al. Cell lineage-specific effects associated with multiple deficiencies of tumor susceptibility genes in Msh2(minus]/−)Rb(+/−) mice. Cancer Res. 2002;62:5134–5138. [PubMed] [Google Scholar]
- 44.Zhou Z, et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.