Abstract
Why do parasites harm their hosts? Conventional wisdom holds that because parasites depend on their hosts for survival and transmission, they should evolve to become benign, yet many parasites cause harm. Theory predicts that parasites could evolve virulence (i.e., parasite-induced reductions in host fitness) by balancing the transmission benefits of parasite replication with the costs of host death. This idea has led researchers to predict how human interventions—such as vaccines—may alter virulence evolution, yet empirical support is critically lacking. We studied a protozoan parasite of monarch butterflies and found that higher levels of within-host replication resulted in both higher virulence and greater transmission, thus lending support to the idea that selection for parasite transmission can favor parasite genotypes that cause substantial harm. Parasite fitness was maximized at an intermediate level of parasite replication, beyond which the cost of increased host mortality outweighed the benefit of increased transmission. A separate experiment confirmed genetic relationships between parasite replication and virulence, and showed that parasite genotypes from two monarch populations caused different virulence. These results show that selection on parasite transmission can explain why parasites harm their hosts, and suggest that constraints imposed by host ecology can lead to population divergence in parasite virulence.
Keywords: coevolution, Danaus plexippus, disease, epidemiology, pathogen
Parasites are arguably the most common life form on earth (1), and understanding their evolution has increasing relevance for predicting their effects on human, agricultural, and wild populations (2–8). By definition, parasites cause harm to their hosts (i.e., they cause virulence), but explaining why they do so remains a challenge for evolutionary biologists. A fundamental question is why parasites that depend on hosts for their own survival and transmission cause disease or even kill their hosts. The most popular evolutionary explanation asserts that virulence is an unavoidable consequence of selection to maximize parasite fitness (9–17). Parasites must replicate within hosts to produce transmission stages, but this also consumes host resources, damages host tissues, and provokes immune clearance, thereby shortening the infectious period over which transmission can take place. Parasites thus face a trade-off between the benefits of increased replication (i.e., increased transmission rate) and the costs (i.e., virulence or immune clearance), resulting in highest fitness at intermediate levels of parasite replication.
This “trade-off hypothesis” underlies many theoretical studies and is advocated to inform public health decisions (6, 7, 13, 18), yet there remains a serious lack of empirical evidence (19, 20). A small number of studies have shown positive relationships between within-host replication and virulence (21) and between virulence and parasite transmission potential (22–24), or have shown optimal transmission at an intermediate level of virulence (25). However, no studies have simultaneously quantified the relationships between within-host parasite replication, virulence, and transmission to examine support for a maximum attainable parasite fitness owing to a trade-off between the costs and benefits of parasite replication. This gap in empirical data from naturally occurring host–parasite systems has led some authors to conclude that the trade-off theory may be too narrow a view of the evolution of parasites (20, 26). Still, a recent study has demonstrated the important role that life-history trade-offs may play in pathogen evolution: for HIV-1 infections, increasing viral loads during the asymptomatic phase both reduced the time to the onset of AIDS and increased the annual transmission rate, such that transmission potential over the course of infection was maximized at intermediate viral loads (27).
We studied the protozoan parasite Ophryocystis elektroscirrha (28), which commonly infects wild populations of the monarch butterfly (Danaus plexippus) (29, 30). Infections occur when caterpillars ingest spores scattered onto eggs or host plant leaves by adult butterflies. Parasites penetrate the gut wall and undergo vegetative replication in the host's hypoderm. After host pupation, the parasite forms spores around the scales of the developing butterflies, and infected adults emerge covered with dormant spores on the outsides of their bodies. Parasite replication is intense, with a single infecting spore sometimes giving rise to over a million progeny (31). The primary route of transmission is from infected females to their offspring when parasite spores are passively transferred to the outside of eggs and host plant leaves during oviposition, although some horizontal transmission occurs (32).
By using this host–parasite system, we quantified relationships between within-host replication, virulence, and transmission, and tested whether parasite fitness was maximized at an intermediate level of replication. To establish genetic relationships between parasite replication and virulence, we conducted a second experiment by using host and parasite genotypes from two North American monarch populations. Our results show that monarch parasites indeed face a trade-off between virulence and transmission, and that parasites from different populations have marked divergence in virulence, as predicted by constraints on transmission and selective forces operating on virulence evolution.
Results
Parasite Replication, Virulence, and Transmission.
We infected monarch butterflies with O. elektroscirrha and quantified survival to the adult stage, mating success, lifespan, fecundity, and parasite transmission. We used the number of parasite spores on infected butterflies (spore load) as our primary measure of parasite replication.
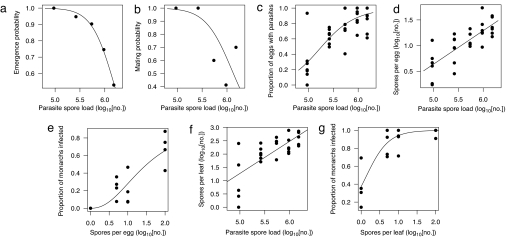
Greater parasite replication reduced host survival to the adult stage, with fewer monarchs emerging successfully from their pupal cases with increasing parasite loads [Figs. 1 and 2a; general linear model (GLM) with binomial error distribution: F1,13 = 34.7, P < 0.001]. Among female monarchs that survived to the adult stage, higher parasite loads reduced mating success (Fig. 2b; GLM with binomial error distribution: F1,13 = 8.35, P = 0.013), in part because higher parasite loads significantly reduced adult female lifespan (linear model: F1,44 = 4.03, P = 0.05). Among mated female monarchs, higher parasite replication rates did not result in lower lifetime fecundity (i.e., the total number of eggs laid; linear model: F1,33 = 0.49, P = 0.49), probably because females laid most of their eggs early on in life (>90% of eggs laid between days 1 and 10 postmating).
Fig. 1.
Parasite-infected monarch stuck to its pupal case. Heavy parasite infection impaired the development of abdominal integuments, such that many infected monarchs were unable to eclose.
Fig. 2.
Relationships between parasite replication, virulence, and transmission. Increasing within-host replication (i.e., parasite spore load) resulted in lower proportions of animals surviving to the adult stage (a) and lower mating probabilities (b). Higher parasite loads also led to increased transmission through higher proportions of monarch eggs that acquired spores (c), and higher numbers of parasites per egg and milkweed leaf (d and f); these higher parasite numbers increased the probability of infection (e and g). Data points are average proportions (a, b, e, and g) or individual monarchs (c, d, and f). Lines are least-squares regression lines (see Table S1 for functions).
Detrimental effects of the parasite on the host may appear maladaptive, but high parasite loads were necessary to increase transmission. Female monarchs with low spore loads (≈105 spores) transferred spores to only 20% of their eggs; this percentage increased to nearly 100% with higher parasite replication (Fig. 2c; GLM with binomial error distribution: F1,31 = 30.7, P < 0.001). The average numbers of spores deposited per egg and onto milkweed leaves on which eggs were laid also increased with spore load (Fig. 2 d and f; linear model: eggs, F1,30 = 47.2, P < 0.001; leaves, F1,25 = 27.3, P < 0.001). This is important because larvae that ingest a higher number of parasites (by feeding on eggs and milkweed leaves with spores) have a higher probability of becoming infected (Fig. 2 e and g; GLM with binomial error distribution: eggs, F1,14 = 31.3, P < 0.001; leaves, F1,14 = 27.7, P < 0.001).
Parasite Lifetime Fitness.
We calculated two measures of parasite lifetime fitness (ω) as a function of spore load (p) by using the least-squares regression lines shown in Fig. 2 and described in supporting information (SI) Table S1. The first measure was a conservative estimate, in which we assumed that parasite transmission occurs exclusively through monarch eggs; it was defined as the averaged proportion of offspring—produced by monarchs with spore load p—that were infected
in which E(p) is the probability of adult emergence (Fig. 2a) and M(p) is the probability of mating (Fig. 2b). Further, T(p) is the proportion of eggs that acquire parasite spores (Fig. 2c) and Ie(de) is the proportion of offspring that will become infected (Fig. 2e) based on dose de(p), which is the average number of spores deposited on a spore-positive egg and is modeled as a function of p (Fig. 2d). The subscript e stands for egg.
Because parasite transmission also occurs through spores deposited onto milkweed leaves where eggs are laid, and because transmission through ingestion of spores on milkweed causes higher infection rates relative to spores on the egg chorion (31), we constructed a less conservative parasite fitness measure as follows:
Here, symbols are analogous to those in Eq. 1, with the exceptions that Il(dl) is now the averaged proportion of offspring that will become infected when ingesting milkweed leaves with parasite spore dose dl(p) (Fig. 2g), and dl(p) in turn is the number of spores deposited on milkweed leaves (Fig. 2f); the new subscript l denotes leaf. Note that the term T(p) remains unaltered; thus, we conservatively assume that parasite spores are only transferred to milkweed leaves when they are also transferred to eggs.
Our fitness measures predict the relative rather than the absolute number of infected offspring because the total fecundity of infected females was independent of parasite spore load. Furthermore, a proportional fitness measure is more relevant across a range of natural background mortality rates of monarch larvae, which will ultimately determine the absolute number of infected offspring that survive.
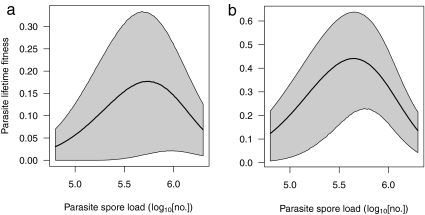
Plotting fitness estimates ωe and ωl against parasite spore load reveals concave fitness curves (Fig. 3) generated by a trade-off between transmission and virulence. Because parasite fitness depends on a series of estimated parameter values that each carry a level of uncertainty (Table S1), 68% confidence regions generated for the predicted fitness curves are broad, especially for the more conservative ωe (Fig. 3a). Despite this uncertainty in parameter estimation, the concavity of the trade-off curves is readily apparent in both the expected values of ω(p) and the confidence regions. Thus, increasing parasite spore loads initially result in greater parasite fitness owing to higher transmission. However, at very high parasite spore loads, the costs of increased within-host replication (i.e., virulence) outweigh the benefits (increased transmission). The resulting trade-off curves suggest that the spore loads maximizing fitness on the basis of transmission through eggs and leaves are similar (respectively, 5.74 and 5.66 on a log10 scale).
Fig. 3.
Relationship between parasite replication and lifetime fitness. Parasite fitness (ω) was calculated as the averaged proportion of offspring predicted to become infected from monarchs with a given parasite load (p), and was based on transmission occurring through spores deposited on either eggs (a) or milkweed leaves (b). Gray areas are 68% confidence regions. Note that these measures of fitness predict the proportions of infected offspring averaged for a range of monarchs with a spore load p; thus, although 100% of offspring produced by surviving and successfully mated monarchs with log10 spore loads >6.0 could become infected, the average parasite fitness is much lower because few monarchs with this spore load emerge or mate.
Population Divergence and Genetic Basis for Virulence.
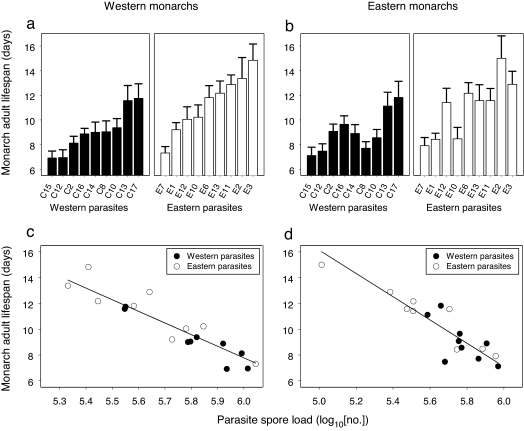
Natural selection requires that relationships between parasite replication and virulence are genetically based. We obtained hosts and parasites from western North America (where monarchs overwinter along the Californian coast) and eastern North America (where monarchs travel to Mexico to overwinter), and infected hosts with 1 of 18 parasite clones. Spore loads on adult butterflies were used as a measure of parasite replication, and virulence was measured as adult longevity (a proxy for the likelihood of host reproduction: successful mating and onset of oviposition). We observed considerable genetic variation in virulence among parasite clones (Figs. 4a and b; ANOVA: F17,710 = 11.2, P < 0.001), and found that parasites from the western population were more virulent than those from the eastern population (Fig. 4 a and b; linear mixed effects model: t1,16 = −2.27, P = 0.038).
Fig. 4.
Genetic relationships between parasite replication and virulence. Parasite clones obtained from the western and eastern North American monarch populations varied in virulence, as indicated by the lifespan of infected monarchs (a and b); western parasites were on average more virulent than eastern parasites. Parasite virulence was related to parasite replication rate (c and d). Data points are parasite clone means; error bars (a and b) are 1 SEM.
This population difference in virulence was related to differences in parasite replication rate (with the effect of parasite source population on virulence nonsignificant when corrected for spore load: linear mixed-effects model: t1,16 = −2.09, P = 0.053). Thus, parasites from the western population were more virulent because they produced higher numbers of spores (Fig. 4 c and d). Our analyses showed that hosts from the two populations did not differ in the virulence they suffered (i.e., host–source population nonsignificant in a linear mixed-effects model: t1,8 = 0.05, P = 0.96). Thus, parasites from the western population were more virulent when tested on monarchs from both eastern and western populations (linear mixed-effects models; western monarchs: t1,16 = −2.24, P = 0.040; eastern monarchs: t1,16 = −2.19, P = 0.044). This demonstrates that average differences in parasite virulence were consistent when tested against sympatric and allopatric hosts.
Across both populations, the average spore load produced by parasite clones was 5.73 ± 0.019 [mean log10(spore load) ± SEM], and the replication rates of the tested parasite clones followed a near-normal distribution around this mean (Fig. S1). Thus, the most commonly observed parasite spore loads were close to those predicted to maximize parasite fitness [Fig. 3; log10(spore load) = 5.74 and 5.66 for egg and leaf transmission, respectively].
Discussion
Our study suggests that parasite virulence can evolve as a consequence of natural selection on parasite transmission. Parasite replication resulted in both costs and benefits to the parasite, and our analyses suggest that parasite lifetime fitness would be maximized at an intermediate replication rate. Importantly, at this level of replication, the parasite causes considerable virulence to the host (approximately a 10% reduction in emergence probability and a 20% reduction in mating probability; Fig. 2). Our finding that the replication rates of natural parasite genotypes fell close to the calculated optima provides further support for the hypothesis that natural selection in this system favors parasite genotypes that cause significant damage to their hosts based on a trade-off between virulence and transmission.
Like another recently published study on bacteria affecting the water flea Daphnia magna (25), our data underscore the importance of understanding the details of a particular host–parasite system when making inferences about virulence evolution (20, 26, 33, 34). In particular, the cost of parasite replication did not simply arise from a shortening of the time over which transmission could take place (e.g., 27), but through reduced survival of hosts to the adult stage as well as reduced female mating probability (which was itself caused by reduced adult longevity). Both of these mechanisms, as well as a detailed quantification of spore transmission and host infection probability, were necessary to reveal a virulence-transmission trade-off.
Population differences in parasite virulence suggest that ecological conditions – by changing the relative costs and benefits of parasite replication - will further determine the adaptive levels of virulence in wild populations. Three explanations could account for virulence differences of parasites in different monarch populations.
First, monarchs in eastern North America travel up to 5,000 km on the round-trip journey to and from their overwintering sites in Mexico (35), whereas monarchs in western North America travel much shorter distances to their overwintering grounds along the Californian coast [≈500 km one-way (35)]. Longer flight distances could increase the cost of parasitism, because high spore loads reduce monarch lifespan (this study) and flight ability (36). In other words, it is conceivable that long-distance migration selects against high parasite virulence by weeding out those monarchs infected with the most virulent parasite genotypes during migration.
Second, ecological conditions tied to monarch migration affect parasite transmission opportunities, which could result in lower or higher virulence (37–40). In the monarch–parasite system, vertical transmission from infected females to their offspring is the most important transmission route, but horizontal transmission can occur when adult monarchs deposit parasite spores on host plants, which are then consumed by unrelated caterpillars. Past studies of spore accumulation on milkweed host plants indicate that horizontal transmission is higher in monarch populations where adults breed year-round and local host densities are high, such as in South Florida and Hawaii (32). Occasional horizontal transmission may be necessary for virulent vertically transmitted parasites to persist (37), and may also select for higher adaptive levels of parasite virulence (but see ref. 38). Such horizontal transmission could further result in a higher frequency of mixed-strain parasite infections, and the resulting within-host competition between parasite genotypes could then select for higher virulence (4, 12, 15).
A third explanation for population divergence in parasite virulence involves the intimate associations between monarchs and their larval host plants. As larvae, monarchs feed exclusively on plant species in the milkweed family (Asclepiadaceae) (41, 42), and monarchs in different populations use different milkweed species (43). Recently, we have shown that host plant species can crucially alter the replication and virulence of O. elektroscirrha (44). Hence, locally occurring larval host plant species could alter the relative costs and benefits of parasite replication and hence the adaptive level of parasite virulence.
Empirical tests of the hypotheses described above will be important to understand how ecological conditions drive the evolution of parasite virulence (45). In a time where anthropogenic disturbance has affected the ecology of many wild host–pathogen interactions, such understanding has increasing relevance for managing disease risks to natural populations (8, 46). Moreover, such understanding will be crucial to validate current theoretical models in which intervention of ecological processes is advocated to drive virulence evolution of human pathogens into beneficial directions (6, 13, 18).
Methods
Experiment 1: Parasite Replication, Virulence, and Transmission.
Larval rearing.
Monarchs were the grand-progeny of wild females captured in Georgia, in May 2006. Monarchs from six noninbred full-sib monarch families were assigned randomly to parasite infection treatments. Groups of 40 replicate larvae were infected with 1, 10, or 100 parasite spores of one of three parasite clones (denoted E1, E11, and E12; derived from the eastern North American migratory population); another 160 larvae were left uninfected to serve as mates for infected females (total n = 520 larvae). Infection and rearing methods are described in ref. 31.
Before adult emergence, pupae were scored for parasite infection by using discoloration of the pupal case on a scale of 0–5, with 0 being uninfected and 5 heavily infected. To translate these pupal infection scores into parasite spore loads, we used a total of 133 monarchs that were monitored at 14°C in the laboratory. Spore loads were quantified by vortexing their bodies in 5 ml of H2O after death and counting spores by using a hemocytometer. Regression analysis showed that adult spore loads were highly correlated with pupal infection scores [log10(sporeload) = 4.21 + 2.53 × log10(pupal infection score); F1,131 = 173, P < 0.001, R2 = 0.57], and we used this relationship to derive estimated spore loads for female monarchs held in outdoor cages (and for which pupal infection scores, but not adult spore loads, had been recorded).
Virulence and transmission measures.
A number of monarchs became stuck to their pupal cases on eclosion, such that they did not emerge successfully, and we recorded this lack of emergence as a first measure of virulence. Of the monarchs that emerged successfully, we randomly selected 51 female monarchs to obtain measurements of virulence and transmission. These were distributed among six mesh outdoor field cages (0.61 m3) with unrelated uninfected males. On mating, females were immediately transferred to individual field cages, where they were provided with an ad libitum 10% honey water solution and a stalk of milkweed host plant (Asclepias incarnata). We replaced milkweed stalks daily and counted the total number of eggs laid per day. Stalks were returned to the laboratory to count the total number of parasite spores on a subset of eggs (n = 6,243) and leaves (n = 163) on which eggs were laid; spores were counted by using a dissecting microscope (60×), and for each female the counts on eggs and leaves were averaged over her lifetime. Females remained in field cages until they died, and we recorded adult longevity as time from eclosion to death in days.
All 51 female monarchs were used to estimate mating probability; 46 of these same monarchs were used to measure adult lifespan (the remaining 5 were removed from their cages before death). Of these 46 monarchs, 35 mated successfully and were used to estimate the number of eggs laid; 33 females laid eggs and were used to calculate the proportion of eggs with parasite spores; 1 female laid a single egg without any parasites on it, leaving 32 females for which the average number of spores deposited per egg could be calculated. We obtained counts of parasites per milkweed leaf for 27 females, excluding several females that laid few eggs.
Statistical analysis.
The aim of the analysis was twofold. First, we analyzed whether parasite spore load (log10-transformed) affected monarch emergence probability, mating probability, adult lifespan, and lifetime fecundity (measures of parasite virulence); and the number of eggs that received parasite spores and the numbers of parasites transferred to eggs and milkweed leaves (measures of parasite transmission). We also analyzed whether higher numbers of parasites per egg and milkweed leaf resulted in a higher infection probability. We used linear models with normal error distributions to test the effect of spore load on monarch adult lifespan, monarch lifetime fecundity, and the numbers of spores transferred to eggs and leaves. GLMs with binomial error distributions were used to test the effect of spore load on monarch emergence probability, monarch mating probability, and the proportion of monarch eggs that received parasite spores. Such GLMs were also used to analyze the effect of the numbers of parasite spores per egg and milkweed leaf on the infection probability of monarchs hatching from those eggs (data for latter analysis derived from ref. 31). Parasite clone was included in all maximal models, but was removed because of insignificance (at the 0.05 level). In analyses of monarch emergence and mating probability, parasite clone was retained (resulting in a total of 15 proportional values on which the analyses were based: 5 infection levels by 3 parasite clones). Significance of model terms was assessed by comparing the explanatory power of models including or excluding these terms (47).
The second aim of the analysis was to assess how the differential effects of parasite spore load on virulence and transmission affected parasite fitness (lifetime transmission) for a given spore load. Denoting log10(parasite spore load) as p, two measures of parasite lifetime transmission ω(p) were calculated as shown in Eqs. 1 and 2. Measure ωe(p) was based on the conservative assumption that parasite transmission occurs exclusively through spore deposition on eggs and the subsequent spore consumption by the hatching caterpillar; Ie(de(p)) thus refers to the probability of infection for a caterpillar ingesting an egg chorion with de(p) parasite spores. Measure ωl(p) assumed that parasite transmission occurs through spore deposition onto milkweed leaves on which eggs are laid, and the subsequent spore ingestion through milkweed consumption. This is a more realistic assumption, because monarch larvae ingest far more leaf material than egg chorion. A second assumption in the calculation of ωl(p) was that transfer of spores to leaves occurs only when spores are also transmitted to eggs: thus, we used T(p) as a measure of the proportion of successful transmission events. Parameters for the functional forms for E(p), M(p), T(p), d(p), and I(d) were estimated by using nonlinear least-squares regression; proportional data were described with Hill functions (see Table S1 for functions, parameter estimates, and standard errors). Sixty-eight percent confidence intervals on ω(p) were calculated by Monte Carlo resampling from the fitted distributions of parameter estimates; 2.5 × 105 replicates were used for each of 100 values of log10(sporeload) in the range 4.8 to 6.3. All analyses were carried out in R version 2.4.0.
Experiment 2: Population Divergence and Genetic Basis for Virulence.
Experimental design.
Monarchs used were the F2 offspring of wild adults collected from overwintering sites of the eastern North American population (Sierra Chincua, Michoacan, Mexico, January 2007) and the western North American population (Pismo Beach, California, February 2007). Five noninbred host families per population were used. We inoculated 5 replicate larvae from each host family with a dose of 10 spores from each of 9 western and 9 eastern parasite clones. Parasite replication was assessed by quantifying parasite spore loads by using the vortex method described earlier. Virulence was quantified as the longevity of adult monarchs held at 14°C (a proxy for the likelihood of host reproduction).
Statistical analysis.
First, we tested whether parasite clones differed in the virulence they caused by using an ANOVA with parasite clone as a fixed factor. Second, we tested whether parasite clones from the western and eastern populations caused different virulence: we used a linear mixed-effects model, with population as a fixed effect and parasite clone nested within population as a random effect (47). We then included parasite spore load (log10-transformed) as a continuous covariate in this model to test whether monarch population remained significant. We repeated these mixed effects analyses for monarchs from the western and eastern populations separately, to test whether similar patterns occurred in sympatric and allopatric hosts. We also analyzed whether monarchs from the two populations suffered different levels of virulence by using a linear mixed-effects model with host population as a fixed effect and monarch family nested within source population as a random effect. All analyses were carried out in R 2.4.0; F values (ANOVA) and t values (mixed models) with the relevant error degrees of freedom are reported.
Supplementary Material
Acknowledgments.
We thank L. Brower, A. Davis, D. Frey, I. Limón, K. Oberhauser, I. Ramirez, N. Vitone, and R. Zubieta for help with obtaining monarchs and parasites; J. Chi, L. Gold, N. Kolleda, B. Ledbetter, M. Maudsley, JR McMillan, C. Norman, E. Osburn, A. Pedersen, R. Rarick, and J. Rushmore for help with experiments; and R. Antia, M. Boots, C. DeCurtis, D. Ebert, C. Lively, A. Pedersen, A. Read, P. Rohani, and a reviewer for comments on the manuscript. This work was supported by Emory University, the University of Georgia, a Netherlands Organisation for Scientific Research TALENT fellowship (to J.C.d.R.), a Marie Curie Outgoing International Fellowship (to J.C.d.R.), and National Science Foundation Grant DEB-0643831 (to S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710909105/DCSupplemental.
References
- 1.Windsor DA. Most of the species on Earth are parasites. Int J Parasitol. 1998;28:1939–1941. doi: 10.1016/s0020-7519(98)00153-2. [DOI] [PubMed] [Google Scholar]
- 2.Antia R, Regoes RR, Koella JC, Bergstrom CT. The role of evolution in the emergence of infectious diseases. Nature. 2003;426:658–661. doi: 10.1038/nature02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thrall PH, Burdon JJ. Evolution of virulence in a plant host-pathogen metapopulation. Science. 2003;299:1735–1737. doi: 10.1126/science.1080070. [DOI] [PubMed] [Google Scholar]
- 4.De Roode JC, et al. Virulence and competitive ability in genetically diverse malaria infections. Proc Natl Acad Sci USA. 2005;102:7624–7628. doi: 10.1073/pnas.0500078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boots M, Mealor M. Local interactions select for lower pathogen infectivity. Science. 2007;315:1284–1286. doi: 10.1126/science.1137126. [DOI] [PubMed] [Google Scholar]
- 6.Dieckmann U, Metz JAJ, Sabelis MA, Sigmund K. Adaptive Dynamics of Infectious Diseases: In Pursuit of Virulence Management. Cambridge, UK: Cambridge Univ Press; 2002. [Google Scholar]
- 7.Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of pathogen virulence. Nature. 2001;414:751–756. doi: 10.1038/414751a. [DOI] [PubMed] [Google Scholar]
- 8.Harvell CD, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 9.Levin S, Pimentel D. Selection of intermediate rates of increase in parasite-host systems. Am Nat. 1981;117:308–315. [Google Scholar]
- 10.Bremermann HJ, Pickering J. A game-theoretical model of parasite virulence. J Theor Biol. 1983;100:411–426. doi: 10.1016/0022-5193(83)90438-1. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 12.Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- 13.Ewald PW. Host-parasite relations, vectors, and the evolution of disease severity. Ann Rev Ecol Syst. 1983;14:465–485. [Google Scholar]
- 14.Antia R, Levin BR, May RM. Within-host population dynamics and the evolution and maintenance of microparasite virulence. Am Nat. 1994;144:457–472. [Google Scholar]
- 15.Van Baalen M, Sabelis MW. The dynamics of multiple infection and the evolution of virulence. Am Nat. 1995;146:881–910. [Google Scholar]
- 16.Bull JJ. Virulence. Evolution (Lawrence, Kans) 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 17.Levin BR. The evolution and maintenance of virulence in microparasites. Emerg Infect Dis. 1996;2:93–102. doi: 10.3201/eid0202.960203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galvani AP. Epidemiology meets evolutionary ecology. Trends Ecol Evol. 2003;18:132–139. [Google Scholar]
- 19.Ebert D, Bull JJ. Challenging the trade-off model for the evolution of virulence: Is virulence management feasible? Trends Microbiol. 2003;11:15–20. doi: 10.1016/s0966-842x(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 20.Koella JC, Turner P. Evolution of parasites. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. London: Oxford Univ Press; 2007. pp. 229–237. [Google Scholar]
- 21.Lipsitch M, Moxon ER. Virulence and transmissibility of pathogens: What is the relationship? Trends Microbiol. 1997;5:31–37. doi: 10.1016/S0966-842X(97)81772-6. [DOI] [PubMed] [Google Scholar]
- 22.Messenger SL, Molineux IJ, Bull JJ. Virulence evolution in a virus obeys a trade-off. Proc R Soc Lond Ser B-Biol Sci. 1999;266:397–404. doi: 10.1098/rspb.1999.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackinnon MJ, Read AF. Genetic relationships between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution (Lawrence, Kans) 1999;53:689–703. doi: 10.1111/j.1558-5646.1999.tb05364.x. [DOI] [PubMed] [Google Scholar]
- 24.Mackinnon MJ, Read AF. Virulence in malaria: An evolutionary viewpoint. Philos Trans R Soc Lond B Biol Sci. 2004;359:965–986. doi: 10.1098/rstb.2003.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen KH, Little TJ, Skorping A, Ebert D. Empirical support for optimal virulence in a castrating parasite. PloS Biol. 2006;4:e197. doi: 10.1371/journal.pbio.0040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebert D, Bull JJ. The evolution and expression of virulence. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. London: Oxford Univ Press; 2007. pp. 153–167. [Google Scholar]
- 27.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: Epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin RE, Myers J. Ophryocystis elektroscirrha sp. n., a neogregarine pathogen of monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer. J Protozool. 1970;17:300–305. [Google Scholar]
- 29.Altizer SM, Oberhauser KS, Brower LP. Associations between host migration and the prevalence of a protozoan parasite in natural populations of adult monarch butterflies. Ecol Entomol. 2000;25:125–139. [Google Scholar]
- 30.Leong KLH, Yoshimura MA, Kaya HK. Occurrence of a neogregarine protozoan, Ophryocystis elektroscirrha McLaughlin and Myers, in populations of monarch and queen butterflies Pan-Pac Entomol. 1997;73:49–51. [Google Scholar]
- 31.De Roode JC, Gold LR, Altizer S. Virulence determinants in a natural butterfly-parasite system. Parasitology. 2007;134:657–668. doi: 10.1017/S0031182006002009. [DOI] [PubMed] [Google Scholar]
- 32.Altizer SM, Oberhauser KS, Geurts KA. Transmission of the protozoan parasite, Ophryocystis electroscirrha, in monarch butterfly populations: Implications for prevalence and population-level impacts. In: Oberhauser KS, Solensky M, editors. The Monarch Butterfly: Biology and Conservation. Ithaca, NY: Cornell Univ Press; 2004. pp. 203–218. [Google Scholar]
- 33.Alizon S, van Baalen M. Emergence of a convex trade-off between transmission and virulence. Am Nat. 2005;165:E155–E167. doi: 10.1086/430053. [DOI] [PubMed] [Google Scholar]
- 34.Ganusov VV, Antia R. Trade-offs and the evolution of virulence of microparasites: Do details matter? Theor Popul Biol. 2003;64:211–220. doi: 10.1016/s0040-5809(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 35.Brower LP. Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857–1995. J Lepidopt Soc. 1995;49:304–385. [Google Scholar]
- 36.Bradley CA, Altizer S. Parasites hinder monarch butterfly flight: Implications for disease spread in migratory hosts. Ecol Lett. 2005;8:290–300. [Google Scholar]
- 37.Lipsitch M, Nowak MA, Ebert D, May RM. The population dynamics of vertically and horizontally transmitted parasites. Proc R Soc Lond Ser B-Biol Sci. 1995;260:321–327. doi: 10.1098/rspb.1995.0099. [DOI] [PubMed] [Google Scholar]
- 38.Lipsitch M, Siller S, Nowak MA. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution (Lawrence, Kans) 1996;50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 39.Day T. Virulence evolution via host exploitation and toxin production in spore-producing pathogens. Ecol Lett. 2002;5:471–476. [Google Scholar]
- 40.Bull JJ, Molineux IJ, Rice WR. Selection of benevolence in a host-parasite system. Evolution (Lawrence, Kans) 1991;45:875–882. doi: 10.1111/j.1558-5646.1991.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 41.Ackery PR, Vane-Wright RI. Milkweed Butterflies: Their Cladistics and Biology. Ithaca, NY: Cornell Univ Press; 1984. [Google Scholar]
- 42.Vickerman DB, de Boer G. Maintenance of narrow diet breadth in the monarch butterfly caterpillar: Response to various plant species and chemicals. Entomol Exp Appl. 2002;104:255–269. [Google Scholar]
- 43.Ladner DT, Altizer S. Oviposition preference and larval performance of North American monarch butterflies on four Asclepias species. Entomol Exp Appl. 2005;116:9–20. [Google Scholar]
- 44.De Roode JC, Pedersen AB, Hunter MD, Altizer S. Host plant species affects virulence in monarch butterfly parasites. J Anim Ecol. 2008;77:120–126. doi: 10.1111/j.1365-2656.2007.01305.x. [DOI] [PubMed] [Google Scholar]
- 45.Dybdahl MF, Storfer A. Parasite local adaptation: Red Queen versus Suicide King. Trends Ecol Evol. 2003;18:523–530. [Google Scholar]
- 46.Altizer S, Harvell D, Friedle E. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol Evol. 2003;18:589–596. [Google Scholar]
- 47.Crawley MJ. Statistical Computing: An Introduction to Data Analysis Using S-Plus. New York: Wiley; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.