Abstract
The neurovascular unit is an emerging concept that emphasizes homeostatic interactions between endothelium and cerebral parenchyma. Here, we show that cerebral endothelium are not just inert tubes for delivering blood, but they also secrete trophic factors that can be directly neuroprotective. Conditioned media from cerebral endothelial cells broadly protects neurons against oxygen-glucose deprivation, oxidative damage, endoplasmic reticulum stress, hypoxia, and amyloid neurotoxicity. This phenomenon is largely mediated by endothelial-produced brain-derived neurotrophic factor (BDNF) because filtering endothelial-conditioned media with TrkB-Fc eliminates the neuroprotective effect. Endothelial production of BDNF is sustained by β-1 integrin and integrin-linked kinase (ILK) signaling. Noncytotoxic levels of oxidative stress disrupts ILK signaling and reduces endothelial levels of neuroprotective BDNF. These data suggest that cerebral endothelium provides a critical source of homeostatic support for neurons. Targeting these signals of matrix and trophic coupling between endothelium and neurons may provide new therapeutic opportunities for stroke and other CNS disorders.
Keywords: brain injury, neurodegeneration, neurovascular, stroke
Over the past decade, intricate molecular mechanisms of neuronal cell death have been dissected in great detail (1–6). However, despite these impressive advances, a clinically proven neuroprotective therapy against CNS injury and neurodegeneration does not exist. This problem is especially obvious in stroke, where a staggering number of clinical trials for neuroprotection have all failed. An emerging consensus in the field now suggests that a singular focus on neurons alone may not suffice. All cell types must be considered within the entire “neurovascular unit,” comprising interactions between neurons, glia, and the cerebral endothelium (7). Thus protecting neurons alone may not work, and all cells and perhaps cell–cell interactions within the brain must be rescued.
The concept of the neurovascular unit especially emphasizes the central importance of homeostatic interactions between endothelium and neuronal parenchyma (8–13). Coupling within the neurovascular unit can be manifested in many ways. At a tissue level, hemodynamic coupling mediates the cerebral blood flow response to neuronal activation (14). Without this coupling, the brain cannot function even if neurons are alive. At a cellular level, interactions between endothelium and neuroblasts promote neurogenesis and brain recovery (15, 16). Stimulation of angiogenesis amplifies neurogenesis, whereas blockade of vascular signals suppresses neurogenesis after stroke (17, 18). Is it possible that additional facets of endothelial-neuronal signaling are essential for supporting neuronal survival? In this study, we explore the hypothesis that matrix-trophic coupling between cerebral endothelium and neurons is fundamentally neuroprotective.
Results
Cerebral Endothelial Cells Are Neuroprotective.
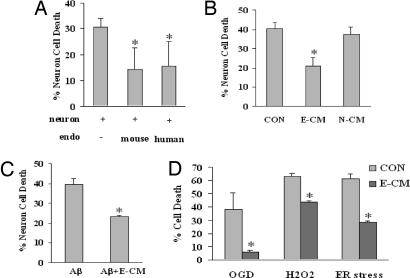
Neurons are known to be especially vulnerable to hypoxia and oxidative stress (1). Exposure to 8 h of hypoxia followed by 16 h of reoxygenation killed primary cortical neurons in cell culture (Fig. 1A). However, when neurons were cocultured in a transwell system together with either primary mouse cerebral endothelial cells or a human brain endothelial cell line, neurotoxicity was significantly reduced (Fig. 1A). Because there is no direct cell–cell contact in our transwell culture system, these data suggest that cerebral endothelial cells may secrete potent neuroprotective factors.
Fig. 1.
Cerebral endothelial cells provide neuroprotection via secretion of BDNF. (A) Coculture of rat cortical neurons with either primary mouse cerebral endothelial cells or a human brain endothelial cell line reduces neurotoxicity after 8 h of hypoxia and 16 h of reoxygenation. (B) Neuroprotection is also obtained by using transfer of E-CM. No protection is observed with N-CM. (C) Neuronal death after 24 h of exposure to 25 μg/ml (6 μM) of amyloid-β 1–40, is significantly reduced by treatment with E-CM. Amyloid-β 1–40 was chosen because this isoform tends to be more commonly associated with brain vasculature than the parenchymal (1–42) forms. (D) In SHSY5Y human neuroblastoma cells, E-CM protects against OGD, 24 h of exposure to 100 μM of H2O2, and 24 h of ER stress using 100 nM thapsigargin. Neurotoxicity was quantified with a standard measurement of LDH release from dying cells. Note that in all control “injury-only” conditions, neurons were treated with equivalent amounts of media derived from empty wells without endothelial cells.
To further extend these coculture observations, we turned to an experimental paradigm previously established to dissect coupling between endothelium and neurogenesis (19). Cerebral endothelial cells were grown separately, and endothelial-conditioned media (E-CM) were then added to primary neuronal cultures subjected to hypoxic injury. Control neurons were treated with “conditioned media” derived from empty wells without endothelial cells. In control neurons, hypoxia led to the expected degree of neurotoxicity. But in neurons that were treated with conditioned media from cerebral endothelial cells, cell death was significantly ameliorated (Fig. 1B). The endothelial specificity of these findings was supported by an additional set of cells where neuroprotection was not observed when the injured neurons were treated with neuron-conditioned media (N-CM), i.e., media collected from uninjured matching neuronal cultures (Fig. 1B).
Because multiple injury mechanisms are triggered after stroke, brain injury, and neurodegeneration, we tested our hypothesis in several different neuronal injury paradigms. The protective effect of E-CM was efficacious against a wide range of insults. These included exposure to amyloid-β (Fig. 1C), oxygen-glucose deprivation (OGD) (Fig. 1D), direct oxidative damage with H2O2 (Fig. 1D), and endoplasmic reticulum (ER) stress (Fig. 1D). Once again, control injury-only neurons were treated with conditioned media derived from empty wells without endothelial cells. Taken together, these results indicate that some soluble factor released by cerebral endothelium comprises a potent protective mediator against multiple forms of neurotoxicity.
Cerebral Endothelial Cells Are Neuroprotective by Secreting Brain-Derived Neurotrophic Factor (BDNF).
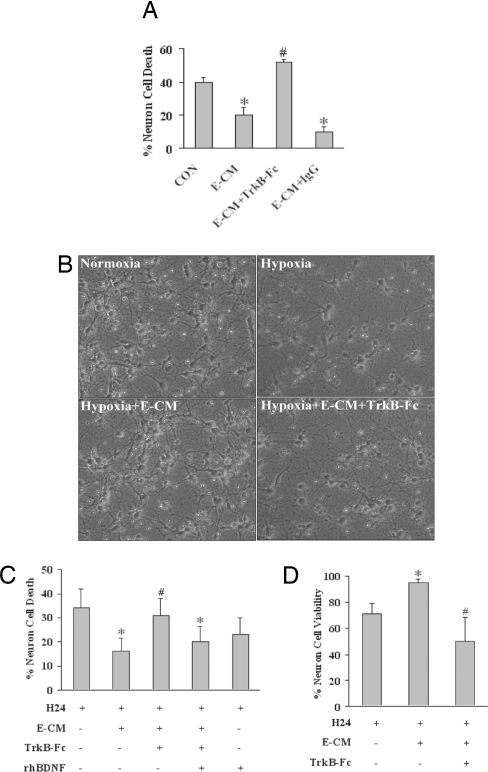
What are cerebral endothelial cells making that is neuroprotective? Cerebral endothelial cells are known to be an important source of BDNF (15, 20–22). In our cell culture systems, cerebral endothelial cells appear to produce BDNF at a rate of ≈50 pg per 106 cells over 24 h. In contrast, BDNF production by cortical neurons was much lower at a rate of ≈1 pg per 106 cells over 24 h. Therefore, we tested the initial hypothesis that BDNF was a soluble factor responsible for neuroprotection in our conditioned-media transfer experiments. The soluble antagonist TrkB-Fc were used to prevent BDNF from binding onto TrkB, the primary BDNF receptor in neurons. Our results showed that TrkB-Fc eliminated the protective effects of E-CM against hypoxia-induced neurotoxicity (Fig. 2A). Because directly adding TrkB-Fc may affect multiple pathways in the recipient neuronal cultures, we sought to further test this hypothesis with another approach. E-CM were filtered with TrkB-Fc to remove BDNF, and then the filtered media were added to neurons before they were subjected to hypoxic injury. Western blots and ELISAs suggested that we effectively removed >99% of all detectable BDNF with this filtering method. When compared against unfiltered media or controls filtered with nonspecific IgG, the neuroprotective effects of TrkB-Fc-filtered E-CM was significantly reduced (Fig. 2 B and C). To further establish BDNF as the primary neuroprotective mediator, we next performed a rescue experiment. When exogenous BDNF was added back to the TrkB-Fc-filtered endothelial media, neuroprotection against hypoxia was mostly restored (Fig. 2C). Finally, we decided to confirm our neurotoxicity data by measuring cell viability instead. We repeated the basic experiments by measuring mitochondrial tetrazolium (MTT)-converting activity as a standard indicator of neuronal cell viability. Once again, endothelial-conditoned media was protective against hypoxia, whereas removing BDNF by filtering the media with TrkB-Fc essentially eliminated the neuroprotection (Fig. 2D).
Fig. 2.
Role of BDNF in E-CM for neuroprotection. (A) E-CM protects against 24 h of hypoxia in primary rat neurons. TrkB-Fc (2 μg/ml) eliminates the neuroprotective properties of E-CM. Treatment with nonspecific IgG (2 μg/ml) has no effects. Neurotoxicity is quantified with the standard LDH release assay. (B) Representative images show primary neurons damaged by 24 h of hypoxia, rescue by E-CM, and blockade of E-CM neuroprotection with TrkB-Fc (2 μg/ml) filtering. (Magnification: 20×20) (C) E-CM neuroprotection against 24 h of hypoxia (H24) is lost when E-CM is filtered with TrkB-Fc (2 μg/ml) to remove BDNF. Addition of exogenous BDNF (10 ng/ml) restores the neuroprotective effect. Positive controls directly treated with exogenous BDNF are also protected. Neurotoxicity is quantified as LDH release from dying cells. (D) The neuroprotective capacity of conditioned media from cerebral endothelial cells is further confirmed with a cell viability assay. Hypoxia for 24 h reduced neuronal viability (MTT conversion). Treatment with E-CM provided significant neuroprotection. Filtering with TrkB-Fc to remove BDNF eliminated the neuroprotective effects of E-CM. *, P < 0.05 between hypoxic neurons alone versus neuron-endothelial cocultures or E-CM-treated neurons; #, P < 0.05 between E-CM versus TrkB-Fc-filtered E-CM conditions.
Nonlethal Levels of Oxidative Stress Suppress Endothelial BDNF Production.
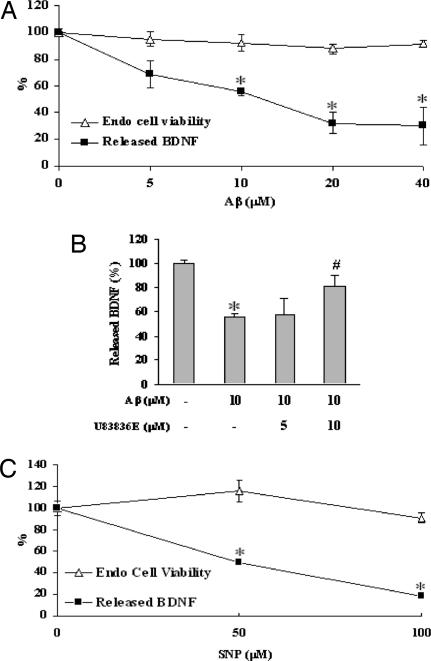
Thus far, our data suggest that BDNF might be a trophic link between cerebral endothelium and neuronal survival. If so, then it is conceivable that endothelial stress and injury could disrupt this potentially important source of trophic neurovascular coupling. To assess this hypothesis, we exposed cerebral endothelial cells to amyloid-β, which is known to damage brain vasculature (13, 23, 24). As expected, increasing concentrations of amyloid-β triggered endothelial cell death. But even at noncytotoxic levels, amyloid-β still appeared to significantly reduce secreted BDNF levels (Fig. 3A). Is oxidative stress an important part of this phenomenon? A pharmacologic approach was used to assess this possibility. Treatment with the potent and relatively specific antioxidant U83836E ameliorated the amyloid-induced reduction in BDNF suppression (Fig. 3B), thus indirectly supporting this idea. To further confirm these findings, endothelial cells were exposed to sodium nitroprusside (SNP) as a nitric oxide donor and well accepted trigger of downstream nitrosative and oxidative stress. At levels of SNP that were not directly cytotoxic, endothelial BDNF production was clearly reduced (Fig. 3C). Taken together, these data suggest that oxidative stress can suppress trophic factor production in cerebral endothelium without outright cell death.
Fig. 3.
Suppression of endothelial BDNF by nonlethal oxidative stress. (A) Exposure of cerebral endothelial cells for 24 h to 5–40 μM amyloid-β 1–40 did not induce detectable cell death in our model system. But BDNF levels in the E-CM are significantly suppressed by 10–40 μM amyloid-β 1–40. Cytotoxicity is assessed with LDH release *, P < 0.05 versus undamaged endothelial cells. (B) Cotreatment of endothelial cells with 5–10 μM antioxidant U83836E prevents the amyloid-induced suppression of BDNF production. *, P < 0.05 versus undamaged endothelial cells. #, P < 0.05 versus cells damaged with 10 μM amyloid-β. (C) Treatment of cerebral endothelial cells with 50–100 μM of the NO donor SNP did not induce any detectable cell death, but significantly reduces BDNF production. Cytotoxicity is quantified as LDH release from dying endothelial cells. *, P < 0.05 versus undamaged cells.
β-1 Integrins and Integrin-Linked Kinase (ILK) Mediate Endothelial BDNF Production.
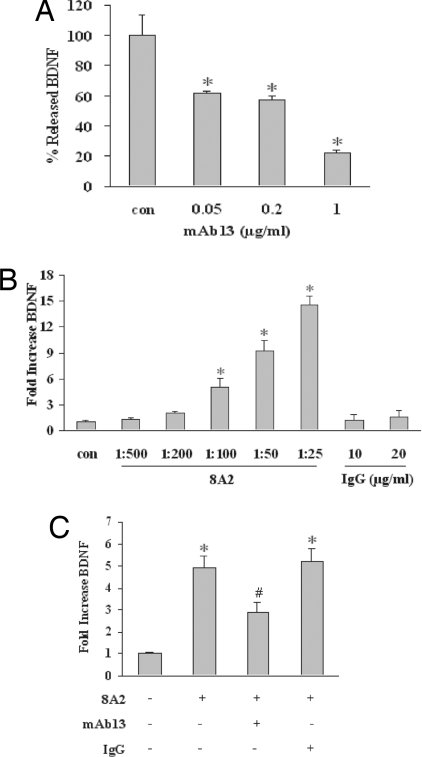
The central idea of trophic coupling between cerebral endothelium and neuronal survival is important because after stroke, brain injury, and perhaps even in neurodegenerarive diseases, neurovascular coupling may be perturbed in many ways (8–13). Recent data suggest that the intercellular matrix mediates neurovascular signaling so that disruption of cell-matrix homeostasis underlies brain cell dysfunction and death (10). If BDNF is important for neurovascular coupling, we next asked how it is regulated in cerebral endothelium. Is it possible that matrix integrity sustains endothelial BDNF? It is known that amyloid and oxidative stress suppresses matrix integrin signaling (25, 26), so we hypothesized that integrins may mediate endothelial trophic responses in our model system. We initially assessed β-1 integrins because these are prominently expressed in endothelial cells (26). Incubation of cerebral endothelial cultures with increasing concentrations of the β-1 blocking antibody mAb13 down-regulated BDNF production in a manner similar to oxidative stress (Fig. 4A). In contrast, stimulation with the potent β-1-activating antibody 8A2 up-regulated BDNF production (Fig. 4B). To further support the specificity of these findings, β-1-activating and -blocking antibodies were added together. Our results showed that mAb13 reduced the elevation in BDNF induced by 8A2 (Fig. 4C). These data suggest that at least in our cell model system β-1 integrins comprise critical matrix regulators of BDNF homeostasis in cerebral endothelium. Therefore, when neurovascular matrix is degraded in CNS disease and injury (10, 26–28), endothelium might no longer be able to produce neuroprotective BDNF.
Fig. 4.
β-1 integrin as a matrix input for BDNF production by cerebral endothelial cells. (A) Exposure of cerebral endothelial cells for 24 h to the β-1 integrin blocking antibody mAb13 (0.05–1 μg/ml) reduces secreted BDNF. *, P < 0.05 versus baseline levels. (B) Conversely, treatment with the β-1 integrin stimulating antibody 8A2 (1:500 to 1:25) increases BDNF production from cerebral endothelial cells. Controls treated with nonspecific IgG shows no response. (C) β-1 blockade with 1 μg/ml mAb13 prevents BDNF production stimulated with 8A2 (1:100). IgG (1 μg/ml) has no effect. *, P < 0.05 versus untreated cells. #, P < 0.05 between 8A2 alone versus 8A2 plus mAb13 conditions. No overt cytotoxicity occurred with these various integrin antibody treatments.
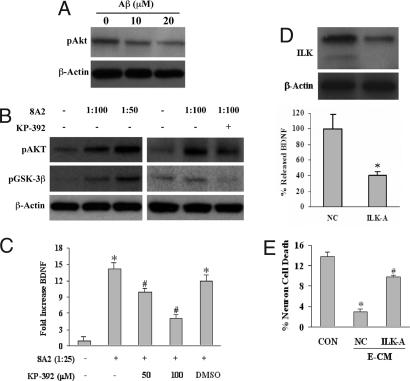
Once endothelial matrix integrins are disrupted, how does the loss of outside-in signaling lead to a down-regulation of BDNF? β-1 integrin signaling typically occurs via ILK activity (29), so ILK was next assessed as a candidate signal in our experimental paradigms. We used both pharmacologic and genetic approaches. First, oxidative stress induced by amyloid-β decreased levels of endothelial phospho-Akt, a downstream target of ILK (Fig. 5A). In contrast, β-1 integrin stimulation with 8A2 increased the phosphorylation of two well established ILK targets, i.e., GSK3-β and Akt. Because both GSK3-β and Akt can, of course, be phosphorylated by many kinases besides ILK, we also performed an inhibitor experiment. The potent and relatively specific ILK inhibitor KP-392 significantly blocked β-1 integrin-stimulated changes in phospho-Akt and phospho-GSK3-β, consistent with the idea that ILK signaling mediates these responses (Fig. 5B). Correspondingly, 8A2-mediated elevations in endothelial BDNF was suppressed by KP-392 as well (Fig. 5C). Next, we used an siRNA approach. Cerebral endothelial cells were transfected with siRNA targeted against ILK (ILK-A) or a scrambled siRNA nonsilencing control (NC). ILK expression was effectively down-regulated by ILK-A (Fig. 5D), and correspondingly, BDNF levels in E-CM were also significantly reduced by ≈60% (Fig. 5D). Finally, if ILK truly underlies BDNF production in cerebral endothelial cells, then we have to predict that down-regulation of ILK signals should negate the neuroprotective effects of E-CM. When conditioned media from ILK-suppressed cerebral endothelium were then added to neuronal cultures, protection against hypoxia was lost (Fig. 5E). Taken together, these data suggest that ILK-mediated BDNF production may mediate neuroprotective matrix-trophic coupling between the vascular and parenchymal compartments in brain.
Fig. 5.
ILK as a mediator to couple neuroprotective matrix-trophic signaling in cerebral endothelial cells. (A) Oxidative stress with amyloid-β 1–40 decreases phospho-Akt, a downstream target of ILK. (B) Activation of β-1 integrins with 8A2 increased phospho-GSK3-β and phospho-Akt, both targets of ILK. These integrin signaling responses are blocked by the ILK inhibitor KP-392 (100 μM). (C) Stimulation of β-1 integrins with 8A2 increases BDNF production by cerebral endothelial cells, and the ILK inhibitor KP-392 blocks this response. *, P < 0.05 versus baseline; #, P < 0.05 between 8A2-treated cells and 8A2 plus KP-392-treated cells. (D) siRNA reduces ILK expression in cerebral endothelial cells (Upper) and decreases production of BDNF (Lower). *, P < 0.05 between ILK siRNA treatment (ILK-A) and nonspecific siRNA controls (NC). (E) Primary neurons are damaged by 24 h of hypoxia (CON). Conditioned media from endothelial cells treated with nonspecific siRNA (NC) is neuroprotective. But when conditioned media is obtained from endothelial cells previously treated with ILK siRNA (ILK-A), neuroprotection is significantly decreased. *, P < 0.05 versus untreated hypoxic neurons; #, P < 0.05 between NC and ILK-A conditions. Neither siRNA nor the drug ILK inhibitors were directly cytotoxic.
Discussion
Our data demonstrate that cerebral endothelial cells are an important source of BDNF that protects neurons from a wide range of neuronal insults including hypoxia, OGD, oxidative damage, ER stress, and amyloid toxicity. Endothelial BDNF is regulated by β-1 integrins that act through ILK. Levels of oxidative stress that do not overtly induce cell death can down-regulate endothelial ILK signaling and BDNF production, thus potentially disrupting this critical source of neurotrophic support. Our present findings are significant because they imply that cerebral endothelial cells do not just comprise inert tubes for delivering blood flow, oxygen, and glucose into brain. Instead, they might additionally serve as active sources of neurotrophic mediators that help defend neuronal tissue against injury and disease. Our TrkB filtering data suggest that BDNF likely plays a dominant role. But the “rescue” experiment where we added back exogenous BDNF after TrkB filtering did not fully prevent neuronal death. This phenomenon implies that other growth factors are involved. In our cerebral endothelial cell system, a gene array detected a wide range of trophic factors, and oxidative stress was able to broadly down-regulate many of these mediators [supporting information (SI) Fig. S1, Fig. S2, and Table S1]. Future studies are warranted to examine the network response of these other mediators in the trophic coupling between brain endothelium and neurons. In vivo, these hypotheses would also be testable by using either inducible brain-endothelial-specific growth factor knockouts or antioxidants that can target brain endothelium without affecting other cell types.
Our study is consistent with the emerging notion that perturbations in the health of cerebral endothelium may mediate progressive neuronal dysfunction. Long-term follow-up of so-called silent strokes reveal that significant cognitive impairment occurs over time (30). In fact, endothelial mediators and risk factors may significantly contribute not only to deterioration after stroke, but also to progressive vascular dementia (31, 32). In Alzheimer's disease, amyloid may trigger oxidative stress in brain endothelium via RAGE receptors, and perturbations in amyloid transport mechanisms further add to a positive feedback loop that damages both neuronal and vascular compartments even more (33). From a clinical perspective, sick cerebrovasculature may eventually lead to sick neurons, even in the absence of primary cell death. Our findings suggest that oxidatively challenged endothelium may lose the ability to provide trophic support to neurons, even in the absence of vascular cell death or blood flow compromise. Thus trying to save neurons alone without preserving this critical endothelial source of BDNF may not be enough for true neuroprotection. The need to protect the vasculature in stroke and neurodegeneration is beginning to be recognized (34, 35). Our data here provide additional evidence as to why this is important.
Beyond neuroprotection, our present findings can also be interpreted more broadly. To date, two forms of coupling and signaling are known to exist between neurons and cerebral endothelium. Fast hemodynamic coupling at the millisecond time scale allows blood vessels to dilate and respond to neuronal activity and metabolic demand (14). On a slower time scale, neurovascular interactions within the stem cell niche contribute to ongoing neurogenesis in adult brain (15–17, 19). Our data here suggest another critical level of neurovascular coupling that may be mediated via matrix and trophic interactions. However, several caveats will require further study. Our initial data support a major role for β-1 integrins, but the dynamic role of other integrins and cell types will need to be carefully dissected. Furthermore, how quickly and how far these intercellular signals propagate remains unknown. Our results implicate oxidative stress as a potential upstream trigger that perturbs this form of trophic coupling. But other pathways may also be triggered simultaneously. For example, matrix metalloproteinases are dysregulated in stroke, trauma, and neurodegeneration (10, 36, 37). Aberrant metalloproteinase activity can degrade neurovascular matrix and interrupt the baseline secretion of endothelial trophic factors. How these matrix-trophic pathways operate in vivo in various brain diseases remains to be elucidated. Finally, for the sake of experimental clarity, we focused on endothelial cells. However, in vivo interactions between the vascular system and the brain parenchyma cannot occur without astrocytes (38). How would matrix-trophic signals “go through” the glial layer? What astrocytic mediators might regulate endothelial gene expression and vice versa? Ultimately, neurovascular signaling cannot be fully dissected without taking astrocytes and other glial cells into consideration.
Our findings demonstrate that endothelial cells can secrete potent neuroprotective factors. We speculate that the cerebral endothelium might even comprise a “neuroprotective organ” within the brain itself. Fundamentally, this new concept may force us to revise our current understandings of neuroprotection. Loss of homeostatic coupling from unhealthy cerebral endothelial cells will lead to dysfunctional neurons. An overwhelming number of clinical neuroprotection trials have failed, perhaps because they only focused on saving neurons. Targeting the cerebral endothelium and salvaging its matrix-trophic signals may broadly provide new therapeutic opportunities against stroke, brain injury, and neurodegeneration.
Experimental Procedures
Cell Cultures.
Primary mouse brain microvascular endothelial cells (39, 40) were cultured from 4-week-old male mice, purified with anti-murine CD31 precoated M-450 Dynabeads (Dynal), plated onto type I collagen-coated multiwell plates (BD Bioscience), and cultured in complete medium consisting of DMEM/F12, 10% horse serum, 10% FCS (Invitrogen), 0.1 mg/ml endothelial cell growth supplement (BD Bioscience), 100 μg/ml Heparin (Sigma), and 100 units/ml penicillin and streptomycin. Mouse endothelial cells were split at a ratio of 1:2 and used at passages 2–3. Additionally, we used a human brain microvascular endothelial cell line with confirmed endothelial characteristics (41). Cells were maintained in complete medium consisting of RPMI medium 1640 (Invitrogen), 10% FBS, 10% NuSerum (BD Biosciences), 1 mM sodium pyruvate, nonessential amino acids, MEM vitamins, and 100 units/ml penicillin and streptomycin. Rat neurons were cultured from embryonic day 17 embryonic cortices, and isolated cells were plated onto poly-d-lysine-coated 12-well plates with medium consisting of NeuroBasal, 2% B27 (Invitrogen), 0.3 mM l-glutamine, and 100 units/ml penicillin and streptomycin. Medium were half changed every 3 days, and cells were used at days in vitro 7–9. The human neuroblastoma cell line SH-SY5Y was obtained from ATCC and cultured in DMEM/F12 with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. For cocultures, cerebral endothelial cells were plated into 12-mm transwell inserts (Corning Costar) coated with type I collagen for mouse primary endothelial cells or human plasma fibronectin (HFN; BD Bioscience) for human endothelial cells, in each complete medium until 70–80% cell density. The day before coculture and hypoxia, endothelial cells were changed to serum-free DMEM/F12, and rat cortical neurons were also starved gradually by half change of medium with mixed medium NeuroBasal/DMEM/F12 (1:1). Right before hypoxia treatment, the transwells with endothelial cells were placed above the neuron cells in fresh serum-free DMEM/F12 and stayed together for hypoxia and after reoxygenation. For conditioned media transfer experiments, cerebral endothelial cells were plated into HFN-coated dishes at 50% cell density and changed to fresh media after 16-h starvation with mixed serum-free medium NeuroBasal/RPMI medium 1640 (1:1). This serum-free media was collected 24 h later and used as E-CM for transfer to rat neurons or SH-SY5Y when assessing neuroprotection.
Cell Treatment Conditions.
Hypoxia was induced with a modular chamber (Billups-Rothenberg) perfused with 90% N2, 5% H2, and 5% CO2 for 30 min at 37°C. The chamber was then sealed and kept at 37°C for indicated time periods for hypoxia. At the end of hypoxia exposure, cells were removed from the chamber and returned to a regular incubator for reoxygenation. Control cultures were incubated in a regular incubator under normoxic condition for the corresponding hypoxic duration. Amyloid-β peptide (Aβ 1–40) (Biosource), preaged at 4°C for 3 days, was added to cells for 24 h as another injury for rat primary neurons after 3-h starvation. Neuroblastoma SH-SY5Y cells were also used to look for neuroprotection of E-CM against OGD, oxidative damage with H2O2, and ER stress. Cells were plated in multiwell plates at 80% density and switched into serum-free DMEM/F12 for 8 h before exposure to stimulations. For OGD, cells were exposed to glucose-free DMEM within a hypoxic chamber for 8 h followed by a return to normoxic condition with fresh DMEM/F12 or E-CM for 12 h. For oxidative stress, 100 μm H2O2 was added for 30 min followed by a change to fresh DMEM/F12 or E-CM for 24 h. For ER stress, cells were exposed to 100 nM thapsigargin (Sigma) for 24 h in DMEM/F12 or E-CM. To assess cerebral endothelial responses, cells were plated into HFN-coated multiwell plates overnight, serum-starved for 8 h, then changed to fresh RPMI medium 1640 with 0.5% FBS plus Aβ 1–40 or SNP (Sigma). Cell death was quantified by using standard MTT and lactate dehydrogenase (LDH) release assays. To examine the role of integrins in our system, we used 8A2 (generous gift from John Harlan, Washington University, St. Louis), mAb13, and the small molecule ILK inhibitor KP-392, in serum-free media RPMI medium 1640. Twenty hours postexposure, the media were collected for measurement. To check the specificities of our β1 integrin antibodies, the stimulating antibody 8A2 was added to the media after 2-h pretreatment with the blocking antibody mAb13. No observable cytotoxicity was detected with these various integrin antibody and ILK inhibitor treatments.
BDNF Procedures and Assays.
E-CM were filtered with recombinant human TrkB-Fc chimera (R&D Systems) to remove BDNF. Briefly, 2.0 μg/ml TrkB-Fc was added to media for 2 h in 4°C with rotation, and equal amounts of normal human IgG (Zymed) were added as control. Protein A-agarose immunoprecipitation reagent was then added for overnight in 4°C with rotation. The media were centrifuged to discard the agarose pellet, and the filtered supernatant was used for conditioned media experiments and BDNF measurement. Recombinant human BDNF (Peprotech) was used for add-back experiments with the filtered conditioned media transfer protocols. BDNF levels were measured by using the BDNF Emax ImmunoAssay System (Promega).
siRNA.
Cerebral endothelial cells were transfected with 100 nM siRNA ILK-A, that specifically targets the ILK gene (42), and NC using 4 μl of Oligofectamine (Invitrogen) according to the manufacturer's instructions. The cells were split at 48 h posttransfection and plated onto HFN-coated dishes. The serum-free media with 24 h-exposure after overnight starvation was collected for conditioned media experiment or BDNF measurement. Note that no significant toxicity was observed for these siRNA treatments overall.
Immunoblotting.
Cells were lysed in lysis buffer (Cell Signaling Technology) and clarified, and protein concentration was determined with the Bradford assay (Bio-Rad). Total lysates of cells (30 μg per lane) were separated in precast 12% Tris-glycine SDS-polyacrylamide gels (Invitrogen), and proteins were transferred to PVDF membrane (Invitrogen). After blocking with 0.2% I-block (Tropix), membranes were incubated overnight at 4°C with indicated primary antibodies, and 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (Amersham). The immune complexes were visualized by enhanced chemiluminescence (Amersham). All immunoblots were repeated for at least three independent experiments.
Statistical Analysis.
All of experiments were done in duplicate or triplicate, repeated two to five times independently. Quantitative data were expressed as mean +/− SD and analyzed with ANOVA followed by Tukey HSD multiple comparisons. Differences of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments.
We thank Drs. Barbara Hempstead, Gregory del Zoppo, and Michael Moskowitz for many helpful discussions and Dr. John Harlan for 8A2 antibody. This work was supported in part by a Bugher award from the American Heart Association and National Institutes of Health Grants R01-NS37074, R01-NS48422, R01-NS53560, R01-NS56458, P50-NS10828, and P01-NS55104.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.I. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801105105/DCSupplemental.
References
- 1.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Chan PH. Future targets and cascades for neuroprotective strategies. Stroke. 2004;35:2748–2750. doi: 10.1161/01.STR.0000143325.25610.ac. [DOI] [PubMed] [Google Scholar]
- 3.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 4.Dawson VL, Dawson TM. Deadly conversations: Nuclear-mitochondrial cross-talk. J Bionenerg Biomembr. 2004;36:287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 6.Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: How brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 9.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 10.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges, and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 11.Park JA, Choi KS, Kim SY, Kim KW. Coordinated interaction of the vascular and nervous systems: From molecule- to cell-based approaches. Biochem Biophys Res Commun. 2003;311:247–253. doi: 10.1016/j.bbrc.2003.09.129. [DOI] [PubMed] [Google Scholar]
- 12.Ward NL, Lamanna JC. The neurovascular unit and its growth factors: Coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–883. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- 13.Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–S233. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 16.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguchi A, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donovan MJ, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, et al. Matrix metalloproteinase-9 in cerebral-amyloid-angiopathy-related hemorrhage. J Neurol Sci. 2005:229–230. 249–254. doi: 10.1016/j.jns.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Yin KJ, Lee JM, Chen SD, Xu J, Hsu CY. Amyloid-β induces Smac release via AP-1/Bim activation in cerebral endothelial cells. J Neurosci. 2002;22:9764–9770. doi: 10.1523/JNEUROSCI.22-22-09764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozzo C, Lombardi G, Santoro C, Canonico PL. Involvement of β(1) integrin in βAP-induced apoptosis in human neuroblastoma cells. Mol Cell Neurosci. 2004;25:1–8. doi: 10.1016/j.mcn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- 27.Gu Z, et al. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297:1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 28.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 29.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): A regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 30.Vermeer SE, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 31.Grammas P, Yamada M, Zlokovic B. The cerebromicrovasculature: A key player in the pathogenesis of Alzheimer's disease. J Alzheimer's Dis. 2002;4:217–223. doi: 10.3233/jad-2002-4311. [DOI] [PubMed] [Google Scholar]
- 32.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–337. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 33.Deane R, et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 34.Cheng T, et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 35.Griffin JH, Zlokovic B, Fernandez JA. Activated protein C: Potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 37.Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 38.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 39.Song L, Pachter JS. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol Anim. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J Neurosci Methods. 2003;130:53–63. doi: 10.1016/s0165-0270(03)00206-1. [DOI] [PubMed] [Google Scholar]
- 41.Callahan MK, et al. CXCR3 marks CD4+ memory T lymphocytes that are competent to migrate across a human brain microvascular endothelial cell layer. J Neuroimmunol. 2004;153:150–157. doi: 10.1016/j.jneuroim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 42.Tan C, et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.