Abstract
The theory of mimicry predicts that selection favors signal refinement in mimics to optimally match the signals released by their specific model species. We provide here chemical and behavioral evidence that a sexually deceptive orchid benefits from its mimetic imperfection to its co-occurring and specific bee model by triggering a stronger response in male bees, which react more intensively to the similar, but novel, scent stimulus provided by the orchid.
Keywords: floral odor, pollination, sex pheromone, signal evolution
Mimicry theory is arguably one of the most illustrative applications of natural selection that has been formulated after Darwin's publication of the Origin of Species (1). Numerous examples of mimicry among unrelated animals have been documented, whereas floral mimicry, despite its frequent occurrence and popularity to the general public, has received comparatively less attention and remains poorly understood (2, 3). A textbook case of mimicry in plants is pollination by sexual deception in orchids that mimic the female mating signals of one or a few insect taxa, resulting in highly specific pollination by males attempting copulation with the flowers (4, 5) [see supporting information (SI) Movie S1]. In this “aggressive” mimicry (6), also called “Pouyannian” mimicry (7) in honor of M. Pouyanne who first described the phenomenon in the genus Ophrys (8), orchids exploit the innate behavioral responses of the dupes (the male insects) to the model species (conspecific female insects). Visual similarity between Ophrys flowers and their associated insects is sometimes very conspicuous, and it has inspired a fanciful “insect-like” nomenclature in the group, starting with Linnaeus who named the fly orchid (Ophrys insectifera) after its overall similarity to flies perching on grass stems (9). However, despite the appearances, visual cues are generally of secondary importance in pollinator attraction for most species of this system (3, 10). Detailed investigations carried out in Australian and European sexually deceptive orchids have shown that orchids attract their specific pollinators with their floral odor, which mimics the sex pheromones of certain species of female insects (3, 11). Furthermore, comparisons of floral scent chemistry between closely related Ophrys species have shown that different relative proportions of the same odor compounds mediate the specific pollinator attraction by the orchids (12). More recently, signal variation in this mimicry system has been reported at the population level in an Ophrys-pollinator pair, where it has been shown that the signal mediating the specific attraction of the male bees varies across populations, in both the model insect species and the orchid mimic (13, 14). Collectively, these recent advances in the field have given us insights in the evolution of Ophrys-pollinator interactions by showing (i) that the relative proportions of the key odor compounds play an important role in signal specificity in this mimicry, and (ii) that population-specific quantitative differences in the key odor signals are found in both the model and the mimic species. When considering the evolution of this plant–pollinator interaction in the light of the mimicry theory, it is therefore expected that natural selection would favor local adaptation in the mimics through a refinement of the chemical mimicry of the orchids' floral scent to optimally match the signal released by their sympatric models (1, 15, 16). In the present study, we have investigated signal similarity between a bee model (Colletes cunicularius, Hymenoptera, Colletidae) and its specific orchid mimic (Ophrys exaltata, Orchidaceae). We have analyzed bees and orchids from 15 different populations across Western Europe, including two distant populations where large numbers of orchids and bees occur in sympatry, to test this “signal refinement hypothesis.” We have assayed manipulated odor bouquets to test the effect of novel bouquets to patrolling C. cunicularius males.
Results
Differences in Odor Signals Between Mimics and Models.
In our study system, the orchid flowers and the female bees produce identical biologically active compounds (data not shown) in similar absolute amounts (orchid: 6.07 ± 0.3 μg; female bees: 7.37 ± 0.73 μg; P = 0.259), which confirms earlier studies (13, 14). However, contrary to theoretical expectations, our results show that the relative proportions of the odor compounds detected by the male bees, the so-called biologically active compounds, differ markedly between orchids and female bees, irrespective of their geographic origin (Fig. 1). Our results further show that the differences between models and mimics are equally present in bees and orchids from sympatric populations (Fig. 2). More specifically, our analyses show that, although the absolute amounts of the three key compounds for the attraction of C. cunicularius males (14) do not differ between orchid and bee odor samples (Fig. 3A) their relative amounts differ significantly (Fig. 3B). These differences in relative proportion of the key odor compounds are also found when all orchid and bee samples are pooled together (Fig. 4).
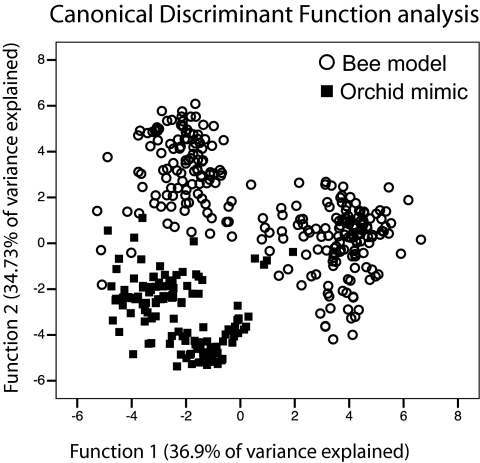
Fig. 1.
Differentiation in odor bouquets between orchid mimics (■) and their specific female bee models (○) sampled across a combination of 13 allopatric and 2 sympatric populations in Western Europe (see Materials and Methods for details). CDF plot of biologically active odor compounds (relative proportions in percentage of the total odor blend): the analysis shows that the proportion patterns of the orchids' floral odor compounds differ significantly from the female bees' sex pheromone, irrespective of the geographic origin of the samples. The CDFs explained 71.1% of the total odor variance among samples (36.9% and 34.3%, respectively; Wilks' λ values: Wλ1 = 0.00018 and Wλ2 = 0.0016, associated P1 and P2 < 0.0001; canonical correlation values: Cc1 = 0.947; Cc2 = 0.943). Overall, 66.5% of all cross-validated samples were assigned correctly to their species/population by the two CDFs.
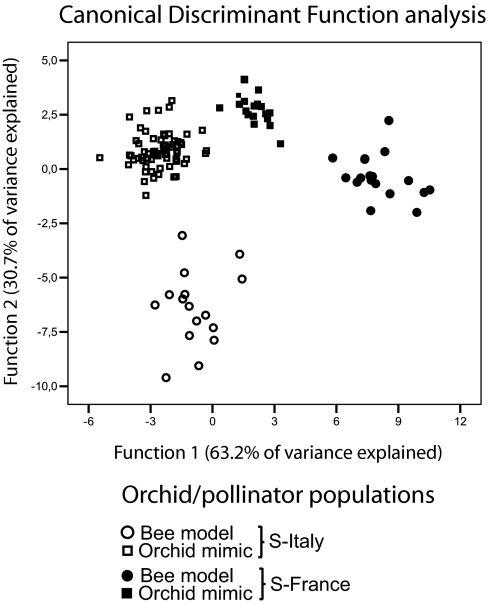
Fig. 2.
Differentiation in odor bouquets between orchid mimics (squares) and their specific female bee models (circles) sampled in sympatry in southern France (filled symbols) and southern Italy (open symbols). CDF plot of biologically active odor compounds (relative proportions, in percentage of the total odor blend): the analysis shows that the relative proportions of the orchids' floral odor differs significantly from the female bees' sex pheromone sampled in sympatry. The discriminatory ability of the CDFs was high, as they explained 93.9% of the total odor variance among samples (63.2% and 30.7%, respectively; Wilks' λ values: Wλ1 = 0.002 and Wλ2 = 0.038, associated P1 and P2 < 0.0001; canonical correlation values: Cc1 = 0.973; Cc2 = 0.947). Overall, 97.5% of all cross-validated samples were assigned correctly to their species/population by the two CDFs.
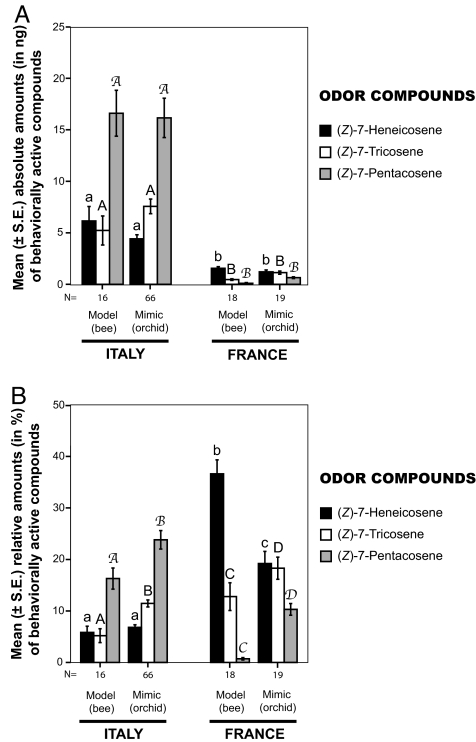
Fig. 3.
Differentiation between the orchid mimics and their sympatric female bee models in emission patterns of the three key odor compounds for the attraction of C. cunicularius males. (A) Mean (± SE) absolute amounts in individual odor extracts (in ng). (B) Mean (± SE) relative proportions (in percentage of the total odor blend). The analysis shows that there is no significant difference in the absolute amounts (in ng) of the three key odor compounds between sympatric orchid mimics and their female bee models, whereas the proportion patterns of these compounds (in percentage of the total odor blend) differ significantly between the models and the mimics. Mann–Whitney U test (α = 0.05), different letters on top of error bars indicate significant differences in odor emission; the numbers of samples analyzed are listed under the columns.
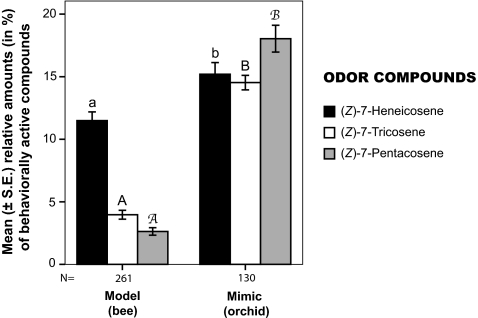
Fig. 4.
Differentiation between the orchid mimics (n = 130) and their female bee models (n = 261) in emission patterns of the three key odor compounds [(Z)-7-C21, (Z)-7-C23, and (Z)-7-C25] for the attraction of C. cunicularius males. Mean (± SE) relative amounts in individual odor extracts, in percentage of the total odor blend, across all 15 populations investigated for this study are shown. The analysis shows that overall there is a significant difference (Mann–Whitney U test: P < 0.01) in the mean relative amounts of the three key odor compounds between orchid mimics and their female bee models, irrespective of the geographic origin of the samples. Different letters on top of error bars indicate significant differences in odor emission; the numbers of samples analyzed are listed under the columns.
The Behavioral Significance of Mimic–Model Dissimilarity in Odor Signals.
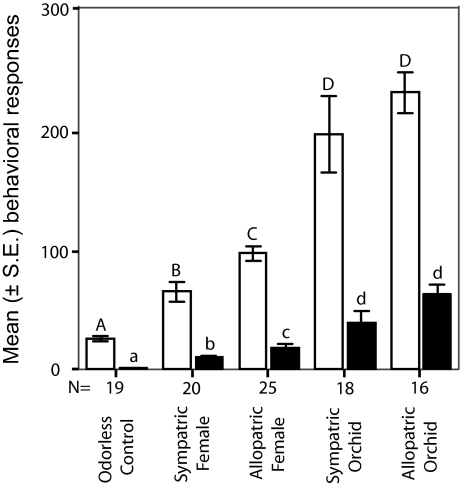
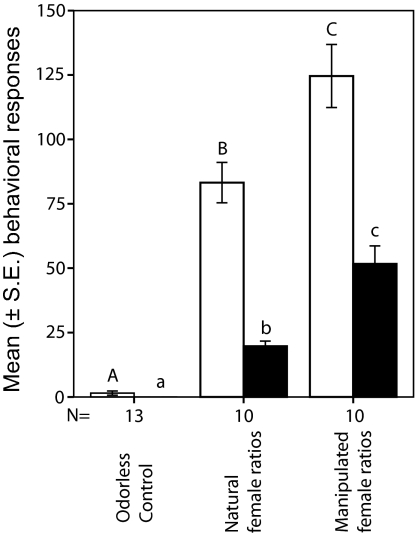
To test whether these quantitative differences in odor blend composition between the bee models and the orchid mimics (Figs. 1–4) have an impact on the attractiveness of the orchids to the targets in this mimicry system (C. cunicularius males), we have performed a series of behavioral experiments. Our results show that male bees prefer odor bouquets of allopatric to sympatric females (Fig. 5), which corroborates behavioral patterns demonstrated recently in this species in different “naïve” populations located in Switzerland and Austria. Our results from the bioassays also show that male bees always preferred the orchids' floral odor to the sex pheromone of the female bees, irrespective of their geographic origin (Fig. 5). Because orchids do not produce significantly different absolute amounts of scent than females (Fig. 3A), these results strongly suggest that the differences in the relative proportions of the key odor compounds (Figs. 1, 2, 3B, and 4), namely the higher proportions of (Z)-7-tricosene and (Z)-7-pentacosene found in the orchids' floral odor extracts (Figs. 3b and 4), are responsible for the higher attractiveness of the orchid blends over the female bees' to patrolling males (Fig. 5). To test this hypothesis, we have manipulated the natural ratios of the three key compounds in natural sex pheromone extracts of local females of C. cunicularius at a study site in France by adding synthetic hydrocarbons to change the female bees' natural proportions into the mean ratios found in orchid floral odor samples (see Materials and Methods for blend composition). Our results confirm that the significantly higher behavioral activity observed in male bees is caused by the deviant ratios of (Z)-7-heneicosene, (Z)-7-tricosene, and (Z)-7-pentacosene, the three key compounds of the female sex pheromone (Fig. 6).
Fig. 5.
Attractiveness of odor bouquets assayed in behavioral experiments with patrolling males of C. cunicularius in the field. The tests show that orchid samples are more attractive than female bee samples; allopatric bee samples are also more attractive than sympatric bee samples. The bars show the mean (± SE) number of inspecting flights (empty bars) and contacts (filled bars) of the male bees with a dummy during 3-min behavioral bioassays. The dummies were scented with odor extracts of orchid flowers (mimic) and attractive female bees (model). We used orchid and bee samples of local (sympatric) and foreign (allopatric) populations. Mann–Whitney U test (α = 0.05), different letters on top of error bars indicate significant differences in attractiveness; the numbers of replicates are listed under the columns.
Fig. 6.
Attractiveness of manipulated odor bouquets assayed in behavioral experiments with patrolling males of C. cunicularius in the field. The bars show the mean (± SE) number of inspecting flights (empty bars) and contacts (filled bars) of the male bees with a dummy during 3-min behavioral bioassays. Extracts of virgin female bees with increased ratios of the key odor compounds [(Z)-7-C21, (Z)-7-C23, and (Z)-7-C25] are significantly more attractive than the natural, nonmanipulated samples. Mann–Whitney U test (α = 0.05), different letters on top of error bars indicate significant differences in attractiveness; the numbers of replicates are listed under the columns.
Discussion
Our study challenges the theoretical expectation that selection should favor an optimal signal match between the mimics and their specific models, and that mimetic imperfection negatively affects the model's attractiveness to the dupes in a specialized mimicry system.
On the contrary, our bioassays show that the pollinator bees actively prefer the floral scent of the “imperfect” mimic, which can be explained by a sensory preference or “receiver bias” toward “novel” signals over more commonly encountered ones (17, 18). As shown in a previous study on C. cunicularius, females use population-specific ratios of their key sex pheromone compounds, and males are more attracted by sex pheromone “dialects” from allopatric populations than from their own (13). Because C. cunicularius usually forms dense “island” aggregations and both sexes can only travel across short distances for mates, foraging resources, or nesting sites, we suggest that populations are probably subjected to inbreeding and male preferences for novel signals might therefore be adaptive and promote outbreeding, e.g., by avoiding sibling mating should the opportunity arise. Such preferences for novel signals are regarded as a common emerging feature in animal cognitive processes, and studies have demonstrated that this phenomenon can be an important driving force behind signal evolution (19, 20). In our orchid-pollinator system, the pollinators' preferences can impose selection on floral odor of the orchids, because the orchids' reproductive success is primarily pollinator-limited (21–23). Specifically, results from a recent study support the hypothesis that the patterns of pollinator-attracting compounds of the orchids' floral odor are under pollinator-mediated selection (22). Pollinator-driven evolution in the orchids does not, however, automatically imply male-driven evolution in female bees. Females of many solitary bee species, including C. cunicularius, are thought to mate only once, and, unlike the orchids, the emerging female bees are presumably not limited in their reproductive success by access to males, because patrolling males typically outnumber the virgin female bees during their reproductive period. We therefore suggest that the mating preferences of C. cunicularius males are unlikely to drive a shift in the females' sex pheromone (13), whereas the pollinators' preference for novel signals (Figs. 5 and 6) have the potential to drive the evolution of the orchids' floral scent (22). Therefore, we hypothesize that this marked difference in the selective pressures acting on the signals of the models versus the mimics has fueled the evolutionary divergence in pollinator-attracting odor signals, which, over time, has resulted in the imperfect chemical mimicry observed between the orchid mimics and their sympatric model species (Figs. 1, 2, 3B, and 4).
Attempts at explaining the evolution of imperfect mimicry have until now mostly involved the “multimodel hypothesis,” postulating that a partial resemblance to multiple models can be beneficial to a mimic (24). In sexually deceptive orchids, however, most species specifically mimic one single model taxon (4, 5). Accordingly, in O. exaltata, C. cunicularius males are the only known pollinator species. Alternatively, several authors (25, 26) have advocated that what appear to be “poor” mimics to human senses might in fact be accurate mimics for the operator's perception. Again, this hypothesis is not relevant for our study, as we compared only the patterns of biologically active odor compounds of orchids and bees that have been shown to attract the males (14). Finally, physiological constraints such as the costs of signal production have been proposed as a mechanism that might impede further improvement of mimic-model resemblance (27). Constraints are, however, also unlikely to play an important role in signal evolution of Ophrys, because the production of the attractive signal, the cuticular wax compounds, and changes in relative rather than absolute amounts of a bouquet imply relatively low costs (10).
Our study reports on an alternative mechanism of pollinators selecting for signal divergence between model and mimic in a specialized floral mimicry system. Because such mechanisms may be more widespread, our study calls for a re-evaluation of the role of signal similarity between mimics and their model species, by paying particular attention to sensory and behavioral ecology of the operators. Such research will lead to a better understanding of patterns of signal evolution in mimicry, especially in cases where imperfect mimicry is actually adaptive.
Materials and Methods
Sample Collection.
A total of 391 individual virgin C. cunicularius females (N) and Ophrys flowers (n) were collected in early spring in 15 different populations across Western Europe, including two [Monte Gargano (Italy) and Cadillon (France)] where both the female bees and the orchids occurred in sympatry. Some samples of the female bees included in this study were collected during a recent study carried out by Vereecken et al. (13) in Austria (Fussach, N = 33; Vienna, N = 12), France (Ondres-plage, N = 20), Italy (Monte Gargano, N = 16) and Switzerland (Neuhausen, N = 56). The other samples were collected in parallel in the following countries: France (Assérac, N = 16; Cadillon, N = 18, n = 19; La Baule, N = 15; La Turballe, N = 18; Monbazin, n = 25; Montferrier, n = 20; Nérac, n = 16; Pornic, N = 25; Pornichet, N = 11), Italy (Monte Gargano, n = 66), and the United Kingdom (Merthyr Mawr, N = 5). Virgin females are easily detected when a cluster of sexually aroused males forms around them directly after they emerge from the soil. All attractive C. cunicularius females were caught with a hand net, stored individually in chilled plastic cups (Eppendorfs), and instantaneously killed by freezing. Epicuticular waxes of the bees were extracted from the body of these females, by dipping them into 400 μl of hexane (HPLC grade) for 1 min. Floral odor samples of O. exaltata were made by extracting waxes from unpollinated flowers in the same way by using 200 μl of hexane (HPLC grade). All extracts were stored at −20°C. Before GC analyses, 100 ng of n-octadecane was added as an internal standard to all samples.
Chemical Analyses.
All samples were analyzed by GC on an Agilent 6890N gas chromatographer equipped with a HP-5 capillary column (30 m × 0.32 mm × 0.25 μm). One-microliter aliquots of the extracts were injected splitless at 50°C (1 min), followed by a programmed increase of oven temperature to 300°C at a rate of 10°C/min; helium was used as the carrier gas. Compounds were identified by comparison of retention times with authentic standard compounds. Additionally, selected samples were analyzed with a gas chromatographer (HP 5890) with a mass selective detector under identical GC conditions, and MS spectra were compared with those of known reference substances (see ref. 14 for details on the MS analysis). The absolute amounts of all behaviorally active compounds were calculated by the internal standard method as described (14). Relative proportions (%) were calculated by dividing the absolute amounts of individual compounds by the sum of all compounds and multiplying by 100.
Behavioral Experiments.
Bioassays were performed in late March and early April in a natural population at Cadillon (France) where several hundred C. cunicularius males were patrolling for emerging females of a nesting/emergence site. All bioassays were conducted between 10 a.m. and 3 p.m., when C. cunicularius males' patrolling activity was at its peak. In all cases, we performed the bioassays starting from the very early days of the male bees' emergence, when very few females had emerged. We found no evidence for changes in the males' behavioral responses to the odor blends tested during the time course of the experiments (data not shown). Because C. cunicularius males patrol fairly localized regions of their nesting/emergence site (28), test spots were changed after each bioassay to assay the responses of different males to the odor blends. The density of bees on the study site was stable over the days of observations. Behavioral responses of male bees toward dummies (black cylindrical plastic beads, 4 × 5 mm, mounted on an insect pin) scented with odor extracts were taped by using a voice recorder for 3 min and classified in two categories: (i) inspecting flights at close range (<10 cm) without any contact with the odor source, and (ii) contacts, from a short pounce to a copulation attempt with the scented dummy. Odor sources were presented individually for each test, and each scented or control dummy was used only once and placed in a male patrolling area after the solvent had evaporated. Dummies treated with solvent only and placed in a male patrolling area after the solvent had evaporated were used as controls and tested independently for their attractiveness after every fifth test.
Statistic Analyses.
Multivariate analyses of variation in all behaviorally active compounds (relative amounts in %) of C. cunicularius females and O. exaltata flowers were performed by canonical discriminant function (CDF) analysis. Because data distributions did not fit normality and the variances were not homogeneous, we used Mann–Whitney U tests (α = 0.05) to evaluate differences in absolute and relative amounts of key odor compounds between the bees and the orchids and to compare the male bees' responses to odor blends. All statistical tests were performed with SPSS 13.0 software.
Preparation of Manipulated Female Sex Pheromone Blend.
Extracts of local females' sex pheromones used for this experiment contained a mean of 3.728 μg (±228 μg) of behaviorally active compounds, with proportions of the key odor compounds as follows: (Z)-7-C21 = 11.3%, (Z)-7-C23 = 3.3%, and (Z)-7-C25 = 1.5%. We used 100 μl of each sex pheromone extract (containing a mean of 0.932 μg of behaviorally active compounds) to test for the attractiveness of natural female ratios. The manipulated extracts were prepared as follows. The following amounts of the three (Z)-7 alkenes (see ref. 14) were added to 100 μl of female extract: 120.46 ng of (Z)-7-C21, 185.17 ng of (Z)-7-C23, and 255.03 ng of (Z)-7-C25 (560 ng of synthetic compounds were added in total). Thus, the relative amounts of these compounds in the manipulated blends were raised to (Z)-7-C21 = 15.2%, (Z)-7-C23 = 14.5%, (Z)-7-C25 = 18.0%, which corresponds to ratios found in floral odor extracts of the orchid O. exaltata. The corresponding increase in the absolute amounts of behaviorally active compounds in the manipulated solution was controlled by using 1.6 times less volume on the dummies during the bioassays (932 ng + 560 ng = 1,492 ng; 1,492 ng/932 ng = 1.6). These bioassays related to this experiment with C. cunicularius males were performed in Assérac (France) using the same protocol as detailed above.
Supplementary Material
Acknowledgments.
We thank J.-C. de Biseau, J. M. Alexander, S. P. M. Roberts, P. Schlüter, and C. Galley for comments on the manuscript; G. Mahé, S. P. M. Roberts, D. Genoud, R. Milhé, R. Souche, and S. Cozzolino for information on wild bee and orchid populations and help in the field; and two anonymous referees for constructive comments and their interest in our study. Financial support was provided by the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture (N.J.V.) and Swiss National Fund Project 3100-068173 (F.P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800194105/DCSupplemental.
References
- 1.Fisher RA. The Genetical Theory of Natural Selection. Oxford: Clarendon; 1930. [Google Scholar]
- 2.Roy B, Widmer A. Floral mimicry: A fascinating yet poorly understood phenomenon. Trends Plants Sci. 1999;4:325–330. doi: 10.1016/s1360-1385(99)01445-4. [DOI] [PubMed] [Google Scholar]
- 3.Schiestl FP. On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- 4.Kullenberg B. Studies in Ophrys Pollination. Uppsala, Sweden: Almquist & Wiksells Boktryckeri; 1961. [Google Scholar]
- 5.Paulus HF, Gack C. Pollinators as prepollinating isolation factors: Evolution and speciation in Ophrys. Isr J Bot. 1990;39:43–79. [Google Scholar]
- 6.Wickler W. Mimicry in Plants and Animals. London: Weidenfeld & Nicholson; 1968. [Google Scholar]
- 7.Pasteur G. A classification review of mimicry systems. Annu Rev Ecol Syst. 1982;13:169–199. [Google Scholar]
- 8.Correvon H, Pouyanne M. A curious case of mimicry in Ophrys. J Soc Nat Horticult France. 1916;4:29–47. [Google Scholar]
- 9.Linnaeus C. Öland and Gotland Journey. London: Academic; 1745. [Google Scholar]
- 10.Schiestl FP, et al. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. [Google Scholar]
- 11.Stökl J, Twele R, Erdmann DH, Franke W, Ayasse M. Comparison of the flower scent of the sexually deceptive orchid Ophrys iricolor and the female sex pheromone of its pollinator Andrena morio. Chemoecology. 2007 doi: 10.1007/s00049-007-0383-y. [DOI] [Google Scholar]
- 12.Schiestl FP, Ayasse M. Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Syst Evol. 2002;234:111–119. [Google Scholar]
- 13.Vereecken NJ, Mant J, Schiestl FP. Population differentiation in female mating signals and male preferences in a solitary bee. Behav Ecol Sociobiol. 2007;61:811–821. [Google Scholar]
- 14.Mant JG, et al. Cuticular hydrocarbons as sex pheromone of the bee Colletes cunicularius and the key to its mimicry by the sexually deceptive orchid Ophrys exaltata. J Chem Ecol. 2005;31:1765–1787. doi: 10.1007/s10886-005-5926-5. [DOI] [PubMed] [Google Scholar]
- 15.Brower L. Mimicry and the Evolutionary Process. Chicago: Chicago Univ Press; 1988. [Google Scholar]
- 16.Poulin R. Evolutionary Ecology of Parasites. Princeton, NJ: Princeton Univ Press; 2006. [Google Scholar]
- 17.Endler JA, Basolo AL. Sensory ecology, receiver biases, and sexual selection. Trends Ecol Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- 18.Ryan MJ. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998;281:1999–2003. doi: 10.1126/science.281.5385.1999. [DOI] [PubMed] [Google Scholar]
- 19.Lynn SK, Cnaani J, Papaj DR. Peak shift discrimination learning as a mechanism of signal evolution. Evolution. 2005;59:1300–1305. [PubMed] [Google Scholar]
- 20.ten Cate C, Rowe C. Biases in signal evolution: Learning makes a difference. Trends Ecol Evol. 2007;22:380–387. doi: 10.1016/j.tree.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Schiestl FP. Floral evolution and pollinator mate choice in a sexually deceptive orchid. J Evol Biol. 2004;17:67–75. doi: 10.1046/j.1420-9101.2003.00650.x. [DOI] [PubMed] [Google Scholar]
- 22.Mant JG, Peakall R, Schiestl FP. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive genus Ophrys? Evolution. 2005;59:1449–1463. [PubMed] [Google Scholar]
- 23.Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: A spasmodic journey to diversification. Biol J Linn Soc. 2005;84:1–54. [Google Scholar]
- 24.Edmunds M. Do Malaysian Myrmarachne associate with particular species of ant? Biol J Linn Soc. 2006;88:645–653. [Google Scholar]
- 25.Howse PE, Allen JA. Satyric mimicry: The evolution of apparent imperfection. Proc R Soc London Ser B. 1994;257:111–114. [Google Scholar]
- 26.Bain RS, Rashed A, Cowper VJ, Gilbert FS, Sherratt TN. The key mimetic features of hoverflies through avian eyes. Proc R Soc London Ser B. 2007;274:1949–1954. doi: 10.1098/rspb.2007.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holen ØH, Johnstone RA. Context-dependent discrimination and the evolution of mimicry. Am Nat. 2006;167:377–389. doi: 10.1086/499567. [DOI] [PubMed] [Google Scholar]
- 28.Peakall R, Schiestl FP. A mark-recapture study of male Colletes cunicularius: Implications for pollination by sexual deception. Behav Ecol Sociobiol. 2004;56:579–584. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.